Abstract
This review describes the progress made in preparing Cochrane systematic reviews of randomized controlled trials for Guillain–Barré syndrome (GBS), chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), multifocal motor neuropathy (MMN) and the demyelinating neuropathies associated with paraproteins. The discovery of antibodies against myelin andaxolemmal glycolipids and proteins has not yet replaced the clinicopathological classificationon which treatment trials have been based. Systematic reviews have endorsed the equivalence of plasma exchange (PE) and intravenous immunoglobulin (IVIg) and the lack of efficacy of steroids in GBS. Systematic reviews have also endorsed the value of steroids, PE and IVIg in CIDP butrandomized controlled trials have only shown benefit from IVIg in MMN. There is a paucity of evidence concerning the efficacy of treatments in paraproteinaemic demyelinating neuropathy apartment from small trials showing short-term benefit from PE or IVIg. There is a lack of good quality controlled trials of immunosuppressive agents in any of these conditions. As the numberof treatment trials increases, Cochrane systematic reviews will be an increasingly valuable resource for summarizing the evidence from randomised controlled trials on which to base clinical practice. They already demonstrate major deficiencies in the existing evidence base.
Keywords: inflammatory neuropathy, paraprotein, randomized controlled trial, systematic review, treatment
Introduction
Classification
By the turn of the millennium the clinicopathological features of GBS and related disorders had been recognized as being more complex than originally envisaged (Table 1). Professsor P. K. Thomas has been prominent among those who have advanced knowledge in the area as the reference list in this review at his Festschrift will testify (Thomas, 1992). The syndrome described by Guillain, Barré & Strohl in 1916 is usually due to acute inflammatory demyelinating polyradiculoneuropathy (Asbury et al. 1969) but may also be caused by acute motor and sensory axonal neuropathy (Feasby et al. 1986) or acute motor axonal neuropathy (Hafer-Macko et al. 1996). The acute inflammatory demyelinating polyradiculoneuropathy variant may overlap with the syndrome of ophthalmoplegia, ataxia and areflexia described by Miller Fisher in 1956 (Fisher, 1956). Formes frustes of Miller Fisher syndrome occur with ophthalmoplegia or sensory neuropathy alone. Recurrent attacks of steroid responsive demyelinating neuropathy described by Austin (Austin, 1958) and P. K. Thomas (Thomas et al. 1969) and then in large series by the Mayo (Dyck et al. 1975) and Sydney (Prineas & McLeod, 1976) groups fit into what is now called chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) (Dyck et al. 1992). This in turn is now being split into subgroups, with a pure motor form, multifocal motor neuropathy with conduction block (Nobile-Orazio, 2001), and a clinically apparently pure sensory form (Oh et al. 1992). The core syndrome is usually a symmetrical sensory and motor disorder with proximal and distal weakness attributed to radiculopathy as well as neuropathy. However, variants are now being described. The onset may be focal and affect the cranial nerves (Waddy et al. 1989) or the upper limbs (Thomas et al. 1996). Persistent asymmetry and conduction block may occur and suchcases have been described as having multifocal acquired demyelinating sensory and motor neuropathy (MADSAM) (Saperstein et al. 2000).
Table 1.
Inflammatory demyelinating neuropathies and related disorders
| Acute (≤ 4 weeks progressive phase) |
| Guillain–Barré syndrome (GBS) |
| acute inflammatory demyelinating polyradiculoneuropathy (AMAN) |
| acute motor axonal neuropathy (AMSAN) |
| acute motor and sensory axonal neuropathy |
| Miller Fisher syndrome |
| Miller Fisher/GBS overlap syndrome |
| acute sensory demyelinating neuropathy |
| acute pandysautonomia |
| Subacute (4–8 weeks progressive phase) |
| subacute inflammatory demyelinating polyradiculoneuropathy |
| Chronic (> 8 weeks progressive phase) |
| chronic inflammatory demyelinating polyradiculoneuropathy (CIDP)* |
| multifocal motor neuropathy with conduction block (MMN) |
| multifocal acquired motor and sensory neuropathy (MADSAM) |
| IgM paraproteinaemic demyelinating neuropathy with antibodies to MAG |
| IgM paraproteinaemic demyelinating neuropathy without antibodies to MAG |
| IgG/IgA paraproteinaemic demyelinating neuropathy |
| solitary or osteosclerotic myeloma with demyelinating neuropathy |
| chronic relapsing axonal neuropathy |
CIDP may be relapsing or progressive; mixed, motor or sensory at onset; involving upper and lower or just upper or lower at onset; proximal and distal, distal or proximal at onset.
Pathogenesis
Changes in classification are beginning to be matched by advances in understanding the immune mechanisms underlying inflammatory neuropathies. In Miller Fisher syndrome 95% of patients have antibodies to ganglioside GQ1b. This ganglioside is more abundant in the ocular motor nerves than other peripheral nerves or spinal roots. Such a distribution would neatly explain the distribution of lesions in the clinical syndrome if it were not for the fact that the optic nerves have a similar high concentration (Chiba et al. 1997). In a mouse phrenic nerve diaphragm model, Miller Fisher sera or IgG antibodies to ganglioside GQ1b will block terminal motor nerve conduction (Plomp et al. 1999; Buchwald et al. 2001) and eventually destroy the nerve terminals in a complement-dependent reaction (O'Hanlon et al. 2001). In acute motor axonal neuropathy, antibodies to a number of different gangliosides have been discovered but the most interesting so far are those directed against ganglioside GD1a (Ho et al. 1999). Recently, Lunn et al. (2000) showed that monoclonal antibodies directed against this ganglioside label the axolemma of rat ventral but not dorsal root axons, consistent with them being the target of the autoimmune reaction in the human disease. Both acute and chronic inflammatory demyelinating polyradiculoneuropathy resemble experimental autoimmune neuritis which is a predominantly T-cell-mediated disorder which may be elicited by T-cell responses to any of the peripheral nerve myelin proteins P0, P2 or PMP22 (Hughes et al. 1999). Proof that the human diseases are due to autoimmunity to myelin proteins has not yet been forthcoming but recently antibody responses to PMP22 have been shown in GBS and CIDP (Gabriel et al. 2000) and to P0 in CIDP (Yan et al. 2001). In multifocal motor neuropathy antibodies to ganglioside GM1 are often present but evidence for their role in pathogenesis is lacking (Nobile-Orazio, 2001). In the demyelinating neuropathy associated with an IgM paraprotein, widely spaced myelin and antibodies to myelin-associated glycoprotein, the antibodies probably cause demyelination (Tatum, 1993) and interfere with Schwann cell axon signalling causing interference with neurofilament phosphorylation and axonal shrinkage (Lunn et al. 2001). Despite these more recent advances, in the absence of an earlier clear understanding of pathogenesis, treatment of the inflammatory neuropathies has had to proceed empirically.
Systematic reviews
Randomized controlled trials are particularly important tools for assessing the value of treatments in conditions such as GBS in which spontaneous improvement is usual and CIDP in which the prognosis is variable. Unless an intervention has a large effect size, clinical experience and cohort studies of new treatments will not provide convincing evidence of efficacy. Unfortunately randomized controlled trials vary in quality and trials of the same intervention in the same condition may reach conflicting conclusions. Furthermore, the number of randomized controlled trials being published is rising exponentially. By the end of 2001 the Cochrane CENTRAL register in the Cochrane Library contained 300 000 references to trials, of which 1500 concerned neuromuscular disease. Coping with this information explosion is already challenging and will become even more difficult. Conventional reviews are subject to bias arising from failure to identify all the randomized controlled trials, inclusion of flawed trials, the opinion of the author and sometimes the lack of rigorous peer review. The Cochrane Collaboration aims to provide systematic reviews, which avoid these sources of bias (Egger et al. 2001). The methods are declared, peer reviewed and published in advance as a protocol. The search for evidence involves a systematic search for randomized trials in all languages using the Cochrane register, which is produced by exhaustively searching MEDLINE, EMBASE, CINAHL and other databases and by hand-searching. The quality of each trial is evaluated according to declared standards with particular attention to qualities such as allocation concealment, which are known to bias outcomes. Where appropriate, data from more than one trial can be combined to summarize the results in a meta-analysis, a single statistical expression of the efficacy of the treatment. Reviews are rigorously refereed, revised and edited before publication on the Cochrane Library. The Library itself is published quarterly electronically and incorporates a system for readers to criticise reviews and for the criticisms and authors' replies to be edited and published. Authors of reviews must undertake to update their reviews at least every 2 years. The Cochrane Neuromuscular Disease Group has completed or is preparing reviews of most of the important interventions for inflammatory demyelinating neuropathy. While this article will grow old, the Cochrane reviews, which it references, should not.
Guillain–Barré syndrome
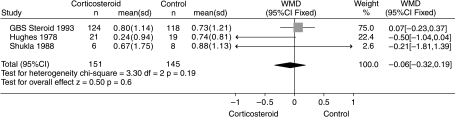
The first treatment tried in GBS was corticosteroids and the Cochrane review identified six eligible randomized trials involving 382 participants (Hughes & van der Meché, 1999). One trial of intravenous methylprednisolone accounted for 243 of the total 382 subjects studied (63%) (Guillain-Barré Syndrome Steroid Trial Group, 1993). This trial did not show a significant difference in any disability-related outcome between the corticosteroid and placebo groups. In the systematic review there was no significant difference between the corticosteroid and control groups for the primary outcome measure, improvement in disability grade on a seven-point scale 4 weeks after randomization. The weighted mean difference of the improvement in the three trials for which this outcome was available showed no difference (Fig. 1). The actual figure was 0.01 (95% CI – 0.27 to 0.29) of a grade in favour of the corticosteroid group. There was also no significant difference between the groups for the secondary outcome measures. Consequently, the balance of evidence does not at present support the use of steroids in GBS. However, the forthcoming results of a Dutch trial comparing intravenous methylprednisolone combined with IVIg to IVIg and placebo will need to be incorporated in this review when they become available and could alter this conclusion. It is surprising that steroids should not be more obviously effective in an acute inflammatory disorder. They are not particularly effective in experimental autoimmune neuritis either (King et al. 1985). It is possible that any beneficial effect of steroids on the inflammatory reaction is counterbalanced by an adverse effect of steroids on denervated muscle (Rich et al. 1998; Rich & Pinter, 2001).
Fig. 1.
Systematic review of steroid treatment for GBS. Improvement in a seven-point disability grade scale 4 weeks after randomization. From Hughes & van der Meché (1999)
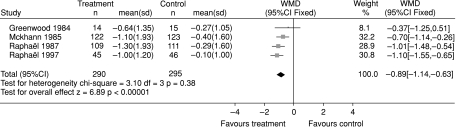
The first treatment shown to be beneficial in GBS was plasma exchange (PE) (Guillain– Barré Syndrome Study Group, 1985; McKhann et al. 1988). The Cochrane review identified six eligible trials involving 649 patients comparing PE to supportive treatment (Raphael et al. 2001). The review showed significant benefit from PE on all the available outcome measures. In the 585 patients for whom the outcome was available, the improvement in disability grade after 4 weeks was 0.9 (95% CI 0.6– 1.4) of a disability grade more with plasma exchange than without (Fig. 2). There were also highly significant differences in favour of PE in time to recover walking with aid, percentage of patients requiring artificial ventilation, duration of ventilation, and full muscle strength recovery and severe sequelae after 1 year. However, there were more patients with infectious events and cardiac arrhythmias in the PE than the control group. The evidence was already sufficient for PE to be regarded as the standard treatment by the late 1980s.
Fig. 2.
Systematic review of PE treatment for GBS. Improvement in a seven-point disability grade scale 4 weeks after randomization. From Hughes et al.(2001b)
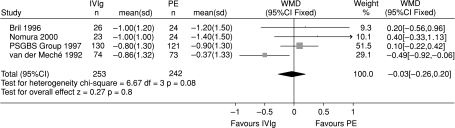
In 1992 the Dutch reported a trial suggesting that IVIg was slightly superior to PE for patients with severe GBS within 2 weeks from the onset of their disease (van der Meché et al. 1992). The Cochrane review will shortly be updated to compare four trials involving 495 patients that compared IVIg with PE (Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group, 1997; van der Meché et al. 1992; Bril et al. 1996; Nomura et al. 2000; Hughes et al. 2001b). There were no significant differences between these treatments in improvement in disability grade after 4 weeks and the 95% confidence intervals were so smallthat they fulfilled the criteria for equivalence set in one of the trials (Fig. 3). There were also no significant differences in time to walk unaided, mortality, or proportion of patients unable to walk without aid after a year. Since IVIg is much simpler to administer it has been adopted as the favoured treatment by most centres. However, there is no directly relevant evidence to guide treatment of mild GBS, children, Miller Fisher syndrome and other variants, and disease which presents more than 2 weeks after onset. The standard dose in the trials has been 0.4 g kg−1 daily for 5 days. Adding IVIg to PE was not helpful in one large trial (Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group, 1997). There is no trial evidence concerning the efficacy of a second dose of IVIg. Such treatment is often offered to those patients who experience a relapse early after treatment.
Fig. 3.
Systematic review of IVIg compared with PE as treatment for GBS. Improvement in a seven-point disability grade scale 4 weeks after randomization. The values for the standard deviation for the Bril 1996 trial, from which the 95% CI has been calculated, were not published and have been imputed. From Hughes et al.(2001b)
These trials have been performed on the populations of patients with GBS who have mostly had acute inflammatory demyelinating polyradiculoneuropathy (Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group, 1997; Hadden et al. 2001). There is some evidence that patients with pure motor neuropathy benefit more from IVIg than from PE but this needs confirmation.
Chronic inflammatory demyelinating polyradiculoneuropathy
Steroids are generally considered helpful in CIDP on the basis of clinical experience. The evidence from clinical trials is confined to one small early trial (Dyck et al. 1982). The significance of the beneficial result reported was lost when the results were re-analysed according to the intention to treat principle (Mehndiratta & Hughes, 2001). Nevertheless, the authors of several large series report benefit in about two-thirds of patients with CIDP. Unfortunately, the doses needed are often large, treatment has to be prolonged for months or years and Cushingoid side-effects are common (van den Bergh, 2001).
Just as in GBS, PE was reported to be beneficial in CIDP (Gross & Thomas, 1981) and this has been confirmed in two small randomized trials (Dyck et al. 1986; Hahn et al. 1996a).
Following the observation of apparent benefit from plasma and then IVIg infusion in a cohort of patients with CIDP (Vermeulen et al. 1985), four randomized trials have been performed in IVIg naïve patients. Three indicated benefit (van Doorn et al. 1990; Hahn et al. 1996b; Thompson et al. 1996; Mendell et al. 2001) and one no difference from placebo (Vermeulen et al. 1993). The trials involved 113 patients and 145 treatments: two had parallel and two crossover designs, which complicates meta-analysis. A Cochrane review concluded that significantly more improvements in disability occurred within 4 weeks after IVIg than after placebo (relative risk 3.2, 95% CI 1.7– 5.8) (Van Schaik et al. 2001). Unfortunately, the treatment effect is short-lived, commonly lasting only 4 weeks and seldom more than 12 weeks, so that treatment, which is very expensive, has to be repeated. Dyck and colleagues undertook a trial comparing PE with IVIg and did not find a significant difference in the short-term effects of these treatments (Dyck et al. 1994). Similarly, a trial comparing IVIg with oral prednisolone also failed to show a significant difference (Hughes et al. 2001a). Both these trials were short-term and the long-term comparative trials needed to decide which of these treatments is superior seem unlikely to be performed. About two-thirds of patients respond to each treatment. Steroids may be preferred because they are much less expensive and more convenient. If patients do not respond or require unreasonably high or prolonged doses they may respond to IVIg. Plasma exchange is more inconvenient and invasive and now usually only considered as a third option. In patients with pure motor CIDP steroids may cause worsening and IVIg is more likely to be effective (Donaghy et al. 1994).
Many different cytotoxic drugs and immunomodulatory treatments have been tried in CIDP but there is little evidence from randomized controlled trials to guide treatment (van den Bergh, 2001). A single trial randomized 30 patients to a low (2 mg kg−1) dose of azathioprine in addition to steroids and showed no benefit after 4 or 9 months on a range of measures of impairment and nerve conduction (Dyck et al. 1985). A single crossover trial of beta interferon 1a 44 µg subcutaneously three times a week for 12 weeks showed no benefit in 10 patients (Hadden et al. 1999). There have been anecdotal reports of beneficial treatment with cyclophosphamide, cyclosporin and mycophenolate but no randomized trials (Hughes et al. 2001c). This is unfortunate because CIDP may remit spontaneously so that controlled trials are essential to assess the value of proposed new treatments.
Multifocal motor neuropathy
Multifocal motor neuropathy (MMN) has been distinguished from CIDP principally by the distribution of weakness, predominantly affecting the upper limbs, absence of sensory impairment, response to IVIg and not steroids, and persistent partial motor conduction block (Nobile-Orazio, 2001). It is a moot point whether MMN is really a separate condition or part of a spectrum that includes MADSAM, symmetrical CIDP and sensory CIDP. From the point of view of treatment the distinction does seem to be significant because patients with MMN rarely respond to treatment with steroids and may worsen. Plasma exchange has also been used without obvious benefit. On the other hand, the response to IVIg is as, or more, marked than in CIDP. No Cochrane review of IVIg is yet available but three small trials support the general experience that IVIg is effective (van den Berg et al. 1995; Federico et al. 2000; Léger et al. 2000). However, the response is not universal, treatment needs to be repeated, as in CIDP, and disability progresses despite continued treatment. As a consequence, many different immunosuppressive drugs, principally cyclophosphamide, have been tried in MMN. A Cochrane review has not identified any randomized trials so that there is no convincing evidence that any particular immunosuppressive agent is effective (Umapathi et al. 2001). Randomized controlled trials to investigate the effect of immunosuppressive treatment in MMN are sorely needed (European Workshop on Multifocal Motor Neuropathy, 2001).
Paraproteinaemic demyelinating neuropathy
Both the paraproteins associated with neuropathy and the neuropathies associated with paraproteins are heterogeneous. This review is particularly concerned with demyelinating neuropathies and not with the radiculopathies due to myelomatous infiltration and collapse of vertebrae, neuropathies due to light chain amyloid deposition or axonal neuropathy. In the treatment of demyelinating neuropathy associated with Waldenstrom's macroglobulinaemia or solitary myelomas or plasmacytomas, treatment of the plasma cell dyscrasia will take precedence and may be rewarded by improvement of the associated neuropathy. Randomized controlled trials focused on the peripheral neuropathy have not been performed and are probably not appropriate. However, patients with monoclonal gammopathy of undetermined significance (MGUS) and neuropathy are asymptomatic apart from the symptoms of their peripheral neuropathy. The associated neuropathy is often demyelinating and may be caused by the antibody activity of the associated paraprotein (Yeung et al. 1991). A fairly stereotyped clinical picture of slowly progressive predominantly sensory neuropathy often associated with tremor is the commonest type and is usually associated with widely spaced myelin and antibodies to carbohydrate epitopes on myelin-associated glycoprotein and sulphated glucuronyl paragloboside (Smith et al. 1983).
In the absence of a more satisfactory classification it is appropriate to consider the treatment of the neuropathies associated with an IgM paraprotein separately from those associated with other paraproteins. Among those with IgM paraproteins are the patients with antibodies to myelin-associated glycoprotein and those with other or no identified antibodies. Reports of the results of treatment often do not define the nature of the antibody activity in the patient populations being treated. The results of treatment with steroids have been disappointing (Yeung et al. 1991) and randomized trials have not been performed (Lunn & Nobile-Orazio, 2001). A double blind crossover trial including 17 patients with IgM paraproteins (Dyck et al. 1991) showed a trend towards more improvement in impairment and compound muscle action potential amplitudes with PE than with sham exchange. There have been two randomized trials of IVIg in IgM paraprotein-associated demyelinating neuropathy. Both were crossover trials in which IVIg was compared with placebo. In the first, two of 11 patients showed significant increases in strength and one other showed improvement in sensation (Dalakas et al. 1996). The second trial included 22 patients. After 4 weeks, 10 of these had improved after IVIg and four after placebo and the mean improvement in disability after IVIg was greater than after placebo (Comi et al. 2002). Immunosuppressive drugs, including cyclophosphamide, azathioprine, fludarabine, rituximab, chlorambucil, melphalan and cyclosporin, and autologous stem cell transplantation have all been used with variable results and no randomized trials (Lunn & Nobile-Orazio, 2001). A randomized double-blind placebo-controlled study in 24 patients did not confirm the efficacy of interferon-alpha suggested by previous open studies (Mariette et al. 2000).
The evidence concerning treatment of demyelinating neuropathy associated with IgG or IgA paraproteins is less complete than that for any of the other diseases included in this review. The features of the associated neuropathy are variable but anecdotal reports suggest that it may respond to the same treatments as CIDP (Bromberg et al. 1992; Bleasel et al. 1993; Simmons et al. 1993). No systematic review of treatment yet exists. The only randomized controlled trial is the trial of PE already mentioned (Dyck et al. 1991) in which 20 patients with IgG or IgA paraproteins showed a significantly greater reduction of weakness with true than with sham exchange. An improved classification and understanding of the pathogenesis of the neuropathies associated with IgG and IgA paraproteins will be necessary before mounting further randomized controlled trials. If the paraprotein is only an incidental finding then the treatments shown to be beneficial in CIDP will be appropriate.
References
- Asbury AK, Arnason BG, Adams RD. The inflammatory lesion in idiopathic polyneuritis. Its role in pathogenesis. Medicine. 1969;48:173–215. doi: 10.1097/00005792-196905000-00001. [DOI] [PubMed] [Google Scholar]
- Austin JH. Recurrent polyneuropathies and their corticosteroid treatment. Brain. 1958;81:157–192. doi: 10.1093/brain/81.2.157. [DOI] [PubMed] [Google Scholar]
- Bleasel AF, Hawke SHB, Pollard JD, McLeod JG. IgG monoclonal paraproteinaemia and peripheral neuropathy. J. Neurol. Neurosurgery Psychiatry. 1993;56:52–57. doi: 10.1136/jnnp.56.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bril V, Ilse WK, Pearce R, Dhanani A, Sutton D, Kong K. Pilot trial of immunoglobulin versus plasma exchange in patients with Guillain– Barré syndrome. Neurology. 1996;46:100–103. doi: 10.1212/wnl.46.1.100. [DOI] [PubMed] [Google Scholar]
- Bromberg MB, Feldman EL, Albers JW. Chronic inflammatory demyelinating polyradiculoneuropathy: Comparison of patients with and without an associated monoclonal gammopathy. Neurology. 1992;42:1157–1163. doi: 10.1212/wnl.42.6.1157. [DOI] [PubMed] [Google Scholar]
- Buchwald B, Bufler J, Carpo M, et al. Combined pre- and postsynaptic action of IgG antibodies in Miller Fisher syndrome. Neurology. 2001;56:67–74. doi: 10.1212/wnl.56.1.67. [DOI] [PubMed] [Google Scholar]
- Chiba A, Kusunoki S, Obata H, MacHinami R, Kanazawa I. Ganglioside composition of the human cranial nerves, with special reference to pathophysiology of Miller Fisher syndrome. Brain Res. 1997;745:32–36. doi: 10.1016/s0006-8993(96)01123-7. [DOI] [PubMed] [Google Scholar]
- Comi G, Roveri L, Swan AV., et al. A randomised controlled trial of IVIg in IgM paraproteinaemic demyelinating neuropathy. J. Neurol. 2002 doi: 10.1007/s00415-002-0808-z. in press. [DOI] [PubMed] [Google Scholar]
- Dalakas MC, Quarles RH, Farrer RG, et al. A controlled study of intravenous immunoglobulin in demyelinating neuropathy with IgM gammopathy. Ann. Neurol. 1996;40:792–795. doi: 10.1002/ana.410400516. [DOI] [PubMed] [Google Scholar]
- Donaghy M, Mills KR, Boniface SJ, et al. Pure motor demyelinating neuropathy: Deterioration after steroid treatment and improvement with intravenous immunoglobulin. J. Neurol. Neurosurgery Psychiatry. 1994;57:778–783. doi: 10.1136/jnnp.57.7.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck PJ, Lais AC, Ohta M, Bastron JA, Okazaki H, Groover RV. Chronic inflammatory polyradiculoneuropathy. Mayo Clin. Proc. 1975;50:621–651. [PubMed] [Google Scholar]
- Dyck PJ, O'Brien PC, Oviatt KF, et al. Prednisone improves chronic inflammatory demyelinating polyradiculoneuropathy more than no treatment. Ann. Neurol. 1982;11:136–141. doi: 10.1002/ana.410110205. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, O'Brien P, Swanson C, Low P, Daube J. Combined azathioprine and prednisone in chronic inflammatory demyelinating polyneuropathy. Neurology. 1985;35:1173–1176. doi: 10.1212/wnl.35.8.1173. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Daube J, O'Brien P, et al. Plasma exchange in chronic inflammatory demyelinating polyradiculoneuropathy. N. Engl. J. Med. 1986;314:461–465. doi: 10.1056/NEJM198602203140801. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Low PA, Windebank AJ, et al. Plasma exchange in polyneuropathy associated with monoclonal gammopathy of undetermined significance. N. Engl. J. Med. 1991;325:1482–1486. doi: 10.1056/NEJM199111213252105. [DOI] [PubMed] [Google Scholar]
- Dyck PJ, Prineas J, Pollard J. Chronic inflammatory demyelinating polyradiculoneuropathy. In: Dyck PJ, Thomas PK, Griffin JW, Low PA, Poduslo JF, editors. Peripheral Neuropathy. Philadelphia: Saunders WB; 1992. [Google Scholar]
- Dyck PJ, Litchy WJ, Kratz KM, et al. A plasma exchange versus immune globulin infusion trial in chronic inflammatory demyelinating polyradiculoneuropathy. Ann. Neurol. 1994;36:838–845. doi: 10.1002/ana.410360607. [DOI] [PubMed] [Google Scholar]
- Egger M, Smith GD, O'Rourke K. Rationale, potentials, and promise of systematic reviews. In: Egger M, Smith GD, Altman DG, editors. Systematic Reviews in Health CareMeta-Analysis in Context. London: BMJ Books; 2001. pp. 3–19. [Google Scholar]
- European Workshop on Multifocal Motor Neuropathy. Report of a European Workshop on Multifocal Motor Neuropathy. Neuromuscular Disorders. 2001;11:309–314. doi: 10.1016/s0960-8966(00)00191-7. [DOI] [PubMed] [Google Scholar]
- Feasby TE, Gilbert JJ, Brown WF, et al. An acute axonal form of Guillain-Barré polyneuropathy. Brain. 1986;109:1115–1126. doi: 10.1093/brain/109.6.1115. [DOI] [PubMed] [Google Scholar]
- Federico P, Zochodne DW, Hahn AF, Brown WF, Feasby TE. Multifocal motor neuropathy improved by IVIg: randomized, double-blind, placebo-controlled study. Neurology. 2000;55:1256–1262. doi: 10.1212/wnl.55.9.1256. [DOI] [PubMed] [Google Scholar]
- Fisher M. Syndrome of ophthalmoplegia, ataxia and areflexia. N. Engl. J. Med. 1956;255:57–65. doi: 10.1056/NEJM195607122550201. [DOI] [PubMed] [Google Scholar]
- Gabriel CM, Gregson NA, Hughes RAC. Anti-PMP22 antibodies in patients with inflammatory neuropathy. J. Neuroimmunol. 2000;104:139–146. doi: 10.1016/s0165-5728(99)00269-6. [DOI] [PubMed] [Google Scholar]
- Gross MLP, Thomas PK. The treatment of chronic relapsing and chronic progressive idiopathic inflammatory polyneuropathy by plasma exchange. J. Neurol. Sci. 1981;52:69–78. doi: 10.1016/0022-510x(81)90135-0. [DOI] [PubMed] [Google Scholar]
- Guillain-Barré Syndrome Study Group. Plasmapheresis and acute Guillain–Barré syndrome. Neurology. 1985;35:1096–1104. [PubMed] [Google Scholar]
- Guillain-Barré Syndrome Steroid Trial Group. Double-blind trial of intravenous methylprednisolone in Guillain–Barré syndrome. Lancet. 1993;341:586–590. [PubMed] [Google Scholar]
- Hadden RDM, Sharrack B, Bensa S, Soudain S, Hughes RAC. Randomized trial of interferon beta-1a in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 1999;53:57–61. doi: 10.1212/wnl.53.1.57. [DOI] [PubMed] [Google Scholar]
- Hadden RDM, Karch H, Hartung H-P, et al. Preceding infections, immune factors and outcome in Guillain–Barré syndrome. Neurology. 2001;56:758–765. doi: 10.1212/wnl.56.6.758. [DOI] [PubMed] [Google Scholar]
- Hafer-MacKo C, Hsieh ST, Li CY, et al. Acute motor axonal neuropathy: An antibody-mediated attack on axolemma. Ann. Neurol. 1996;40:635–644. doi: 10.1002/ana.410400414. [DOI] [PubMed] [Google Scholar]
- Hahn AF, Boulton CF, Pillay N, et al. Plasma-exchange therapy in chronic inflammatory demyelinating polyneuropathy (CIDP): a double-blind, sham-controlled, cross-over study. Brain. 1996a;119:1055–1066. doi: 10.1093/brain/119.4.1055. [DOI] [PubMed] [Google Scholar]
- Hahn AF, Bolton CF, Zochodne D, Feasby TE. Intravenous immunoglobulin treatment (IVIg) in chronic inflammatory demyelinating polyneuropathy (CIDP): a double-blind placebo-controlled cross-over study. Brain. 1996b;119:1067–1078. doi: 10.1093/brain/119.4.1067. [DOI] [PubMed] [Google Scholar]
- Ho TW, Willison HJ, Nachamkin I, et al. Anti-GD1a antibody is associated with axonal but not demyelinating forms of Guillain–Barré syndrome. Ann. Neurol. 1999;45:168–173. doi: 10.1002/1531-8249(199902)45:2<168::aid-ana6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Hughes RAC, Gregson NA, Hadden RDM, Smith KJ. Pathogenesis of Guillain–Barré syndrome. J. Neuroimmunology. 1999;100:74–97. doi: 10.1016/s0165-5728(99)00195-2. [DOI] [PubMed] [Google Scholar]
- Hughes RAC, Van Der Meche FGA. The Cochrane Library (4) Oxford: Update Software; 1999. Corticosteroid treatment for Guillain–Barré syndrome (Cochrane Review) [Google Scholar]
- Hughes RAC, Bensa S, Willison HJ, et al. Randomized controlled trial of intravenous immunoglobulin versus oral prednisolone in chronic inflammatory demyelinating polyradiculoneuropathy. Ann. Neurol. 2001a;50:195–201. doi: 10.1002/ana.1088. [DOI] [PubMed] [Google Scholar]
- Hughes RAC, Raphael J-C, Swan AV, Van Doorn PA. The Cochrane Library (3) Oxford: Update Software; 2001b. Intravenous immunoglobulin for Guillain–Barré syndrome (Cochrane Review) [DOI] [PubMed] [Google Scholar]
- Hughes RAC, Swan AV, Van Doorn PA. The Cochrane Library (4) Oxford: Update Software; 2001c. Cytotoxic drugs and interferons for chronic inflammatory demyelinating polyradiculoneuropathy (Protocol for a Cochrane Review) [Google Scholar]
- King RHM, Craggs RI, Gross MLP, Thomas PK. Effects of glucocorticoids on experimental allergic neuritis. Exp. Neurol. 1985;87:9–19. doi: 10.1016/0014-4886(85)90129-3. [DOI] [PubMed] [Google Scholar]
- Léger J-M, Chassande B, Musset L, Meininger V, Bouche P, Baumann N. Intravenous imunoglobulin in the treatment of multifocal motor neuropathy with persistent conduction blocks: a randomized double-blind placebo controlled study. Brain. 2000;124:145–153. doi: 10.1093/brain/124.1.145. [DOI] [PubMed] [Google Scholar]
- Lunn MP, Crawford TO, Hughes RA, Griffin JW, Sheikh KA. Anti-MAG antibodies alter neurofilament spacing: a contribution to the pathogenesis of IgM anti-MAG paraproteinaemic neuropathy? J. Peripheral Nervous System. 2001;6:158. [Google Scholar]
- Lunn MP, Johnson LA, Fromholt SE, et al. High-affinity anti-ganglioside IgG antibodies raised in complex ganglioside knockout mice: reexamination of GD1a immunolocalization. J. Neurochem. 2000;75:404–412. doi: 10.1046/j.1471-4159.2000.0750404.x. [DOI] [PubMed] [Google Scholar]
- Lunn MPT, Nobile-Orazio E. The Cochrane Library (4) Oxford: UpdateSoftware; 2001. Immunotherapy for IgM anti-Myelin-Associated Glycoprotein paraprotein-associated peripheral neuropathies (Protocol for a Cochrane Review) [Google Scholar]
- Mariette X, Brouet JC, Chevret S, et al. A randomised double blind trial versus placebo does not confirm the benefit of alpha-interferon in polyneuropathy associated with monoclonal IgM. J. Neurol. Neurosurgery Psychiatry. 2000;69:279–280. doi: 10.1136/jnnp.69.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM, Griffin JW, Cornblath DR, et al. Plasmapheresis and Guillain–Barré Syndrome: analysis of prognostic factors and the effect of plasmapheresis. Ann. Neurol. 1988;23:347–353. doi: 10.1002/ana.410230406. [DOI] [PubMed] [Google Scholar]
- Mehndiratta MM, Hughes RAC. The Cochrane Library (3) Oxford: Update Software; 2001. Corticosteroids for chronic inflammatory demyelinating polyradiculoneuropathy (Cochrane Review) [DOI] [PubMed] [Google Scholar]
- Mendell JR, Barohn RJ, Freimer ML, et al. Randomized controlled trial of IVIg in untreated chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 2001;56:445–449. doi: 10.1212/wnl.56.4.445. [DOI] [PubMed] [Google Scholar]
- Nobile-Orazio E. Multifocal motor neuropathy. J. Neuroimmunol. 2001;115:4–18. doi: 10.1016/s0165-5728(01)00266-1. [DOI] [PubMed] [Google Scholar]
- Nomura T, Hamaguchi K, Hattori T, Satou T, Mannen T, et al. A randomized controlled trial comparing intravenous immunoglobulin and plasmapheresis in Guillain–Barré syndrome. Neurol. Therapeutics. 2000;18:69–81. [Google Scholar]
- O'Hanlon GM, Plomp JJ, Chakrabarti M, et al. Anti-GQ1b ganglioside antibodies mediate complement-dependent destruction of the motor nerve terminal. Brain. 2001;124:893–906. doi: 10.1093/brain/124.5.893. [DOI] [PubMed] [Google Scholar]
- Oh SJ, Joy JL, Kuruoglu R. ‘Chronic sensory demyelinating neuropathy’: Chronic inflammatory demyelinating polyneuropathy presenting as a pure sensory neuropathy. J. Neurol. Neurosurgery Psychiatry. 1992;55:677–680. doi: 10.1136/jnnp.55.8.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain–Barré syndrome. Lancet. 1997;349:225–230. [PubMed] [Google Scholar]
- Plomp JJ, Molenaar PC, O'Hanlon GM, et al. Miller Fisher anti-GQ1b antibodies: alpha-latrotoxin-like effects on motor end plates. Ann. Neurol. 1999;45:189–199. doi: 10.1002/1531-8249(199902)45:2<189::aid-ana9>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Prineas JW, McLeod JG. Chronic relapsing polyneuritis. J. Neurol. Sci. 1976;27:427–458. doi: 10.1016/0022-510x(76)90213-6. [DOI] [PubMed] [Google Scholar]
- Raphael J-C, Chevret S, Hughes RAC, Annane D. The Cochrane Library (2) Oxford: Update Software; 2001. Plasma Exchange for Guillain–Barré Syndrome (Cochrane Review) [DOI] [PubMed] [Google Scholar]
- Rich MM, Pinter MJ. Sodium channel inactivation in an animal model of acute quadriplegic myopathy. Ann. Neurol. 2001;50:26–33. doi: 10.1002/ana.1016. [DOI] [PubMed] [Google Scholar]
- Rich MM, Pinter MJ, Craner SD, Barchi RL. Loss of electrical excitability in an animal model of acute quadriplegic myopathy. Ann. Neurol. 1998;43:171–179. doi: 10.1002/ana.410430207. [DOI] [PubMed] [Google Scholar]
- Saperstein DS, Amato AA, Wolfe GI, et al. Multifocal acquired demyelinating motor and sensory motor neuropathy: the Lewis–Sumner syndrome. Muscle Nerve. 2000;22:560–566. doi: 10.1002/(sici)1097-4598(199905)22:5<560::aid-mus2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Simmons Z, Albers JW, Bromberg MB, Feldman EL. Presentation and initial clinical course in patients with chronic inflammatory demyelinating polyradiculoneuropathy: Comparison of patients without and with monoclonal gammopathy. Neurology. 1993;43:2202–2209. doi: 10.1212/wnl.43.11.2202. [DOI] [PubMed] [Google Scholar]
- Smith IS, Kahn SN, Lacey BW, et al. Chronic demyelinating neuropathy associated with benign IgM paraproteinaemia. Brain. 1983;106:169–195. doi: 10.1093/brain/106.1.169. [DOI] [PubMed] [Google Scholar]
- Tatum AH. Experimental paraprotein neuropathy, demyelination by passive transfer of human IgM anti-myelin-associated glycoprotein. Ann. Neurol. 1993;33:502–506. doi: 10.1002/ana.410330514. [DOI] [PubMed] [Google Scholar]
- Thomas PK, Lascelles RG, Hallpike JF, Hewer RL. Recurrent and chronic relapsing Guillain-Barré polyneuritis. Brain. 1969;92:589–606. doi: 10.1093/brain/92.3.589. [DOI] [PubMed] [Google Scholar]
- Thomas PK. The Guillain-Barré syndrome: no longer a simple concept. J. Neurol. 1992;239:361–362. doi: 10.1007/BF00812150. [DOI] [PubMed] [Google Scholar]
- Thomas PK, Claus D, Jaspert A, et al. Focal upper limb demyelinating neuropathy. Brain. 1996;119:765–774. doi: 10.1093/brain/119.3.765. [DOI] [PubMed] [Google Scholar]
- Thompson N, Choudhary P, Hughes R, Quinlivan R. A novel trial design to study the effect of intravenous immunoglobulin in chronic inflammatory demyelinating polyradiculoneuropathy. J. Neurol. 1996;243:280–285. doi: 10.1007/BF00868527. [DOI] [PubMed] [Google Scholar]
- Umapathi T, Leger J-M, Nobile-Orazio E, Hughes RAC. The Cochrane Library (4) Oxford: Update Software; 2001. Immunosuppressants for treating multifocal motor neuropathy (Protocol for a Cochrane Review) [Google Scholar]
- van den Berg LH, Kerkhoff H, Oey PL, et al. Treatment of multifocal motor neuropathy with high dose intravenous immunoglobulins: a double blind, placebo controlled study. J. Neurol. Neurosurgery Psychiatry. 1995;59:248–252. doi: 10.1136/jnnp.59.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bergh PYK. Intravenous immunoglobulin (IVIG) in the treatment of chronic demyelinating polyradiculoneuropathy. Acta Neurol. Belg. 2001;101:147–151. [PubMed] [Google Scholar]
- van der Meché FGA, Schmitz PIM Dutch Guillain-Barré Study Group. A randomized trial comparing intravenous immune globulin and plasma exchange in Guillain–Barré syndrome. N. Engl. J. Med. 1992;326:1123–1129. doi: 10.1056/NEJM199204233261705. [DOI] [PubMed] [Google Scholar]
- van Doorn PA, Brand A, Strengers PF, Meulstee J, Vermeulen M. High-dose intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyneuropathy: a double-blind, placebo-controlled, crossover study (see comments) Neurology. 1990;40:209–212. doi: 10.1212/wnl.40.2.209. [DOI] [PubMed] [Google Scholar]
- Van Schaik IS, Winer JB, De Haan R, Vermeulen M. The Cochrane Library (4) Oxford: Update Software; 2001. Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy (Protocol for a Cochrane Review) [DOI] [PubMed] [Google Scholar]
- Vermeulen M, Van Der Meche FGA, Speelman JD, Weber A, Busch HFM. Plasma and gamma-globulin infusion in chronic inflammatory polyneuropathy. J. Neurol. Sci. 1985;70:317–326. doi: 10.1016/0022-510x(85)90173-x. [DOI] [PubMed] [Google Scholar]
- Vermeulen M, Van Doorn PA, Brand A, Strengers PFW, Jennekens FGI, Busch HFM. Intravenous immunoglobulin treatment in patients with chronic inflammatory demyelinating polyneuropathy: adouble blind, placebo controlled study. J. Neurol., Neurosurgery Psychiatry. 1993;56:36–39. doi: 10.1136/jnnp.56.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddy HM, Misra VP, King RHM, Thomas PK, Middleton L, Ormerod IEC. Focal cranial nerve involvement in chronic inflammatory demyelinating polyneuropathy: clinical and MRI evidence of peripheral and central lesions. J. Neurol. 1989;236:400–405. doi: 10.1007/BF00314898. [DOI] [PubMed] [Google Scholar]
- Yan WX, Archelos JJ, Hartung HP, Pollard JD. P0 protein is a target antigen in chronic inflammatory demyelinating polyradiculoneuropathy. Ann. Neurol. 2001;50:286–292. doi: 10.1002/ana.1129. [DOI] [PubMed] [Google Scholar]
- Yeung KB, Thomas PK, King RHM, et al. The clinical spectrum of peripheral neuropathies associated with benign monoclonal IgM, IgG and IgA paraproteinaemia. Comparative clinical, immunological and nerve biopsy findings. J. Neurol. 1991;238:383–391. doi: 10.1007/BF00319857. [DOI] [PubMed] [Google Scholar]