Abstract
While the signals that direct neural crest cells to choose the glial lineage and generate Schwann cell precursors are still obscure, studies both in vivo and in vitro indicate that the survival and differentiation of these cells to form Schwann cells is regulated by at least two signals, neuregulin-1 and endothelin. We know little about the signals that cause some immature Schwann cells to choose myelin differentiation, while other cells form non-myelinating cells. Three transcription factors, Sox-10, Oct-6 and Krox-20, have been shown to play key roles in the Schwann cell lineage. The transcription factor Krox-20 has been identified as a major target of the signals that induce myelin differentiation. Gene transfer experiments in vitro show that this protein has a remarkable ability to promote a large number of phenotypic changes in immature Schwann cells that characterize the transition of these cells to myelinating cells. Furthermore, Krox-20 shows important functional interactions with neuregulin and transforming growth factor β (TGFβ), two factors that have been implicated in the regulation of myelination in postnatal nerves. Another signal of importance in developing peripheral nerves, Desert Hedgehog, secreted by Schwann cells directs formation of the peripheral nerve connective tissue sheaths. Ongoing gene screening experiments are likely to reveal new genes of interest in this system.
Keywords: Desert hedgehog, Krox-20, neuregulin, Sox-10, TGFβ
The origin of Schwann cells
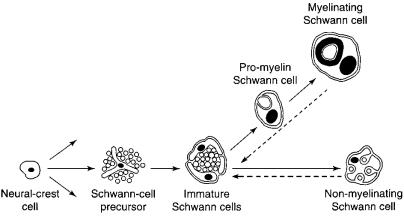
It has been known for many years that the large majority of Schwann cells in vertebrate peripheral nerves are generated from the neural crest, a transient group of cells that delaminates from the neural tube during embryonic development (Fig. 1). In the trunk region, cells of the neural crest also give rise to other major cell types, including the neurones of the sensory, sympathetic and parasympathetic ganglia, chromaffin cells and melanocytes.
Fig. 1.
The main stages in the development of Schwann cells from the neural crest. The last of the three main transitions in this lineage is readily reversible as indicated by the stippled arrows.
Much remains to be learnt about the regulation of gliogenesis from the neural crest. Some prospective glial cells already appear to have entered the glial lineage at the onset of crest migration while other cells are likely to start glial development later as they migrate ventrally along the neural tube. It is not clear what cell-extrinsic signals are important for inducing glial development from crest cells nor whether the signals responsible for the early and late entry to the glial lineage are the same. Two growth factor signals have been shown by in vitro and in vivo experiments to be involved in regulating early Schwann cell development in embryonic nerves. These are β-neuregulin-1 and endothelin (Brennan et al. 2000; Garratt et al. 2000). It is unlikely, however, that these factors have a role in the initial establishment of the PNS glial lineage, i.e. that they are part of an instructive signalling mechanism that directs multipotent crest cells to start developing as glial cells rather than as neurones or melanocytes.
In vitro, neuregulin blocks neuronal development from crest cells while Schwann cells (identified by expression of glial fibrillary acidic protein (GFAP)) develop readily with or without neuregulin (Shah et al. 1994). In agreement with this, gliogenesis within dorsal root ganglia (DRGs) (the generation of satellite cells) proceeds normally in mutant mice in which neuregulin signalling has been inactivated (Garratt et al. 2000). Both of these observations show that neuregulin is not needed for the formation of glia from the neural crest. Nevertheless, mice deficient in β-neuregulin-1 or its receptors erbB3 and erbB4 show a striking PNS glial phenotype. This is the near absence of Schwann cell precursors, and consequently Schwann cells at later stages, from peripheral nerves. A major reason for the inability to generate Schwann cell precursors and Schwann cells is likely to be the fact that Schwann cell precursors from early peripheral nerves require β-neuregulin for survival (see below) (Jessen et al. 1994; Dong et al. 1995, 1999). Mice with mutations in the neuregulin signalling pathway, however, also show deficiencies in the development of the sympathetic ganglia, which is likely to be due to a failure of sympathogenic neural crest cells to migrate to the appropriate site in the mesenchyme ventro-lateral to the dorsal aorta, although the crest cells that form the DRGs migrate normally (Garratt et al. 2000). Therefore, β-neuregulin-1 appears to be required for some aspects of neural crest migration. In vitro β-neuregulin-1 can promote Schwann cell migration (Morris et al. 1999; Meintanis et al. 2001), although β-neuregulin-1 was not found to promote migration of satellite cells/Schwann cells from embryo day (E) 12 ganglia in similar experiments (Morris et al. 1999). While the evidence relating to migration in this context is therefore somewhat contradictory, it clearly remains possible that impaired migration of progenitors into nerves contributes to the paucity of Schwann cell precursors in peripheral nerves of β-neuregulin-1 mutants.
The transcription factor Sox-10, which is initially expressed in the earliest migrating neural crest cells, appears to be intimately involved in the development of glia from the crest (Kuhlbrodt et al. 1998; Southard-Smith et al. 1998; Peirano et al. 2000; Wegner, 2000; Britsch et al. 2001; Sonnenberg-Riethmacher et al. 2001; Paratore et al. 2001). In Sox-10-deficient mice early peripheral glial are missing (Britsch et al. 2001). This applies equally to satellite cells within DRGs and Schwann cell precursors in peripheral nerves, both of which were identified by expression of brain-specific fatty acid binding protein in these experiments. In contrast, DRG sensory neurones, identified by expression of β-III tubulin and other markers, are initially generated in normal numbers, although they die later (Britsch et al. 2001; Sonnenberg-Riethmacher et al. 2001). On the basis of these experiments it is unlikely that Sox-10 has a major function in undifferentiated crest cells prior to their choice between neuronal and glial development, and points to a particular function for Sox-10 in glial development. Mice with mutations in the Sox-10 gene also have deficiencies in pigmentation and in the enteric nervous system, indicating a widespread role for this gene in the development of neural crest derivatives. Interestingly, the major myelin protein P0 is also a transcriptional target of Sox-10 (Peirano et al. 2000).
A link between Sox-10 and erbB3 expression exists. Both genes are widely expressed in early neural crest cells, are maintained in glial lineage cells and down-regulated in neuronal lineage cells as differentiation occurs. Expression of erbB3 is regulated by Sox-10 since very low expression of this gene is seen in late neural crest cells and their derivatives in Sox-10 mutants. Consequently, neuregulin does not support the survival of these cells in vitro (Paratore et al. 2001). Despite this, initial expression of erbB3 in the crest is not controlled by Sox-10, since normal erbB3 expression is initially seen in emerging neural crest cells in Sox-10 mutants (Britsch et al. 2001) (Fig. 2).
Fig. 2.
The main changes in the expression of proteins and lipids that take place as the population of immature Schwann cells diverges to generate myelinating and non-myelinating cells during development. Note that myelination involves a combination of down-regulation and up-regulation of molecular expression. The generation of non-myelinating cells involves many fewer molecular changes. Hereditary demyelinating neuropathies related to abnormal expression of some of the myelin-associated proteins are indicated. CMT: Charcot–Marie–Tooth disease; PMD: Pelizaeus–Merzbacher disease. For references see text and references therein.
Schwann cell precursors
To investigate how neural crest cells develop into Schwann cells, we initially used the rat sciatic nerve as a model (Fig. 3). The majority of experiments on peripheral myelination and regeneration have used this system, and it is well characterized at both the morphological and molecular level. More recently, in view of the availability of transgenic mice with peripheral nerve defects, we have also studied the normal development of mouse sciatic nerves. In the rat, axons project out into the rat hind limb between E13 and 14, while in the mouse axons extend into the limb 2 days earlier, between E11 and E12 (Reynolds et al. 1991). At this stage, the glial cells in the nerve are intimately associated with axons that are vigorously growing towards their target tissues. Unlike older nerves, where within a nerve fascicle axon-Schwann cell units are surrounded by abundant fibrous connective tissue containing blood vessels, within the E14 rat or E12 mouse nerve there is no significant connective tissue and Schwann cell precursors are found both inside and at the edge of the nerve. The cells possess extensive processes that contact and form junctions with one another. They surround large groups of axons, most of which are of similar size at this age, and divide the nerve into territories. To investigate the properties of these cells, we excised sciatic nerves from rats or mice at these ages, E14 and E12, respectively, dissociated the cells from axons and placed them in tissue culture. We found that they differed from Schwann cells in a large number of properties (Jessen et al. 1994; Dong et al. 1995; Jessen & Mirsky, 1999). Notably, they underwent programmed cell death within 20 h in defined medium under conditions that supported full survival of neonatal Schwann cells (i.e. at moderate cell density (2–4000 cells per coverslip)), they clustered together in pavement-like arrays, did not express cytoplasmic S-100 protein, the 04 antigen or GFAP and were much more motile than Schwann cells. Furthermore, fibroblast growth factor 2 (FGF2), a Schwann cell mitogen, is not mitogenic for rat Schwann cell precursors. The precursors also express the transcription factor AP-2_, which is down-regulated in Schwann cells (Stewart et al. 2001). We concluded that embryonic nerves contained at least two distinct cell types in the embryonic period: Schwann cell precursors, present in the rat nerve at E14 and 15 (mouse nerve E12 and 13), and Schwann cells, found in the rat nerve from E16 onwards (mouse nerve E14 onwards), which form the majority of cells in the nerve by E18. Subsequent work has revealed more clearly the distinct molecular phenotype of Schwann cell precursors, defining a number of differences between these cells and migrating neural crest cells from which they derive (Table 1). The Schwann cell precursor is thus the first stage in the progression from neural crest cells to the eventual generation of myelin- and non-myelin-forming Schwann cells in postnatal nerves (Jessen et al. 1994). In most respects the properties of the mouse Schwann cell precursors are similar to those of rat (Dong et al. 1999).
Fig. 3.
Electron micrograph of transverse sections of mouse sciatic nerve showing the nerve sheaths in wild-type (+/+) and Desert Hedgehog null (−/−) animals. In the epineurium (EP) of the wild-type mouse, numerous collagen fibrils and a fibroblast can be seen. The perineurium (P) consists of compacted layers of flattened cells. The endoneurium (EN) is filled with collagen fibrils. The epineurium of the null nerve contains very little collagen, while the perineurium is disorganized and the layers are uncompacted. A reduction in endoneurial collagen fibrils is also evident.
Table 1.
Some of the main differences between migrating crest cells and Schwann cell precursors in rat and mouse
| Migrating crest cellsa | Schwann cell precursors |
|---|---|
| Neuregulin-β does not promote survival1 | Neuregulin-β promotes survival2 |
| P0 negative3,4 | P0 positive3 |
| PMP22 negative4 | PMP22 positive4 |
| PLP negative5 | PLP positive5 |
| GAP-43 negative6 | GAP-43 positive6 |
| CD9 negative5 | CD9 positive5 |
Migrating crest refers to crest cells in vivo that are on a level with the dorsal third of the neural tube (i.e. it does not refer to cells found further ventrally at the level of ganglionic condensation) and/or crest cells in vitro on day 1 of outgrowth from neural tube explants.
unpublished observations (A. Brennan, E. Calle, R. Mirsky, K.R. Jessen)
The survival of Schwann cell precursors and generation of Schwann cells: the role of β-neuregulins and endothelins
Schwann cell precursors show an intimate morphological relationship with axons and these cells fail to survive when they are deprived of axonal contact by dissociation. This suggests that the survival of Schwann cell precursors in developing nerves might be supported by axon-derived signals. We made several observations in vitro that showed that neurones or neurone-derived signals not only rescued precursors from apoptotic cell death, but also allowed them to convert to Schwann cells on schedule (i.e. after 4 days in culture, equivalent to E18 in vivo). Thus conditioned medium from postnatal day 1 DRG neurones, close proximity to neurites in sparse cultures of DRG neurones, or exposure to axonal membranes isolated from cultured DRG neurones all rescued precursors from death (Jessen et al. 1994; Dong et al. 1995). The dependence of Schwann cell precursors on axonal survival signals was confirmed in vivo using chick embryos (Ciutat et al. 1996). We identified the activity associated with axonal membranes and present in the DRG conditioned medium as β-neuregulin-1 since incubation with a soluble hybrid protein that contains the extracellular domain of the erbB4 receptor, a highly specific high-affinity receptor for neuregulin-1, removed the survival activity (Dong et al. 1995). Furthermore, several isoforms of β-neuregulin-1 mimicked the effect of the neurone-conditioned medium. They supported Schwann cell precursor survival and promoted the generation of Schwann cells from precursors on schedule in vitro. The main conclusions from this work, i.e. that precursor survival and Schwann cell generation depended on a signal from axons, and that a major component of this signal was β-neuregulin-1, have been confirmed by in vivo experiments that involve the inactivation of neuregulin signalling in genetically modified mice (above).
There is high expression of neuregulin-1 mRNA in both DRG and ventral horn motor neurones in rat embryos at E14 through to adult life. It is therefore expressed at the right time and place not only to act as a signal from neurones to precursors but also to play a role at later stages of development (Marchionni et al. 1993; Bermingham-McDonogh et al. 1997).
The survival and progression of Schwann cell precursors to Schwann cells is regulated in vitro and in vivo by another family of factors, endothelins. Endothelins allow rat Schwann cell precursor survival in culture in the absence of axons, an effect promoted by insulin-like growth factor (IGF) (Brennan et al. 2000). Unlike β-neuregulins, endothelins do not induce DNA synthesis in precursors. In the presence of endothelin alone, Schwann cell precursors generate Schwann cells much more slowly in vitro than they do in the presence of β-neuregulin. Also, in the combined presence of β-neuregulin and endothelin, Schwann cell generation is significantly slower than in β-neuregulin alone. These findings suggest that endothelin acts as a negative regulator of the precursor/Schwann cell transition. We confirmed this in vivo using the spotting lethal rat. The action of endothelins on Schwann cell precursors is mediated by endothelin B receptors, which are expressed in developing peripheral nerves. In spotting lethal rats, which have functionally inactive endothelin B receptors, generation of Schwann cells, monitored by examining the appearance of the Schwann cell marker S-100, is accelerated as predicted from the in vitro studies. This indicates that endothelins have a role in the timing of Schwann cell generation not only in vitro but in vivo as well (Brennan et al. 2000). FGF2 may also be involved in the timing of Schwann cell generation since FGF2 accelerates the generation of Schwann cells from mouse Schwann cell precursors in the presence of neuregulin-1 (Dong et al. 1999).
Schwann cell survival signals
While Schwann cell precursors are dependent on paracrine signals from neurones for survival, Schwann cells in the distal stumps of adult animals survive for several months in the absence of axons, although their number gradually declines and they become less responsive to extrinsic signals (Grinspan et al. 1996; Trachtenberg & Thompson, 1996; Li et al. 1998). Schwann cell survival for an extended period in the absence of axons is crucial for nerve regeneration because Schwann cells in the distal stump provide both trophic factors and adhesive substrates that promote axonal growth. A major reason for their ability to survive under these conditions (under which Schwann cell precursors would die) is the presence of autocrine survival circuits in Schwann cells which enable them to support their own survival (Meier et al. 1999). Autocrine survival circuits are not present in Schwann cell precursors. Since the Schwann cell survival signal is not mitogenic and does not support precursor survival, it is unlikely to be β-neuregulin-1. We have identified major components of the autocrine loop as IGF-2, neurotrophin-3 (NT3) and platelet-derived growth factor-BB (PDGF-BB). Schwann cells have receptors for these factors and they support survival when applied in very low concentrations. Furthermore, antibodies to these factors block the Schwann cell survival activity in Schwann cell conditioned medium. Longer-term survival is promoted by culture on a laminin substrate, although laminin alone does not support survival (Meier et al. 1999). Another factor secreted by denervated Schwann cells, leukaemia inhibitory factor (LIF), can also promote Schwann cell survival in the presence of other growth factors (Dowsing et al. 1999). In the neonatal period, axons are also likely to provide important survival signals, since transection of neonatal nerves results in increased Schwann cell death within the body of the nerve and death of all teloglia at the neuromuscular junction. This is rescued by application of exogenous β-neuregulin-1 (Grinspan et al. 1996; Syroid et al. 1996; Trachtenberg & Thompson, 1996). In adult nerves, however, there is no significant Schwann cell death following transection (Trachtenberg & Thompson, 1996). In summary, Schwann cell precursors do not have autocrine survival circuits and their survival is acutely dependent on axonal β-neuregulin, while Schwann cells can rescue themselves by secretion of factors including IGF-2, PDGF-BB, NT3 or LIF. Importantly, Schwann cells can therefore survive in the absence of axons, although axonal β-neuregulin is likely to provide additional survival support in the perinatal period.
In addition to positive survival signals, factors that actively promote apoptosis may also play a role in Schwann cell death after injury or infection. Nerve growth factor (NGF) acting via the p75 neurotrophin receptor promotes cell death in several systems including Schwann cells. After nerve transection in neonatal p75 neurotrophin receptor-deficient mice, less Schwann cell death than normal occurs, and cultured Schwann cells from these mice survived better than normal Schwann cells when deprived of serum and growth factors (Frade & Barde, 1999; Soilu-Hanninen et al. 1999; Syroid et al. 2000; Hirata et al. 2001). The balance between survival and death in response to NGF in Schwann cells is mediated by an adaptor protein, receptor interacting protein 2, which protects cells from NGF-mediated cell death (Khursigara et al. 2001). We have found that TGFβs have similar effects on neonatal Schwann cells both in vitro and in vivo (Parkinson et al. 2001). Application of TGFβ1 kills Schwann cells by apoptosis in culture and increases cell death in transected but not in normal neonatal nerves. In vitro, the apoptotic effect is completely blocked by the combined presence of β-neuregulin-1 and the autocrine signals IGF-2, PDGF-BB and NT3. TGFβ1 signals via c-Jun – N-terminal kinase and phosphorylation of c-Jun, since adenoviral expression of dominant-negative c-Jun inhibits TGFβ induced apoptosis, while retroviral expression of constitutively active v-Jun promotes cell death. Myelinating Schwann cells from postnatal day 4 nerves are resistant to TGFβ-induced apoptosis, and this is related to a failure to phosphorylate c-Jun and expression of Krox-20 (D. B. Parkinson et al. unpubl. obs. and see below). In older nerves all Schwann cells become resistant to cell death (Grinspan et al. 1996; Parkinson et al. 2001).
Schwann cell differentiation and Krox-20
Although the molecular identity of the axonal signal(s) that induce the myelinating or non-myelinating phenotype in Schwann cells is unknown, there is significant information on the intracellular signals and transcription factors in Schwann cells that are involved in myelination. Based on experiments using targeted mutagenesis in mice, three transcription factors are known to be important in development of the glial lineage. The first of these, Sox-10 has been discussed above. The other two, Oct-6 (also known as SCIP and Tst-1) and Krox-20, are involved in the myelination programme. In Oct-6 null mice myelination is severely delayed (Bermingham et al. 1996; Jaegle et al. 1996), while in Krox-20 null mice myelination fails completely. Although Schwann cells null for Krox-20 form a 1 : 1 relationship to axons, a prerequisite for myelination, and make up to one and a half wraps around the axon, compact myelin is not formed (Topilko et al. 1994; Topilko & Meijer, 2001). In normal mice, Krox-20 is expressed selectively by Schwann cells that have been signalled to myelinate (from E16 onwards) while Oct-6 is expressed in all Schwann cells in late embryogenesis and in the early postnatal period, with highest expression in pro-myelin cells (Blanchard et al. 1996; Arroyo et al. 1998). Schwann cells in peripheral nerves of Oct-6 null mice do not express Krox-20 at the appropriate time, whereas Schwann cells in nerves of Krox-20 null mice express Oct-6 on schedule and maintain it at higher than normal levels postnatally. In these mice rates of Schwann cell proliferation and death are also abnormally high in the perinatal period (Zorick et al. 1999; Topilko & Meijer, 2001). Taken together these results suggest that Oct-6 (or later in development a related transcription factor, perhaps Brn-5) (Wu et al. 2001) is required developmentally for Krox-20 expression, although it is unlikely to regulate Krox-20 directly. Krox-20, in turn, is required for the progression of myelination which includes the down-regulation of Oct-6 in myelinating cells.
Mutations in Krox-20 are associated with both Charcot-Marie-Tooth and Dejerine-Sottas neuropathies (Warner et al. 1998, 1999; Timmerman et al. 1999; Boerkoel et al. 2001), emphasizing the importance of Krox-20 for the myelination programme. Furthermore, Krox-20 mRNA and protein levels fall sharply on nerve transection in common with axonally regulated myelin genes, and are re-induced on axonal regeneration and myelination or in cultured cells by cAMP elevation (Murphy et al. 1996; Zorick et al. 1999; Parkinson et al. 2001; Topilko & Meijer, 2001). Experiments using gene array technology to detect target genes that are up-regulated by enforced Krox-20 expression in cultured Schwann cells have reinforced the central role of Krox-20. Under these conditions mRNA for genes involved in myelin lipid synthesis and several myelin proteins, including P0, periaxin, myelin basic protein and peripheral myelin protein22 (PMP22), are strongly up-regulated, as are many other genes of unknown function in the Schwann cell (Nagarajan et al. 2001).
We have recently been enforcing expression of Krox-20 in cultured Schwann cells in the absence of axons with the aim of revealing events that lie downstream of Krox-20 in the signalling cascade that leads to myelination. We find that Krox-20 is sufficient to organize a surprisingly large set of phenotypic changes, all of which are associated with the transition from proliferating cells to quiescent myelinating cells. These include activation of the myelin proteins periaxin and P0, and down-regulation of L1, a marker of non-myelinating and immature cells. Cells that express Krox-20 also acquire resistance to TGFβ-mediated cell death which, as discussed above, is a feature that accompanies myelination. Importantly, Krox-20 blocks β-neuregulin-induced proliferation in Schwann cells, thus linking together changes in molecular phenotype associated with myelin formation with changes in responsiveness to the main axonal mitogen. Krox-20 also induces expression of a myelin gene, periaxin, in a heterologous type of cell, NIH-3T3 fibroblasts. Thus expression of Krox-20 has profound effects on the interpretation of extrinsic signals received by Schwann cells and is sufficient to co-ordinate a widespread set of changes associated with myelination. In the ability to cause a withdrawal from the cell cycle and activate gene expression of a differentiation gene in a heterolgous cell type where the gene is not normally expressed, Krox-20 shows a functional similarity to master regulatory genes previously described in some other cell types such as MyoD, neural bHLH factors or PPARγ (Davis et al. 1987; Tontonoz et al. 1994; Lee et al. 1995; Lo et al. 1998; Sabourin & Rudnicki, 2000; D. B. Parkinson et al. unpubl. obs.).
Schwann cells control perineurium formation
Clearly a major aspect of Schwann cell biology concerns the analysis of how Schwann cells respond to extrinsic signals such as survival factors, mitogens, differentiation and death signals, as outlined above. It should not be overlooked, however, that in addition to being signalling targets, developing Schwann cells have an important role as a source of developmental signals in embryonic and neonatal nerves (Jessen & Mirsky, 1999). Signals derived from Schwann cells or their precursors are likely to regulate neurofilament phosphorylation and thereby axonal architecture, Na-channel clustering, and the survival of embryonic DRG and motor neurones in addition to Schwann cell survival as mentioned previously. One striking example of the signalling function of Schwann cells is the control of perineurium formation.
The perineurial sheath surrounds peripheral nerves and acts as a diffusion barrier to protect Schwann cells and axons in the endoneurium (Bunge et al. 1989; Olsson, 1990). We have found that a molecule secreted by Schwann cells, Desert Hedgehog, a member of the Hedgehog family of signalling molecules, is important for the formation of not only the perineurium, but also of the endo- and epineurial connective tissue (Parmantier et al. 1999) (Fig. 4). Desert Hedgehog transcripts can be detected by in situ hybridization in developing nerves. The mRNA for the Desert Hedgehog receptor Patched (Bitgood & McMahon, 1995; Stone et al. 1996) is found in the mesenchyme surrounding the nerve at the time when the perineurium starts to form, indicating that Desert Hedgehog molecules from Schwann cells signal to the surrounding connective tissue cells to organize the perineurium (Parmantier et al. 1999). This idea was borne out by examining a Desert Hedgehog knockout mouse (Bitgood et al. 1996). In these mice the perineurium is abnormally thin and the cells appear to be ‘wavy’, rather than taut as in a normal perineurium, and have a basal lamina that is discontinuous. The collagen sheath of the epineurium is also sparse and even absent, while there appear to be numerous perineurial cells in the endoneurium which divide the nerve into mini-fascicles. The nerve–tissue barrier is defective in terms of permeability to proteins and migratory cells, and the tight junctions between perineurial cells are abnormal and immature (Parmantier et al. 1999). Thus not only do Schwann cells and their precursors signal to neurones and themselves, but they are also involved in fashioning the connective tissue sheaths of the nerves. Homozygous mutation of the Desert Hedgehog gene in humans produces a similar phenotype to knockout of the gene in mice (Umehara et al. 2000). Thus signals from Schwann cells also control the formation of the nerve connective tissue sheaths.
Fig. 4.
A schematic representation of nerve sheath formation showing the role of Desert Hedgehog (Dhh) in this process.
Acknowledgments
The work carried out in the authors’ laboratory and described in this article was supported by the Wellcome Trust and Medical Research Council. We thank past and present members of the laboratory for their contributions and Mrs D. Bartram for editorial assistance during the preparation of this article.
References
- Arroyo EJ, Bermingham JR, Jr, Rosenfeld MG, Scherer SS. Promyelinating Schwann cells express Tst-1/SCIP/Oct – 6. J. Neurosci. 1998;18:7891–7902. doi: 10.1523/JNEUROSCI.18-19-07891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham JR, Scherer SS, O’Connell S, Arroyo E, Kalla KA, Powell FL, Rosenfeld MG. Tst-1/Oct – 6/SCIP regulates a unique step in peripheral myelination and is required for normal respiration. Genes Dev. 1996;10:1751–1762. doi: 10.1101/gad.10.14.1751. [DOI] [PubMed] [Google Scholar]
- Bermingham-Mcdonogh O, Xu Y-T, Marchionni MA, Scherer SS. Neuregulin expression in PNS neurons: isoforms and regulation by target interactions. Mol. Cell. Neurosci. 1997;10:184–195. doi: 10.1006/mcne.1997.0654. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, Mcmahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell–cell interaction in the mouseembryo. Dev. Biol. 1995;172:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- Bitgood MJ, Shen L, Mcmahon AP. Sertoli cell signaling by Desert hedgehog regulates the male germline. Current Biol. 1996;6:298–304. doi: 10.1016/s0960-9822(02)00480-3. [DOI] [PubMed] [Google Scholar]
- Blanchard AD, Sinanan A, Parmantier E, Zwart R, Broos L, Meijer D, Meier C, Jessen KR, Mirsky R. Oct – 6 (SCIP/Tst-1) is expressed in Schwann cell precursors, embryonic Schwann cells, and postnatal myelinating Schwann cells. Comparison with Oct – 1, Krox-20 and Pax-3. J. Neurosci. Res. 1996;46:630–640. doi: 10.1002/(SICI)1097-4547(19961201)46:5<630::AID-JNR11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Boerkoel CF, Takashima H, Bacino CA, Daentl D, Lupski JR. EGR2 mutation R359W causes a spectrum of Dejerine-Sottas neuropathy. Neurogenetics. 2001;3:153–157. doi: 10.1007/s100480100107. [DOI] [PubMed] [Google Scholar]
- Brennan A, Dean CH, Zhang AL, Cass DT, Mirsky R, Jessen KR. Endothelins control the timing of Schwann cell generation in vitro and in vivo. Dev. Biol. 2000;227:545–557. doi: 10.1006/dbio.2000.9887. [DOI] [PubMed] [Google Scholar]
- Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, et al. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge MB, Wood PM, Tynan LB, Bates ML, Sanes JR. Perineurium originates from fibroblasts: demonstration in vitro with a retroviral marker. Science. 1989;243:222–231. doi: 10.1126/science.2492115. [DOI] [PubMed] [Google Scholar]
- Ciutat D, Calderó J, Oppenheim RW, Esquerda JE. Schwann cell apoptosis during normal development and after axonal degeneration induced by neurotoxins in the chick embryo. J. Neurosci. 1996;16:3979–3990. doi: 10.1523/JNEUROSCI.16-12-03979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Dong Z, Brennan A, Liu N, Yarden Y, Lefkowitz G, Mirsky R, Jessen KR. NDF is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron. 1995;15:585–596. doi: 10.1016/0896-6273(95)90147-7. [DOI] [PubMed] [Google Scholar]
- Dong Z, Sinanan A, Parkinson D, Parmantier E, Mirsky R, Jessen KR. Schwann cell development in embryonic mouse nerves. J. Neurosci. Res. 1999;56:334–348. doi: 10.1002/(SICI)1097-4547(19990515)56:4<334::AID-JNR2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Dowsing BJ, Morrison WA, Nicola NA, Starkey GP, Bucci T, Kilpatrick TJ. Leukemia inhibitory factor is an autocrine survival factor for Schwann cells. J. Neurochem. 1999;73:96–104. doi: 10.1046/j.1471-4159.1999.0730096.x. [DOI] [PubMed] [Google Scholar]
- Frade JM, Barde YA. Genetic evidence for cell death mediated by nerve growth factor and the neurotrophin receptor p75 in the developing mouse retina and spinal cord. Development. 1999;126:683–690. doi: 10.1242/dev.126.4.683. [DOI] [PubMed] [Google Scholar]
- Garratt AN, Britsch S, Birchmeier C. Neuregulin, a factor with many functions in the life of a Schwann cell. Bioessays. 2000;22:987–996. doi: 10.1002/1521-1878(200011)22:11<987::AID-BIES5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Grinspan JB, Marchionni MA, Reeves M, Coulaloglou M, Scherer SS. Axonal interactions regulate Schwann cell apoptosis in developing peripheral nerve: neuregulin receptors and the role of neuregulins. J. Neurosci. 1996;16:6107–6118. doi: 10.1523/JNEUROSCI.16-19-06107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn L, Suter U, Sommer L. P0 and PMP22 mark a multipotent neural crest-derived cell type that displays community effects in response to TGF-beta family factors. Development. 1999;17:3781–3794. doi: 10.1242/dev.126.17.3781. [DOI] [PubMed] [Google Scholar]
- Hirata H, Hibasami H, Yoshida T, Ogawa M, Matsumoto M, Morita A, et al. Nerve growth factor signaling of p75 induces differentiation and ceramide-mediated apoptosis in Schwann cells cultured from degenerating nerves. Glia. 2001;36:245–258. doi: 10.1002/glia.1113. [DOI] [PubMed] [Google Scholar]
- Jaegle M, Mandemakers W, Broos L, Zwart R, Karis A, Visser P, et al. The POU factor Oct – 6 and Schwann cell differentiation. Science. 1996;273:507–510. doi: 10.1126/science.273.5274.507. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Brennan A, Morgan L, Mirsky R, Kent A, Hashimoto Y, Gavrilovic J. The Schwann cell precursor and its fate. A study of cell death and differentiation during gliogenesis in rat embryonic nerves. Neuron. 1994;12:509–527. doi: 10.1016/0896-6273(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. Schwann cells and their precursors emerge as major regulators of nerve development. Trends Neurosci. 1999;22:402–410. doi: 10.1016/s0166-2236(98)01391-5. [DOI] [PubMed] [Google Scholar]
- Khursigara G, Bertin J, Yano H, Moffett H, Distefano PS, Chao MV. A prosurvival function for the p75 receptor death domain mediated the caspase recruitment domain receptor-interacting protein 2. J. Neurosci. 2001;21:5854–5863. doi: 10.1523/JNEUROSCI.21-16-05854.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M. Sox10, a novel transcriptional modulator in glial cells. J. Neurosci. 1998;18:237–250. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M-J, Brennan A, Blanchard A, Zoidl G, Dong Z, Tabernero A, et al. P0 is constitutively expressed in the rat neural crest and embryonic nerves and is negatively and positively regulated by axons to generate non-myelin-forming and myelin-forming Schwann cells, respectively. Mol. Cell. Neurosci. 1997;8:336–350. doi: 10.1006/mcne.1996.0589. [DOI] [PubMed] [Google Scholar]
- Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, Weintraub H. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science. 1995;268:836–844. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- Li H, Wigley C, Hall SM. Chronically denervated rat Schwann cells respond to GGF in vitro. Glia. 1998;24:290–303. [PubMed] [Google Scholar]
- Lo L, Tiveron MC, Anderson DJ. MASH1 activates expression of the paired homeodomain transcription factor Phox2a, and couples pan-neuronal and subtype-specific components of autonomic neuronal identity. Development. 1998;125:609–620. doi: 10.1242/dev.125.4.609. [DOI] [PubMed] [Google Scholar]
- Marchionni MA, Goodearl ADJ, Chen MS, Bermingham-McDonogh O, Kirk C, Hendricks M, et al. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- Meier C, Parmantier E, Brennan A, Mirsky R, Jessen KR. Developing Schwann cells acquire the ability to survive without axons by establishing an autocrine circuit involving IGF, NT-3 and PDGF-BB. J. Neurosci. 1999;19:3847–3859. doi: 10.1523/JNEUROSCI.19-10-03847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintanis S, Thomaidou D, Jessen KR, Mirsky R, Matsas R. The neuron-glia signal β-neuregulin promotes Schwann cell motility via the MAPK pathway. Glia. 2001;34:39–51. [PubMed] [Google Scholar]
- Morris JK, Kin W, Hauser C, Marchuk Y, Getman D, Lee KF. Rescue of the cardiac defect in erbB2 mutant mice reveals essential roles of erbB2 in peripheral nervous system development. Neuron. 1999;23:273–283. doi: 10.1016/s0896-6273(00)80779-5. [DOI] [PubMed] [Google Scholar]
- Murphy P, Topilko P, Schneider-Manoury S, Seitanidou T, Baron Van Evercooren A, Charnay P. The regulation of Krox-20 expression reveals important steps in the control of peripheral glial cell development. Development. 1996;122:2847–2857. doi: 10.1242/dev.122.9.2847. [DOI] [PubMed] [Google Scholar]
- Nagarajan R, Svaren J, Le N, Araki T, Watson M, Milbrandt J. EGR2 mutations in inherited neuropathies dominant-negatively inhibit myelin gene expression. Neuron. 2001;30:355–368. doi: 10.1016/s0896-6273(01)00282-3. [DOI] [PubMed] [Google Scholar]
- Olsson Y. Microenvironment of the peripheral nervous system under normal and pathological conditions. Crit. Rev. Neurobiol. 1990;5:265–311. [PubMed] [Google Scholar]
- Parkinson DB, Dong Z, Bunting H, Whitfield J, Meier C, Marie H, et al. Transforming growth factor β (TGFβ) mediates Schwann cell death in vitro and in vivo. examination of c–Jun activation, interactions with survival signals, and the relationship of TGFβ mediated death to Schwann cell differentiation. J. Neurosci. 2001;21:8572–8585. doi: 10.1523/JNEUROSCI.21-21-08572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmantier E, Lynn B, Lawson D, Turmaine M, Sharghi Namini S, et al. Schwann cell-derived Desert Hedgehog controls the development of peripheral nerve sheaths. Neuron. 1999;23:713–724. doi: 10.1016/s0896-6273(01)80030-1. [DOI] [PubMed] [Google Scholar]
- Peirano RI, Goerich DE, Riethmacher D, Wegner M. Protein zero gene expression is regulated by the glial transcription factor Sox10. Mol. Cell. Biol. 2000;20:3198–3209. doi: 10.1128/mcb.20.9.3198-3209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds ML, Fitzgerald M, Benowitz LI. GAP-43 expression in developing cutaneous and muscle nerves in the rat hindlimb. Neuroscience. 1991;41:201–211. doi: 10.1016/0306-4522(91)90210-f. [DOI] [PubMed] [Google Scholar]
- Sabourin LA, Rudnicki MA. The molecular regulation of myogenesis. Clin. Genet. 2000;57:16–25. doi: 10.1034/j.1399-0004.2000.570103.x. [DOI] [PubMed] [Google Scholar]
- Shah NM, Marchionni MA, Isaacs I, Stroobant P, Anderson DJ. Glial growth factor restricts mammalian neural crest stem cells to a glial fate. Cell. 1994;77:349–360. doi: 10.1016/0092-8674(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Soilu-Hanninen M, Ekert P, Bucci T, Syroid D, Bartlett PF, Kilpatrick TJ. Nerve growth factor signaling through p75 induces apoptosis in Schwann cells via a Bcl-2-independent pathway. J. Neurosci. 1999;19:4828–4838. doi: 10.1523/JNEUROSCI.19-12-04828.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg-Riethmacher E, Miehe M, Stolt CC, Goerich DE, Wegner M, Riethmacher D. Development and degeneration of dorsal root ganglia in the absence of the HMG-domain transcription factor Sox10. Mechanisms Dev. 2001;109:253–265. doi: 10.1016/s0925-4773(01)00547-0. [DOI] [PubMed] [Google Scholar]
- Southard-Smith EM, Kos L, Pavan WJ. Sox 10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nature Genet. 1998;18:60–64. doi: 10.1038/ng0198-60. [DOI] [PubMed] [Google Scholar]
- Stewart HJS, Brennan A, Rahman M, Zoidl G, Mitchell PJ, Jessen KR, et al. Developmental regulation and overexpression of the transcription factor AP-2, a potential regulator of the timing of Schwann cell generation. Eur. J. Neurosci. 2001;14:363–372. doi: 10.1046/j.0953-816x.2001.01650.x. [DOI] [PubMed] [Google Scholar]
- Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, et al. The tumor-suppressor gene patched encodes a candidate receptor for Sonic hedgehog. Nature. 1996;384:129–134. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- Syroid DE, Maycox PR, Burrola PG, Liu N, Wen D, Lee K-F, Lemke G, Kilpatrick TJ. Cell death in the Schwann cell lineage and its regulation by neuregulin. Proc. Natl. Acad. Sci. USA. 1996;93:9229–9234. doi: 10.1073/pnas.93.17.9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syroid DE, Maycox PJ, Soilu-Hanninen M, Petratos S, Bucci T, Burrola P, et al. Induction of postnatal Schwann cell death by the low-affinity neurotrophin receptor in vitro and after axotomy. J. Neurosci. 2000;20:5741–5747. doi: 10.1523/JNEUROSCI.20-15-05741.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman V, De Jonghe P, Ceuterick C, De Vriendt E, Lofgren A, Nelis E, et al. Novel missense mutation in the early growth response 2 gene associated with Dejerine–Sottas syndrome phenotype. Neurology. 1999;52:1827–1832. doi: 10.1212/wnl.52.9.1827. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Topilko P, Meijer D. Transcription factors that control Schwann cell development and myelination. In: Jessen KR, Richardson WD, editors. Glial Cell Development. Basic Principles and Clinical Relevance. 2. Oxford: Oxford University Press; 2001. pp. 223–244. [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, et al. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371:796–799. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT, Thompson WJ. Schwann cell apoptosis at developing neuromuscular junctions is regulated by glial growth factor. Nature. 1996;379:174–177. doi: 10.1038/379174a0. [DOI] [PubMed] [Google Scholar]
- Umehara F, Tate G, Itoh K, Yamaguchi N, Douchi T, Mitsuya T, et al. A novel mutation of desert hedgehog in a patient with 46, XY partial gonadal dysgenesis accompanied by minifascicular neuropathy. Am. J. Human. Genet. 2000;67:1302–1305. doi: 10.1016/s0002-9297(07)62958-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner LE, Garcia CA, Lupski JR. Hereditary peripheral neuropathies: clinical forms, genetics, and molecular mechanisms. Annu. Rev. Med. 1999;50:263–275. doi: 10.1146/annurev.med.50.1.263. [DOI] [PubMed] [Google Scholar]
- Warner LE, Mancias P, Butler IJ, McDonald CM, Keppen L, Koob KG, et al. Mutations in the early growth response 2 (EGR2) gene are associated with hereditary myelinopathies. Nature Genet. 1998;18:382–384. doi: 10.1038/ng0498-382. [DOI] [PubMed] [Google Scholar]
- Wegner M. Transcriptional control in myelinating glia: the basic recipe. Glia. 2000;29:118–123. [PubMed] [Google Scholar]
- Wu R, Jurek M, Sundarababu S, Weinstein DE. The POU. gene Brn-5 is induced by neuregulin and is restricted to myelinating Schwann cells. Mol. Cell. Neurosci. 2001;17:683–695. doi: 10.1006/mcne.2000.0957. [DOI] [PubMed] [Google Scholar]
- Zorick TS, Syroid DE, Brown A, Gridley T, Lemke G. Krox-20 controls SCIP expression, cell cycle exit and susceptibility to apoptosis in developing myelinating Schwann cells. Development. 1999;126:1397–1406. doi: 10.1242/dev.126.7.1397. [DOI] [PubMed] [Google Scholar]