Abstract
Exposure to stressful stimuli is known to activate the peripheral sympathetic nervous system and the adrenal gland. In this study, we evaluated the effects of single or repeated bouts of exposure to a readily measurable stressful stimulus (loud noise) on the catecholamine content and ultrastructure of the rat adrenal medulla. In particular, we measured tissue levels of dopamine, noradrenaline, adrenaline and metabolites. In parallel studies, we evaluated the fine ultrastructure of catecholamine cells, including a detailed study of catecholamine granules and a morphometric analysis of adrenaline and noradrenaline medullary cells. Animals were exposed either to a single (6 h) session of loud (100 dBA) noise, or to this noise stimulus repeated every day for 21 consecutive days. There was a marked correlation between biochemical indexes of catecholamine activity and the ultrastructural morphometry of specific catecholamine granules. Exposure to loud noise for 6 h induced a parallel increase in dopamine, noradrenaline, adrenaline and their metabolites, a polarization and an increased numerical density of noradrenaline and adrenaline granules in the cells. After repeated noise exposure, noradrenaline levels were significantly higher than in controls, and adrenaline decreased significantly. In addition, adrenaline cells also exhibited ultrastructural alterations consisting of wide homogeneous cytoplasmic areas and large, pale vesicles.
Keywords: adrenal medulla, adrenaline, catecholamine, noise exposure, noradrenaline, ultrastructural morphometry
Introduction
Stressful stimuli produce increased catecholamine (CA) release in specific brain areas (Borrell et al. 1980) and lead to the activation of the hypothalamic–pituitary–adrenal axis (HPA) and the sympathetic nervous system (Axelrod & Reisine, 1984). As a consequence, most stressful conditions result in a marked release of noradrenaline (NA) and adrenaline (A) into the bloodstream (McCarty, 1985; Kvetnansky et al. 1992). These CA derive both from sympathetic ganglia (NA only) and from the adrenal medulla (both NA and A).
Despite the fact that the early response to a stressful stimulus is quite stereotyped (Kvetnansky et al. 1998), repeated intermittent application of stressful agents might result in heterogeneous and contrasting effects. For instance, repeated exposure to stressful stimuli has been reported to decrease (Basset et al. 1973; Borrell et al. 1980; Murison et al. 1986), to increase (Tache et al. 1976; Baron & Brush, 1979; Majer et al. 1986; Vogel & Jensh, 1988; Soldani et al. 1999) or even not to change (Baron & Brush, 1979; Osborne & Seggie, 1980; Kant et al. 1983, 1985) levels of certain hormones and neurotransmitters. These findings represent either tolerance (i.e. ‘adaptation’) or ‘sensitization’ (i.e. reverse tolerance) to the stimulus.
In order to correlate a given response with a specific stimulus, it is necessary to keep all the experimental variables as constant as possible.
It is critical to apply a stressful stimulus which is highly reproducible.
Furthermore, analysis of the effects induced by the stimulus should include morphofunctional changes of target organs measured ‘in situ’, instead of relying solely on indirect effects (such as CA plasma levels) which do not necessarily depend on the activity of target organs.
To fulfil the first criterion, we selected exposure to loud noise which is a readily measurable and highly reproducible stressful stimulus; its experimental application thus allows comparison of data and reduces experimental bias. Furthermore, exposure to loud noise in humans occurs daily, either during work or accidentally, and is therefore potentially relevant for environmental medicine.
As regards the second criterion, we carried out an ultrastructural and biochemical study of the adrenal medulla in order to measure directly, in situ, the morphofunctional changes induced by the application of loud noise.
The literature dealing with the effects of noise exposure on the HPA and sympathetic nervous system mainly concerns either behavioural or biochemical studies (Beardwood et al. 1975; Kraicer et al. 1977; Borrel et al. 1980; Armario et al. 1984; Alario et al. 1987). A few morphological investigations have been reported but even these never correlate in situ biochemical analysis. For instance, we found that brief exposure to loud noise causes time-dependent ultrastructural changes in the adrenal gland (Pellegrini et al. 1997). Also, in a recent ultrastructural study (Soldani et al. 1999), we compared the sensitivity in various cortical zones of the adrenal gland after repeated noise exposure.
In the present study, we correlated the fine structure of A and NA cells with morphometric analysis of CA granules and tissue levels of dopamine (DA), A, NA and metabolites. The time-course of these morphofunctional changes in the adrenal medulla induced by loud noise offers a new experimental setting to investigate either tolerance or sensitization in response to chronic stressful stimuli.
Materials and methods
Animals
Eighty male Wistar rats weighing 200–250 g were used. Animals were housed in the animal facility, fed ad libitum and kept under closely controlled environmental conditions (12 h light/dark cycle, lights on between 07:00 and 19:00 hours; room temperature 21 °C). Animals were treated in accordance with the Guidelines for Animal Care and Use of the National Institutes of Health. All possible efforts were made to minimize animal suffering and to reduce the number of animals used.
Experimental procedure
Experiments were carried out in late spring/early summer. The sound stimulus was produced by two loudspeakers (15 W), installed at a distance of 40 cm on two opposite sides of the cage and driven by a white-noise generator (0–26 kHz). A precision sound level meter (Quest Electronic 215) was used to adjust the intensity of sound to 100 dBA uniformly in the cage. Rats were randomly assigned to eight groups, each of 10 rats. Two experimental groups (A and B), intended for biochemical assay and transmission electron microscopy, respectively, were exposed to noise for 6 h (brief exposure); groups C and D were exposed 6 h daily for 21 days (repeated exposure), and were killed to permit biochemical assay and transmission electron microscopy, respectively. To avoid the influence of cage stress on the evaluation of the effects due to noise exposure, control rats (groups E; F; G; H) were maintained in the same cage as experimental animals but were moved during times of noise exposure to comparable cages which were protected from noise.
Assay of catecholamines
Immediately after noise, exposed and control animals were killed by decapitation between 08:30 and 09.00 hours. Both adrenal glands from each animal were removed and dissected to isolate the medulla. The specimens were sonicated in 0.6 mL of ice-cold 0.1 mperchloric acid and an aliquot of homogenate (50 µL) was assayed for protein (Lowry et al. 1951). After centrifugation at 8000 g for 10 min, 20 µL of the clear supernatant was injected into an HPLC system to measure levels of DA, NA, A, vanilylmandelic acid (VMA), dihydroxymandelic acid (DOMA), dihydroxyphenylacetic acid (DOPAC), 3,4-dihydroxyphenylethylglycole (DOPEG) and 3-methoxy, 4-hydroxyphenylethylglycole (MOPEG). In order to evaluate the turnover of the neurotransmitters, we calculated the ratio VMA/NA (Turnover Index, TI). Furthermore, to compare the amount of CA release during brief (B) and repeated (prolonged, P) noise exposure in baseline conditions (C), we calculated the ratio VMAB/VMACor VMAP/VMAC(Release Index, RI). These values refer to the levels of extracellular metabolite VMA either in control rats (VMAC), or in animals briefly (VMAB) or chronically (VMAP) exposed to noise. The HPLC system consisted of a reversed-phase column (250 × 4.5 mm, C18, Beckman, Palo Alto, CA, USA) and two electrochemical detectors (analytical cell, ESA, 5011). The potentials of the two electrodes were maintained at +0.35 and −0.35 V as previously described (Fornai et al. 1999a, b). The mobile phase consisted of a citrate-phosphate buffer (0.04 mcitric acid, 0.06 mNa2HPO4·2H2O) containing 0.1 mmEDTA, 0.6 mmsodium 1-heptanesulphonate and 10% methanol. The flow rate was 1 mL min−1.
Transmission electron microscopy
Immediately after noise exposure, animals were anesthetized with an intraperitoneal (i.p.) injection of chloral hydrate (4 mL kg−1) and thoracotomized; subsequently they were fixed by perfusion (2% glutaraldehyde in 0.11 mcacodylate buffer, pH 7.2, 550 mosmol) through the left ventricle. Following the method described by Tomlinson & Coupland (1990), both adrenal glands of each animal were removed from their pericapsular fat and immersed in the same fixative solution. Each gland was cut under a stereomicroscope in order to obtain slices of the medullary part. After 3 h fixation, specimens were post-fixed in buffered 1% osmium tetroxide for 1 h, dehydrated in ethanol and embedded in Epon-araldite. Thin sections were cut with an LKB ultramicrotome, stained with uranyl acetate and lead citrate and examined by use of a Jeol JEM 100 SX transmission electron microscope.
Ultrastructural morphometry
This study aimed to correlate, at the ultrastructural level, the granular content and localization of both A and NA storing cells with medullary levels of CA and metabolites. Cells were classified as A or NA based on the size of the cell and, on the distribution of electron density within the chromaffin granules (Coupland, 1965; Tomlinson et al. 1987; Kobayashi & Coupland, 1993) which results from a differential fixation of A and NA. We selected specific cell types (either NA or A) and then used a constant field of observation measuring 12 µm2. Because the distribution of CA granules is not homogeneous within A/NA cells, we selected a defined cell compartment to perform ultrastructural counts. In accordance with the suggestions made in a previous elegant morphometric study of the adrenal medulla (Tomlinson et al. 1987), we assessed the granules in rectangular areas placed either in the perinuclear position (excluding the Golgi area) or apposed to the plasma membrane. The larger size of A compared with NA cells meant that, when we measured granules in the perinuclear position within A cells, we took micrographs of subcellular fields which were more distant from those located in the perimembrane position compared with NA cells. We chose this procedure because it takes into account both the concentration of CA granules and the polarization of granule density close to the plasma membrane. This was relevant because stimulation of the adrenal medulla was expected to modify intracellular granule trafficking, due to an alteration in granule secretion. Counts were carried out from 100 pictures (magnification ×15 000) taken of both A- and NA-storing cells from each animal belonging to the various groups.
Furthermore, for each cell (A/NA), we measured the amount (expressed as a percentage) of granules situated in the perinuclear position compared with those close to the plasma membrane. Deviations from the percentage mean were expressed as percentage of SEM. We correlated granule position after brief or repeated noise exposure compared with controls. We define the ‘Polarization Index’ (PI) as the ratio between the number of perimembrane and perinuclear granules. In order to measure PI, we selected the same zones, close to the perinuclear position and the plasma membrane. One of the long sides of the rectangles corresponded either to the nuclear membrane (perinuclear areas, PN) or to the plasma membrane (perimembrane areas, PM). For each count the same number of PN and PM areas was considered. Of course, movement of granules from PN to PM areas means a longer distance in A compared with NA cells. However, the promotion of CA release implies comparable granule movement in both cell types because a similar proportion of granule was found in PN and PM zones in basal conditions in A and NA cells (see Results).
Data analysis
For CA assays, a standard curve was prepared using known amounts of DA, and metabolites (Sigma Chemicals Co., St Louis, MO, USA), dissolved in perchloric acid (0.1 m) containing a constant amount (10 pg µL−1) of the internal standard (dihydroxybenzylamine, DBA), as used for the dialysis samples. The standard curve for each compound (NA, A, DA, DOPAC, DOMA, DOPEG, MOPEG, VMA) was calculated using regression analysis of the ratios of the peak areas (compound area/DBA area) for various concentrations of each compound recorded at the reducing electrode. Results from control and noise-exposed rats are expressed as the means ± SEM of values obtained from groups of 10 rats. The effects of each treatment on NA, A, DA and their metabolites (DOPAC, DOMA, DOPEG, MOPEG and VMA) were compared using anovawith Sheffè's post hoc analysis. Data from morphometric analysis were expressed as the mean ± SEM of 100 counts per animal (n= 10). Differences between groups were compared using anovawith Sheffè's post hoc analysis. For each comparison, the null hypothesis (H0) was rejected when P< 0.05.
Results
Effects of noise exposure on tissue catecholamine content
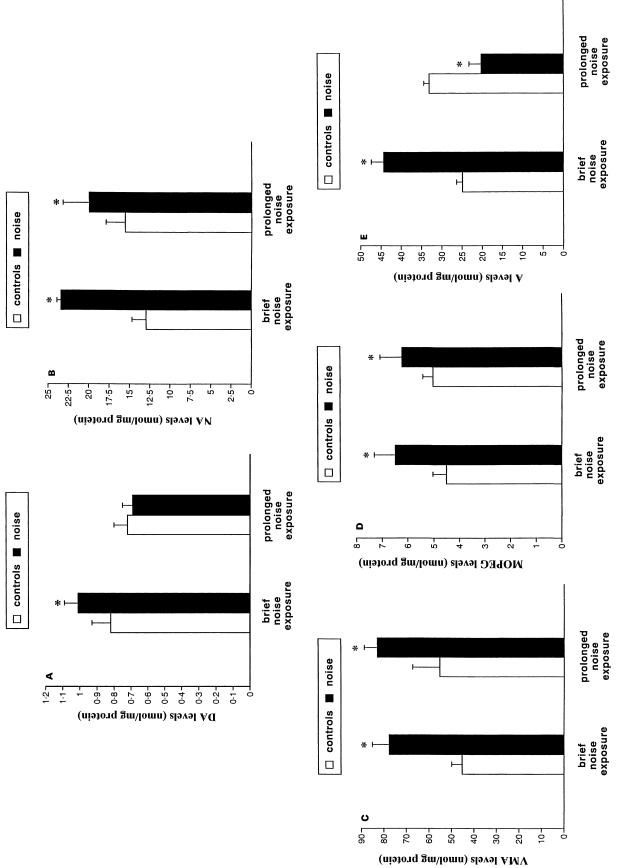
Among the CA metabolites for which we plotted a standard curve, DOMA, DOPAC and DOPEG were always under the detection limit of the method, which required a signal-to-noise ratio of 5 : 1 corresponding to an amount of 1.0 pmol mL−1. Dopamine content in the adrenal medulla was significantly enhanced by brief noise exposure, but not by repeated noise (Figure 1A). In contrast, medullary NA (Figure 1B), VMA (Figure 1C) and MOPEG (Figure 1D) were all increased after both brief and repeated noise stress in comparison with controls. Levels of medullary A were increased after brief noise exposure, but were reduced after repeated noise exposure compared with controls (Figure 1E). During brief noise exposure, we did not find any change in the TI compared with controls, but repeated noise exposure led to a significant increase in the TI (Table 1). This suggests that the increase in CA release observed after brief noise exposure (see Table 1 and Figure 1C) occurs together with a comparable increase in CA synthesis. In contrast, measurement of CA release by the RI showed that CA cells increased their release after both short and repeated noise exposure, although release was higher after brief compared with repeated noise exposure (Table 1).
Fig. 1.
Effects of noise exposure on CA content in the adrenal medulla. (A) Dopamine (DA); (B) noradrenaline (NA); (C) vanilmandelic acid (VMA); (D) methoxyhydroxyphenylethylglycole (MOPEG); (E) adrenaline (A) levels in the adrenal medulla in baseline conditions (□) or following noise exposure (▪). Brief noise exposure consisted of a single session (6 h) of loud (100 dBA) noise, while prolonged noise exposure consisted of consecutive sessions repeated for 21 days. Values are given in nmol/mg of medullary protein. Each group was composed of 10 rats and data represent the mean ± SEM of each group. Comparisons between groups were carried out by using anova with Sheffè's post-hoc analysis. P< 0.05 compared with baseline levels.
Table 1.
Effects of brief and repeated noise exposure on Turnover Index (TI; VMA/NA) and Release Index (RI; VMA stimulated/VMA control)
| Controls | Brief Noise | Control | Repeated Noise | |
|---|---|---|---|---|
| TI | VMAc/NAc | VMAB/NAB | VMAC/NAC | VMAR/NAR |
| 3.49 ± 0.08 | 3.31 ± 0.05 | 3.55 ± 0.05 | 4.15 ± 0.07* | |
| RI | VMAB/NAc | VMAR/NAC | ||
| 1.71 ± 0.05 | 1.50 ± 0.07 |
The ratio between medullary levels of the extracellular metabolite VMA and NA represents an estimate of the functional activity of the gland (Turnover Index, TI). This ratio measures both newly synthesized NA and the amount of CA released. The ratio between medullary levels of the extracellular metabolite VMA in different conditions represents the changes in the functional activity of the gland after brief and prolonged noise exposure compared with baseline conditions (Release Index, RI). This index is related to the amount of NA released. C; B; R refer to the level of the compounds in controls, after brief and prolonged noise exposure, respectively. Values are the ratios between the means of 10 animals per group.
P< 0.05 compared with controls.
Effects of noise exposure on the ultrastructure and ultrastructural morphometry of the adrenal medulla
Both types of chromaffin cells were observed in control rats. In particular, in agreement with classic literature (Coupland, 1965; Tomlinson et al. 1987; Kobayashi & Coupland, 1993), A cells possessed mild electron-dense granules, while NA cells showed granules with a peripheral electron density (not shown).
Similarly, the proportion of A and NA cells was similar to that reported in the literature, with NA cells accounting for roughly 20% of the total population of the adrenal medulla.
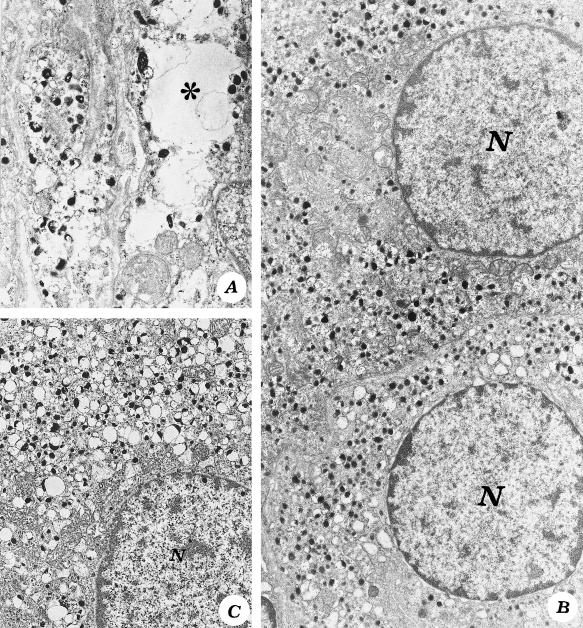
After 6 h of noise exposure, several NA cells exhibited slight alterations consisting of scattered empty cytoplasmic areas (Figure 2A) alternating with areas in which CA granules were densely packed (Figure 2B), while A-storing cells still maintained a normal ultrastructure (not shown). In NA and A cells, brief noise exposure induced a significant increase in the numerical density of NA granules compared with controls (Table 2).
Fig. 2.
Noradrenaline cells after noise exposure. (A) After brief noise exposure, a NA cell shows areas of empty cytoplasm (*) (×12 000), alternating with areas of densely packed granules (B, ×9500). After repeated noise exposure, granules increase homogeneously within the cytoplasm (C, ×10 000). N = nucleus.
Table 2.
Effects of brief and repeated noise exposure on the numerical density and distribution of catecholamine granules in the adrenaline (A) and noradrenaline (NA) cells of the adrenal medulla
| A cells | NA cells | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Brief noise | Control | Repeated noise | Control | Brief noise | Control | Repeated noise | |
| Number CA granules per µm2cytoplasm | 3.1 ± 0.2 | 4.4 ± 0.1* | 3.2 ± 0.1 | 2.2 ± 0.1* | 3.3 ± 0.2 | 4.1 ± 0.1* | 3.4 ± 0.2 | 3.8 ± 0.1* |
| Perinuclear granules (PN%) | 42 ± 3 | 42 ± 3 | 43 ± 3 | 39 ± 4 | 41 ± 4 | 38 ± 2 | 42 ± 4 | 43 ± 4 |
| Perimembrane granules (PM%) | 58 ± 3 | 58 ± 3 | 58 ± 3 | 61 ± 6 | 59 ± 3 | 61 ± 3 | 58 ± 3 | 57 ± 5 |
| Polarization index (PM/PN) | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.6 ± 0.1* | 1.4 ± 0.1 | 1.6 ± 0.1* | 1.4 ± 0.1 | 1.3 ± 0.1 |
Percentage of granules situated in the perinuclear (PN) and perimembrane (PM) position in baseline conditions (controls) or after different amounts (brief or repeated) of noise exposure in A or NA cells of the adrenal medulla. Perinuclear and PM areas were defined as rectangles with the long side opposite either the nuclear or the plasma membrane, respectively (see methods). The polarization Index is an integrated number indicating intracellular granule distribution. It is calculated by the ratio between the amount of CA granules located in the PM position and the amount of granules in the PN position (PI = PM/PN). Even in baseline conditions (controls) there is a physiological polarization of CA granules towards the plasma membrane, which is similar for NA and A cells. However, there is a different response between A and NA cells after noise exposure. While brief noise exposure increases the polarization of NA granules only, prolonged noise exposure results in opposite effects leading to increased polarization of A granules. Values indicating granule number represent the mean ± SEM obtained from 100 counts of 10 animals per group. Values for PM and PN are expressed as the percentage of the mean ± SEM percentage of 10 animals per group. Values for PI represent the ratio between the mean PM and PN ± SEM obtained from 100 counts of 10 animals per group. Comparisons between groups have been carried out using anovawith Sheffe's post hoc analysis.
P< 0.05 compared with controls.
After repeated noise exposure, NA cells were filled with rather uniformly distributed NA granules (Figure 2C), but A-storing cells showed various ultrastructural changes. These consisted of wide homogeneous cytoplasmic areas, in which A granules were absent, alternating with zones in which granules were scattered and pale (Figure 3A). In addition, in A cells of animals exposed to repeated noise, we found large non-homogeneous vesicles (Figure 3B,C).
Fig. 3.
Adrenaline cells after repeated noise exposure. (A) (×13 600) The cytoplasm of an A cell shows scattered granules (arrows) alternated with big vacuoles at low (B, ×7000) and high (C, ×14 500) magnification. Arrowheads indicate the zone between two vacuoles. N = nucleus.
The number of NA granules was still higher than in controls, while A granules decreased significantly (Table 2).
Also, the subcellular granule distribution (PN and PM areas) was influenced by various durations of noise exposure. In controls, there was a different distribution of CA granules between PN and PM zones, which was similar for both A and NA cells. However, after brief noise the percentage of NA granules in the PM position increased significantly, which led to an increase in the PI (Table 2), but the pattern of distribution of A granules did not change, and neither did the PI (Table 2). After repeated noise exposure, polarization of NA granules was no longer evident, but A granules moved significantly towards the plasma membrane, leading to an increased PI (Table 2).
Discussion
After brief and repeated exposure to loud noise, biochemical and ultrastructural data indicate that both NA- and A-storing cells of the adrenal medulla undergo morphofunctional changes which follow a different time course. These changes involve both the functional ultrastructure of A/NA-storing cells, and medullary levels of CA, their precursors and metabolites.
In particular, we found a marked correlation between biochemical measurements and ultrastructural morphometry, showing that biochemical indexes of sustained CA release are associated with polarization of CA granules towards the plasma membrane.
In agreement with classic literature (Coupland, 1965; Tomlinson et al. 1987; Kobayashi & Coupland, 1993), in baseline conditions, A cells represent roughly 80% of the total population of the gland and are much larger (three-fold volume) compared with the NA-storing cells. This corresponds to a higher baseline A content, which is twice that of NA.
As shown in a previous correlative study (Tomlinson et al. 1987), although A cells are more abundant than NA cells (4 : 1) and have a greater volume (3 : 1), the difference between medullary A and NA is smaller than expected: if the CA concentrations were similar in both cell types, one would expect the A content to exceed 12-fold (4 × 3) the medullary NA content. However, the difference is less than 2 : 1. This can be explained by several considerations:
Noradrenaline cells have a higher concentration of granules than A cells; this was shown by calculating the number of granules per µm2, and was also found by Tomlinson et al. (1987), who calculated the number of granules per volume unit.
The concentration of NA molecules in each granule is greater than that of A cells (Winkler & Westhead, 1980; Phillips, 1982; Ciolkowski et al. 1992).
Whereas A represents the final storage product in A-storing cells, NA is the final product in NA-storing cells but is also the precursor of A in A-storing cells. This might lead to an overestimation of NA cells based on the measurement of NA content. However, this is unlikely in that DA (a CA precursor) is present to such a low level to make it unlikely that even NA when acting as an A precursor might significantly contribute to the total NA level assayed in the adrenal medulla.
There are sympathetic nerve endings in the medullary gland that are partly involved in the innervation of smooth muscle fibres of blood vessels (Coupland, 1965; Parker et al. 1993). These nerve terminals might significantly contribute to medullary NA levels.
Despite these morphofunctional differences between A and NA cells, we found a similar polarization of CA granules in basal conditions; the proportion of PM NA granules was similar to that of A granules, and in both cases far in excess of the PN granules, which, again, was similar for both CA granules.
After brief noise exposure, the ultrastructure of NA-storing cells was only slightly modified. In some cytoplasmic areas NA granules were either absent or rarely present; in others, NA granules were densely packed. Our morphometric study, however, revealed a marked polarization of the granules.
Although the total number of NA granules per µm2increased, the proportion of PM NA granules compared with PN NA granules also increased compared with controls, leading to a high PI. These ultrastructural changes were associated with a significant increase in NA levels after 6 h of noise exposure. However, this increase in NA was associated with an increase in the metabolic precursor DA, as well as in the extracellular metabolite VMA. This suggests that brief noise stimulation leads to an increase in NA synthesis and NA storage, as well as NA release. In particular, the ratio of VMA levels in stimulated rats vs. VMA levels in basal conditions increased to an extent comparable to the PI calculated by ultrastructural morphometry. This suggests that the increase in functional activity in the adrenal medulla, measured as CA release, correlates with specific ultrastructural indexes which can be measured.
The increase in PI shows an ultrastructural scenario in which CA granules are more densely packed beneath the plasma membrane. This cellular response could prepare a number of CA granules to be readily released by moving granules toward the membrane. However, we should take into consideration the difficulty in relating the count of granules to their catecholamine content and the fact that newly formed, immature granules might possess different ultrastructural features and diverse release mechanisms, leading to potential discrepancy in the morphofunctional correlation.
The ultrastructure of A cells was not modified after brief noise exposure; similarly, we did not measure any change in the PI. The increase in A levels in the medulla was associated with a homogeneous increase in the numerical density of A granules in the various subcellular compartments. This suggests a different regulation of membrane trafficking/granule distribution between A and NA cells after brief noise stimulation, despite a similar increase in CA levels.
After repeated, intermittent noise exposure, the ultrastructure of NA cells did not show any change, and morphometric analysis did not reveal changes in the proportion of PM and PN NA granules (PI similar to controls). Nonetheless, the numerical density of NA granules and NA concentration both remained significantly higher than in controls, as occurred after brief noise exposure. However, DA levels were similar to controls despite an increased amount of the extracellular metabolite VMA.
These findings suggest a new equilibrium in the metabolic pathway for CA synthesis. This contrasts with the increased DA levels after brief noise exposure. Because DA is the immediate precursor of NA, an increase in DA levels suggests an unbalanced increase in the activity of the enzymatic pathway, in which enzymes located upstream of DA (DOPA-decarboxylase, and tyrosine-hydroxylase, TH) increase their activity more than downstream enzymes: DA-β-hydroxylase (which transforms DA into NA) or phenylethanolamine-N-methyltransferase (PNMT), which converts NA into A. This is consistent with the finding that TH represents the slow, rate-limiting step in CA biosynthesis (Kopin, 1985). This is also in line with data showing a differential modulation of CA biosynthetic enzymes concomitant with an increase in TH occurring immediately after and up to 6 h following stress exposure, which was not accompanied by a sudden increase in PNMT. In contrast, prolonging the time of stress exposure resulted in an increased activity of both enzymes (Bhatnagar et al. 1995).
After repeated noise exposure, A cells underwent marked subcellular modifications consisting of the appearance of giant pale vesicles, suggesting a potential dysfunction of this component of the gland. Morphometric analysis indicated a significant decrease in granule density compared with baseline, which was related to depletion of medullary A levels compared with controls. By contrast, there was an increase in the PI which could represent the need to sustain an adequate A release despite the loss of A levels.
In short, biochemistry and ultrastructural morphometry both suggest a different trafficking and distribution of NA and A granules within their cells after brief and repeated noise exposure.
In terms of the significance of the present data to the integrative aspect of physiological responses to stress, we hypothesize a differential response in the two components of the gland to repeated noise stimulation. NA cells show sustained hyperactivity with an onset of tolerance following repetitive stimuli. In contrast, A cells apparently become habituated to the stress, with reduced levels of A. However, it remains unclear whether the morphofunctional changes observed in A cells after repeated noise exposure represent tolerance following repeated stimulation or, because the ultrastructural appearance of the secretory apparatus appears disfunctional, whether they represent early stages of endocrine degeneration. In line with this it would be interesting to evaluate A plasma levels.
Acknowledgments
This work was supported by a MURST (Ministero della Università e della Ricerca Scientifica e Tecnologica) grant.
References
- Alario P, Gamallo A, Beato MJ, Trancho G. Body weight gain, food intake and adrenal development in chronic noise stressed rats. Physiol. Behav. 1987;40:29–30. doi: 10.1016/0031-9384(87)90181-8. [DOI] [PubMed] [Google Scholar]
- Armario A, Castellanos JM, Balash J. Adaptation of anterior pituitary hormones to chronic noise stress in rats. Behav. Neural. Biol. 1984;41:71–76. doi: 10.1016/s0163-1047(84)90745-3. [DOI] [PubMed] [Google Scholar]
- Axelrod J, Reisine TD. Stress hormones: their interaction and regulation. Science. 1984;224:452–459. doi: 10.1126/science.6143403. [DOI] [PubMed] [Google Scholar]
- Baron S, Brush FR. Effects of acute and chronic restraint and estrus cycle on pituitary-adrenocortical function in the rat. Hormones Behav. 1979;12:218–224. doi: 10.1016/0018-506x(79)90004-7. [DOI] [PubMed] [Google Scholar]
- Basset JR, Cairncross LD, King MG. Parameters of novelty, shock predictability and response contingenity in corticosterone release in the rat. Physiol. Behav. 1973;37:559–561. doi: 10.1016/0031-9384(73)90060-7. [DOI] [PubMed] [Google Scholar]
- Beardwood CJ, Mundell CA, Utian WH. Gonadotropin excretion in response to audiostimulation of human subjects. Am. J. Obstetr. Gynaecol. 1975;121:682–687. doi: 10.1016/0002-9378(75)90473-1. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Mitchell JB, Betito K, Boksa P, Meaney P. Effects of chronic intermittent cold stress on pituitary adrenocortical and sympathetic adrenomedullary functioning. Physiol. Behav. 1995;4:633–639. doi: 10.1016/0031-9384(94)00161-8. [DOI] [PubMed] [Google Scholar]
- Borrell J, Torrellas A, Guaza C, Borrell S. Sound stimulation and its effects on the pituitary-adrenocortical function and brain catecholamines in rats. Neuroendocrinology. 1980;31:53–59. doi: 10.1159/000123050. [DOI] [PubMed] [Google Scholar]
- Ciolkowski EL, Cooper BR, Jankowski JA, Jorgensen JW, Wightman RM. Direct observation of epinephrine and norepinephrine cosecretion from individual adrenal medullary chromaffin cells. J. Am. Chem. Soc. 1992;114:2815–2821. [Google Scholar]
- Coupland RE. Electron microscopic observations on the structure of the rat adrenal medulla. II. Normal innervation. J. Anat. 1965;99:255–272. [PMC free article] [PubMed] [Google Scholar]
- Fornai F, Chen K, Giorgi FS, Gesi M, Alessandrì MG, Shih JC. Striatal dopamine metabolism in monoamine oxidase B-deficient mice: a brain dialysis study. J. Neurochem. 1999a;73:2434–2440. doi: 10.1046/j.1471-4159.1999.0732434.x. [DOI] [PubMed] [Google Scholar]
- Fornai F, Saviozzi M, Piaggi S, Gesi M, Corsini GU, Malvaldi G, et al. Localization of a glutathione-dependent dehydroascorbate reductase within the central nervous system of the rat. Neuroscience. 1999b;94:937–948. doi: 10.1016/s0306-4522(99)00349-8. [DOI] [PubMed] [Google Scholar]
- Kant GJ, Bunnel BN, Mougey EH, Pennington LL, Meyrhoff JL. Effect of repeated stress on pituitary cyclic AMP and plasma prolactin, corticosterone and growth hormone in male rats. Pharmacol. Biochem. Behav. 1983;18:967–971. doi: 10.1016/s0091-3057(83)80022-7. [DOI] [PubMed] [Google Scholar]
- Kant GJ, Eggleston T, Landmann-Roberts L, Kenion CC, Driver GC, Meyrhoff JL. Habituation to repeated stress is stressor specific. Pharmacol. Biochem. Behav. 1985;22:631–634. doi: 10.1016/0091-3057(85)90286-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Coupland RE. Morphological aspects of chromaffin tissue: the differential fixation of adrenaline and noradrenaline. J. Anat. 1993;183:223–235. [PMC free article] [PubMed] [Google Scholar]
- Kopin IJ. Catecholamine metabolism: basic aspects and clinical significance. Pharmacol. Rev. 1985;37:333–364. [PubMed] [Google Scholar]
- Kraicer GJ, Berand G, Lywood DW. Pars intermedia ACTH and MSH contents: effects of adrenalectomy, gonadectomy and a neurotropic (noise) stress. Neuroendocrinology. 1977;23:352–367. doi: 10.1159/000122684. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Golstein DS, Weise VK, Holmes C, Szemerdi K, Bagdy G, et al. Effects of handling or immobilization on plasma levels of 3,4-dihydroxyphenylalanine, catecholamines, and metabolites in rats. J. Neurochem. 1992;58:2296–2302. doi: 10.1111/j.1471-4159.1992.tb10977.x. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Pacak K, Sabban EL, Kopin IJ, Golstein DS. Stressor specificity of peripheral catecholaminergic activation. Adv. Pharmacol. 1998;42:556–560. doi: 10.1016/s1054-3589(08)60811-x. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;19:265–275. [PubMed] [Google Scholar]
- Majer SF, Ryan SM, Barksdale CM, Kalin NH. Stressor controllability and pituitary-adrenal system. Behav. Neurosci. 1986;100:669–674. doi: 10.1037//0735-7044.100.5.669. [DOI] [PubMed] [Google Scholar]
- McCarty R. Sympathetic-adrenal medullary and cardiovascular responses to acute cold stress in adult and aged rats. J. Autonomic Nervous System. 1985;12:15–22. doi: 10.1016/0165-1838(85)90037-2. [DOI] [PubMed] [Google Scholar]
- Murison R, Overmier JB, Skoglund EJ. Serial stressor: prior exposure to a stressor modulates its later effectiveness on gastric ulceration and corticosterone release. Behav. Neural. Biol. 1986;45:185–195. doi: 10.1016/s0163-1047(86)90761-2. [DOI] [PubMed] [Google Scholar]
- Osborne B, Seggie J. Behavioural, corticosterone and prolactin responses to novel environments in rats with fornix transections. J. Comparative Physiol. Psychol. 1980;94:536–546. doi: 10.1037/h0077688. [DOI] [PubMed] [Google Scholar]
- Parker TL, Kesse WK, Mohamed AA, Afework M. The innervation of the mammalian adrenal gland. J. Anat. 1993;183:265–276. [PMC free article] [PubMed] [Google Scholar]
- Pellegrini A, Soldani P, Gesi M, Lenzi P, Natale G, Paparelli A. Effect of varying noise stress duration on rat adrenal gland: an ultrastructural study. Tissue Cell. 1997;29:597–602. doi: 10.1016/s0040-8166(97)80060-2. [DOI] [PubMed] [Google Scholar]
- Phillips JH. Dynamic aspect of chromaffin granule structure. Neuroscience. 1982;7:1595–1609. doi: 10.1016/0306-4522(82)90017-3. [DOI] [PubMed] [Google Scholar]
- Soldani P, Gesi M, Lenzi P, Natale G, Fornai F, Pellegrini A, et al. Long-term exposure to noise modifies rat adrenal cortex ultrastructure and corticosterone plasma levels. J. Submicroscopic Cytol. Pathol. 1999;31:441–448. [PubMed] [Google Scholar]
- Tache Y, Du Ruisseau P, Tache J, Seyle H, Collu R. Shift in adenohypophyseal activity during chronic intermittent immobilization of rats. Neuroendocrinology. 1976;22:325–336. doi: 10.1159/000122641. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Coupland RE. The innervation of the adrenal gland. IV. Innervation of the rat adrenal medulla from birth to old age. A descriptive and quantitative morphometric and biochemical study of the innervation of chromaffin cells and adrenal medullary neurons in Wistar rats. J. Anat. 1990;169:209–236. [PMC free article] [PubMed] [Google Scholar]
- Tomlinson A, Durbin J, Coupland RE. A quantitative analysis of rat adrenal chromaffin tissue: morphometric analysis at tissue and cellular level correlated with catecholamine content. Neuroscience. 1987;20:895–904. doi: 10.1016/0306-4522(87)90250-8. [DOI] [PubMed] [Google Scholar]
- Vogel WH, Jensh R. Chronic stress and plasma catecholamine and corticosterone levels in male rats. Neurosci. Lett. 1988;87:183–188. doi: 10.1016/0304-3940(88)90167-x. [DOI] [PubMed] [Google Scholar]
- Winkler H, Westhead E. The molecular organization of the adrenal chromaffin granules. Neuroscience. 1980;5:1803–1823. doi: 10.1016/0306-4522(80)90031-7. [DOI] [PubMed] [Google Scholar]