Abstract
We have analysed the surface antigen phenotype of a human embryonic stem (hES) cell line (H7) and the changes that occur upon differentiation induced by retinoic acid, hexamethylene bisacetamide and dimethylsulphoxide. The undifferentiated stem cells expressed Stage Specific Embryonic Antigen-3 (SSEA3), SSEA4, TRA-1-60, and TRA-1-8 but not SSEA1. In these characteristics they closely resemble human embryonal carcinoma (EC) cells derived from testicular teratocarcinomas, and are distinct from murine EC and ES cells. The undifferentiated cells also expressed the liver/bone/kidney isozyme of alkaline phosphatase detected by antibody TRA-2-54, the class 1 major histocompatability antigens, HLA-ABC, and the human Thy1 antigen. Differentiation of hES cells was induced by retinoic acid, HMBA and DMSO with the appearance of various cell types including neurons and muscle cells. The surface antigens characteristically expressed by hES cells were down-regulated following induction of differentiation and other antigens appeared, notably several ganglioside glycolipids detected by antibodies VIN-IS-56 (GD3 and GD2), VIN-2PB-22 (GD2), A2B5 (GT3) and ME311 (9-O-acetyl-GD3). Whereas the expression of HLA was slightly down-regulated upon differentiation, its expression was strongly induced by interferon-γ in both the undifferentiated and the differentiated cells, although the induction in the differentiated cultures was considerably stronger than in the stem cells. In all of these features the human ES cells, and their pattern of differentiation, resembled the pluripotent human EC cell line NTERA-2 although clearly the range of cells generated by the hES cells was considerably greater.
Keywords: stem cells, surface antigens
Introduction
EC cells are the malignant stem cells of teratocarcinomas, a subset of germ cell tumours that may contain many embryonic and extra-embryonic tissues, and apparently recapitulate many aspects of cell differentiation that occur during normal embryogenesis, albeit in a disorganized manner. Both spontaneous and experimentally induced teratocarcinomas occur in the laboratory mouse (Solter & Damjanov, 1979). By now it is well documented that murine EC cells isolated from such teratocarcinomas closely resemble cells of the inner cell mass from the blastocyst stage of the early mouse embryo, and embryonic stem (ES) cells cultured directly from explanted blastocysts (Martin, 1980). For example, when mouse EC and ES cells are placed into a blastocyst they can participate in embryonic development, and contribute to normal tissues in the resulting chimeric embryo (Brinster, 1974; Papaioannou et al. 1975). ES cells form chimeras much more efficiently than EC cells, but this may well reflect the accumulation of genetic and epigenetic changes that occur in EC cells as they adapt to efficient tumour growth.
Until the recent derivation of human ES cell lines, human EC cell lines provided the best model for studying cell differentiation pertinent to human embryonic development (Andrews, 1998). For example, the human teratocarcinoma-derived cell line NTERA2 is composed of pluripotent EC cells that differentiate in response to retinoic acid, yielding a variety of cell types including functional, post-mitotic neurons (Andrews et al. 1984b; Andrews, 1984; Rendt et al. 1989; Pleasure et al. 1992; Squires et al. 1996). This differentiation is characterized by marked changes in gene expression (Ackerman et al. 1994) but most notably by the activation of HOX genes in a retinoic acid concentration-dependent manner (Simeone et al. 1990). However, changes in the expression of cell surface antigens provide a particularly powerful means of monitoring differentiation, not only because antigen expression can be readily assessed on single cells in complex differentiating populations, but because subsets of viable cells corresponding to different stages of differentiation, or different lineages, can be isolated in order to analyse their individual properties and capacity for further differentiation (Fenderson et al. 1987).
Human EC cells, like NTERA2, are characterized by expression of the globoseries glycolipid antigens SSEA3 and SSEA4, and by a lack expression of a lactoseries oligosaccharide antigen, SSEA1 (Andrews et al. 1982, 1984b, 1996). They also express the keratan sulphate-related antigens, TRA-1-60 and TRA-1-81 (Andrews et al. 1984a; Badcock et al. 1999) and the tissue non-specific alkaline phosphatase-related antigens TRA-2-49 and TRA-2-54 (Andrews et al. 1984c). Differentiation induced by retinoic acid is marked by the down-regulation of all of these antigens, and by the appearance of other antigens including SSEA1 and several ganglioseries glycolipid antigens, which segregate on different subsets of cells (Fenderson et al. 1987). If differentiation is induced by another agent, hexamethylene bisacetamide (HMBA), the EC-specific antigens are similarly down-regulated, but the antigens induced by retinoic acid do not appear, consistent with the notion that retinoic acid and HMBA induce differentiation along distinct lineages (Andrews et al. 1990).
The recent derivation of ES cell lines from human embryos (Thomson et al. 1998; Reubinoff et al. 2000) now provides additional tools for investigating the molecular processes that guide human development and the causes of infertility and birth defects when these mechanisms operate incorrectly. Further, human ES cells could provide a potential source of ‘normal’ differentiated cell types for transplantation therapies and drug discovery.
Initial studies have confirmed the anticipated similarities between human ES and EC cells, and significant differences in antigen expression and other properties between human ES and EC cells and their murine counterparts (Andrews, 1998). We have now examined the changes in surface antigen expression that occur during the differentiation of human ES cells induced by retinoic acid, HMBA or DMSO, or by culture in the absence of feeders. As anticipated, a number of changes that occur during EC cell differentiation, notably down-regulation of stem-cell-specific markers, also occurred during the differentiation of ES cells. There were also some differences in the patterns of antigens induced, mostly likely because of the greater diversity of differentiated cell types that appear during ES cell differentiation.
Materials and methods
Cell culture
The H7 human ES (hES) cell line, described by Thomson et al. (1998), was cultured on mouse embryonic fibroblast (MEF) feeder cells, mitotically inactivated using Mitomycin-C, as previously described. However, the cells were cultured in DMEM-SR medium (Gibco-BRL) supplemented with 4 ng mL−1 bFGF (Gibco-BRL) and 20% ‘Serum Replacement’ (SR) (Gibco-BRL) instead of fetal calf serum. Briefly, MEFs isolated from 13-day mouse embryos (Robertson, 1987) were treated with 10 µg mL−1 mitomycin-C (Sigma Aldrich) for 2.5 h. Subsequently, the treated cells were washed with PBS, harvested with trypsin-EDTA and reseeded at 104 cells per cm2 on gelatin-treated tissue culture dishes. For passaging, the H7 hES cells were treated with 1 mg mL−1 Collagenase type IV (Sigma Aldrich) in DMEM:F12 for 8–10 min at 37 °C and then detached by scraping using glass beads (Andrews et al. 1984b), washed by centrifugation, and re-plated onto inactivated MEFs. In some experiments, H7 hES cells were plated on tissue culture dishes that had been pretreated with poly D-lysine and Matrigel (Becton-Dickinson) in the absence of inactivated MEFs. Differentiation was induced by adding 10−5 m all trans-retinoic acid (RA) (Eastman Kodak), 3 mm hexamethylene bisacetamide (HMBA) (Sigma Aldrich), or 1% dimethylsulphoxide (DMSO) as described by Andrews (1984), Andrews et al. (1990) and Andrews et al. (1986), respectively. Human interferon-γ (IFNγ) was purchased from Sigma Aldrich.
NTERA2 cL.D1 human EC cells (Andrews et al. 1984b) were cultured as previously described and induced to differentiate by treatment with 10−5 m all-trans retinoic acid (RA) (Eastman Kodak) (Andrews, 1984).
Surface antigen expression
Cell surface antigen expression was assessed by immunofluorescence detected by flow cytofluorimetry after harvesting cultures as single cell suspensions using trypsin-EDTA, as previously described (Andrews et al. 1987a, 1987b; Fenderson et al. 1987). The following monoclonal antibodies were used to detect surface antigen expression: MC631, anti-Stage Specific Embryonic Antigen-3 (SSEA3) (Shevinsky et al. 1982), MC813-70, anti-Stage Specific Embryonic Antigen-4 (SSEA4) (Kannagi et al. 1983), MC480, anti-Stage Specific Embryonic Antigen-1 (SSEA1) (Solter & Knowles, 1978), TRA-1-60 and TRA-1-81 (Andrews et al. 1984a), TRA-2-54, anti-liver/kidney/bone alkaline phosphatase (Andrews et al. 1984c), A2B5 (Eisenbarth et al. 1979; Fenderson et al. 1987), ME311 (Thurin et al. 1985; Fenderson et al. 1987), VIN-IS-56 and VIN-2B-22 (Andrews et al. 1990), N901 (Griffin et al. 1983) and anti-Thy1 (McKenzie & Fabre, 1981), W6/32 (Barnstable et al. 1978), BBM1 (Brodsky et al. 1979). TRA-1-85 (Williams et al. 1988), which detects a pan-human antigen, was used to monitor contamination with mouse feeder cells.
Results
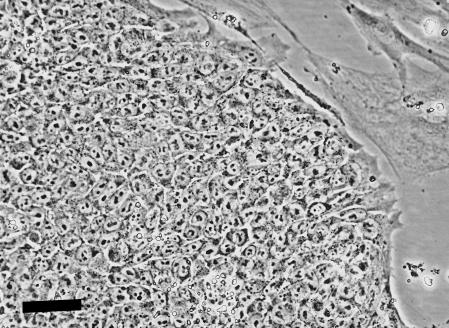
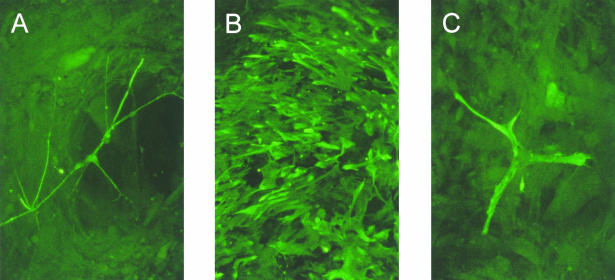
Cultures of H7 hES cells grown on mouse embryo fibroblast feeders contained many colonies of cells that closely resembled human EC cells (Fig. 1). If the cells were plated onto tissue culture dishes precoated with poly D-lysine and Matrigel, but without MEFs, many of the cells appeared to differentiate and many colonies of cells with markedly different morphologies appeared, although occasional colonies of cells retaining an ES phenotype persisted. If all-trans-retinoic acid was included into the cultures, either grown on feeder layers, or after plating on Matrigel, even more marked differentiation occurred and a variety of cell types, for example neurons, glia and muscle cells, could be detected by immunofluorescence staining with appropriate antibodies (Fig. 2).
Fig. 1.
Phase contrast photomicrograph of an undifferentiated human ES cell colony. The cells resembled human EC cells morphologically, forming tight colonies composed of cells with high nucleus/cytoplasm ratios and containing few prominent nucleoli. Scale bar = 50 µm.
Fig. 2.
Neurons, glial and muscle cells detected by immunofluorescence with antibodies to neurofilaments (A), GFAP (B) and desmin (C) were found in the hES H7 cultures grown in the presence of 10−5 m retinoic acid.
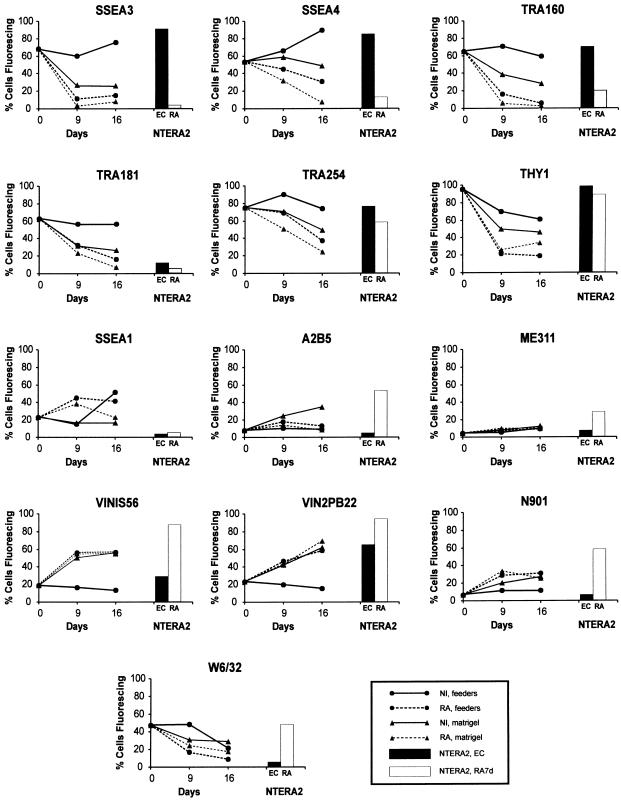
We then examined the expression of a panel of antigens previously analysed on human EC cells (Fig. 3). As anticipated from previous reports, the hES cells from stock cultures, at the time of reseeding, strongly expressed the markers that also characterize undifferentiated EC cells, namely SSEA3, SSEA4, TRA-1-60 and TRA-1-81. They also strongly expressed Thy1, and the liver/bone/kidney isozyme of alkaline phosphatase detected by reactivity with antibody TRA-2-54. By contrast, they expressed relatively low levels of SSEA1. These characteristics are typical of the surface antigen phenotype of human EC cells and distinct in certain respects from murine EC cells. The proportion of feeder cells at the time of assay, monitored by reactivity with an antibody to a pan-human antigen, TRA-1-85, was negligible (< 2%) (data not shown).
Fig. 3.
Changes in surface antigen expression by hES H7 cells when cultured on MEF feeders or Matrigel, in the presence or absence of 10−5 m retinoic acid. For comparison typical antigen expression by NTERA2 EC cells and NTERA2 cells induced to differentiate with retinoic acid for 7 days are also shown.
During subsequent culture, expression of SSEA3, SSEA4, TRA-1-60, TRA-1-81, TRA-2-54 and Thy1 remained high when the ES cells were cultured on MEFs in the absence of retinoic acid. However, these antigens were down-regulated when the cells were plated on Matrigel in the absence of MEFs, or when they were cultured in the presence of retinoic acid, whether on MEFs or on Matrigel. Such changes are consistent with the differentiation of the cells, as suggested by morphological examination, and are comparable to changes seen during the differentiation of human EC cells.
The kinetics of disappearance of these antigens differed from one another. In particular, SSEA3 disappeared more quickly than SSEA4, a result which we have consistently found before in the case of differentiating human EC cells (Fenderson et al. 1987). TRA-1-60 and TRA-1-81 also disappeared rapidly, whereas TRA-2-54 and Thy1 tended to disappear relatively slowly. Whether this reflects persistent expression on a subset of differentiating cells or a slower turnover of the antigens was not assessed. A further notable point was that although the EC/ES markers were down-regulated when the cells were cultured on Matrigel in the absence of retinoic acid, a significant proportion of cells that continued expressing these antigens nevertheless persisted, suggesting that at least during short-term culture the presence of MEFs is not an absolute requirement for maintenance of an undifferentiated phenotype.
Differentiation of the NTERA2 EC cell line is marked by transient up-regulation of SSEA1 and a strong up-regulation of gangliosides, notably GT3 detected by A2B5, all consistent with a marked propensity of these cells to differentiate in a neuroectodermal direction (Fenderson et al. 1987). In the case of hES cells, the gangliosides GD3 and GD2 detected by antibodies VIN-IS-56 and VIN-2PB-22 were also up-regulated when the cells were cultured either without feeders or after addition of retinoic acid, but only relatively small numbers of cells expressed antigens detected by A2B5 (GT3) and ME311 (9-O-acetyl-GD3). In fact the greatest induction of these antigens appeared to occur without retinoic acid, when the cells were plated on Matrigel. SSEA1(+) cells were seen after culture on Matrigel and, even more after the addition of retinoic acid, although their numbers subsequently declined. The antigen detected by antibody N901, CD56, a version of NCAM, was up-regulated after addition of retinoic acid and to some extent after culture on Matrigel in the absence of retinoic acid.
The presence or absence of bFGF in the medium during differentiation had no significant effect on the pattern of antigen expression (data not shown).
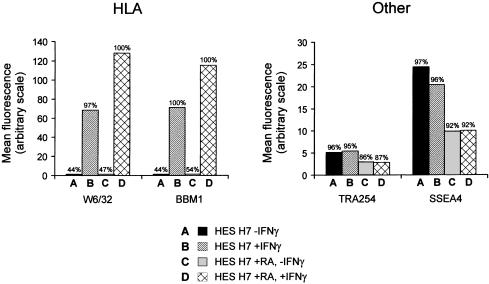
The class 1 Major Histocompatibility Complex (MHC) antigens (HLA) are commonly expressed by human EC cells, in contrast to their absence from murine EC and ES cells (Andrews, 1998). HLA-A, B, C, the class 1 MHC antigen detected by antibody W6/32, was expressed by the undifferentiated H7 hES cells (Fig. 3), and some down-regulation was noted after differentiation induced with retinoic acid or on Matrigel or indeed on extended cultures. In human EC cells, HLA is strongly inducible by IFNγ without any obvious effects on differentiation, and is inducible to a higher level after differentiation. We have now found that the same is true of the H7 hES cells (Fig. 4). Thus, HLA-A, B, C, detected by W6/32, and β2 microglobulin, detected by antibody BBM1, were both much more strongly expressed after culture of the cells for 3 days in the presence of IFNγ. However, considerably stronger expression was noted on cells that had been pretreated with retinoic acid. The effect was specific to HLA; the expression of other surface antigens, for example, TRA-2-54 and SSEA4, was not affected by culture in the presence of IFNγ suggesting that there was no effect on the state of differentiation of the cells.
Fig. 4.
The effect of IFNγ on the expression of HLA (HLA-A,B,C and β2 microglobulin) and the antigens (alkaline phosphatase and SSEA4) by hES H7 cells. The histograms show the mean fluorescence intensity of cells after staining with antibodies W6/32 (anti HLA-A,B,C), BBM1 (anti-β2 microglobulin), TRA-2-54 (anti-liver/bone/kidney alkaline phosphatase) and MCX813-70 (anti-SSEA4); the percentage of cells fluorescing is indicated above each bar.
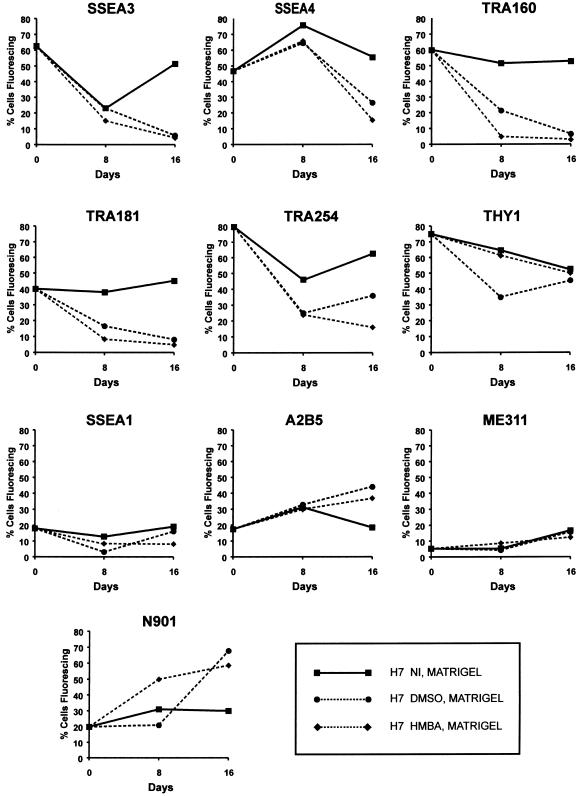
Although retinoic acid is, perhaps, the most widely used agent to induce differentiation of EC cells and ES cells, in both the mouse and human, other agents are also known to cause undifferentiated pluripotent cells to differentiate. Notably, HMBA induces differentiation of mouse (Jakob et al. 1978) and human EC cells (Andrews et al. 1990), while DMSO which induces differentiation of mouse EC cells (McBurney et al. 1982) has very little effect on, at least, the NTERA-2 human EC cell line (Andrews et al. 1986). When H7 hES cells were cultured in the presence of 1% DMSO or 3 mm HMBA (Fig. 5) after plating on Matrigel, without MEFs, significant differentiation was indicated by changes in antigen expression. All the antigens characteristic of human EC and ES cells, SSEA3, SSEA4, TRA-1-60, TRA-1-81, TRA 2–54 and Thy1, were strongly down-regulated in cultures exposed to the DMSO and HMBA. Again, SSEA3 disappeared rather more rapidly than SSEA4. At the same time antigens detected by antibodies A2B5, ME311 and N901 were also all induced by both DMSO and HMBA. Indeed, A2B5 reactivity appeared to be induced to a greater extent by DMSO than by retinoic acid. Neither agent induced SSEA1 to any significant extent. Thus, as with EC cells, HMBA and DMSO appear to induce differentiation along lineages to some extent distinct from those induced by retinoic acid.
Fig. 5.
Surface antigen expression by hES. H7 cells were cultured on Matrigel in the presence, or absence, of 3 mm HMBA or 1% (v/v) DMSO.
Discussion
It has not always been evident that EC and ES cells in humans are related cell types. Although in the laboratory mouse EC cells do closely resemble ES cells, and may be considered their malignant counterparts, human EC cells differ in many respects from murine EC and ES cells. Thus the cell surface antigen phenotype of human EC cells is typically SSEA1(–) but SSEA3(+) and SSEA4(+), differing from that of murine EC cells, which are typically SSEA1(+) but SSEA3(–) and SSEA4(–) (Andrews, 1998). A further difference is the definite but low level expression of the class 1 MHC antigens by human EC cells, but not by mouse EC cells (Andrews et al. 1981, 1982, 1984b, 1996). Because of these differences from mouse EC and ES cells it was unclear whether human ES cells derived from human embryos would prove distinct from human EC cells from teratocarcinomas, or whether human ES and EC cells would resemble one another, so that the differences from the mouse would represent differences between the embryos of the two species.
Recent reports, as well as the present study, confirm the similarity of human EC and ES cells, and the existence of differences between comparable cells of humans and mice. Thus, human EC and ES cells are both characterized by their expression of SSEA3, SSEA4, TRA-1-60, TRA-1-81 antigens, as well as the liver/bone/kidney isozyme of alkaline phosphatase (detected by TRA-2-54) and human Thy1 antigen. We have now also demonstrated that these antigens are down-regulated during the differentiation of human ES cells, and that several antigens induced during the differentiation of human EC cells are also induced during the differentiation of ES cells. On the other hand, there are some differences. Whereas several gangliosides are induced during differentiation of human ES cells as detected by antibodies VIN-15-56 and VIN-2PB-22 (GD3 and GD2), other ganglioside antigens that are strongly expressed during NTERA2 human EC differentiation, GT3 detecting A2B5 and 9-O-acetyl-GD3 detecting ME311, were only induced to relatively low levels. Most likely, this reflects rather more heterogeneity within the differentiating human ES cultures. The marked ganglioside expression is most likely particularly associated with neuroectodermal differentiation which occurs in both hES and NTERA2 cultures. However, whereas the hES cells clearly generate muscle and mesodermal derivatives, there is no evidence that NTERA2 is capable of mesodermal differentiation (Gokhale et al. 2000)
The expression of HLA by the human ES cells and its tendency for slight down-regulation upon differentiation is another feature that they share in common with human EC cells. In both cases HLA is strongly induced by IFNγ while amongst the differentiated cultures, the induction of HLA by IFNγ is considerably greater than in the undifferentiated cells. Indeed, IFNγ can also induce the expression of MHC antigens in some mouse EC cells, even though expression is generally not detectable in the absence of this inducer. In both murine and human EC cells it has been shown that these cells only exhibit a partial response to IFNγ without a fully fledged antiviral response (Andrews et al. 1987).
Surface antigen markers provide invaluable tools for monitoring the progress of differentiation and isolate subsets of cells to explore their functions and capacity for further differentiation. Many of the antigens we have studied are associated with carbohydrate epitopes, some linked with glycolipids (e.g. the SSEA series), some with glycoproteins (TRA-1-60 and TRA-1-81). Others are associated with protein epitopes, notably TRA-2-54 (alkaline phosphatase), Thy1 and N901 (NCAM, CD56). The function of the carbohydrate antigens is particularly uncertain in the light of differences in expression between corresponding human and mouse cells. Despite the lack of a clear function for the carbohydrate antigens, a considerable number of genes are devoted to encoding glycosyltransferases, and the expression of these antigens during embryogenesis and cell differentiation is very carefully controlled. It has been suggested that the core structures of these carbohydrates, rather than the terminal structures commonly recognized as epitopes by the various antibodies used in these studies, are the key molecules to affect cell behaviour (Fenderson et al. 1987). Although there are differences in expression of SSEA1, SSEA3 and SSEA4 between mouse and human, globoseries structures are a common feature of these cells in both species: although murine EC cells do not express SSEA3 and SSEA4 they do strongly express the Forsman antigen, another globoseries structure that is absent from human cells (Willison et al. 1982). Some carbohydrate antigens, however, have been shown to have a function. For example, the Lewis-X (Lex) structure recognized as SSEA1 (Gooi et al. 1981) may be involved in compaction at the morula stage of mouse embryo development (Bird & Kimber, 1984; Fenderson et al. 1984). A role for the carbohydrate structures has also been postulated in axon guidance in the developing nervous system (Dodd & Jessell, 1986), and we previously proposed that the ability of cytomegalovirus to induce inappropriate expression of SSEA1 might provide a partial explanation for its ability to disrupt neural development in infected fetuses (Andrews et al. 1989).
Our present study highlights both similarities and differences between EC and ES cells, from humans, and between these cell types in humans and mice. EC cells are caricatures of ES cells, adapted for tumour growth, and indeed many human EC cells do not differentiate significantly, presumably reflecting the selective advantage of accumulated mutations that interfere with differentiation (Andrews & Goodfellow, 1980; Duran et al. 2001). Nevertheless, the similarities of differentiation demonstrated by surface antigen changes between human EC cells that do differentiate, like NTERA-2, and hES cells is notable. The patterns induced by RA and HMBA are comparable, even if DMSO does not seem to be an effective inducer of the EC cells. It is also notable that neuroectoderm differentiation is a common feature of EC and hES differentiation, even though the hES cells evidently generate a greater diversity of cell types. Therefore, while it is clear that studies of hES cells can provide insights into the process of development in a way pertinent to human embryogenesis, human EC cells can in some circumstances provide convenient surrogates, lessons from which can be applied to understanding ES cell biology.
Acknowledgments
This work was supported in part by a grant 016150 from the Wellcome Trust.
Reference
- Ackerman SL, Knowles BB, Andrews PW. Gene regulation during neuronal and non-neuronal differentiation of NTERA2 human teratocarcinoma-derived stem cells. Mol. Brain Res. 1994;200:157–162. doi: 10.1016/0169-328x(94)90293-3. [DOI] [PubMed] [Google Scholar]
- Andrews PW, Goodfellow PN. Antigen expression by somatic cell hybrids of a murine embryonal carcinoma cell with thymocytes and L cells. Somat. Cell Genet. 1980;6:271–284. doi: 10.1007/BF01538801. [DOI] [PubMed] [Google Scholar]
- Andrews PW, Knowles BB, Goodfellow PN. A human cell surface antigen defined by a monoclonal antibody and controlled by a gene on chromosome 12. Somat. Cell Genet. 1981;7:435–443. doi: 10.1007/BF01542988. [DOI] [PubMed] [Google Scholar]
- Andrews PW, Goodfellow PN, Shevinsky L, Bronson DL, Knowles BB. Cell surface antigens of a clonal human embryonal carcinoma cell line: Morphological and antigenic differentiation in culture. Int. J. Cancer. 1982;29:523–531. doi: 10.1002/ijc.2910290507. [DOI] [PubMed] [Google Scholar]
- Andrews PW. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev. Biol. 1984;103:285–293. doi: 10.1016/0012-1606(84)90316-6. [DOI] [PubMed] [Google Scholar]
- Andrews PW, Banting GS, Damjanov I, Arnaud D, Avner P. Three monoclonal antibodies defining distinct differentiation antigens associated with different high molecular weight polypeptides on the surface of human embryonal carcinoma cells. Hybridoma. 1984a;3:347–361. doi: 10.1089/hyb.1984.3.347. [DOI] [PubMed] [Google Scholar]
- Andrews PW, Damjanov I, Simon D, Banting G, Carlin C, Dracopoli NC, et al. Pluripotent embryonal carcinoma clones derived from the human teratocarcinoma cell line Tera-2: Differentiation in vivo and in vitro. Lab. Invest. 1984b;50:147–162. [PubMed] [Google Scholar]
- Andrews PW, Meyer LJ, Bednarz KL, Harris H. Two monoclonal antibodies recognizing determinants on human embryonal carcinoma cells react specifically with the liver isozyme of human alkaline phosphatase. Hybridoma. 1984c;3:33–39. doi: 10.1089/hyb.1984.3.33. [DOI] [PubMed] [Google Scholar]
- Andrews PW, Oosterhuis JW, Damjanov I. Cell lines from human germ cell tumours. In: Robertson EJ, editor. Teratocarcinomas and Embryonic Stem Cells: a Practical Approach. Oxford: IRL Press; 1987a. pp. 207–248. [Google Scholar]
- Andrews PW, Trinchieri G, Perussia B, Baglioni C. Induction of class 1 major histocompatibility complex antigens in human teratocarcinoma cells by interferon without induction of differentiation, growth inhibition or resistance to viral infection. Cancer Res. 1987b;47:740–746. [PubMed] [Google Scholar]
- Andrews PW, Gönczöl E, Plotkin SA, Dignazio M, Oosterhuis JW. Differentiation of TERA 2 human embryonal carcinoma cells into neurons and HCMV – permissive cells: induction by agents other than retinoic acid. Differentiation. 1986;31:119–126. doi: 10.1111/j.1432-0436.1986.tb00392.x. [DOI] [PubMed] [Google Scholar]
- Andrews PW, Gönczöl E, Fenderson BA, Holmes EH, O’Malley G, Hakomori S-I, et al. Human cytomegalovirus induces stage-specific embryonic antigen-1 in differentiating human teratocarcinoma cells and fibroblasts. J. Exp. Med. 1989;169:1347–1359. doi: 10.1084/jem.169.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PW, Casper J, Damjanov I, Duggan-Keen M, Giwercman A, Hata JI, et al. Comparative analysis of cell surface antigens expressed by cell lines derived from human germ cell tumours. Int. J. Cancer. 1996;66:806–816. doi: 10.1002/(SICI)1097-0215(19960611)66:6<806::AID-IJC17>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Andrews PW. Teratocarcinomas and human embryology: pluripotent human EC cell lines. Acta Pathol. Microbiol. Immunol. Scand. 1998;106:158–168. doi: 10.1111/j.1699-0463.1998.tb01331.x. [DOI] [PubMed] [Google Scholar]
- Andrews PW, Nudelman E, Hakomori S-i, Fenderson BA. Different patterns of glycolipid antigens are expressed following differentiation of TERA-2 human embryonal carcinoma cells induced by retinoic acid, hexamtehylene bisacetamide (HMBA) or bromodeoxyuridine (BUdR) Differentiation. 1990;43:131–138. doi: 10.1111/j.1432-0436.1990.tb00439.x. [DOI] [PubMed] [Google Scholar]
- Badcock G, Pigott C, Goepel J, Andrews PW. The Human Embryonal Carcinoma Marker Antigen TRA-1-60 Is A Sialylated Keratan Sulphate Proteoglycan. Cancer Res. 1999;59:4715–4719. [PubMed] [Google Scholar]
- Barnstable CJ, Bodmer WF, Brown F, Galfe G, Milstein C, Williams AF, et al. production of monoclonal antibodies to groupA erythrocytes, HLA, and other human cell surface antigens – new tools for genetic analysis. Cell. 1978;14:9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Bird JM, Kimber SJ. Oligosaccharides containing fucose linked α (1→3) and α (1→4) to N-acetylglucosamine cause decompaction of mouse morulae. Dev. Biol. 1984;104:449–460. doi: 10.1016/0012-1606(84)90101-5. [DOI] [PubMed] [Google Scholar]
- Brinster RL. The effect of cells transferred into the mouse blastocyst on subsequent development. J. Exp. Med. 1974;140:1049–1056. doi: 10.1084/jem.140.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky FM, Parham P, Barnstable CJ, Crumpton MJ, Bodmer WF. Monoclonal antibodies for analysis of the HLA system. Immunol. Rev. 1979;47:3–61. doi: 10.1111/j.1600-065x.1979.tb00288.x. [DOI] [PubMed] [Google Scholar]
- Dodd J, Jessell TM. Cell surface glycoconjugates and carbohydrate-binding proteins: possible recognition signals in sensory neurone development. J. Exp. Biol. 1986;124:225–238. doi: 10.1242/jeb.124.1.225. [DOI] [PubMed] [Google Scholar]
- Duran C, Talley PJ, Walsh J, Pigott C, Morton I, Andrews PW. Hybrids of pluripotent and nullipotent human embryonal carcinoma cells: partial retention of a pluripotent phenotype. Int. J. Cancer. 2001;93:324–332. doi: 10.1002/ijc.1355. [DOI] [PubMed] [Google Scholar]
- Eisenbarth GS, Walsh FS, Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc. Natl. Acad. Sci. USA. 1979;76:4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenderson BA, Zehavi U, Hakormori S. a MULTIVALENT LACTO-N-fucopentaose III-lysyllysine conjugate decompacts preimplantation-stage mouse embryos, while the free oligosacharide is ineffective. J. Exp. Med. 1984;160:1591–1596. doi: 10.1084/jem.160.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenderson BA, Andrews PW, Nudelman E, Clausen H, Hakomori S-i. Glycolipid core structure switching from globo- to lacto- and ganglio-series during retinoic acid-induced differentiation of TERA-2-derived human embryonal carcinoma cells. Dev. Biol. 1987;122:21–34. doi: 10.1016/0012-1606(87)90328-9. [DOI] [PubMed] [Google Scholar]
- Gokhale PJ, Giesberts AN, Andrews PW. Brachyury is Expressed by Human Teratocarcinoma Cells in the Absence of Mesodermal Differentiation. Cell Growth Differentiation. 2000;11:157–162. [PubMed] [Google Scholar]
- Gooi HC, Feizi T, Kapadia A, Knowles BB, Solter D, Evans MJ. Stage-specific embryonic antigen involves α1→3 fucosylated type 2 blood group chains. Nature (London) 1981;292:156–158. doi: 10.1038/292156a0. [DOI] [PubMed] [Google Scholar]
- Griffin JD, Hercend T, Beveridge R, Schlossman SF. Characterization of an antigen expressed by human nature killer cells. J. Immunol. 1983;130:2947–2951. [PubMed] [Google Scholar]
- Jakob H, Dubois P, Eisen H, Jacob F. Effects de l’hexamethylenebisacetamide sur la differenciation de cellules de carcinome embryonaire. CR Séance Acad. Sci. (III) 1978;286:109–111. [PubMed] [Google Scholar]
- Kannagi R, Cochran NA, Ishigami F, Hakomori S-i, Andrews PW, Knowles BB, Solter D. Stage-specific embryonic antigens (SSEA-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983;2:2355–2361. doi: 10.1002/j.1460-2075.1983.tb01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GR. Teratocarcinomas and mammalian embryogenesis. Science. 1980;209:768–775. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]
- McBurney MW, Jones-Villeneuve EMV, Edwards MKS, Anderson PS. Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature. 1982;299:165–167. doi: 10.1038/299165a0. [DOI] [PubMed] [Google Scholar]
- McKenzie JL, Fabre JW. Human Thy1: Unusual localisation and possible functional significance in lymphoid tissues. J. Immunol. 1981;126:843–850. [PubMed] [Google Scholar]
- Papaioannou VE, Mcburney MW, Gardner RL, Evans MJ. Fate of teratocarcinoma cells injected into early mouse embryos. Nature. 1975;258:70–73. doi: 10.1038/258070a0. [DOI] [PubMed] [Google Scholar]
- Pleasure SJ, Page C, Lee VM-Y. Pure, post-mitotic, polarized human neurons derived from Ntera2 cells provide a system for expressing exogenous proteins in terminally differentiated neurons. J. Neurosci. 1992;12:1802–1815. doi: 10.1523/JNEUROSCI.12-05-01802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendt J, Erulkar S, Andrews PW. Presumptive neurons derived by differentiation of a human embryonal carcinoma cell line exhibit tetrodotoxin-sensitive sodium currents and the capacity for regenerative responses. Exp. Cell Res. 1989;180:580–584. doi: 10.1016/0014-4827(89)90087-6. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat. Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Robertson EJ. Embryo derived stem cell lines. In: Robertson EJ, editor. Teratocarcinomas and Embryonic Stem Cells: a Practical Approach. Oxford: IRL Press; 1987. pp. 71–112. [Google Scholar]
- Shevinsky L, Knowles BB, Damjanov I, Solter D. Monoclonal antibody to murine embryos defines a stage-specific embryonic antigen expressed on mouse embryos and human teratocarcinoma cells. Cell. 1982;30:697–705. doi: 10.1016/0092-8674(82)90274-4. [DOI] [PubMed] [Google Scholar]
- Simeone A, Acampora D, Arcioni L, Andrews PW, Boncinelli E, Mavilio F. Sequential activation of human HOX2 homeobox genes by retinoic acid in human embryonal carcinoma cells. Nature. 1990;346:763–766. doi: 10.1038/346763a0. [DOI] [PubMed] [Google Scholar]
- Solter D, Damjanov I. Teratocarcinoma and the expression of oncodevelopmental genes. Meth. Cancer Res. 1979;18:277–332. [Google Scholar]
- Solter D, Knowles BB. Monoclonal antibody defining a stage specific mouse embryonic antigen (SSEA1) Proc. Natl. Acad. Sci. USA. 1978;75:5565–5569. doi: 10.1073/pnas.75.11.5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires PE, Wakeman JA, Chapman H, Kumpf S, Fidock MD, Andrews PW, Dunne MJ. Regulation of intracellular Ca2+ in response to muscarinic and glutamate receptor agonists during the differentiation of NTERA2 human embryonal carcinoma cells into neurons. Eur. J. Neurosci. 1996;8:783–793. doi: 10.1111/j.1460-9568.1996.tb01263.x. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Thurin J, Herlyn M, Hindsgaul O, Stromberg N, Marklsson K, Elder D, et al. Proton NMR and fast atom bombardment mass spectrometry analysis of the melanoma-associated ganglioside 0-O-acetyl-GD3. J. Biol. Chem. 1985;260:14556–14563. [PubMed] [Google Scholar]
- Williams BP, Daniels GL, Pym B, Sheer D, Povey S, Okubo Y, et al. Biochemical and genetic analysis of the OKa blood group antigen. Immunogenetics. 1988;27:322–329. doi: 10.1007/BF00395127. [DOI] [PubMed] [Google Scholar]
- Willison KR, Karol RA, Suzuki A, Kundu SK, Marcus DM. Neutral glycolipids antigens as developmental markers of mouse teratocarcinoma and early embryos: An immunologic and chemical analysis. J. Immunol. 1982;129:603–609. [PubMed] [Google Scholar]