Abstract
Abstract
Embryonic stem (ES) cells are unique cells derived from the inner cell mass of the mammalian blastocyst. These cells are immortal and pluripotent, retain their developmental potential after prolonged culture, and can be continuously cultured in an undifferentiated state. Many in vitro differentiation systems have been developed for mouse ES cells, including reproducible methods for mouse ES cell differentiation into haematopoietic and neural precursors, cardiomyocytes, insulin-secreting cells, endothelial cells and various other cell types. The derivation of new human ES cell lines provides the opportunity to develop unique models for developmental research and for cell therapies. In this review we consider the derivation and spontaneous differentiation of human ES cells.
Keywords: cell lines, embryo, embryoid bodies, stem cell technology
Introduction
During early mammalian embryonic development there is a short period when each cell of the developing embryo possesses the capacity to differentiate into every cell type of the adult body. After gastrulation, the embryos consist of specialized precursors of all three germ layers; thus the pluripotency of each individual cell is lost.
Embryonic stem (ES) cells are unique cells derived from the inner cell mass of the mammalian blastocyst. These cells are pluripotent and immortal, and can be continuously cultured in an undifferentiated state. The first mammalian ES cell lines were derived from mouse blastocyst in 1981 (Evans & Kaufman, 1981; Martin, 1981). Mouse ES cells have been shown to be able to integrate into all embryonic germ layers, including the germ line, following injection into blastocysts (Bradley et al. 1984) and develop into chimaeric animals. Some mouse ES lines can form entire viable fetuses and newborns when injected into heat-treated blastocysts or tetraploid embryos (Nagy et al. 1993; Amano et al. 2000).
Many in vitro differentiation systems have been developed for mouse ES cells. In recent years several groups have reported the generation of reproducible models of in vitro-directed differentiation of mouse ES cells into haematopoietic stem cell-like cells. Nakano et al. (1994) demonstrated their ability to generate haematopoietic precursor cells from ES cells by co-culturing these cells with stromal cells combined with various cytokines. Similarly, Keller’s group proved the existence of a common precursor for primitive erythrocytes and other cells of the haematopoietic lineages that developed within cultured embryoid bodies (EBs) (Kennedy et al. 1997).
Mouse ES cells can also be directed in controlled systems to differentiate into different neural precursors. McKay’s group developed in vitro conditions to induce the differentiation of mouse ES cells into glial and neural precursors, which can contribute to brain development after transplantation into rat embryo brains (Brüstle et al. 1997, 1999). Other directed differentiation models include using genetic manipulation in mouse ES cells to generate relatively pure populations of cardiomyocytes or insulin-secreting cells (Klug et al. 1996; Soria et al. 2000).
ES cell lines and ES cell-like lines have also been derived from other species, including other rodents such as the golden hamster (Doetschman et al. 1988), rat (Iannaccone et al. 1994) and rabbit (Giles et al. 1993; Graves & Moreadith, 1993), several domestic animal species (Notarianni et al. 1991; Sims & First, 1994; Wheeler, 1994; Mitalipova et al. 2001) and two non-human primate species (rhesus monkey and marmoset) (Thomson et al. 1995, 1996). Only the ES cells from mouse, rat, hamster and bovine have been shown to be able to create chimaeric animals following injection into blastocysts. However, only these single publications claimed successful derivation of ES-like cell lines from rodents (Doetschman et al. 1988; Iannaccone et al. 1994).
In this review we consider the derivation and spontaneous differentiation of human ES cells.
Derivation of human es cell lines
The first human ES cell lines were derived by Thomson et al. (1998). Five human ES cell lines were derived that fulfilled the criteria proposed by Thomson for the definition of primate ES cells: ‘(1) Derivation from the preimplantation embryo, (2) prolonged undifferentiated proliferation, and (3) stable developmental potential after prolonged culture to form derivatives of all three embryonic germ layers’ (Thomson & Marshall, 1998). For ethical reasons, the ability of human ES cells to contribute to embryonic development in chimaeric embryos cannot be examined. A year after the original report, Reubinoff and colleagues reported the derivation of two additional human ES cell lines (Reubinoff et al. 2000).
ES cell lines are usually derived by immunosurgery. In this process the trophoblast layer of the blastocyst is selectively removed, and the intact inner cell mass is further cultured on mitotically inactivated mouse embryonic fibroblasts (MEFs). This process is illustrated in Fig. 1. In our laboratory two human ES cell lines, I-3 and I-6, have been derived using rabbit anti-human whole antiserum (generously provided by Prof J. A. Thomson, University of Wisconsin), and a further pluripotent line, I-4, was derived by gentle removal of the trophoblast with 27G needles. Five donated frozen-thawed blastocysts were used to develop these three lines; Thomson et al. (1998) and Reubinoff et al. (2000) derived five lines from 14 blastocysts and two lines from four blastocysts, respectively. Thus, the overall efficiency of human ES cell line derivation is 43%, in contrast to the approximate 30% success rates in creating mouse ES cell lines (Robertson, 1987). The three ES lines developed in our laboratory have been grown continuously for over a year (more than 60 passages); I-6 is still continuously growing and has reached 77 passages. After more than 20 passages of continuous culture, karyotype analysis revealed that I-4 and I-3 are normal XX lines and I-6 has a normal XY karyotype. The lines were found to be strongly positive to surface markers typical for ES cells, stage-specific embryonic antigen 4 (SSEA4), TRA-1-60 and TRA-1-81 (examples illustrated in Fig. 2), with weakly positive staining for SSEA3 and negative staining for SSEA1 (the antibodies were generously provided by Prof P. Andrews, University of Sheffield). These results are consistent with the characteristics reported regarding existing human ES lines (Thomson et al. 1998; Reubinoff et al. 2000). Following injection into SCID-beige mice, the I-3 and I-6 lines created teratomas that contained representative tissues from all three embryonic germ layers. The I-4 line has still not been examined. All three lines formed EBs after their removal from the feeder layer or when grown in crowded cultures for more than 3 weeks. Thus these ES cell lines fulfil the criteria of human ES cells lines (Thomson et al. 1998; Reubinoff et al. 2000).
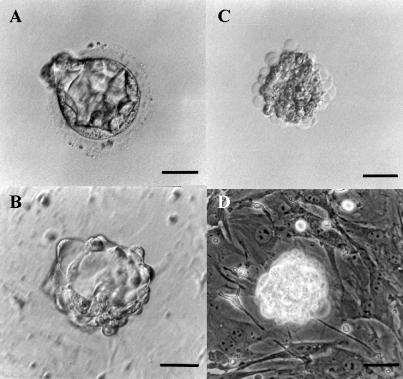
Fig. 1.
Immunosurgery of a human blastocyst for the derivation of human ES cell line. (A) Donated human embryo produced by in vitro fertilization at the blastocyst stage. (B) Human blastocyst after zona pellucida removal by Tyrode’s solution, during exposure to rabbit anti-human whole antiserum. (C) Embryo after exposure to guinea-pig complement. (D) Intact inner cell mass immediately after immunosurgery on mitotically inactivated mouse embryonic fibroblast feeder layer. Scale bar = 50 µm.
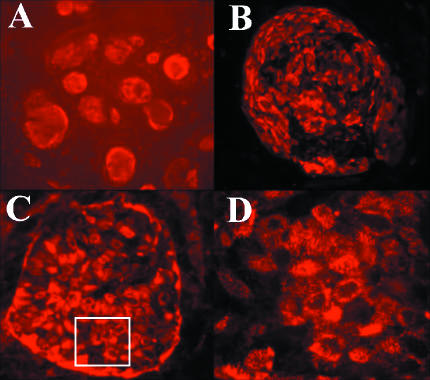
Fig. 2.
Fluorescent immunostaining of human ES cells. (A) Immunostaining of I-3 human ES cell colonies with anti-TRA-1-60 antibodies. ×5. (B) Immunostaining of I-6 human ES cell colony with anti-SSEA4 antibodies. ×20. (C) Immunostaining of I-6 human ES cell colony with anti-TRA-1-81. ×20. (D) High-power picture from the marked area in (C). ×63.
Tissue culture of embryonic stem cells
The classic methods used for culturing human ES cells have not changed dramatically since these procedures were described for mouse and primate ES cells (Robertson, 1987; Marshall et al. 2001). Human ES cells are cultured on mitotically inactivated MEFs using medium supplemented with 20% fetal bovine serum (FBS). Although primate ES cells require much more meticulous care than mouse ES cells (Marshall et al. 2001), they can be cultured in large numbers, frozen and thawed, using 10% DMSO together with 20% FBS or 30% serum replacer, with reasonable survival rates, or frozen with vitrification, which permits full recovery of the frozen colonies (Reubinoff et al. 2001a; Xu et al. 2001).
Further improvements in the basic culture methods used for human ES cell cultures have come from the ability to grow human ES cells under serum-free conditions, using serum replacer supplemented with basic fibroblast growth factor (bFGF). Under these better defined culture conditions, the clonality of the ES cells is higher than in media containing FBS (Amit et al. 2000).
Murine ES cells can be cultured without an MEF feeder layer, but with the addition of leukaemia inhibitory factor to the growth media. In contrast, initially it was impossible to grow human ES cells using the same feeder-free conditions (Thomson et al. 1998; Reubinoff et al. 2000). Recently, a scientific group from the Geron Corporation (Menlo Park, CA, USA) reported details of a unique culture system for growing human ES cells in feeder-free conditions (Xu et al. 2001). In their system human ES cells are grown on Matrigel matrix (Becton Dickinson, Bedford, MA, USA) with 100% MEF-conditioned medium supplemented with bFGF. Although that system still requires massive growth of MEFs for the production of conditioned medium, it has opened the door for the large-scale growth of human ES cells and may serve as the basis for completely MEF-free culture systems for human ES cells in the future.
Apart from the improved culture system, the serum-free growth conditions described above have been found to be suitable for the clonal derivation of human ES single-cell clones (Amit et al. 2000). To date, single-cell clones have been derived from five of the original reported parental ES cell lines: H-1, H-9, H-13 (Thomson et al. 1998), I-3 and I-6 (unpublished data). All single-cell clones fulfil the criteria described for primate ES cell lines (Thomson & Marshall, 1998). Besides proving that the parental ES cell lines consist of ‘true ES’ and not a combination of several committed multipotent cells, the single-cell clones form homogeneous populations of cells, which are easier to grow and manipulate.
Differentiation
In vivo, human ES cells have been shown to differentiate into representative tissues derived from all three embryonic germ layers, including bone tissue, cartilage tissue, striated muscle, gut-like structures, structures resembling fetal glomeruli, neural rosettes, etc. (Thomson et al. 1998; Reubinoff et al. 2000). In teratomas, human ES cells can also create more complex and well-organized organ-like structures, which requires co-operation between cells and tissues derived from different germ layers. An example of such an organ is seen in Fig. 3, which shows a salivary gland-like structure, including the exocrine secretory units and ducts and associated myoepithelial cells.
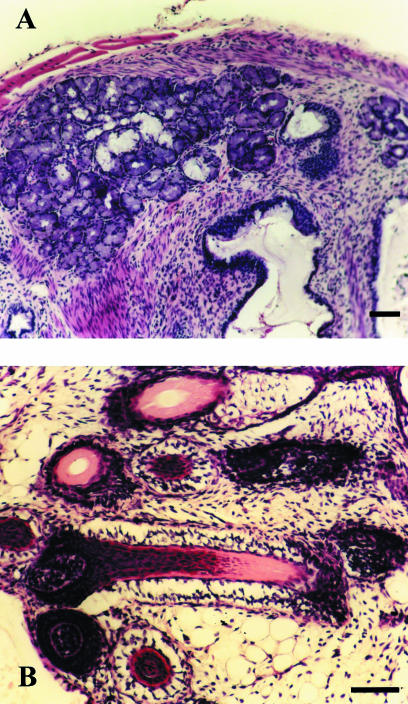
Fig. 3.
Differentiated human ES cells from subclone H-9.2.4 in teratomas. (A) Mixed salivary gland with serous and mucous secretory cells (H&E). (B) Group of developing hair follicles (H&E). Scale bar = 50 µm.
Such teratomas – involving models of ES cell differentiation – can be used to demonstrate the pluripotency of ES cell lines, and to explore the effects of genetic manipulation on the ES cells and their developmental potential; however, they cannot serve as a controlled system for ES cell differentiation. Controlled direct differentiation systems for human ES cells have to be developed in vitro. The first step in creating such systems involved creating a reproducible method for efficient formation of EBs from human ES cells (Itskovitz-Eldor et al. 2000). Formation of EBs was found to be an important step in in-vitro differentiation of mouse ES cells into haematopoietic cells (Keller, 1995), cardiomyocytes (Klug et al. 1996) and other cell types. As with murine ES cells, when human ES cells are cultured in suspension they spontaneously create EBs, including cystic EBs. Although these EBs have been found to be somewhat less organized than mouse ES cell-derived EBs, they do contain derivatives of the three embryonic germ layers (Itskovitz-Eldor et al. 2000). Some of the existing ES cell lines, however, do not create EBs (Reubinoff et al. 2000). The reason for this may be technical, or it may reflect differences in the developmental potential between different human ES cell lines. This subject needs further examination.
The next step in developing differentiation methods for human ES was to examine the abilities of eight different growth factors to induce differentiation of human ES cells (Schuldiner et al. 2000). Each of the growth factors used has a different effect on the differentiation of human ES cells, but none was able to direct homogeneous differentiation into a specific cell type. Nevertheless, that study demonstrated the possibility of manipulating human ES cell differentiation.
To date, several studies have been published on specific differentiation of human ES cells. Assady et al. (2001) demonstrated the ability of human ES cells to differentiate into insulin-secreting cells. These cells developed in growing EBs and immunostained positively for insulin. They also were shown to secrete insulin into the growth medium, and expressed beta-cell-specific genes. The insulin-secreting cells did not show a response to increasing concentrations of glucose and the ability of these cells to normalize glycaemia in diabetic mice was not examined. The conditions presented may provide the direction for further studies on the capacity of human ES cells to form insulin-secreting cells.
A reproducible method, based on spontaneous differentiation, was established by Kehat et al. (2001) for differentiation of human ES cells into cardiomyocytes. Cardiomyocytes derived from human ES cells demonstrated many characteristic features of cardiomyocytes, including typical myofibrillar organization consistent with early stage cardiomyocytes, positive staining with specific cardiomyocytic markers including anti-cardiac myosin heavy chain, anti-α-actinin, anti-desmin, and also expressed specific cardiac genes. In addition, the cardiomyocytes derived from human ES cells demonstrated physiological cardiac features. These innovative models can supply insight into early cardiac differentiation.
The first directed differentiation system for human ES cell differentiation into haematopoietic precursors was developed by Kaufman et al. (2001). These authors demonstrated that about 1–2% of examined cells showed CD34+/CD38− phenotype consistent with early haematopoietic cells.
Human ES cells have been shown to differentiate into neural precursors in serum-free conditions (Reubinoff et al. 2000). These give rise to neural cells expressing specific mature neural surface markers, including neurofilament protein and β-tubulin, and cells that synthesize glutamate and contain glutamic acid decarboxylase and the GABAA receptor α2 subunit. Other studies on the differentiation of human ES cells into neural precursors have demonstrated the incorporation of these precursors into brain tissue during brain development in a rat model (Reubinoff et al. 2001b; Zhang et al. 2001).
Overall, these studies have established the basic groundwork needed for creating models of differentiation of human ES cells, and they present the possibility of creating systems of human ES differentiation resembling the well-developed model existing for mouse ES cells.
Genetic manipulation
One of the most important milestones in the use of mouse ES cells for research has been the ability to manipulate these cells genetically. Efficient stable transfection methods may be needed for creating pure populations of cells, directed differentiation, marking the cells so that they can be recognized in histological slides, and examining the role of specific genes in embryonic development.
Surprisingly, human ES cells are relatively easy to transfect. Several promoters and transfection agents have been found to be useful, with different success rates, in transfecting human ES cells (Eiges et al. 2001). Our data have shown that stably transfected subclones can hold the transfected plasmids for more than a year in continuous culture, and after freezing/thawing cycles. The genes inserted were expressed both in undifferentiated cells and in cells differentiated in an EBs system (Fig. 4) or in teratomas (data not shown). Overall, these findings suggest that genetic manipulation may be introduced into human ES cells.
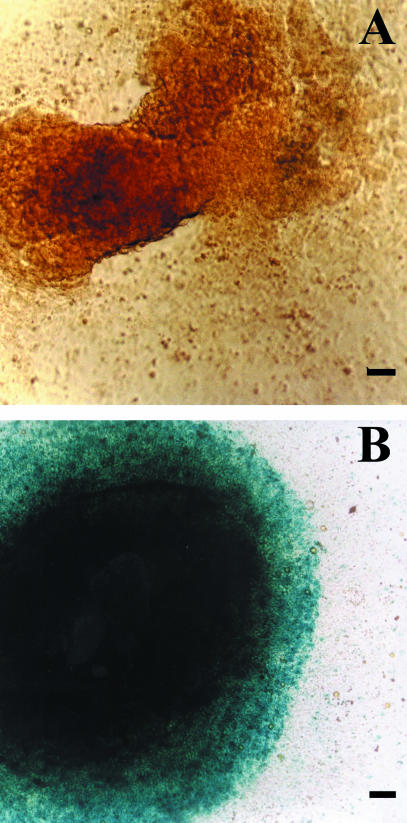
Fig. 4.
Attached EBs stained for Lac Z. (A) Negative control: EB from H-9.2 clone. (B) H-9.2.4 stably transfected (using FuGENE™6 transfection reagent, Roche, Germany) subclone expressing Lac Z gene, under phosphoglycerate kinase promoter control. Scale bar = 50 ∝m.
Conclusions
From their derivation in 1998 to date, human ES cells have been demonstrated to be able to create EBs and teratomas, and to differentiate into haematopoietic cells, insulin-secreting cells, cardiomyocytes and neurons. The basic methodology for large-scale culture and more defined culture conditions of human ES cells have been developed. Initial results indicate that human ES cells will be available for genetic manipulations. Thus there is growing evidence that human ES cells may be useful for cell therapies, tissue engineering and as a unique research tool for early human development and differentiation processes.
Acknowledgments
We thank Kohava Shariki, Victoria Margolitz and Ludmilla Mazor for technical assistance. We thank Professor Raymond Coleman for invaluable advice and assistance, and Ruth Singer for editing the manuscript. The research done in our laboratory was partly supported by the Fund for Medical Research and Development of Infrastructure and Health Services, Rambam Medical Center.
References
- Amano T, Nakamura K, Tani T, Kato Y, Tsunoda Y. Production of mice derived entirely from embryonic stem cells after injecting the cells into heat treated blastocysts. Theriogenology. 2000;200:1449–1458. doi: 10.1016/S0093-691X(00)00287-9. [DOI] [PubMed] [Google Scholar]
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL, Tzukerman M. Insulin production by human embryonic stem cells. Diabetes. 2001;50:1691–1697. doi: 10.2337/diabetes.50.8.1691. [DOI] [PubMed] [Google Scholar]
- Bradley A, Evans M, Kaufman MH, Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–256. doi: 10.1038/309255a0. [DOI] [PubMed] [Google Scholar]
- Brüstle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, et al. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- Brüstle O, Spiro AC, Karram K, Choudhary K, Okabe S, Mckay RDG. In vitro-generated neural precursors participate in mammalian brain development. Proc. Natl Acad. Sci. USA. 1997;94:14809–14814. doi: 10.1073/pnas.94.26.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetschman T, Williams P, Maeda N. Establishment of hamster blastocyst-derived embryonic stem (ES) cells. Dev. Biol. 1988;127:224–227. doi: 10.1016/0012-1606(88)90204-7. [DOI] [PubMed] [Google Scholar]
- Eiges R, Schuldiner M, Drukker M, Yanuka O, Itskovitz-Eldor J, Benvenisty N. Establishment of human embryonic stem cell-transfected clones carrying a marker for undifferentiated cells. Current Biol. 2001;11:514–518. doi: 10.1016/s0960-9822(01)00144-0. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Giles JR, Yang X, Mark W, Foote RH. Pluripotency of cultured rabbit inner cell mass cells detected by isozyme analysis and eye pigmentation of fetuses following injection into blastocysts or morulae. Mol. Reprod. Dev. 1993;36:130–138. doi: 10.1002/mrd.1080360203. [DOI] [PubMed] [Google Scholar]
- Graves KH, Moreadith RW. Derivation and characterization of putative pluripotential embryonic stem cells from preimplantation rabbit embryos. Mol. Reprod. Dev. 1993;36:424–433. doi: 10.1002/mrd.1080360404. [DOI] [PubMed] [Google Scholar]
- Iannaccone PM, Taborn GU, Garton RL, Caplice MD, Brenin DR. Pluripotent embryonic stem cells from the rat are capable of producing chimeras. Dev. Biol. 1994;163:288–292. doi: 10.1006/dbio.1994.1146. [DOI] [PubMed] [Google Scholar]
- Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Ynuka O, Amit M, et al. Differentiation of human embryonic stem cells into embryoid bodies comprising the three embryonic germ layers. Mol. Med. 2000;6:88–95. [PMC free article] [PubMed] [Google Scholar]
- Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Haematopoietic colony-forming cells derived from human embryonic stem cells. Proc. Natl Acad. Sci. USA. 2001;98:10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J. Clin. Invest. 2001;108:407–414. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller GM. In vitro differentiation of embryonic stem cells. Current Opinion Cell Biol. 1995;7:862–869. doi: 10.1016/0955-0674(95)80071-9. [DOI] [PubMed] [Google Scholar]
- Kennedy M, Firpo M, Choi K, Wall C, Robertson S, Kabrun N, Keller G. A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature. 1997;386:488–493. doi: 10.1038/386488a0. [DOI] [PubMed] [Google Scholar]
- Klug MG, Soonpaa MH, Koh GY, Field LJ. Genetically selected cardiomyocytes from differentiating embryonic stem cells form stable intracardiac grafts. J. Clin. Invest. 1996;98:216–224. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall VS, Waknitz MA, Thomson JA. Isolation and maintenance of primate embryonic stem cells. Meth. Mol. Biol. 2001;158:11–18. doi: 10.1385/1-59259-220-1:11. [DOI] [PubMed] [Google Scholar]
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl Acad. Sci. USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitalipova M, Beyhan Z, First NL. Pluripotency of bovine embryonic stem cell line derived from precompacting embryos. Cloning. 2001;3:59–67. doi: 10.1089/15204550152475563. [DOI] [PubMed] [Google Scholar]
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl Acad. Sci. USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T, Kodama H, Honjo T. Generation of lymphohaematopoietic cells from embryonic stem cells in culture. Science. 1994;265:1098–1101. doi: 10.1126/science.8066449. [DOI] [PubMed] [Google Scholar]
- Notarianni E, Galli C, Laurie S, Moor RM, Evans MJ. Derivation of pluripotent, embryonic cell lines from the pig and sheep. J. Reprod. Fertil.-Supplement. 1991;43:255–260. [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong C, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nature Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Itsykson P, Turetsky T, Pera MF, Reinhartz E, Itzik A, et al. Neural progenitors from human embryonic stem cells. Nature Biotechnol. 2001a;19:1134–1140. doi: 10.1038/nbt1201-1134. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Vajta G, Trounson AO. Effective cryopreservation of human embryonic stem cells by the open pulled straw vitrification method. Human Reprod. 2001b;16:2187–2194. doi: 10.1093/humrep/16.10.2187. [DOI] [PubMed] [Google Scholar]
- Robertson EJ. Embryo-derived stem cell lines. In: Robertson EJ, editor. Teratocarcinomas and Embryonic Stem Cells: a Practical Approach. Oxford: IRL Press; 1987. pp. 71–112. [Google Scholar]
- Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc. Natl Acad. Sci. USA. 2000;97:11307–11312. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims M, First NL. Production of calves by transfer of nuclei from cultured inner cell mass cells. Proc. Natl Acad. Sci. USA. 1994;91:6143–6147. doi: 10.1073/pnas.91.13.6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria B, Roche E, Berná G, León-Quinto T, Reig JA, Martín F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes. 2000;49:157–162. doi: 10.2337/diabetes.49.2.157. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Becker RA, Hearn JP. Isolation of a primate embryonic stem cell line. Proc. Natl Acad. Sci. USA. 1995;92:7844–7848. doi: 10.1073/pnas.92.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Hearn JP. Pluripotent cell lines derived from common marmoset (Callithrix jacchus) blastocysts. Biol. Reprod. 1996;55:254–259. doi: 10.1095/biolreprod55.2.254. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Marshall VS. Primate embryonic stem cells. Current Topics Dev. Biol. 1998;38:133–165. doi: 10.1016/s0070-2153(08)60246-x. [DOI] [PubMed] [Google Scholar]
- Wheeler MB. Development and validation of swine embryonic stem cells: a review. Reprod. Fertil. Dev. 1994;6:563–568. doi: 10.1071/rd9940563. [DOI] [PubMed] [Google Scholar]
- Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nature Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- Zhang S-C, Wernig M, Duncan ID, Brüstle O, Thomson JA. In Vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nature Biotechnol. 2001;19:1129–1133. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]