Abstract
Folds of dura mater, the falx cerebri and tentorium cerebelli, traverse the vertebrate endocranial cavity and compartmentalize the brain. Previous studies suggest that the tentorial fold has adopted an increasingly important role in supporting the increased load of the cerebrum during human evolution, brought about by encephalization and an adaptation to bipedal posture. Ontogenetic studies of the fetal tentorium suggest that its midline profile rotates inferoposteriorly towards the foramen magnum in response to disproportionate growth of the cerebrum. This study tests the hypothesis that differential growth of the cerebral and cerebellar components of the brain underlies the inferoposterior rotation of the tentorium cerebelli during human fetal development. Brain volumes and tentorial angles were taken from high-resolution magnetic resonance images of 46 human fetuses ranging from 10 to 29 gestational weeks. Apart from the expected increases of both supratentorial and infratentorial brain volumes with age, the results confirm previous studies showing a significant relative enlargement of the supratentorial volume. Correlated with this enlargement was a rotation of the midline section of the tentorium towards the posterior cranial base. These findings support the concept that increases of supratentorial volume relative to infratentorial volume affect an inferoposterior rotation of the human fetal tentorium cerebelli. These results are discussed in the context of the role played by the tentorium cerebelli during human evolution and underline implications for phylogenetic and ontogenetic models of encephalization.
Keywords: dura folds, encephalization, evolution, fetal
Introduction
The dural folds of meningeal dura mater traverse the endocranial cavity and structurally support, as well as compartmentalize, the brain by bridging different elements of the skull (Moss et al. 1956; Moss, 1958, 1963; Klintworth, 1967, 1968; Bull, 1969; Friede, 1981). As such, differential expansion amongst the brain compartments during development and evolution, and the consequent effect of this on dural fold morphology, are integral to our understanding of the mechanical influences of brain growth on the skull. In this paper, the relationship between the tentorium cerebelli and the human fetal brain is investigated. This framework is subsequently used to explore the wider implications for studies of encephalization (brain growth).
The tentorium cerebelli is of particular interest with regard to enlargement of infratentorial and supratentorial parts of the brain, approximating the cerebellum plus brainstem and cerebrum, respectively. This dural fold varies considerably amongst mammals, typically consisting of two bilateral meningeal sheets separated by the brain in small mammals and a single fused sheet above the cerebellum in larger mammals (Klintworth, 1968). Amongst adult carnivores and cetaceans, the tentorium is also often partially or completely ossified, resembling bone formation in the endosteal dura mater (Klintworth, 1968; Nojima, 1988). The modern human tentorium is fused along the midline, has the largest surface area in relation to body size amongst mammals and occasionally exhibits modest degrees of mineralization, though not explicitly ossification (Bull, 1969; Saldino & Chiro, 1974; Lang, 1991). The large surface area, which covers the contents of the posterior cranial fossa, is anchored to the cranial base and is continuous with the falx cerebri superiorly and falx cerebelli inferiorly. This configuration furnishes infratentorial components of the human brain with some degree of mechanical independence from supratentorial parts, by partially dissipating the incident load of the cerebrum directly to the cranial base via the tentorium and to other parts of the skull via the falx cerebri (Bull, 1969). The importance of this weight-bearing role played by the human tentorium is widely recognized in the clinical literature (e.g. Mayer, 1920; Osborn, 1977; Ropper, 1986, 1989; Inao et al. 1993; Fisher, 1995; Wijdicks & Miller, 1997).
Previous studies suggest that the hominin tentorium adopted an increasingly important role in supporting the phylogenetic increase of supratentorial brain load resulting from cerebral growth (Klintworth, 1967; Bull, 1969). Disproportionate increases of the supratentorial brain, in particular the cerebrum, compared with the infratentorial brain, have been noted across extant primate species (e.g. Stephan et al. 1981; Semendeferi et al. 1997; Rilling & Insel, 1998; Semendeferi & Damasio, 2000). These interspecific findings demonstrate that differential growth of the cerebrum is greatest amongst the hominoids, and point to a general evolutionary trend of increasing supratentorial load. With specific regard to human evolution, Bull (1969) suggests that the overall load incident on the tentorium was further increased with additional expansion of the cerebrum, especially the occipital lobes, and the adoption of obligatory bipedalism, thereby shifting more of the gravitational load of the cerebrum (i.e. weight) directly onto the surface of the tentorium. Indeed, the modern human skull demonstrates bony morphologies at the attachments of the dural folds to the skull, for example at the petrous crests, which are augmented in comparison with those of other primates and are consistent with an increased loading regime on the tentorium cerebelli (Moss, 1958; Aiello & Dean, 1990).
Not surprisingly, the tentorium does not remain entirely unperturbed by the forces incident upon it. The midline ridge of the tentorium is more inferoposteriorly orientated towards the foramen magnum in modern humans than it is amongst non-human primates and mammals in general (Huxley, 1863; Klintworth, 1968; Bull, 1969). With this in mind, Bull (1969) hypothesized that while the human tentorium partly dissipated the overall load brought about by phylogenetic differential growth of the cerebrum and an adaptation to bipedalism, it also responded to this load and pivoted inferoposteriorly about its anterior attachments towards the foramen magnum. This, in turn, would have altered the nature of load distribution across the skull, giving feedback into the interplay between load dissipation, tentorial displacement and load distribution. This hypothesis and its potential implications for the study of skull architecture in association with brain growth remains to be fully explored.
Questions such as the above have traditionally been tested via interspecific analyses and with material from the fossil record. However, there are good reasons for advocating a prenatal ontogenetic framework for evaluating the relationship between differential encephalization and tentorial orientation. Firstly, because phylogeny is grounded on the accumulative variations of successive ontogenies (Garstang, 1922; De Beer, 1958) a given mechanistic explanation for evolutionary change can be equally well tested during ontogeny as it is with phylogenetic proxies such as extant interspecific samples (see also Gould, 1977). Secondly, increases of brain size during prenatal ontogeny are considerable, giving clear a priori statements as to related changes of morphology that are decisive in testing the hypothesis. Thirdly, the compounding of postural with brain size influences on tentorial orientation is limited to a greater extent during intra-uterine gestation than it is, for example, during postnatal development or with interspecific samples. Thus, fetal studies are particularly suited to testing the differential encephalization part of the hypothesis. Indeed, findings from prenatal human studies already indicate that the midline position of the tentorium rotates inferoposteriorly towards the foramen magnum during development (Hochstetter, 1939; Keith, 1948). Furthermore, Moss et al. (1956) suggest that this process of rotation is associated with differential growth of the fetal brain. Recent studies demonstrate differential growth of the fetal supratentorial brain (e.g. Koop et al. 1986; Guihard-Costa & Larroche, 1990; Jeffery, 1999; Jeffery & Spoor, in press), but the relationship with tentorial orientation remains to be evaluated in a statistically meaningful way. The present study aims to test the hypothesis that prenatal differential enlargement of the supratentorial brain, compared with the infratentorial brain, drives tentorial rotation. This predicts a significant negative correlation of the angle between the midline tentorium and posterior cranial base with increases of supratentorial volume relative to infratentorial volume. It is worth outlining possible alternatives with which to consider this statement in context. These include that the tentorium rotates posteriorly independently of differential brain growth due to, as yet, undefined determinants or that differential encephalization is accommodated elsewhere in the skull and there is no rotation, with the fetal configuration retained into adulthood.
Subjects and methods
Sample
Fifty formalin-fixed human fetuses from the D. V. Davies museum collection housed at the Department of Anatomy and Developmental Biology, University College London, and collections at the Department of Anatomy, Queen Mary and Westfield College, London, were imaged with high-resolution magnetic resonance imaging (hrMRI; see Jeffery & Spoor, in press). The sample consists mostly of spontaneous and therapeutic abortuses accumulated by the late Professor D. V. Davies, formerly of the Department of Anatomy, St Thomas's Medical School, London. Specimens were screened for external malformations prior to scanning and images were subsequently screened for indications of internal abnormalities. Four individuals were excluded from the study because of internal anomalies seen in the hrMR images, giving a final sample size of n= 46 (see Table 1). Exclusions were for indications of neurological abnormalities, specifically one case of cerebral hypertrophy (011A), and bony irregularities such as warping of the basioccipital (UMSCL-222), deformation of the frontal bone (UMSCL-299) and orbital malformation (UMSCL-96). In order to provide a pragmatic chronological order to the sample, estimated gestational ages (EGA) were computed to the nearest tenth of a week from measures of biparietal diameter (BPD) using the regression equation reported by Chitty et al. (1994) for mean exocranial ultrasound measurements. This approach preserves the distinction between individuals obtained from measures of BPD while simultaneously providing a more readily interpreted axis, i.e. that of ontogenetic time. However, it is important to note that age variables computed in this way are subject to several caveats and are thus used with considerable caution. Measures for computing age other than BPD (see, for example, Sherwood et al. 2000) were impractical because of previous invasive works on the material. Specimens ranged from about 10 to about 29 weeks gestation. The sample distribution with regard to BPD is neither significantly skewed nor kurtotic (skew = −0.08, ns; kurtic = −0.25, ns), indicating that the sample is normally distributed.
Table 1.
Sample: head size, computed age and imaging parameters
| Code | Biparietal diameter (mm) | Estimated age (weeks) | Field of view (mm) | Slice thickness (mm) |
|---|---|---|---|---|
| UMSCL-163 | 15.0 | 10.1 | 40 | 0.31 |
| UMSCL-39 | 15.0 | 10.1 | 31 | 0.24 |
| QMWF49 | 19.0 | 11.5 | 40 | 0.31 |
| UMSCL-61 | 21.0 | 12.1 | 40 | 0.31 |
| QMWF7 | 24.0 | 13.1 | 40 | 0.31 |
| UMSCL-299* | 24.0 | 13.2 | 40 | 0.31 |
| QMWF34 | 25.5 | 13.5 | 40 | 0.31 |
| UMSCL-164 | 27.0 | 14.0 | 50 | 0.39 |
| UMSCL-96* | 29.0 | 14.1 | 50 | 0.39 |
| 011A* | 32.0 | 15.4 | 50 | 0.39 |
| UMSCL-165 | 32.0 | 15.4 | 64 | 0.50 |
| UMSCL-94 | 33.0 | 15.7 | 50 | 0.39 |
| Z101 | 35.0 | 16.3 | 64 | 0.50 |
| UMSCL-63 | 37.0 | 16.8 | 64 | 0.50 |
| UMSCL-92 | 37.0 | 16.8 | 64 | 0.50 |
| UMSCL-216 | 38.5 | 17.2 | 64 | 0.50 |
| QMW35 | 40.0 | 17.6 | 64 | 0.50 |
| QMW28 | 40.0 | 17.6 | 80 | 0.63 |
| UMSCL-223 | 40.0 | 17.6 | 64 | 0.50 |
| UMSCL-86 | 42.0 | 18.2 | 64 | 0.50 |
| UMSCL-91 | 42.0 | 18.2 | 64 | 0.50 |
| UMSCL-287 | 42.0 | 18.2 | 80 | 0.63 |
| Z102 | 43.0 | 18.4 | 64 | 0.50 |
| UMSCL-220 | 43.0 | 18.4 | 80 | 0.63 |
| UMSCL-222* | 44.0 | 18.7 | 64 | 0.50 |
| UMSCL-221 | 44.0 | 18.7 | 80 | 0.64 |
| UMSCL-210 | 45.0 | 19.0 | 80 | 0.63 |
| ZN1 | 45.0 | 19.0 | 80 | 0.63 |
| UMSCL-166 | 46.0 | 19.2 | 80 | 0.63 |
| UMSCL-215 | 46.0 | 19.2 | 80 | 0.63 |
| UMSCL-212 | 47.0 | 19.5 | 80 | 0.63 |
| UMSCL-207 | 48.0 | 19.8 | 80 | 0.63 |
| UMSCL-218 | 49.0 | 20.1 | 80 | 0.63 |
| UMSCL-214 | 49.0 | 20.1 | 80 | 0.63 |
| UMSCL-211 | 49.0 | 20.1 | 80 | 0.63 |
| UMSCL-297 | 50.0 | 20.3 | 80 | 0.63 |
| UMSCL-209 | 52.0 | 20.9 | 80 | 0.63 |
| UMSCL-292 | 52.0 | 20.9 | 80 | 0.63 |
| UMSCL-286 | 54.0 | 21.5 | 80 | 0.63 |
| UMSCL-281 | 57.0 | 22.3 | 80 | 0.63 |
| UMSCL-301 | 57.5 | 22.5 | 80 | 0.63 |
| UMSCL-284 | 60.5 | 23.4 | 80 | 0.63 |
| UMSCL-242 | 62.0 | 23.8 | 102.4 | 0.80 |
| UMSCL-248 | 64.0 | 24.5 | 85 | 0.66 |
| UMSCL-232 | 64.0 | 24.5 | 89.6 | 0.70 |
| UMSCL-229 | 66.0 | 25.1 | 102.4 | 0.80 |
| UMSCL-238 | 67.0 | 25.5 | 102.4 | 0.80 |
| UMSCL-275 | 69.5 | 26.3 | 102.4 | 0.80 |
| UMSCL-240 | 70.0 | 26.5 | 102.4 | 0.80 |
| UMSCL-303 | 77.0 | 29.2 | 115.2 | 0.90 |
Exclusions from study (see main body of text). Specimens with codes starting with UMSCL are from University College London; codes starting with Z, QMW or 01 refer to specimens from Queen Mary and Westfield College, London. All specimens were scanned with a 256 × 256-pixel matrix and square fields of view.
Angular measures
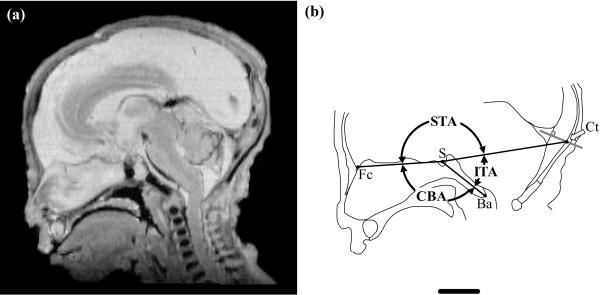
Tentorial orientations were computed with landmark co-ordinates taken from midline images (Figure 1a). The infratentorial angle (ITA) was measured from basion (Ba), sella (S) to the calvarial attachment of the tentorium cerebelli (Ct). These landmarks were used to allow for comparisons with studies on dry skulls using the internal occipital protuberance as an equivalent to Ct (see Huxley, 1863). In the fetus this protuberance is inconsistently discerned. Thus Ct was defined here as the midline point on the endocranial surface of the calvaria where it is intersected by a line that is (1) projected from a point equidistant along the posterior margin of the confluence of sinuses and (2) perpendicular to the posterior margin of the confluence of sinuses (Figure 1b).
Fig. 1.
Midline (a) hrMR image of a human fetal head and (b) schematic illustration of the measurements taken. Landmarks shown in (b) are foramen caecum, Fc; sella, S; basion, Ba; and calvarial attachment of tentorium, Ct. Angular measurements shown are supratentorial angle (STA), infratentorial angle (ITA) and cranial base angle (CBA). Also shown is a line (light grey) projected from the midpoint along, and perpendicular to, the posterior midline surface of the confluence of sinuses. The intersection of this line with the endocranial surface of the calvaria defines the position of Ct. Scale bar = 10 mm.
A previous investigation of the sample reveals that the cranial base angle (CBA), measured between foramen caecum (Fc), S and Ba, increases with fetal age (Jeffery & Spoor, in press). Superimposition of this basicranial retroflexion on ITA from the shared chord Ba-S was offset by calculating an additional angle, the supratentorial angle (STA), as the number of degrees remaining after subtracting ITA and measures of CBA (see Figure 1b and Table 2). All angular measures were recorded to the nearest degree.
Table 2.
Landmarks, angles and volume measurements
| Measure | Abbreviation | Definition |
|---|---|---|
| Landmarks | ||
| Basion | Ba | The midline point on the anterior margin of the foramen magnum |
| Calvarial attachment of tentorium | Ct | The midline point on the endocranial surface of the calvaria where it is intersected by a line projected perpendicular to and equidistant along the posterior surface of the confluence of sinuses |
| Foramen caecum | Fc | The midline point marking the pit between the fetal crista galli and the endocranial wall of the frontal bone |
| Sella | S | The centre of the sella turcica in the midline |
| Angles | ||
| Cranial base angle | CBA | Angle between the anterior cranial base (Fc-S) and posterior cranial base (S-Ba) |
| Infratentorial angle | ITA | Angle between the posterior cranial base (S-Ba) and the base plane of the tentorium (S-Ct) |
| Supratentorial angle | STA | Angle between the anterior cranial base (Fc-S) and base plane of the tentorium (S-Ct) |
| Volumes | ||
| Index of differential encephalization | IDE | Sum of voxels in the endocranial cavity below the level of the tentorium cerebelli (infratentorial) divided by the sum of voxels above the tentorium (supratentorial) |
Volume measures
To consider relative brain proportions and their interactions with dural fold architecture, whole endocranial and infratentorial volumes were measured. Volumes were measured by outlining the endocranial region of interest in sagittal images. The sum of pixel areas from relevant slices was subsequently multiplied by slice thickness to give volumes. Infratentorial volumes were measured by outlining the endocranial area below the tentorium cerebelli while supratentorial volumes were computed by subtracting infratentorial volume from whole endocranial volume. All absolute volume measurements were recorded to the nearest 10 mm3. An index of differential encephalization (IDE) was computed to three decimal places with infratentorial volume as the numerator and supratentorial volume as the denominator (see Jeffery & Spoor, in press). This number of decimal places was used to conserve information provided in the absolute volumes measured. Landmarks and measures are summarized in Table 2.
Statistical analysis
Simple bivariate relationships were evaluated with Spearman rank correlation coefficients (rrank) and Model II reduced major axis (RMA) regressions. Trends that are more complex were evaluated with second-order polynomial curve fittings. t-tests were used to test the statistical significance of rank correlation coefficients and RMA regressions. In all significance tests, a level of P< 0.05 was used to reject null hypotheses.
Results
Reliability of the study was evaluated with a one-way analysis of variance (anova) of repeated measurements taken five times over five consecutive days for four randomly selected individuals (Table 3). These results showed that errors incurred among repeated measurements are negligible in comparison with the biological variation between individuals. In other words, the null hypothesis that values for repeated measures from one individual fetus are the same was accepted (P< 0.01). The angular measurements and the relative brain proportions of the fetal sample at regular gestational intervals are given in Table 4.
Table 3.
anovaresults for five sets of repeated measures from four randomly selected individuals
| Measure | MS within groups groups | MS between groups | F | P |
|---|---|---|---|---|
| Cranial base angle | 1.85 | 49.20 | 26.58 | < 0.001 |
| Infratentorial angle | 1.56 | 43.24 | 27.69 | < 0.001 |
| Supratentorial angle | 0.67 | 4.11 | 6.10 | < 0.01 |
| Supratentorial volume ×10−3 | 0.48 | 2669.38 | 5571.89 | < 0.001 |
| Infratentorial volume ×10−3 | 0.06 | 30.15 | 524.50 | < 0.001 |
MS within groups, variation between repeated measures; MS between groups, variation between individuals; F, critical variance ratio; P, probability significance that repeated measures are different.
Table 4.
Means and standard deviations of examined variables at regular intervals of estimated age
| Age (weeks) | n | ITA (°) | SD | STA (°) | SD | CBA (°) | SD | IDE | SD |
|---|---|---|---|---|---|---|---|---|---|
| 10.1–12 | 3 | 89.7 | 9.1 | 137.3 | 10.0 | 133.0 | 4.4 | 0.2267 | 0.0461 |
| 12.1–14 | 4 | 79.5 | 13.8 | 148.8 | 12.5 | 131.8 | 2.8 | 0.1758 | 0.0393 |
| 14.1–16 | 2 | 63.0 | 5.7 | 161.0 | 4.2 | 136.0 | 1.4 | 0.1515 | 0.0431 |
| 16.1–18 | 7 | 54.6 | 3.7 | 167.9 | 4.9 | 137.6 | 3.5 | 0.1176 | 0.0285 |
| 18.1–20 | 12 | 55.5 | 3.4 | 170.3 | 6.8 | 134.2 | 4.5 | 0.0954 | 0.0152 |
| 20.1–22 | 7 | 53.6 | 5.7 | 170.7 | 5.8 | 135.7 | 3.2 | 0.0929 | 0.0102 |
| 22.1–24 | 4 | 51.8 | 2.2 | 171.3 | 4.1 | 137.0 | 3.6 | 0.0943 | 0.0117 |
| 24.1–26 | 4 | 49.8 | 3.5 | 169.3 | 1.5 | 141.0 | 4.2 | 0.0815 | 0.0166 |
| 26.1–29.2 | 3 | 48.3 | 2.3 | 170.7 | 2.5 | 141.0 | 1.0 | 0.0787 | 0.0025 |
Temporal trends of variables were evaluated with plots against estimated gestational age. Plots in Figure 2 demonstrate both linear and curvilinear trajectories in relation to age. These were evaluated with RMA and polynomial regressions accordingly. Cranial base angle increases linearly by some 9°, whilst IDE decreases in a curvilinear fashion by some 70%. Figure 2 also shows curvilinear decreases for ITA and increases for STA, representing a 30° variation of tentorial orientation over the period investigated. Statistics for these line fittings are given in Table 5. The curvilinear variation of STA, which takes into account base retroflexion, shows a similar, but reversed, pattern of variation to IDE with a marked rate of change early in fetal life that gradually tapers off. This finding suggests that inferoposterior rotation of the tentorium is contemporaneous with increases of supratentorial volume relative to infratentorial volume. These temporal variations are summarized in Figure 3.
Fig. 2.
Bivariate plot showing infratentorial angle (ITA), supratentorial angle (STA), cranial base angle (CBA) and index of differential encephalization (IDE) against estimated gestational age. Values of IDE are multiplied by a factor of 500 for illustrative purposes only. Also shown are the polynomial and RMA regression fittings for these plots. The polynomial regression line for ITA against age is given in grey. The rate of change of all of these variables, with the exception of cranial base angle, gradually decreases towards the end of the period investigated.
Table 5.
Statistics for second-order polynomials of the form Y= a+ bX+ cX2
| Comparison | a | b | c | R2 |
|---|---|---|---|---|
| STA against EGA | 28.02 | 12.93 | −0.29 | 0.78 |
| ITA against EGA | 198.90 | −12.75 | 0.27 | 0.82 |
| IDE against EGA | 0.55 | −0.040 | 0.001 | 0.82 |
STA, supratentorial angle; ITA, infratentorial angle; IDE, index of differential encephalization; EGA, estimated gestational age.
Fig. 3.
Schematic illustration of the temporal trends observed in relation to estimated gestational age: cranial base angle (CBA; broken grey line) and supratentorial angle (STA; solid grey line) increase with fetal age; supratentorial volume (light grey area) increases in relation to infratentorial volume (dark grey area) with age; infratentorial angle (ITA; broken black line) decreases with age.
The relationship between differential encephalization and STA was further evaluated with a direct bivariate comparison (Figure 4). This plot illustrates a linear trend with a significant negative slope and the correlation between STA and IDE is highly significant (P< 0.001, Table 6). These findings indicate that the tentorium cerebelli rotates towards the posterior cranial base in association with increases of supratentorial volume relative to infratentorial volume. This result is consistent with the hypothesis that differential encephalization is associated with tentorial rotation during prenatal human development.
Fig. 4.
Bivariate plot of supratentorial angle against the index of differential encephalization. RMA regression line fitting is shown.
Table 6.
Reduced major axes (RMA) statistics for linear line fittings
| Comparison | Slope | 95% conf. int. of slope | Intercept | Rrank | |
|---|---|---|---|---|---|
| CBA against EGA | 0.98 | 0.72 > 1.24 | 117.49 | 0.43 | < 0.01 |
| STA against IDE | −251.70 | −291.96 > −211.44 | 194.32 | −0.62 | < 0.001 |
CBA, cranial base angle; EGA, estimated gestational age; STA, supratentorial angle; IDE, index of differential encephalization; Rrank, rank correlation coefficient; P, significance of correlation.
Discussion
Results from the present study are in close agreement with those from previous investigations. The finding here and reported in Jeffery & Spoor (in press), that the cranial base angle opens out, i.e. retroflexes, during human fetal development is consistent with that of other studies (e.g. Kvinnsland, 1971; Dimitriadis et al. 1995). It is not, however, consistent with those studies reporting that the angle remains unchanged (e.g. Ford, 1956; Burdi, 1965) or flexes (e.g. Erdoglija, 1989; Van Den Eynde et al. 1992). The pattern of differential encephalization with gestational age, also detailed by Jeffery & Spoor (in press), is congruous with the scale of volumetric changes reported by Jenkins (1921) and Koop et al. (1986). There are, however, notable absolute differences at specific intervals of age due to methodology. For example, both Jenkins and Koop et al. computed volumes from tissue sections that are susceptible to shrinkage and Jenkins excludes the ventricular system from his measures – the ventricles occupy a large proportion of overall brain volume in the embryo and early fetus. More marked is the congruity with the process of prenatal differential encephalization described by Guihard-Costa & Larroche (1990). The authors show that infratentorial mass decreases as a proportion of total brain mass during early fetal life. They also demonstrate that the rate of this proportional change gradually slows with age and that eventually the decrease reverts in later prenatal life to an increase, i.e. infratentorial mass enlarges in relation to supratentorial mass. The asymptotic-like transition from this negative to positive slope lies between the 24th and 26th weeks of gestation and is contemporaneous with the period of reduced differential encephalization shown here in Figure 2. Rakic & Sidman (1970) note that the cerebellum, being the greater part of the infratentorial volume, commences growth later but completes growth earlier than most other parts of the fetal brain. This is achieved by way of a late fetal period of rapid growth leading to sizeable increases of surface area, mass and width at rates exceeding that of the forebrain (Noback & Moss, 1956; Dobbing & Sands, 1973). These data indic-ate that differential infratentorial enlargement may succeed differential supratentorial enlargement during human fetal development.
The results reported here, which show that the fetal tentorium rotates towards the posterior cranial base, closely matches that portrayed in the figures of Hochstetter (1939). These figures depict large increases of supratentorial angle during early fetal life and smaller increases towards the end of the period investigated by Hochstetter, about the 18th week. Similar increases and a gradual rate decrease, almost to an asymptote, were observed here. Results from the present study also show that this tentorial rotation is closely linked with a similar pattern of differential brain growth. This is consistent with the contention of Moss et al. (1956) that rotation corresponds to changes of ‘brain topography’. Moss et al. further suggest that once a state of topographical equilibrium is achieved between infratentorial and supratentorial components, the orientation of the tentorium remains essentially unchanged thereafter. It is not clear from the results presented in this paper, which only cover part of the gestational period, whether or not tentorial orientation would respond to differential infratentorial growth later in fetal life. Perhaps the lag in cerebellar growth (see above references) affects a decrease of supratentorial angle or is adequately accommodated elsewhere by, for example, widening of the posterior cranial fossa (see Ford, 1956). Interestingly, congenital malformations of the brain resulting in disproportionate increases of infratentorial size during development, such as Dandy–Walker syndrome, can displace the midline ridge of the tentorium and the confluence of sinuses superiorly (Castan et al. 1975; Lang, 1991). This would correspond to a decrease of supratentorial angle as defined here. Notwithstanding such conjecture, the findings presented here can only be seen as congruous with the hypothesis that tentorial rotation, towards the foramen magnum, is closely linked with differential supratentorial enlargement for the period investigated, i.e. from about 10 to 29 gestational weeks. Even then, it cannot be assumed that this association amounts to causation since it is possible that equivalent changes of these variables occur independently as the result of different processes or mechanisms.
The ontogenetic model, as discussed above, shows an initial period of differential supratentorial encephaization associated with posteroinferior tentorial rotation followed by a period of differential infratentorial encephalization, possibly in association with anterosuperior tentorial rotation. Given that successive such patterns of ontogenetic variation can underlie evolutionary changes, it seems reasonable to suggest that analogous variations could well have occurred during phylogeny. Indeed, studies of primate brain volumes demonstrate differential supratentorial enlargement and show that modern humans possess one of the smallest infratentorial brain sizes relative to supratentorial brain size (Stephan et al. 1981; Semendeferi et al. 1997; Rilling & Insel, 1998; Semendeferi & Damasio, 2000). Furthermore, there is evidence of an evolutionary inferoposterior rotation of the tentorium cerebelli. As early as 1863, Huxley showed that the angle of the tentorium cerebelli relative to the posterior cranial base is more acute in baboons and modern humans than in lemurs. More recently, Klintworth (1967) and Bull (1969) observed that the modern human tentorium is one of the most inferoposteriorly rotated amongst primates and mammals in general. That there are indications of interspecific as well as ontogenetic differential encephalization and tentorial rotation, with modern humans being perhaps the most derived, raises the possibility that comparative differences in the fetal relationship accumulated over phylogenetic time contributed, at least in part, to the apomorphic configuration seen in adult modern humans. However, it should be noted that interspecific trends do not necessarily reflect evolutionary histories of change (see Harvey & Pagel, 1991) and that the present study only examines part of the period over which ontogenetic change can occur. In particular, it is important to reiterate that the null hypothesis has yet to be systematically tested with either late fetal or postnatal material. It seems likely that interspecific differences during these later developmental stages, like for example differences of differential infratentorial enlargement, may also have contributed to the unique condition seen in modern humans, suggesting a more complex process than discussed above.
In addition to differential encephalization, changes of posture during human evolution are said to have further influenced the overall load on the tentorium and thus altered its relative position within the skull (Bull, 1969). Amongst modern humans, the baseline of the midline tentorium, drawn from its anterior sphenoid to posterior occipital attachments, lies almost perpendicular to the gravity vector in a bipedal stance. Thus, the weight of the cerebrum on the tentorium is comparatively greater in modern humans than it is, for example, in quadrupedal primates where the tentorium runs more obliquely to the vector of gravity. It therefore appears that the orientation of the tentorium has an uncommon potential for elucidating not one but two derived features of the modern human condition, namely an enlarged brain and obligatory bipedalism. The significance of tentorial configuration for studies of human evolution remains to be explored.
Perhaps the major implication of this paper is a practical one, concerning the approach used to study encephalization. The tentorium cerebelli and falx cerebri compartmentalize and structurally support the brain in three parts, two supratentorial and one infratentorial. These contain the two cerebral hemispheres and the cerebellum plus brainstem. Moreover, this general configuration is found throughout all primates (Klintworth, 1968), many of which are used in studies to represent various primitive stages of the modern human condition. Despite this, recent key studies examining, for example, the effects of phylogenetic encephalization on skull base morphology, model the brain as a single amorphous volume or, at best, model the subdivisions of the brain independently (e.g. Ross & Ravosa, 1993; Ross & Henneberg, 1995; Spoor, 1997). This paper questions such an approach of generalizing encephalization since it is shows variations in the orientation of the dural folds and proportions of the brain can potentially influence the load, and hence biomechanical demands, placed on different elements of the skull. The relationship between basicranial architecture and measures of differential encephalization have recently been explored elsewhere for fetal development (Jeffery & Spoor, in press) and across extant adult primates (Lieberman et al. 2000).
In conclusion, the findings confirm that during human fetal life the supratentorial compartment of the endocranial cavity enlarges disproportionately and that the midline orientation of the tentorium cerebelli rotates towards the foramen magnum. It is also substantiated that these changes are concomitant and suggested that insights into this trend, and the possible influence on the structural demands placed on the skull by the brain, will likely prove useful in understanding human development and evolution. Further concerted study is therefore warranted with material from the later stages of development, the fossil record and via interspecific analyses.
Acknowledgments
This work depended on the support and guidance of Dr Fred Spoor, University College London. Thanks are also due to Dr Sam Cobb, Mr William Harcourt-Smith and two anonymous referees for their helpful comments. Support from the Medical Research Council, the Wellcome Trust and the University of London Intercollegiate Research Service scheme at Queen Mary and Westfield College is acknowledged.
References
- Aiello L, Dean C. Human Evolutionary Anatomy. London: Academic Press; 1990. [Google Scholar]
- Bull JW. Tentorium cerebelli. Proc. Royal Soc. Medicine. 1969;62:1301–1310. doi: 10.1177/003591576906201242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdi AR. Sagittal growth of the nasomaxillary complex during the second trimester of human prenatal development. J. Dental res. 1965;44:112–125. doi: 10.1177/00220345650440010401. [DOI] [PubMed] [Google Scholar]
- Castan P, Castan-Tarbouriech E, Bouzige JC. Cerebral angiography of hydrocephalic infants: techniques, indications, and results. In: Salamon G, editor. Advances in Cerebral Angiography. Berlin: Spinger; 1975. pp. 300–310. [Google Scholar]
- Chitty LS, Altman DG, Henderson A, Campbell S. Charts of fetal size: 2. Head measurements. Br. J. Obstetrics Gynaecol. 1994;101:35–43. doi: 10.1111/j.1471-0528.1994.tb13007.x. [DOI] [PubMed] [Google Scholar]
- De Beer G. Embryos and Ancestors. Oxford: Clarendon Press; 1958. [Google Scholar]
- Dimitriadis AS, Haritanti-Kouridou A, Antoniadis K, Ekonomou L. The human skull base angle during the second trimester of gestation. Neuroradiology. 1995;37:68–71. doi: 10.1007/BF00588524. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Quantitative growth and development of human brain. Arch. Dis Childhood. 1973;48:757–767. doi: 10.1136/adc.48.10.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdoglija LJ. Dynamics of the cranial base angle changes during the second trimester of the normal intrauterine growth and development. Bilten Udruzenja Ortodonata Jugoslavije. 1989;22:7–14. [PubMed] [Google Scholar]
- Fisher CM. Brain herniation: a revision of classical concepts. Can. J. Neurol. Sci. 1995;22:83–91. doi: 10.1017/s0317167100040142. [DOI] [PubMed] [Google Scholar]
- Ford EHR. The growth of the foetal skull. J. Anat. 1956;90:63–72. [PMC free article] [PubMed] [Google Scholar]
- Friede H. Normal development and growth of the human neurocranium and cranial base. Scand. J. Plastic Reconstructive Surgery. 1981;15:163–169. doi: 10.3109/02844318109103431. [DOI] [PubMed] [Google Scholar]
- Garstang W. The theory of recapitulation: a critical restatement of the biogenetic law. Zool. J. Linnean Soc. London. 1922;35:81–101. [Google Scholar]
- Gould SJ. Ontogeny and Phylogeny. London: Harvard University Press; 1977. [Google Scholar]
- Guihard-Costa AM, Larroche JC. Differential growth between the fetal brain and its infratentorial part. Early Human Dev. 1990;23:27–40. doi: 10.1016/0378-3782(90)90126-4. [DOI] [PubMed] [Google Scholar]
- Harvey PH, Pagel MD. The Comparative Method in Evolutionary Biology. Oxford: Oxford University Press; 1991. [Google Scholar]
- Hochstetter F. Über die Entwicklung und Differenzierung de Hüllen des Menschlichen Hehirns. Morph. Jahrb. 1939;83:359–494. [Google Scholar]
- Huxley TH. On some fossil remains of man. In: Rhys E, editor. Huxley's Essays. London: J.M. Dent & Sons Inc; 1863. pp. 111–150. Everyman Library, 1905. [Google Scholar]
- Inao S, Kuchiwaki H, Kanaiwa H, Sugito K, Banno M, Furuse M. Magnetic resonance imaging assessment of brainstem distortion associated with a supratentorial mass. J. Neurol., Neurosurgery Psychiatry. 1993;56:280–285. doi: 10.1136/jnnp.56.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery N. Fetal Development and Evolution of the Human Cranial Base. PhD thesis: University College London. 1999. [Google Scholar]
- Jeffery N, Spoor F. Brain size and the human cranial base: a prenatal perspective. Am. J. Phys. Anthropol. (in press) [DOI] [PubMed]
- Jenkins GB. Relative weight and volume of the component parts of the brain of the human embryo at different stages of development. Contributions Embryol. 1921;29:41–60. [Google Scholar]
- Keith A. Human Embryology and Morphology. Baltimore: Williams & Wilkins; 1948. [Google Scholar]
- Klintworth GK. The ontogeny and growth of the human tentorium cerebelli. Anat. Record. 1967;158:433–441. doi: 10.1002/ar.1091580407. [DOI] [PubMed] [Google Scholar]
- Klintworth GK. The comparative anatomy and phylogeny of the tentorium cerebelli. Anat. Record. 1968;160:635–642. doi: 10.1002/ar.1091600312. [DOI] [PubMed] [Google Scholar]
- Koop M, Rilling G, Herrmann A, Kretschmann HJ. Volumetric development of the fetal telencephalon, cerebral cortex, diencephalon, and rhombencephalon including the cerebellum in man. Bibliotheca Anat. 1986;28:53–78. [PubMed] [Google Scholar]
- Kvinnsland S. The sagittal growth of the foetal cranial base. Acta Odontol. Scand. 1971;29:699–715. doi: 10.3109/00016357109026542. [DOI] [PubMed] [Google Scholar]
- Lang J. Clincal Anatomy of the Posterior Cranial Fossa and its Foramina. New York: Georg Thieme Verlag; 1991. [Google Scholar]
- Lieberman DE, Ross CF, Ravosa MJ. The primate cranial base: ontogeny, function and integration. Yearb. Phys. Anthropol. 2000;43:117–169. doi: 10.1002/1096-8644(2000)43:31+<117::aid-ajpa5>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- Mayer A. Herniation of the brain. Arch. Neurol. Psychiatry. 1920;4:387–400. [Google Scholar]
- Moss ML, Noback CR, Robertson GG. Growth of certain human fetal cranial bones. Am. J. Anat. 1956;98:191–204. doi: 10.1002/aja.1000980203. [DOI] [PubMed] [Google Scholar]
- Moss ML. The pathogenesis of artificial cranial deformation. Am. J. Phys. Anthropol. 1958;16:269–286. doi: 10.1002/ajpa.1330160302. [DOI] [PubMed] [Google Scholar]
- Moss ML. Morphological variations of the crista galli and medial orbital margin. Am. J. Phys. Anthropol. 1963;18:259–264. doi: 10.1002/ajpa.1330210208. [DOI] [PubMed] [Google Scholar]
- Noback CR, Moss ML. Differential growth of the human brain. J. Comparative Neurol. 1956;105:539–551. doi: 10.1002/cne.901050306. [DOI] [PubMed] [Google Scholar]
- Nojima T. Developmental pattern of the bony falx and bony tentorium of spotted dolphins (Stenella-attenuata) and the relationships between degree of development and age. Mar. Mammal Sci. 1988;4:312–322. [Google Scholar]
- Osborn AG. The medical tentorium and incisura: normal and pathological anatomy. Neuroradiology. 1977;13:109–113. doi: 10.1007/BF00339844. [DOI] [PubMed] [Google Scholar]
- Rakic P, Sidman RL. Histogenesis of cortical layers in human cerebellum, particularly the lamina dissecans. J. Comparative Neurol. 1970;139:473–500. doi: 10.1002/cne.901390407. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Insel TR. Evolution of the cerebellum in primates: differences in relative volume among monkeys, apes and humans. Brain Behav. Evol. 1998;52:308–314. doi: 10.1159/000006575. [DOI] [PubMed] [Google Scholar]
- Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. N. Engl. J. Med. 1986;314:953–958. doi: 10.1056/NEJM198604103141504. [DOI] [PubMed] [Google Scholar]
- Ropper AH. A preliminary MRI study of the geometry of brain displacement and level of consciousness with acute intracranial masses. Neurology. 1989;39:622–627. doi: 10.1212/wnl.39.5.622. [DOI] [PubMed] [Google Scholar]
- Ross CF, Ravosa MJ. Basicranial flexion, relative brain size, and facial kyphosis in nonhuman primates. Am. J. Phys. Anthropol. 1993;91:305–324. doi: 10.1002/ajpa.1330910306. [DOI] [PubMed] [Google Scholar]
- Ross C, Henneberg M. Basicranial flexion, relative brain size, and facial kyphosis in Homo sapiens and some fossil hominids. Am. J. Phys. Anthropol. 1995;98:575–593. doi: 10.1002/ajpa.1330980413. [DOI] [PubMed] [Google Scholar]
- Saldino RM, Di Chiro G. Tentorial calcification. Radiology. 1974;111:207–211. doi: 10.1148/111.1.207. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Damasio H, Frank R, Van Hoesen GW. The evolution of the frontal lobes: a volumetric analysis based on three-dimensional reconstructions of magnetic resonance scans of human and ape brains. J. Human Evol. 1997;32:375–388. doi: 10.1006/jhev.1996.0099. [DOI] [PubMed] [Google Scholar]
- Semendeferi K, Damasio H. The brain and its main anatomical subdivisions in living hominoids using magnetic resonance imaging. J. Human Evol. 2000;38:317–332. doi: 10.1006/jhev.1999.0381. [DOI] [PubMed] [Google Scholar]
- Sherwood RJ, Meindl RS, Robinson HB, May RL. Fetal age: methods of estimation and effects of pathology. Am. J. Phys. Anthropol. 2000;113:305–315. doi: 10.1002/1096-8644(200011)113:3<305::AID-AJPA3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Spoor F. Basicranial architecture and relative brain size of Sts 5. (Australopithecus africanus) and other Plio-Pleistocene hominids. South African J. Sci. 1997;93:182–187. [Google Scholar]
- Stephan H, Frahm H, Baron G. New and revised data on volumes of brain structures in insectivores and primates. Folia Primatol. 1981;35:1–29. doi: 10.1159/000155963. [DOI] [PubMed] [Google Scholar]
- Van Den Eynde B, Kjaer I, Solow B, Graem N, Kjaer TW, Mathiesen M. Cranial base angulation and prognathism related to cranial and general skeletal maturation in human fetuses. J. Craniofacial Genet. Dev. Biol. 1992;12:22–32. [PubMed] [Google Scholar]
- Wijdicks EF, Miller GM. MR imaging of progressive downward herniation of the diencephalon. Neurology. 1997;48:1456–1459. doi: 10.1212/wnl.48.5.1456. [DOI] [PubMed] [Google Scholar]