Abstract
Layer 7 is one of the retinorecipient layers of the avian optic tectum. However, little information is available about the neuronal organization of this layer and its implications for visual function. Golgi impregnation was used to investigate the retinal input to and the neuronal architecture of layer 7 of the chick optic tectum, which forms a narrow band between the two cell-dense layers 6 and 8. Anterograde tracers were also used to investigate the afferent and efferent connections of layer 7, in both the light and the electron microscope, together with GABA immunogold labelling. Three types of radial neuron were defined according to the origin and course of their axons. The perikarya of these neurons were situated in tectal layers 10–11. The principal dendrites of these radial neurons ascended to the tectal surface and gave rise to dendritic side-branches in layer 7. These dendritic side-branches received asymmetric synapses from the terminations of retinal fibre arborisations. Type 2 radial neurons, whose axons arose from the deep pole of the perikaryon or occasionally from a basal dendrite, were shown to project to the nucleus isthmi pars magnocellularis, which has previously been demonstrated to be GABAergic and to project to glomerulus-like complexes in tectal layers 4–5. In these layers, the dendritic branches of layer 13 neurons that project to the nucleus rotundus have previously been shown to receive retinal fibre input. Therefore, the retinal input to layer 7 may be able to modulate the transmission of information to the visual thalamus, by way of a feedback loop to layers 4–5 of the tectum involving the nucleus isthmi pars magnocellularis.
Keywords: biotinylated dextran-amine, isthmic nuclei, modulatory feed-back, Phaseolous vulgaris leucoagglutinin, ultrastructure
Introduction
The arborization of retinal fibres in the avian optic tectum was first described by Ramón y Cajal (1911), following investigation of Golgi-impregnated material. The greater proportion of retinal fibres was found to terminate in layers 2, 3, 4–5 and 7, and these were referred to as the optic layers of the tectum. Ramón y Cajal's (1911) observations have subsequently been augmented by the results of other light and electron microscope studies (Hayes & Webster, 1975; Hunt & Webster, 1975; Angaut & Repérant, 1976; Hunt & Künzle, 1976a, b; Repérant & Angaut, 1977). The terminals of retinotectal fibres have previously been identified and characterized in the electron microscope as large terminals filled with round synaptic vesicles, which establish asymmetric synapses in the external layers (2, 3 and 4–5) of the optic tectum (Hayes & Webster, 1975; Angaut & Repérant, 1976). These terminals contact the dendrites of radial projection neurons and local circuit neurons as well as the horizontal neurons of layer 4. Furthermore, small, simple glomerulus-like synaptic complexes have been described in the external layers of the avian optic tectum (Hayes & Webster, 1975; Angaut & Repérant, 1976).
Ultrastructural evidence has revealed that retino-tectal terminals establish asymmetric synapses with PHA-L-labelled terminal portions of layer 13 projection neuron (ganglion cells of Ramón y Cajal) dendrites within the neuropil of the radial columns of layers 4–5 (Tömböl, 1998; Tömböl & Németh, 1999). These findings support earlier electrophysiological evidence for direct contact between retinal fibres and dendritic endings of layer 13 projection neurons in the avian optic tectum (Hardy et al. 1982, 1985). Layer 13 projection neurons have been demonstrated to possess ‘bottlebrush’ dendritic endings and form large dendritic fields (Luksch et al. 1998). Their output is largely directed at the ipsilateral nucleus rotundus, although some projects to the contralateral rotundus (Ngo et al. 1994). The synapses in tectal layers 4–5 are organized into glomerulus-like complexes (Tömböl & Németh, 1999) and there is evidence that visual transmission is modulated by GABAergic terminals. The isthmic nuclei may play an important modulatory role in visual processing within the optic tectum, possibly involving delayed inhibition (Bagnoli et al. 1989; Felix et al. 1994; Wang et al. 1995), with positive and negative feedback loops working together to permit orientation to one of several moving visual stimuli simultaneously present in the visual field (Wang et al. 1995).
Although retinal fibres have been demonstrated to terminate in layer 7, little detailed information is available about the precise nature of their connections within this retinorecipient layer of the optic tectum. Therefore, Golgi-impregnated preparations of the chick optic tectum were investigated to characterize in detail the neuronal structures lying within/traversing layer 7 and the relationship between retinal terminals and these neuronal structures. This was complemented by the iontophoresis of anterograde tracers into the optic nerve/chiasm and optic tectum in order to investigate the precise nature of the arborization and synaptic contacts of retinal fibres in layer 7 and of tectal efferents associated with layer 7, using light and electron microscopy. GABA-immunogold labelling was also employed to investigate the organization of putative inhibitory synapses within layer 7 of the optic tectum.
Materials and methods
Golgi impregnation
Three, day-old and 25, month-old light-reared domestic chicks (Hunnia broilers) were given a lethal dose of anaesthetic (Nembutal, Serva) and perfused through the left cardiac ventricle with a rapid flush of 10–30 mL of physiological saline, followed by 500 mL of 4% paraformaldehyde in 0.1 mphosphate buffer (pH 7.4). Each brain was then removed from its skull and immersed in similar fixative for 1 day prior to further processing. Serial, coronal slices (5–6 mm thick) were cut freehand through the optic tecta. The slices were then impregnated using a modification of the rapid Golgi technique (Valverde, 1962). Serial 100-µm sections were cut from the unembedded impregnated slices on a sliding microtome, mounted onto glass slides and examined in a light microscope. They were compared with reference sets of coronal sections of chick optic tecta stained with luxol fast blue and cresyl violet, to aid histological orientation.
Anterograde tracing
Four, 14-day-old chicks (Hunnia broilers) were deeply anaesthetized by intraperitoneal injection of Equithesin (Davies & Horn, 1983; 0.025 mL/10 g body weight) and mounted in a stereotaxic instrument. The ear bars were set 8.5 mm above and posterior to a horizontal bar placed at the angle between the upper and lower beak, to give an orientation comparable to that used for preparation of the atlas of 2-week-old chick brain by Kuenzel & Masson (1988). The scalp was incised to expose the cranium, which was opened together with the dura mater, to reveal the left side of the optic tectum. A glass capillary cannula (outside diameter approximately 50 µm) was lowered 1.0–1.5 mm vertically below the dorsal surface of the optic tectum, under visual control. The anterograde tracer Phaseolus vulgaris leucoagglutinin (PHA-L; Vector Laboratories; 2.5% in 0.1 mphosphate buffer, pH 7.3) was then iontophoresed into the tectum, using a direct current of 5–8 mA cycling on and off every 7 s for a period of 15–20 min. The cannula was left in situ for 5–10 min after iontophoresis, to minimize tracer reflux along the entry tract as the cannula was withdrawn. The dura mater and skull flaps were then replaced, the scalp closed and each chick allowed to recover.
Fourteen to 16 days after iontophoresis, the chicks were given a lethal dose of anaesthetic and perfused through the left ventricle with a flush of 50–100 mL of physiological saline. This was followed by 500 mL of fixative (4% paraformaldehyde, 0.05% glutaraldehyde and 0.2% picric acid in 0.1 mphosphate buffer, pH 7.3) delivered over 30 min. All perfusion fluids were at room temperature. The brains were then removed from their cranial cavities and sequentially immersed in buffered 10% and 20% sucrose solutions until they sank. Tissue blocks comprising the optic tecta, the remainder of the mesencephalon and parts of the diencephalon were then quenched in liquid nitrogen and serial coronal vibratome sections (50 µm) cut through the optic tecta. The sections were then immunolabelled according to the protocol of Gerfen & Sawchenko (1984). Free-floating sections were incubated with the PHA-L antiserum (Vector Laboratories, diluted 1 : 200 in 0.05 mTRIS buffered saline, pH 7.4) for 48 h at 4 °C and then reacted with the avidin–biotin complex (Vectakit Elite, Vector Laboratories, diluted 1 : 100 in TRIS-buffered saline) for 2 h at room temperature. The immunoprecipitate was visualised with 3,3-diaminobenzidine (Sigma; 0.025%) intensified with nickel ammonium sulphate (Merck; 0.05%), in the presence of 0.001% hydrogen peroxide diluted in TRIS-buffered saline. The sections were then mounted on glass slides, counterstained with 0.5% neutral red (diluted in 0.1 macetate buffer, pH 4.7) and examined in a light microscope.
The anterograde tracer biotinylated dextran-amine (BDA; Vector Laboratories; 20% diluted in physiological saline) was delivered by iontophoresis into the optic nerve or optic chiasma of four, 3-week-old chicks. The roofs of the orbit and optic canal were opened on the left side, BDA was delivered under visual control using a similar procedure to that for iontophoresis of PHA-L and the chicks were allowed to recover. Five to 6 days later, the chicks were perfused and serial vibratome sections obtained following the PHA-L protocol. The sections were then reacted with the avidin–biotin complex, visualized and examined as described above.
Some sections destined for electron microscopy were post-fixed with osmium tetroxide (1% diluted in distilled water) for 10 min, mounted on glass slides and covered in Durcupan (ACM, Fluka). They were examined in a light microscope and areas that contained BDA-labelled terminals in layer 7 of the optic tectum were microdissected and re-embedded in Durcupan. Ultrathin sections were then cut and investigated in a JEOL 1200 EMX electron microscope.
GABA immunogold labelling
Two, 26-day-old chicks were given a lethal dose of anaesthetic and perfused with 10–30 mL of physiological saline followed by 500 mL of 4% paraformaldehyde and 2% glutaraldehyde in 0.1 mphosphate buffer (pH 7.3). The brains were then removed from the skulls and immersed in similar fixative for 2–3 days. Blocks of tissue were cut from the optic tecta and processed into Durcupan. Ultrathin sections were cut and treated for post-embedding GABA immunogold labelling, following the method of Somogyi & Hodgson (1985). The ultrathin sections were mounted on Formvar-coated nickel grids and pretreated with 1% periodic acid and 1% sodium periodate (in distilled water) to etch the resin and remove the osmium. They were then sequentially placed in 10% normal goat serum (in TRIS-buffered saline at pH 7.6) for 20–30 min, in rabbit primary GABA antiserum (Sigma) diluted 1 : 2000 in normal goat serum for 4 h and then in goat anti-rabbit IgG-gold conjugate (15 nm particle size, British Biocell International) diluted 1 : 20 in TRIS-buffered saline (pH 8.2) for 2 h. Between each step, the grids were washed three times in Milipore filtered distilled water. Finally, the sections were stained with uranyl acetate and lead citrate. Some vibratome sections of BDA-labelled tecta were also processed into Durcupan, ultrathin sections cut and processed for post-embedding GABA immunogold labelling. The ultrathin sections were then examined in a Jeol 1200 EMX electron microscope.
All experiments were carried out in accord with the ethics guidelines of Semmelweis University Medical School.
Results
Golgi architecture
In 1-month-old chicks, the relatively thick retinal fibres descended from the surface of the optic tectum into layer 7 (Figures 1 and 2a), where they immediately ramified with their branches extending parallel to the tectal surface. Each retinal fibre ramified into three or four wavy branches, forming an arborization of 100–120 µm in length. Each branch emitted short and very thin preterminals that were studded along their course with numerous, relatively large, terminal bulbs. These were densely arranged in a variety of patterns including ‘bunch of grapes-like’ clusters. Such terminals were distributed throughout the entire width of layer 7 and their main target appeared to be the dendritic side-branches of radial neurons, but they may also contact the dendrites of other tectal neurons that traverse layer 7 and the dendrites of horizontal neurons intrinsic to layer 7. The branching pattern of retinal fibres in layer 7 of the 1-day-old chick optic tectum was similar to that in 1-month-old birds, with the exception that in 1-day-old chicks the arborizations were smaller and the preterminals and terminal bulbs were sparser and more simply organized, with very few ‘bunch of grapes-like’ clusters (Figure 2c).
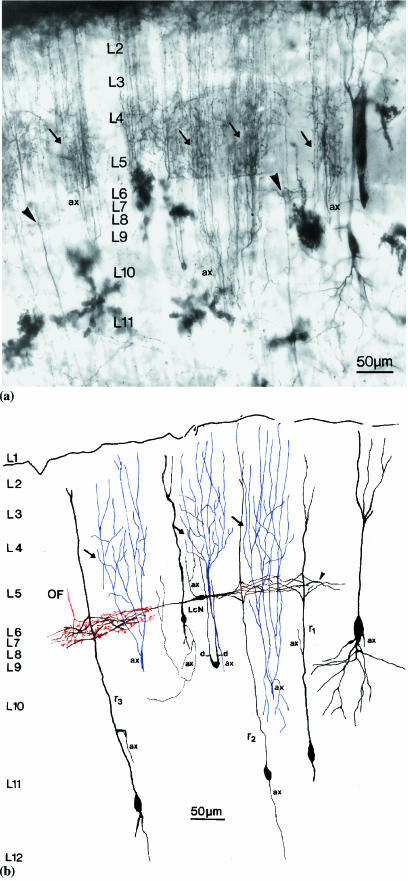
Fig. 1.
(a) A light photomicrograph of a section of Golgi-impregnated optic tectum from a 1-month-old chick, showing one focal plane of the 100-µm-thick preparation. The dark upper margin of the micrograph is the retinal fibre layer at the tectal surface. Arrows indicate the axon arborizations of isthmic nucleus neurons in layers 2–5. These axon arborizations exhibit denser branchings in layers 4–5 than in the more superficial layers 2–3. Layer 7 (L7) is indicated traversing the centre of the micrograph and it contains the dendritic side-branches (arrowheads) of radial neurons. Layers 6 and 8 appear as narrow, lighter bands above and below the narrow layer 7, respectively. A large impregnated neuronal perikaryon is visible in layers 9–10 (right) and a small impregnated perikaryon is visible in layer 10 (centre). The axonal stem of an isthmic neuron is indicated (ax). Golgi-impregnated protoplasmic astrocytes are visible as large stellate structures in the internal layers of the optic tectum. (b) A semi-schematic drawing of the Golgi-impregnated optic tectum in Figure 1(a). Focusing through the section allowed more detail to be drawn than is visible in the photographic plane of Figure 1(a). Arrows indicate the branching of axons (blue, ax) from isthmic nucleus neurons. The optic fibre (OF) arborization (red in L7), a horizontally extending local circuit neuron (LcN) and the dendritic side-branches (arrowhead) of types 1 (r1), 2 (r2) and 3 (r3) radial neurons are illustrated in layer 7. Impregnated axons are labelled (ax). LcNs, one with an ascending axon and another with dendrites (d), are illustrated in layer 9.
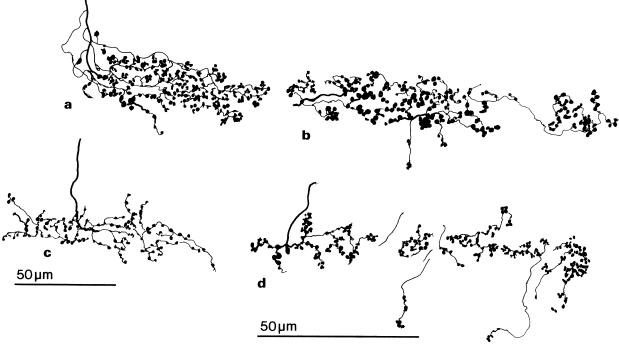
Fig. 2.
Camera lucida drawings of the optic fibre arborizations in layer 7 of the optic tectum. (a) Golgi-impregnated arborizations from a 1-month-old chick. (b) BDA-labelled arborizations from a 3-week-old chick. (c) Golgi-impregnated arborizations from a 1-day-old chick. (d) BDA-labelled arborizations from a 3-week-old chick.
A variety of ascending and descending structures were present in layer 7 of the optic tectum, including large dendritic shafts, thin and very thin dendritic branches, axons and axon branches (Figure 1a,b). The dendritic shafts are the principal dendrites of radial and other types of neurons whose perikarya are located in deeper layers of the tectum. Fine and very fine dendritic branches of layer 13 projection neurons also traversed layer 7 of the tectum. Both thick and thin, ascending and descending axons were observed to cross layer 7 of the tectum. The thick axons developed characteristic terminal arborizations that extended across the external layers of the optic tectum (Figure 1a,b). These thick axons have previously been demonstrated to originate from neurons in the isthmic nuclei. (Hunt & Künzle, 1976a; Tömböl et al. 1995; Tömböl, 1998). The thin axons with fine, more or less beaded axon branches varied a little in thickness. They are predominantly axons and axon branches of local circuit neurons (unpublished observations).
Small, fusiform/ovoid neurons were the only neuronal type intrinsic to layer 7 of the optic tectum (Figures 1b and 3). The long axis of their perikarya was orientated parallel to the tectal layer and their main dendrites emanated from each pole. The smooth dendrites were relatively long in comparison with the perikaryon and ramified poorly, entirely within layer 7 of the tectum. The axons of these neurons ascended from layer 7 and branched in the immediately more superficial layers, but their complete arborization and termination pattern could not be visualized.
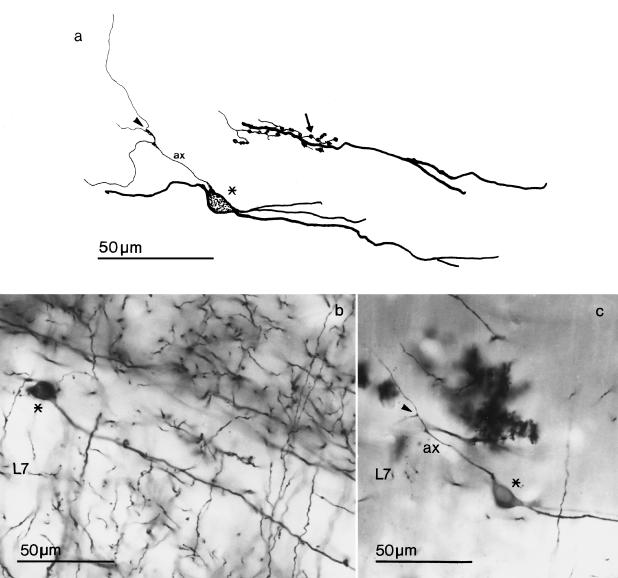
Fig. 3.
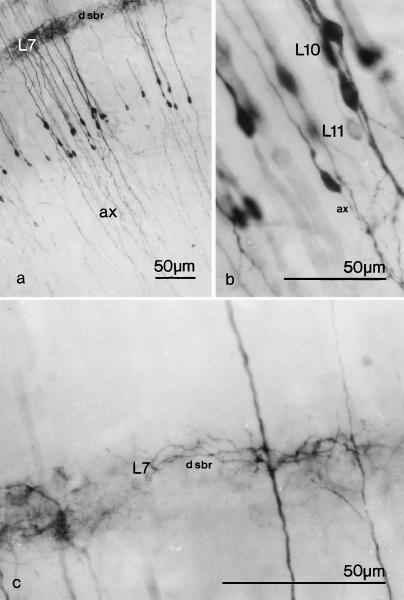
Horizontal local circuit neurons in layer 7 (L7) of the optic tectum. (a) Camera lucida drawings; (b) and (c) photomicrographs of Golgi-impregnated optic tectum. The local circuit neuron drawn in Figure 3(a) is that illustrated in Figure 3(c). The perikarya are indicated by asterisks and give rise to axons (ax) that branch (arrowheads) relatively close to their origin. The arrow indicates optic terminals in contact with a dendrite of a horizontal local circuit neuron.
Characteristic radial neurons with perikarya in layers 10–11 of optic tectum were occasionally completely impregnated (Figure 1b). Their radial dendrites ascended to divide into two or three apical rami below the tectal surface and gave rise to dendritic side-branches that originated in layer 7, or just superficial or deep to it (Figures 1 and 4). All of these side-branches developed their ramifications within layer 7 and appeared to be contacted by retinal fibres. The side-branches of a radial dendrite (Figures 5 and 6c) extended for 80–100 µm within layer 7 of the tectum and their small branches gave rise to a few processes and occasionally spines. Radial neurons could be classified into three types, principally on the basis of the origin and course of their axons. The axons of type 1 radial neurons arose from the ascending radial dendrite and travelled towards the tectal surface. Typically, the axons of this type of radial neuron did not impregnate well, but some ascended and appeared to enter the retinal fibre layer. The perikarya of type 1 radial neurons ranged from 11 to 13 µm in length. The ovoid perikarya of type 2 radial neurons were generally smaller (10–12 µm in length) and their axons arose either directly from the deep pole of the perikaryon or more rarely from a basal dendrite and traversed the internal layers of the optic tectum to enter the stratum album centrale. The ovoid perikaryon of type 3 radial neurons was 11–12 µm in length and its axon arose from the ascending radial dendritic stem and ‘hooked’ downwards to descend into the stratum album centrale. The perikaryon of type 3 radial neurons also gave rise to a dendrite from its deep pole that bifurcated or branched and descended into the deep layers of optic tectum. All three types of radial neuron established connections with the retinal fibres in layer 7 of the optic tectum by means of their profusely ramified dendritic side-branches. This synaptic field was arranged parallel to the tectal surface and occupied a large proportion of layer 7.
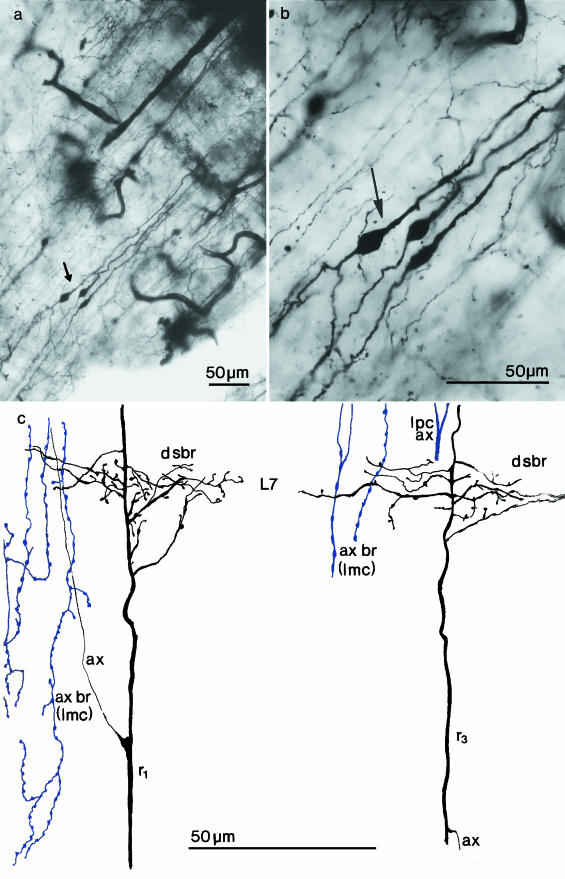
Fig. 4.
(a, b) Photomicrographs of Golgi-impregnated radial neurons (arrows) with ascending and descending dendrites in the 1-month-old chick optic tectum. (c) Camera lucida drawings of dendritic sections from type 1 (r1) and type 3 (r3) radial neurons illustrating the origin, branching and course of dendritic side-branches (d sbr) given off in layer 7 (L7). The origin of the axons (ax) of these radial neurons is demonstrated. The varicose fibres are a few sections of axonal branching (ax br) of nucleus isthmi pars magnocellularis (Imc) neurons; lpc ax = the branching stem of an axon from the nucleus isthmi pars parvocellularis.
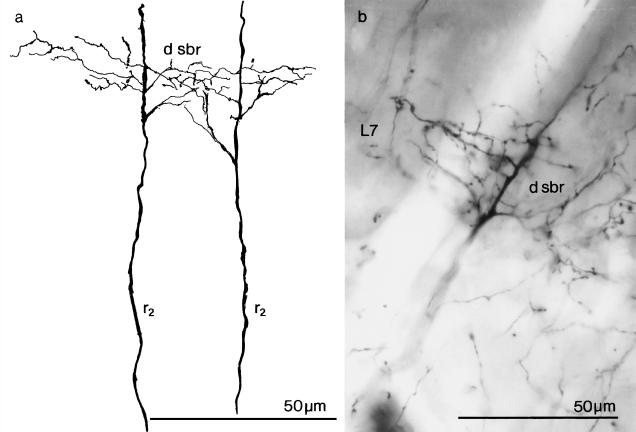
Fig. 5.
The dendritic side-branches (d sbr) of type 2 (r2) radial neurons in layer 7 (L7) of the optic tectum of a 1-month-old chick. (a) A camera lucida drawing and (b) a photomicrograph of Golgi-impregnated material.
Fig. 6.
(a–c) Photomicrographs at increasing magnification of type 2 (r2) radial neurons from 1-month-old chick optic tectum, labelled with PHA-L. Labelled axons are indicated (ax) and dendritic side branches (d sbr) can be seen in layer 7 (L7). Their perikarya are located in layers 10–11 (L10, L11).
Light microscope tracer studies
Following iontophoresis of the anterograde tracer PHA-L into the optic tectum, neuronal perikarya were labelled in the vicinity of the injection site. The dendrites and axons of type 2 radial neurons were labelled with PHA-L and these neurons displayed all of the characteristic morphological features revealed by Golgi impregnation. Their dendritic side-branches were clearly observed to extend into and occupy a large proportion of layer 7 of the tectum and their axons descended directly from the deep pole of the perikaryon or a basal dendrite into the stratum album centrale (Figure 6). The axons of type 2 radial neurons were occasionally traced to their terminations, which were always found to be in the isthmic nuclei. The axons of types 1 and 3 radial neurons with their characteristic origins were not observed in the PHA-L immunolabelled preparations.
Iontophoresis of the anterograde tracer BDA into the optic nerve or chiasm of 3-week-old chicks resulted in labelling of axon terminals in layer 7 of the optic tectum. All of the BDA-labelled axons that entered layer 7 had axon ramifications and terminal bulbs solely within layer 7. The arborization pattern and terminal bulb arrangement of these retinal terminals revealed by BDA labelling (Figure 2b,d) was similar to that found in the Golgi-impregnated optic tectum of 1-month-old chicks (Figure 2a), as was the extent of the optic fibre arborizations (100–120 µm in length).
Electron microscope observations
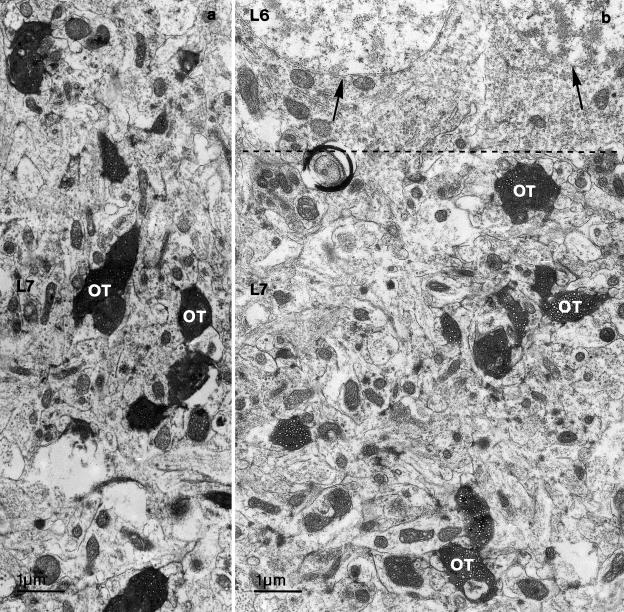
Electron microscope preparations of BDA-labelled optic tectum revealed structures in layer 7 that were similar to those present in Golgi-impregnated material. Sections of both thick and thin ascending dendritic stems were present, together with branches of both thick and thin descending and ascending axons that were intermingled with dendritic side-branches of radial neurons. In a few sections, the soma and dendritic profiles of small fusiform intrinsic neurons were also present. Between the perikarya of layer 6 and layer 8 neurons, BDA-labelled and unlabelled optic terminal profiles were disseminated throughout layer 7 of the optic tectum (Figure 7). The BDA-labelled optic terminals varied in size and shape depending on the plane of the ultrathin section through the terminal bulbs. The largest BDA-labelled retinal terminal profiles measured 2 µm in diameter. Both the BDA-labelled retinal terminals and unlabelled terminals in layer 7 contained round synaptic vesicles that, typically, virtually filled them. These terminals established asymmetric synapses with one or more dendritic profiles (Figure 8).
Fig. 7.
(a, b) Electron photomicrographs of dense BDA-labelled optic fibre terminals (OT) in layer 7 (L7) of the optic tectum. The border between layer 6 (L6) and L7 is indicated by a dotted line. Neurons, in L6, are indicated by arrows.
Fig. 8.
An electron photomicrograph showing dense BDA-labelled optic fibre terminals in layer 7 of the optic tectum, establishing synaptic contacts (arrows) with several dendritic profiles.
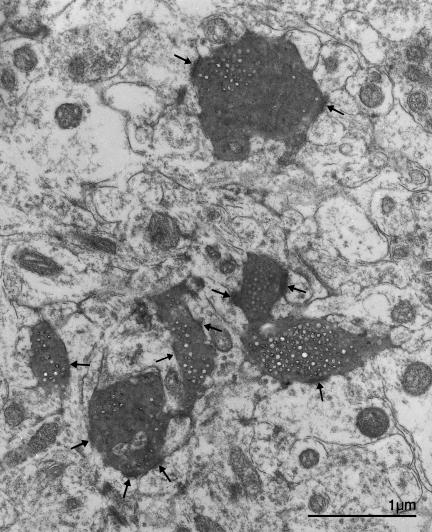
Although the application of post-embedding GABA immunogold labelling revealed GABA-immunoreactive elements in layer 7 of the optic tectum, GABA-immunopositive terminals were not observed on the small dendritic profiles near to the synapses established by retinal terminals (Figure 9). The small, GABA-positive dendritic profiles present in the neuropil may belong to the small, ovoid GABA-positive cells of layer 7. These small dendritic profiles typically exhibited a course parallel to the tectal surface or an oblique course in layer 7 and they are innervated by retinal terminals. Retinal fibre synapses were very rare on the vertically arranged thick radial dendritic stems that traverse layer 7 and no complex glomerulus-like synaptic specializations were observed in the neuropil of layer 7 of the chick optic tectum.
Fig. 9.
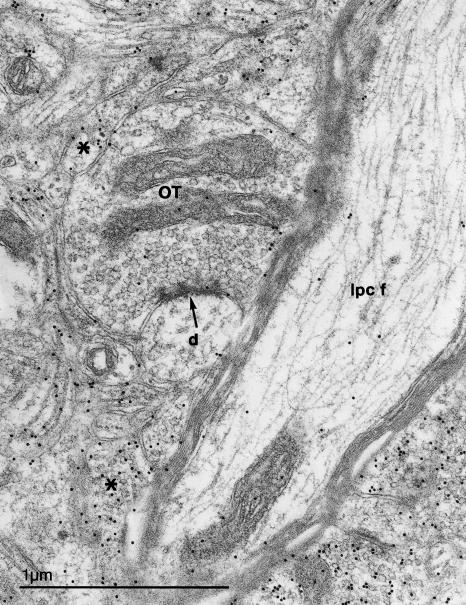
An electron micrograph of neuropil in layer 7 of the optic tectum, showing an optic fibre terminal (OT) making an asymmetric synapse (arrow) with a small dendrite (d). GABA-immunogold labelled profiles that are probably the dendrites of small, fusiform neurons are indicated by asterisks. Nearby is a thick, myelinated GABA-immunonegative ascending fibre (Ipc f) that has the characteristics of an axon from the parvocellular isthmic nucleus.
Discussion
Analysis of layer 7 in Golgi preparations of the chick optic tectum in the present study revealed the presence of ascending principal dendrites, which give rise to side-branches that ramify profusely within it. These ascending principal dendrites originate from radial neurons whose perikarya lie in tectal layers 10–11 and give rise to tectal efferents. The axons of type 1 radial neurons described in this study ascend towards the tectal surface and enter the retinal fibre layer. The course of these axons suggests that they may give rise to tectal commissure projections. However, the axons of type 1 radial neurons were not labelled in the PHA-L anterograde tracing experiments and so could not be traced further.
The results of Golgi impregnation and PHA-L anterograde tracing experiments performed in the current study, indicate that axons of type 2 radial neurons with dendritic side branches in layer 7 originate from the deep pole of the perikaryon, descends toward the central white matter of the tectum and project to the nucleus isthmi magnocellularis. The results of previous PHA-L tracer experiments have revealed a projection from the optic tectum to the isthmic nuclei, but the nature of the projection neurons involved was not identified (Tömböl et al. 1995). The type 3 radial neurons with dendritic side-branches in layer 7 described in the present Golgi study have also previously been demonstrated to send their axons to the isthmic nuclei (Ramón y Cajal, 1911; Hunt & Künzle, 1976a, b). These neurons could not be identified in PHA-L-labelled preparations because their axons were not labelled with the anterograde tracer in either the current or previous (Tömböl et al. 1995) studies. The reason why the axons of type 1 and 3 radial neurons were not anterogradely labelled with PHA-L is not clear, but it may be due to the curved nature of their axonal origins. However, the axons of type 3 radial neurons have been retrogradely labelled following HRP injections into the isthmic nuclei (Hunt & Künzle, 1976a, b). Although terminal arborizations and terminal bulbs of retinal fibres were present in layer 7 of the chick optic tectum at 1 day of age, their development over the first month of life suggests that the modulation and transmission of visual information in layer 7 is refined during this period.
The principal visual input from the retina in birds enters the optic tectum (Cowan et al. 1961; Hayes & Webster, 1975; Hunt & Webster, 1975; Hunt & Künzle, 1976a, b), where the majority of fibres arborize radially and terminate directly on the terminal portions of the dendrites of layer 13 projection neurons in layers 4–5 (Tömböl, 1995, 1998;Tömböl & Németh, 1999). The main target of these layer 13 projection neurons is the nucleus rotundus of the visual thalamus (Karten, 1965; Hodos & Karten, 1966; Revzin & Karten, 1966; Karten & Hodos, 1970;Benowitz & Karten, 1976; Ngo et al. 1994). Glomerulus-like synaptic complexes involving retinal terminals surrounded by glial processes have been reported to be present in the outer layers of the optic tectum (Hayes & Webster, 1975; Angaut & Repérant, 1976). Within these glomerulus-like complexes there are also GABAergic terminals, which establish symmetrical synapses with the terminal portions of projection neuron dendrites (Tömböl & Németh, 1999). Thus, transmission of retinal information via projection neuron dendrites in layers 4–5 of the optic tectum is likely to be modulated within the glomerulus-like complexes. The GABAergic terminals within the glomerulus-like complexes may originate from neurons in the isthmic nuclei (Tömböl, 1998; Tömböl & Németh, 1999), or from the horizontal neurons of layers 4–5 or other local circuit neurons whose axon branches arborize in this layer of the tectum (unpublished observations).
In contrast to isthmic neurons, all of the other GABAergic local circuit neurons that may be candidates for involvement in the layer 4–5 glomerulus-like complexes receive direct retinal innervation, but little is known about the distribution of their axon arborizations within the layers of the optic tectum. However, the current Golgi and anterograde tracer studies together with the results of earlier HRP retrograde tracer studies (Hunt & Künzle, 1976a, b) have demonstrated that isthmic neurons receive indirect retinal input via types 2 and 3 radial neurons whose perikarya lie in layers 10–11. Retinal axon terminals arborize parallel to the tectal surface in layer 7 of the optic tectum and establish simple axo-dendritic synapses with the side branches of types 2 and 3 radial neuron ascending dendrites. The axons of types 2 and 3 radial neurons then project to the isthmic nuclei. The isthmic nuclei in turn feedback upon layers 4–5 of the optic tectum (Wang & Frost, 1991; Felix et al. 1994). The morphological differences between the synaptic organisation of retinal terminals in tectal layers 4–5 and 7, and the connections between layers 7 and 4–5 via the isthmic nuclei are illustrated in Figure 10. There is physiological evidence to suggest that neurons in the nucleus isthmi pars parvocellularis utilize glycine as a neurotransmitter (Wang & Frost, 1991), whereas there are GABAergic neurons in the nucleus isthmi pars magnocellularis that project back to the tectum where they take part in the glomerulus-like complexes in layers 4–5 (Tömböl & Németh, 1998, 1999;Tömböl, 1998). Thus, type 2 and 3 radial neurons may play an important role in eliciting feedback from the isthmic nuclei to the optic tectum, facilitating modulation of the transmission of visual information. However, further studies of neuronal connectivity within the optic tectum, particularly of the axon arborizations and terminals of the numerous types of local circuit neurons, will be required to elucidate how this modulation occurs.
Fig. 10.
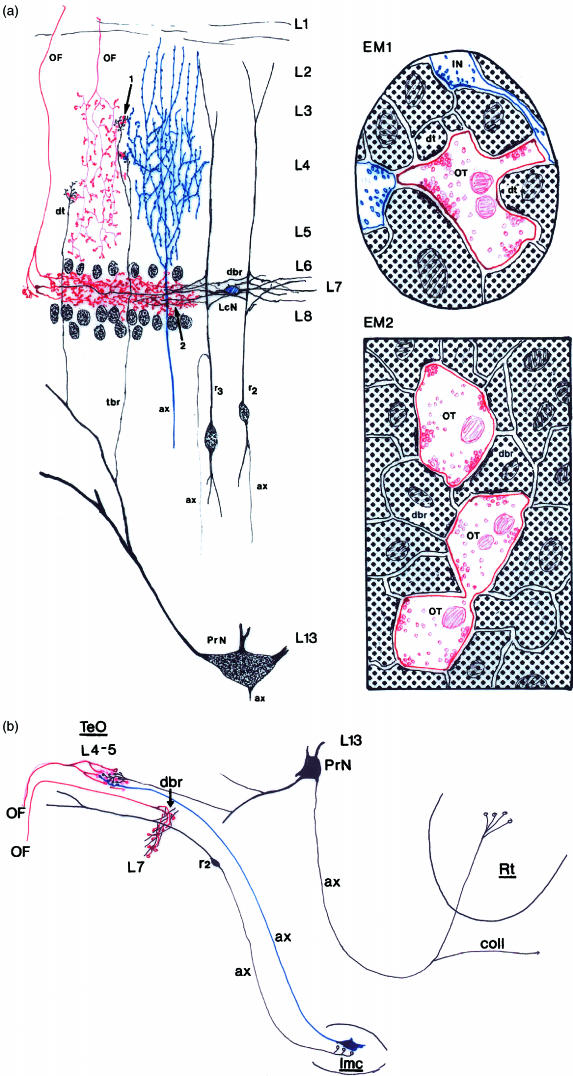
(a) A schematic representation of structures in the optic tectum that are involved in the transmission of visual information in layers 4–5 (relayed to the nucleus rotundus of the visual thalamus) and in layer 7 (relayed to the isthmic nuclei). Arrow 1 indicates an optic terminal in layers 4–5, where the terminal dendrites (dt) of the projection neuron (PrN) and parts of the axon arborization of neurons from the magnocellular isthmic nucleus (ax, blue coloured) are in close contact. This direct connection between optic fibres and projection neurons transmits visual information to the nucleus rotundus. However, the transmission of visual information is modified by putative inhibitory GABAergic terminals (IN) as illustrated in the schematic diagram of ultrastructure (EM1). Arrow 2 indicates the optic terminals and the ramification of radial neuron dendritic side branches in layer 7 (L7) of the optic tectum. EM2 schematically represents the connection between optic fibre terminals (or) and dendritic side-branches (dbr) of radial neuron dendrites. The optic fibre terminals establishes asymmetric synapses with the dendritic side-branches. This connection may give rise to the negative feedback loop to layers 4–5 of the optic tectum via the isthmic nuclei. (b) A diagram that illustrates the ‘reflex arc’ between layers 7 and 4–5 of the optic tectum. The afferent limb is formed by the axon of a radial neuron and the efferent limb by an isthmic neuron; ax = axon, lmc = nucleus isthmipars magrocellularis, L = layer, LcN = local circuit neuron, OF = optic fibres, Rt = nucleus rotundus, r2and r3= type 2 and 3 radial neurons, coll = axon collateral to contralateral Rt.
Acknowledgments
We would like to express our thanks to Jozsef Kiss for preparation of the photomicrographs. This work was supported by OTKA grant T023461 from the Hungarian Academy.
References
- Angaut P, Repérant J. Fine structure of optic fibre termination layers in pigeon optic tectum: a Golgi and electron microscope study. Neuroscience. 1976;1:93–105. doi: 10.1016/0306-4522(76)90003-8. [DOI] [PubMed] [Google Scholar]
- Bagnoli P, Fontanesi GA, Streit P, Domenici L, Alesci R. Changing distribution of GABA-like immunoreactivity in pigeon visual areas during the early posthatching period and effects of retinal removal on tectal GABAergic systems. Visual Neurosci. 1989;3:491–508. doi: 10.1017/s0952523800009846. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Karten HJ. Organization of the tectofugal visual pathway in the pigeon: a retrograde transport study. J. Comp. NeUrol. 1976;167:503–520. doi: 10.1002/cne.901670407. [DOI] [PubMed] [Google Scholar]
- Cowan WM, Adamson L, Powell TPS. An experimental study of the avian visual system. J. Anat. 1961;95:545–563. [PMC free article] [PubMed] [Google Scholar]
- Davies DC, Horn G. Putative cholinergic afferents to the chick hyperstriatum ventrale: a combined acetylcholinesterase and retrograde fluorescence labelling study. Neurosci. Lett. 1983;38:103–107. doi: 10.1016/0304-3940(83)90024-1. [DOI] [PubMed] [Google Scholar]
- Felix D, Wu G-Y, Wang SH-R. GABA as an inhibitory transmitter in the pigeon isthmo-tectal pathway. Neurosci. Lett. 1994;129:212–214. doi: 10.1016/0304-3940(94)90394-8. [DOI] [PubMed] [Google Scholar]
- Gerfen CH, Sawchenko PE. An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: immunohistochemical localisation of an axonally transported plant lectin Phaseolus vulgaris leucoagglutinin (PHA-L) Brain res. 1984;290:219–238. doi: 10.1016/0006-8993(84)90940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy O, Leresche N, Jassik-Gerschenfeld D. The spatial organization of the excitatory regions in the visual receptive fields of pigeon's optic tectum. Exp. Brain res. 1982;46:59–68. doi: 10.1007/BF00238098. [DOI] [PubMed] [Google Scholar]
- Hardy O, Leresche N, Jassik-Gerschenfeld D. Morphology and laminar distribution of electrophysiologically identified cells in pigeon's optic tectum: an intracellular study. J. Comp. NeUrol. 1985;233:390–404. doi: 10.1002/cne.902330308. [DOI] [PubMed] [Google Scholar]
- Hayes BP, Webster KE. An electron microscopic study of retino-receptor layers in the pigeon optic tectum. J. Comparative NeUrol. 1975;162:447–466. doi: 10.1002/cne.901620404. [DOI] [PubMed] [Google Scholar]
- Hodos W, Karten HJ. Brightness and pattern discrimination deficits in the pigeon after lesions in the nucleus rotundus. Exp. Brain res. 1966;2:151–167. doi: 10.1007/BF00240403. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Webster KE. The projection of the retina upon optic tectum of the pigeon. J. Comp. NeUrol. 1975;162:433–446. doi: 10.1002/cne.901620403. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Künzle H. Observations of the projections and intrinsic organization of pigeon optic tectum: an autoradiographic study based on anterograde and retrograde axonal and dendritic flow. J. Comp. NeUrol. 1976a;170:153–172. doi: 10.1002/cne.901700203. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Künzle H. Selective uptake and transport of label within three identified neuronal system after injection of 3H-GABA into the pigeon optic tectum: an autoradiographic and Golgi study. J. Comp. NeUrol. 1976b;170:173–190. doi: 10.1002/cne.901700204. [DOI] [PubMed] [Google Scholar]
- Karten HJ. Projection of the optic tectum of the pigeon (Columba livia) Anat. Rec. 1965;151:369. [Google Scholar]
- Karten HJ, Hodos W. Telencephalic projections of the nucleus rotundus in the pigeon, Columba livia. J. Comp. NeUrol. 1970;140:35–51. doi: 10.1002/cne.901400103. [DOI] [PubMed] [Google Scholar]
- Kuenzel WJ, Masson M. A Stereotaxic Atlas of the Brain of the Chick (Gallus Domesticus) Baltimore: The Johns Hopkins University Press; 1988. [Google Scholar]
- Luksch H, Cox K, Karten HJ. Bottlebrush dendritic endings and large dendritic fields: motion-detecting neurons in the tectofugal pathway. J. Comp. NeUrol. 1998;396:399–414. [PubMed] [Google Scholar]
- Ngo TD, Davies DC, Egedi GY, Tömböl T. A Phaseolus lectin anterograde tracing study of the tectorotundal projections in the domestic chick. J. Anat. 1994;184:129–136. [PMC free article] [PubMed] [Google Scholar]
- Ramón y, Cajal S. Histologie Du Système Nerveux de L’homme et Des Vertébrès (Translated by L. Azoulay) Paris: Maloine; 1911. pp. 196–212. Vol. II. [Google Scholar]
- Repérant J, Angaut P. The retinotectal projections in the pigeon. An experimental optical and electron microscope study. Neuroscience. 1977;2:119–140. doi: 10.1016/0306-4522(77)90073-2. [DOI] [PubMed] [Google Scholar]
- Revzin AM, Karten HJ. Rostral projections of the optic tectum and the nucleus rotundus in the pigeon. Brain res. 19661967;5:264–276. [Google Scholar]
- Somogyi P, Hodgson AJ. Antiserum to amino-butiric acid. III. Demonstration of GABA in Golgi-impregnated neurons and in conventional electron microscopic sections in cat striate cortex. J. Histochem. Cytochem. 1985;33:249–257. doi: 10.1177/33.3.2579124. [DOI] [PubMed] [Google Scholar]
- Tömböl T. Golgi and EM-Golgi Study on the Avian Tectum Opticum. Eur. J. Neurosci. Suppl. 1995;8:76. [Google Scholar]
- Tömböl T, Egedi GY, Németh A. Some data on connections of neurons of nuclei isthmi of the chicken. J. Brain res. 1995;36:501–508. [PubMed] [Google Scholar]
- Tömböl T. Golgi and electron-microscopic Golgi-GABA immunostaining study of the avian optic tectum. Acta Anat. 1998;162:209–225. doi: 10.1159/000046436. [DOI] [PubMed] [Google Scholar]
- Tömböl T, Németh A. GABA immunohistological observations, at the electron microscopic level, of the neurons of isthmic nuclei in chicken, Gallus domesticus. Cell Tissue res. 1998;291:255–266. doi: 10.1007/s004410050995. [DOI] [PubMed] [Google Scholar]
- Tömböl T, Németh A. Direct connections between dendritic terminals of tectal ganglion cells and glutamate-positive terminals of presumed optic fibres in layers 4–5 of the optic tectum of Gallus domesticus. A light- and electron microscopic study. Neurobiology (Budapest) 1999;7:45–66. [PubMed] [Google Scholar]
- Valverde F. Intrinsic organization of the amygdaloid complex. Trab. Inst. Cajal Invest Biol. 1962;54:291–314. [Google Scholar]
- Wang YC, Frost BJ. Visual response characteristics of neurons in the nucleus isthmi magnocellularis and nucleus isthmi parvocellularis of pigeons. Exp. Brain res. 1991;87:624–633. doi: 10.1007/BF00227087. [DOI] [PubMed] [Google Scholar]
- Wang SH-R, Wu G-Y, Felix D. Avian Imc-tectal projection is mediated by acetylcholine and glutamate. Neuroreport. 1995;6:757–760. doi: 10.1097/00001756-199503270-00013. [DOI] [PubMed] [Google Scholar]