Abstract
Mice heterozygously deficient in the peripheral myelin adhesion molecule P0 (P0+/− mice) are models for some forms of Charcot–Marie–Tooth (CMT) neuropathies. In addition to the characteristic hallmarks of demyelination, elevated numbers of CD8-positive T-lymphocytes and F4/80-positive macrophages are striking features in the nerves of these mice. These immune cells increase in number with age and progress of demyelination, suggesting that they might be functionally related to myelin damage. In order to investigate the pathogenetic role of lymphocytes, the myelin mutants were cross-bred with recombination activating gene 1 (RAG-1)-deficient mice, which lack mature T-and B-lymphocytes. The immunodeficient myelin mutants showed a less severe myelin degeneration. The beneficial effect of lymphocyte-deficiency was reversible, since demyelination worsened in immunodeficient myelin-mutants when reconstituted with bone marrow from wild-type mice. Ultrastructural analysis revealed macrophages in close apposition to myelin and demyelinated axons. We therefore cross-bred the P0+/− mice with spontaneous osteopetrotic (op) mutants deficient in the macrophage colony-stimulating factor (M-CSF), hence displaying impaired macrophage activation. In the corresponding double mutants the numbers of macrophages were not elevated in the peripheral nerves, and the demyelinating phenotype was less severe than in the genuine P0+/− mice, demonstrating that macrophages are also functionally involved in the pathogenesis of genetically mediated demyelination. We also examined other models for inherited neuropathies for a possible involvement of immune cells. We chose mice deficient in the gap junction component connexin 32, a model for the X-linked form of CMT. Similar to P0-deficient mice, T-lymphocytes and macrophages were elevated and macrophages showed a close apposition to degenerating myelin. We conclude that the involvement of T-lymphocytes and macrophages is a common pathogenetic feature in various forms of slowly progressive inherited neuropathies.
Keywords: demyelination, lymphocytes, macrophages, myelin, Schwann cells
Introduction
Demyelination leads to a substantial functional impairment of the nervous system, reflecting the fact that the myelin sheath is an important phylogenetic invention to maintain the axon's functional and structural integrity (Martini, 2001). Major causes for demyelination are autoimmune inflammation, toxic agents, metabolic dysfunction, and mutations in genes related to the myelin sheath or other components of the nervous system (Reilly, 2000; Young & Suter, 2001). Meanwhile, mutations in more than 10 genes have been identified that can lead to disorders in the peripheral nervous system (Reilly, 2000; Young & Suter, 2001). Mutations in genes encoding for the myelin components PMP22, P0 and connexin 32 are clinically most relevant, since they were found in the majority of patients with inherited demyelinating neuropathies. The biological effects of these genes in myelination have been studied (for reviews see Martini & Schachner, 1997; Werner et al. 1998; Abrams et al. 2000; Martini, 2000; Müller, 2000; Ressot & Bruzzone, 2000; Young & Suter, 2001). However, the cellular and molecular processes involved in myelin damage as a result of partial or complete gene inactivation have not been characterized in the intact organism so far.
In order to get an insight into the pathomechanisms of inherited demyelination, we investigated mice heterozygously deficient in P0, which express only 50% of the wild-type dose of P0 and are an established animal model for a slowly progressing demyelinating form of inherited neuropathies (Martini et al. 1995). These mice initially show normal myelination, followed by slowly progressing myelin degeneration starting at the age of approximately 4 months. Demyelination is accompanied by an increase in the number of T-lymphocytes and macrophages in peripheral nerves (Schmid et al. 2000; Carenini et al. 2001). By cross-breeding experiments using mouse mutants deficient in either functional lymphocytes or activated macrophages, the infiltrating immune cells were identified as substantial mediators of primarily gene-related demyelination (Schmid et al. 2000; Carenini et al. 2001). Since in other myelin mutants myelin damage is also accompanied by an elevation of immune cells, the functional involvement of these cells seems to be a widespread phenomenon with relevance for putative treatment strategies for some forms of inherited demyelination.
Lymphocytes as mediators of inherited demyelination
Mice heterozygously deficient in P0 have been described as suitable models for some forms of CMT (Martini et al. 1995). Myelin forms almost normally for approximately 4 months, followed by a progressive demyelinating neuropathy in motor, but not sensory, nerves (Martini et al. 1995; Shy et al. 1997; Martini, 2000; Schmid et al. 2000; Samsam et al. 2001, for review). Typical pathological features are demyelinated axons, unusually thin myelin (reflecting incomplete remyelination), and supernumerary Schwann cells reminiscent of onion bulbs in human neuropathies. In line with the demyelinating phenotype were prolonged F-wave latencies of the compound muscle action potential in small foot muscles in response to sciatic nerve stimulation (Martini et al. 1995).
A striking and initially unexpected feature was the presence of CD8-positive T-lymphocytes in the endoneurium of these mutants (Shy et al. 1997; Schmid et al. 2000). The number of these immune cells increased with time and progress of demyelinating neuropathy, suggesting that they might be functionally related to the disease. In order to investigate the functional roles of these cells, the myelin mutants were cross-bred with mice deficient in mature T- and B-lymphocytes, i.e. RAG-1-deficient mice. The double mutants showed a less severe myelin degeneration in the absence of lymphocytes (compare Fig. 1A with Fig. 1D). This improvement of myelin maintenance manifested in a reduction of F-wave latencies reflecting improved nerve conduction properties (Schmid et al. 2000). A comparable amelioration of the demyelinating phenotype was achieved when P0-mutants were cross-bred with mice deficient in the α-subunit of the T-cell receptor lacking αβ-T-lymphocytes (Schmid et al. 2000). In order to investigate whether the beneficial effect of lymphocyte-deficiency is reversible, we reconstituted the myelin/RAG-1–/– mutants with bone marrow from mice with an intact immune system. This transfer of functional intact immune cells led to an aggravation of the myelin phenotype similar to that found in genuine P0+/− mice (Fig.1; Mäurer et al. 2001). Thus T-lymphocytes are functionally involved in the primarily genetically mediated demyelination.
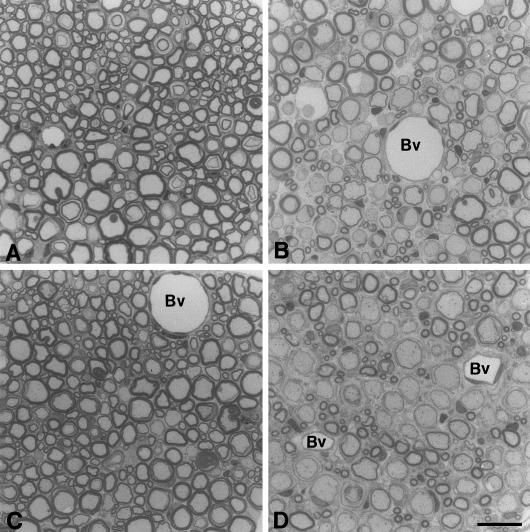
Fig. 1.
Semi-thin sections of ventral spinal roots from P0+/− /RAG-1−/− mice that received no bone marrow (A), or bone marrow from wild-type mice (B), or bone marrow from RAG-1−/− mice (C). For comparison, ventral spinal roots from genuine P0+/− mice are shown (D). Note that demyelination is mild when no bone marrow (A) or bone marrow from RAG-1−/− mice had been transplanted (C), whereas in P0+/− /RAG-1−/− mutants that had received bone marrow from wt mice (B) demyelination is similarly severe as in genuine P0+/− mice (D). Bv, blood vessel. Scale bar in D (for A-D): 20 μm.
Macrophages as mediators of inherited demyelination
T-lymphocytes are not the only immune cells that show an increased frequency in the peripheral nerves of the myelin mutants. We also found an increased number of macrophages within the peripheral nerves of P0+/− mice. At 6 months of age, they are elevated by a factor of 3–4 and outnumber T-lymphocytes by a factor of approximately 20 (Fig. 2A,B). Electron microscopy and immunoelectron microscopy revealed that some macrophages had entered the endoneurial tubes either contacting demyelinated axons or myelin that is morphologically still intact (Fig. 2C,D). Since this apposition of macrophages with endoneurial tubes was highly suggestive of an involvement in degeneration and resembled macrophage–myelin interaction in inflammatory neuropathies (Ballin & Thomas, 1969; Lampert, 1969; Ho et al. 1998; Smith, 1999), we cross-bred the myelin mutants with spontaneous mutants deficient in the macrophage-colony-stimulating factor (M-CSF), hence displaying impaired macrophage activation. In the P0-deficient double mutants, the number of macrophages was not elevated in the demyelinating nerves (Carenini et al. 2001). In addition, the demyelinating phenotype was less severe than in genuine P0+/− mice (Fig. 3). Since M-CSF receptor was found exclusively associated with macrophages but never with Schwann cells or endoneurial fibroblasts within the peripheral nerves (Fig. 4), the ameliorated demyelination in the absence of M-CSF is most probably due to an impaired macrophage activation. Thus we conclude that macrophages are functionally involved in genetically mediated demyelination (Carenini et al. 2001).
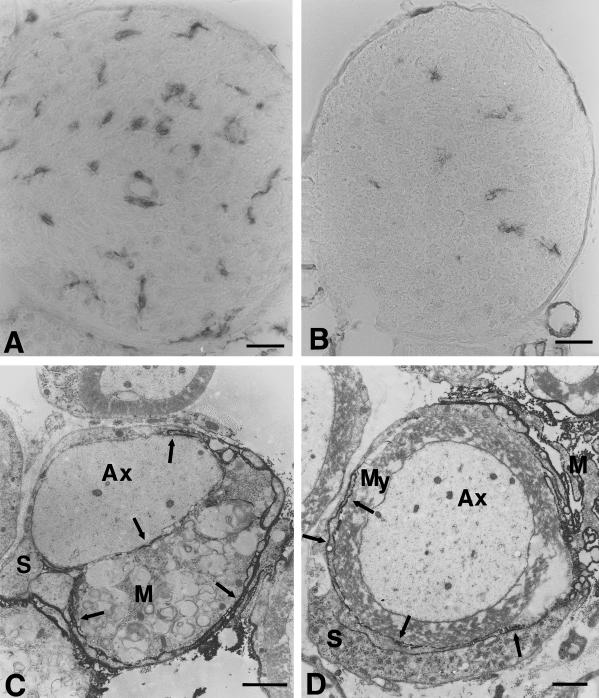
Fig. 2.
(A,B) Immunohistological localization of macrophages in femoral quadriceps nerves of P0+/− (A) and P0+/+ mice (B) at the age of 6 months using antibodies to F4/80. In quadriceps nerves of P0+/− mice the number of macrophages is clearly elevated when compared to P0+/+ mice. Note the larger size of the cells and the close vicinity of two cells to an endoneurial blood vessel in the nerve of the mutant (A). (C,D) Immunoelectron microscopic localization of F4/80-positive macrophages in peripheral nerves of 6-month-old P0+/− mice. (C) An F4/80-positive macrophage (M), containing myelin debris, is in close apposition to a demyelinated axon. Arrows indicate electron-dense immunoreaction product. Axon (Ax), Schwann cell (S). (D) A slender, immunoreactive process (arrows) of an F4/80-positive macrophage (M) has penetrated in between the pericaryon of a Schwann cell (S) and its normal appearing myelin sheath (My). Scale bars: 20 μm (for A and B); 1.5 μm (for C and D).
Fig. 3.
(A,B) Electron microscopy of ventral roots of 6-month-old P0+/− op/wt (heterozygous osteopetrotic (op) mice with wildtype-like M-CSF expression; A) and of P0+/− op/op littermates (homozygous op mice which are M-CSF-deficient; B). In many fibres of P0+/− op/wt mice, there is profound demyelination when compared to P0+/− op/op littermates deficient in M-CSF. Scale bars: 5 μm.
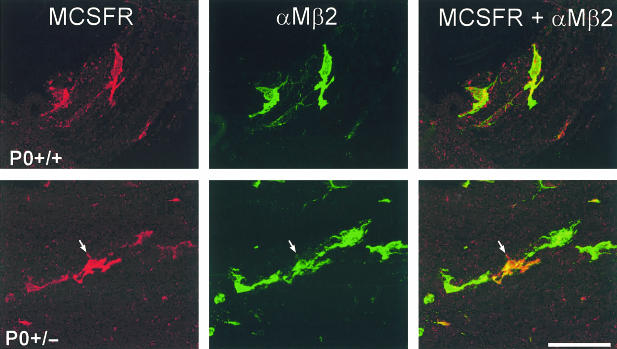
Fig. 4.
Cellular localization of the M-CSF receptor (MCSFR, red) immunoreactivity in teased fibre preparations from ventral roots of P0+/+ and P0+/− mice using antibodies to αMβ2 integrin (green) as a marker for peripheral nerve macrophages. αMβ2-negative cells, such as the adjacent Schwann cells, were never labelled. Note the particularly strongly labelled M-CSFR-immunoreactive macrophage in the P0+/− mutant (arrow). Scale bar: 50 μm.
Involvement of immune cells in inherited demyelination: evidence for a widespread phenomenon
A similarly slowly progressing demyelinating pathology as in P0+/− mice is found in mice hemizygously or homozygously deficient in the gap junction protein connexin (Cx) 32 (also designated GJB1), an established model for the X-linked dominant form of CMT caused by mutations in the Cx32 gene (Anzini et al. 1997; Martini, 1997; Scherer et al. 1998; Martini, 2000, for review). Although the typical pathological features of Cx32-mutants, such as enlarged periaxonal collars, abnormal non-compacted myelin domains and the superimposed axonopathy are different from the features seen in P0+/− mice, the overall disease progression is similar. Therefore, we set out to investigate whether immune cells might be involved in the demyelinating neuropathy of Cx32-deficient mice. We quantified the T-lymphocytes and macrophages in the demyelinating peripheral nerves of Cx32-mutants and investigated the association of macrophages and demyelinating fibres by immunoelectron microscopy. Although the main pathological features are different from those found in P0+/− mice, we found that T-lymphocytes and macrophages increased during demyelination in Cx32-deficient mice (Kobsar et al. 2002). Even more striking was the finding that macrophages were tightly associated with demyelinated axons and degenerating myelin (Kobsar et al. 2002; Fig. 5). Thus an increase in immune cells and their functional involvement in demyelination might be a common pathological pathway in slowly progressing demyelinating disorders.
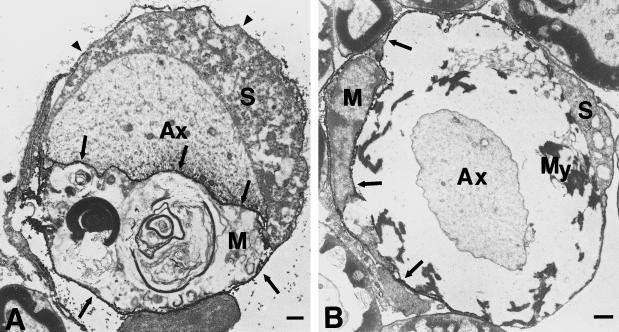
Fig. 5.
Immunoelectron microscopy of F4/80-positive macrophages in ventral roots of 6-month-old Cx32−/− mice. (A) A demyelinated axon (Ax) is in close contact to an F4/80-positive macrophage. Arrows demarcate F4/80-related immunoprecipitate. Note that the surface and the basement membrane of the Schwann cell (S; arrowheads) and the axon-Schwann cell interface are not labelled. (B) An F4/80-positive macrophage (M) has penetrated the Schwann cell basal lamina and contacts poorly preserved and partially vacuolized myelin (My). Note well preserved myelin of the fibres not in contact with the macrophage. Arrows demarcate F4/80-related immunoprecipitate. Ax, myelinated axon; S, F4/80-negative Schwann cell. Scale bars: 1 μm.
Discussion and conclusions
Our studies clearly demonstrate that inherited demyelination is modulated by the immune system. This concept is novel, and the underlying mechanisms are not yet understood. A striking observation was that elevated numbers of macrophages occur in the nerves of myelin mutants already at the age of 2 months, i.e. approximately 2 months before overt demyelination. T-lymphocytes then follow approximately 2–4 months later. This raises the question of the signals that ‘lure’ the immune cells into the nerves and lead to their activation. Interesting candidates for attraction and activation of macrophages are Schwann cell-related factors. It is possible that Schwann cells react to the consequences of reduced gene dosage by unknown signals and start to secrete chemokines/cytokines before demyelinating features are obvious (Fig. 6). One possible candidate is M-CSF, since its absence results in a lack of increase in macrophages in the mutant nerves (Carenini et al. 2001). In addition, chemokines, and in particular MCP-1, are of interest. MCP-1 is up-regulated by Schwann cells of injured nerves (Toews et al. 1998; Taskinen & Röyttä, 2000; Subang & Richardson, 2001) and inactivation of its specific chemokine receptor CCR2 leads to a reduction of macrophage influx into transected sciatic nerves (Siebert et al. 2000). Moreover, MCP-1 is also up-regulated when demyelination is induced by the gliotoxin lysophosphatidylcholine (LPC) and MCP-1-specific antibodies reduce LCP-mediated demyelination (Ousman & David, 2001). Finally, experimental autoimmune encephalomyelitis (EAE) is highly reduced in MCP-1-deficient mice (Huang et al. 2001). Indeed, by using a microarray approach, we recently identified MCP-1 message in nerve homogenates from young P0+/− mice (Mäurer & Martini, unpublished observations).
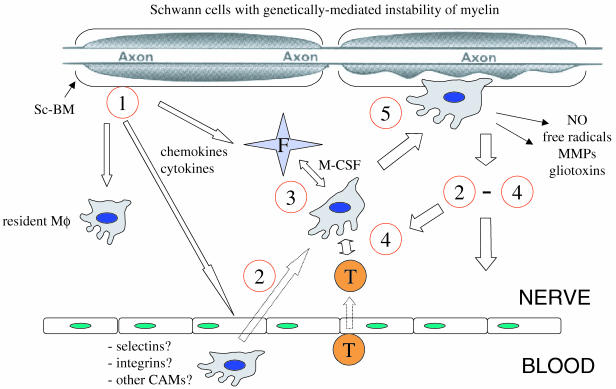
Fig. 6.
Hypothetical sequence of events in peripheral nerves of mice with inherited demyelination leading to superimposed immune attack. 1, pathogenesis starts with myelin instability due to myelin-related mutations in Schwann cell genes. Schwann cells react to myelin instability by unknown signals and secrete chemokines/cytokines. 2, Schwann cell-derived chemokine secretion may chemotactically attract monocytes/macrophages, in part by activating adjacent endothelial cells, and lead to proliferation of resident macrophages (Mö). As a novel player, endoneurial fibroblasts (F) may be also activated by the Schwann cell-derived chemokine secretion. 3, macrophages may interact with endoneurial fibroblasts (F) and become activated by fibroblast-derived M-CSF. 4, activated macrophages present antigens and/or secrete cytokines that attract and activate T-lymphocytes (T) which in turn activate more macrophages and T-cells. 5, macrophages penetrate Schwann cell basal laminae (Sc-BM), phagocytose myelin and secrete gliotoxic factors.
Alternatively or additionally, it is possible that the Schwann cells indirectly induce macrophage-activating agents in other cells within the peripheral nerve such as endothelial cells and/or endoneurial fibroblasts (Fig. 6). In this context, it is worth mentioning that in the bone, osteoblasts (specialized fibroblasts) activate adjacent osteoclasts (specialized macrophages) by secreting M-CSF (Teitelbaum, 2000). Indeed, endoneurial fibroblasts and macrophages are often tightly associated with each other in the demyelinating nerves (Martini and Mäurer, unpublished observations) and we could definitively show that M-CSF is involved in macrophage activation in our animal model (Carenini et al. 2001).
In myelin mutant mice, T-lymphocytes appear to be attracted by macrophages, possibly by cytokines (Fig. 6). This is clearly reflected by the observation that in P0+/− mice with compromised macrophage activation no elevation of T-lymphocyte numbers occurs (Carenini et al. 2001). On the other hand, in RAG-1-deficient P0+/− mutants, absence of T-lymphocytes causes only a very mild elevation of macrophages (Schmid et al. 2000) reflecting the complex cross-talk of immune elements in demyelinating nerves (Fig. 6).
Another relevant question is whether the detrimental effect of the T-lymphocytes is antigen-specific. Splenocytes from P0+/− mice show a clear proliferative response upon exposition with peripheral myelin components, such as P0 (Schmid et al. 2000). P0 has been identified as a target antigen in chronic inflammatory demyelinating polyradiculoneuropathy in humans (Yan et al. 2001), suggesting that the protein could be an auto-antigen under various pathological conditions in the PNS. However, further experiments are necessary to prove an antigen-specific reaction in our myelin mutants. One approach could be the transfer of bone marrow from transgenic mouse donors generating monospecific, non-neural, virus-specific T-lymphocytes (Pircher et al. 1989) into the P0+/− /RAG-1–/– mice. The P0+/− /RAG-1–/– mice show an ameliorated demyelinating neuropathy due to an absence of T-lymphocytes (Mäurer et al. 2001). The effect of T-lymphocytes that are not able to interact with neural antigens would then decisively argue in favour or against an antigen-specific effect of T-lymphocytes in P0+/− mice.
In summary, we could clearly show that both T-lymphocytes and macrophages contribute to the demyelinating neuropathy in P0+/− mice. In addition, we have provided evidence that these mechanisms might also be found in other genetically mediated nerve disorders. Indeed, in a variety of CMT patients, corticosteroid treatment (Dyck et al. 1982; Bird & Sladky, 1991; Crawford & Griffin, 1991; Rajabally et al. 2000) or plasma exchange (Malandrini et al. 1999) lead to an alleviation of particularly rapidly progressing symptoms. It is possible that such cases reflect a more aggressive impact of immune cells on top of the primarily genetically mediated disorder. Less conspicuous and more chronically progressing immune reactions being more comparable to our mouse models may have been unnoticed in the majority of cases, particularly since the active phase of demyelination of CMT occurs during childhood (Gabreëls-Festen et al. 1992; Vital et al. 1992; Thomas, 1999), when nerve biopsies are rarely performed.
It is plausible to assume that similar immune mechanisms as a consequence of neural mutations might also occur in the CNS. In an animal model for globoid cell leucodystrophy (Krabbe’s disease), a spontaneous null mutant for galactosyltransferase, a strong reduction in brain macrophages and activated microglial cells could be obtained by cross-breeding these mice with mice deficient in the MHC class II complex. Most strikingly, this reduction was associated with a substantial improvement of myelin deficiency and clinical phenotype (Matsushima et al. 1994). Moreover, in mice either overexpressing or being deficient in PLP, the major protein of CNS myelin, increased numbers of microglial cells are visible (Anderson et al. 1998; Griffiths et al. 1998). In addition, a recent study in an MBP mutant, the Long Evans shaker rat, shows that genetically related dys- and demyelination is in space and time correlated with microglial activiation, proliferation and myelin phagocytosis (Zhang et al. 2001). In humans, the clinical outcome of some leukodystrophies, in particular adrenoleukodystrophy, is substantially modulated by inflammatory reactions upon the primary gene defect (Berger et al. 2001). This might lead us to the provocative view that possibly some forms of multiple sclerosis might be initially caused by mutations in neural-related genes that then manifest as an inflammatory disorder.
Our combined observations and their possible relevance for severe human demyelinating disorders should now stimulate research on the mechanisms involved. Due to its histological simplicity, the peripheral nerve might be an ideal paradigm for studying the immunological events downstream from the glia-related mutations. Of particular interest should be the cross-talk between the mutant glial cells, other endoneurial elements and the immune cells themselves. Modern tools derived from molecular biology, such as expression arrays, differential display analysis and the availability of the appropriate knockout mutants should enable us to understand these pathogenetic mechanisms which have not been exhaustively considered so far. Based on these activities, it should be possible to design appropriate therapeutic strategies to interfere with the progress of the still incurable demyelinating disorders of both the peripheral and central nervous system.
Acknowledgments
We are grateful to Professor K. V. Toyka for critical reading of the manuscipt, Professor R. Gold and Dr H. Hofstetter for many stimulating discussions and H. Blazyca and Carolin Kiesel for excellent technical assistance. The studies in the laboratory of R.M. are supported by the Deutsche Forschungsgemeinschaft (SFB 581; Priority Programme ‘Microglia’ MA1053/3), Gemeinnützige Hertie-Stiftung (GHS2/476/98), by the German Ministry of Research (Interdisziplinäres Zentrum für klinische Forschung, project C-6), by the Schweizerische Nationalfonds and by local research funds of the University of Würzburg.
References
- Abrams CK, Oh S, Ri Y, Bargiello TA. Mutations in connexin 32: the molecular and biophysical bases for the X-linked form of Charcot-Marie-Tooth disease. Brain Res. Rev. 2000;32:203–214. doi: 10.1016/s0165-0173(99)00082-x. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Schneider A, Barrie BA, Klugmann MMC, Culloch MC, Kirkham D, et al. Late-onset neurodegeneration in mice with increased dosage of the proteolipid protein gene. J. Comp. Neurol. 1998;394:506–519. doi: 10.1002/(sici)1096-9861(19980518)394:4<506::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Anzini P, Neuberg DHH, Schachner M, Nelles E, Willecke K, Zielasek J, Toyka KV, Suter U, Martini R. Structural abnormalities and deficient maintenance of peripheral nerve myelin in mice lacking the gap junction protein connexin 32. J. Neurosci. 1997;17:4545–4551. doi: 10.1523/JNEUROSCI.17-12-04545.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballin RH, Thomas PK. Electron microscope observations on demyelination and remyelination in experimental allergic neuritis I Demyelination. J. Neurol. Sci. 1969;8:1–18. doi: 10.1016/0022-510x(69)90037-9. [DOI] [PubMed] [Google Scholar]
- Berger J, Moser HW, Forss-Petter S. Leukodystrophies: recent developments in genetics, molecular biology, pathogenesis and treatment. Curr. Opin. Neurol. 2001;14:305–312. doi: 10.1097/00019052-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Bird SJ, Sladky JT. Corticosteroid-responsive dominantly inherited neuropathy in childhood. Neurology. 1991;41:437–439. doi: 10.1212/wnl.41.3.437. [DOI] [PubMed] [Google Scholar]
- Carenini S, Mäurer M, Werner A, Blazyca H, Toyka KV, Schmid CD, et al. The role of macrophages in demyelinating peripheral nervous system of mice heterozygously deficient in P0. J. Cell Biol. 2001;152:301–308. doi: 10.1083/jcb.152.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford TO, Griffin JW. Morphometrical and ultrastructural evaluation of the sural nerve in children with Charcot-Marie-Tooth: implication for pathogenesis and treatment. Ann. Neurol. 1991;30:500. [Google Scholar]
- Dyck PJ, Swanson CJ, Low PA, Bartleson JD, Lambert EH. Prednisone-responsive hereditary motor and sensory neuropathy. Mayo Clin. Proc. 1982;57:239–246. [PubMed] [Google Scholar]
- Gabreëls-Festen AAWM, Joosten EMG, Gabreëls FJM, Jennekens FGI, Janssen-van Kempen TW. Early morphological features in dominantly inherited demyelinating motor and sensory neuropathy (HMSN type I) J. Neurol. Sci. 1992;107:145–154. doi: 10.1016/0022-510x(92)90282-p. [DOI] [PubMed] [Google Scholar]
- Griffiths I, Klugmann M, Anderson T, Yool D, Thomson C, Schwab MH, et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280:1610–1613. doi: 10.1126/science.280.5369.1610. [DOI] [PubMed] [Google Scholar]
- Ho TW, McKhann GM, Griffin JW. Human autoimmune neuropathies. Ann. Rev. Neurosci. 1998;21:187–226. doi: 10.1146/annurev.neuro.21.1.187. [DOI] [PubMed] [Google Scholar]
- Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J. Exp. Med. 2001;193:713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobsar I, Mäurer M, Ott T, Martini R. Macrophage-related demyelination in peripheral nerves of mice deficient in the gap junction protein connexin 32. Neurosci. Lett. 2002;320:17–20. doi: 10.1016/s0304-3940(02)00015-0. [DOI] [PubMed] [Google Scholar]
- Lampert PW. Mechanism of demyelination in experimental allergic neuritis. Laboratory Invest. 1969;20:127–138. [PubMed] [Google Scholar]
- Malandrini A, Villanova M, Dotti MT, Frederico A. Acute inflammatory neuropathy in Charcot-Marie-Tooth disease. Neurology. 1999;52:859–861. doi: 10.1212/wnl.52.4.859. [DOI] [PubMed] [Google Scholar]
- Martini R, Zielasek J, Toyka KV, Giese KP, Schachner M. Protein zero (P0)-deficient mice show myelin degeneration in peripheral nerves characteristic of inherited human neuropathies. Nat. Genet. 1995;11:281–286. doi: 10.1038/ng1195-281. [DOI] [PubMed] [Google Scholar]
- Martini R. Animal models for inherited peripheral neuropathies. J. Anat. 1997;191:321–336. doi: 10.1046/j.1469-7580.1997.19130321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R, Schachner M. Molecular bases of myelin formation as revealed by investigations on mice deficient in glial cell surface molecules. Glia. 1997;19:298–310. [PubMed] [Google Scholar]
- Martini R. Animal models for inherited peripheral neuropathies: Chances to find treatment strategies? J. Neurosci. Res. 2000;61:244–250. doi: 10.1002/1097-4547(20000801)61:3<244::AID-JNR2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Martini R. The effect of myelinating Schwann cells on axons. Muscle Nerve. 2001;24:456–466. doi: 10.1002/mus.1027. [DOI] [PubMed] [Google Scholar]
- Matsushima GK, Taniike M, Glimcher LH, Grusby MJ, Frelinger JA, Suzuki K, et al. Absence of MHC class II molecules reduces CNS demyelination, microglial/macrophage infiltration, and twitching in murine globoid cell leukodystrophy. Cell. 1994;78:645–656. doi: 10.1016/0092-8674(94)90529-0. [DOI] [PubMed] [Google Scholar]
- Mäurer M, Schmid CD, Bootz F, Zielasek J, Toyka KV, Oehen S, et al. Bone marrow transfer from wild-type mice reverts the beneficial effect of genetically-mediated immune deficiency in myelin mutants. Mol. Cell. Neurosci. 2001;17:1094–1101. doi: 10.1006/mcne.2001.0990. [DOI] [PubMed] [Google Scholar]
- Müller HW. Tetraspan myelin protein PMP22 and demyelinating peripheral neuropathies: new facts and hypotheses. Glia. 2000;29:182–185. doi: 10.1002/(sici)1098-1136(20000115)29:2<182::aid-glia12>3.3.co;2-b. [DOI] [PubMed] [Google Scholar]
- Ousman SS, David S. MIP-1alpha, MCP-1, GM-CSF, and TNF-alpha control the immune cell response that mediates rapid phagocytosis of myelin from the adult mouse spinal cord. J. Neurosci. 2001;21:4649–4656. doi: 10.1523/JNEUROSCI.21-13-04649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher HP, Bürki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- Rajabally Y, Vital A, Ferrer X, Vital C, Julien J, Latour P, et al. Chronic inflammatory demyelinating polyneuropathy caused by HIV infection in a patient with asymptomatic CMT1A. J. Periph. Nerv. Syst. 2000;5:158–162. doi: 10.1046/j.1529-8027.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- Reilly MM. Classification of the hereditary motor and sensory neuropathies. Curr. Opin. Neurol. 2000;13:561–564. doi: 10.1097/00019052-200010000-00009. [DOI] [PubMed] [Google Scholar]
- Ressot C, Bruzzone R. Connexin channels in Schwann cells and the development of the X-linked form of Charcot-Marie-Tooth disease. Brain Res. Rev. 2000;32:192–202. doi: 10.1016/s0165-0173(99)00081-8. [DOI] [PubMed] [Google Scholar]
- Samsam M, Frei R, Marziniak M, Martini R, Sommer C. Impaired sensory function in heterozygous P0 knockout mice is associated with nodal changes in sensory nerves. J. Neurosci. Res. 2001 doi: 10.1002/jnr.10115. in press. [DOI] [PubMed] [Google Scholar]
- Scherer SS, Xu Y-T, Nelles E, Fischbeck K, Willecke K, Bone LJ. Connexin32-null mice develop demyelinating peripheral neuropathy. Glia. 1998;24:8–20. doi: 10.1002/(sici)1098-1136(199809)24:1<8::aid-glia2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Schmid CD, Stienekemeier M, Oehen S, Bootz F, Zielasek J, Gold R, et al. Immune deficiency in mouse models for inherited peripheral neuropathies leads to improved myelin maintenance. J. Neurosci. 2000;20:729–735. doi: 10.1523/JNEUROSCI.20-02-00729.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shy ME, Arroyo E, Sladky J, Menichella D, Jiang H, Xu W, et al. Heterozygous P0 knock-out mice develop a peripheral neuropathy that resembles chronic inflammatory demyelinating polyneuropathy. J. Neuropath. Exp. Neurol. 1997;56:811–821. [PubMed] [Google Scholar]
- Siebert H, Sachse A, Kuziel WA, Maeda N, Bruck W. The chemokine receptor CCR2 is involved in macrophage recruitment to the injured peripheral nervous system. J. Neuroimmunol. 2000;110:117–185. doi: 10.1016/s0165-5728(00)00343-x. [DOI] [PubMed] [Google Scholar]
- Smith ME. Phagocytosis of myelin in demyelinative disease: a review. Neurochem. Res. 1999;24:261–268. doi: 10.1023/a:1022566121967. [DOI] [PubMed] [Google Scholar]
- Subang MC, Richardson PM. Influence of injury and cytokines on synthesis of monocyte chemoattractant protein-1 mRNA in peripheral nervous tissue. Eur. J. Neurosci. 2001;13:521–528. doi: 10.1046/j.1460-9568.2001.01425.x. [DOI] [PubMed] [Google Scholar]
- Taskinen HS, Röyttä M. Increased expression of chemokines (MCP-1, MIP-1alpha, RANTES) after peripheral nerve transection. J. Peripher. Nerv. Syst. 2000;5:75–81. doi: 10.1046/j.1529-8027.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- Thomas PK. Overview of Charcot-Marie-Tooth disease 1A. Ann. New York Acad. Sci. 1999;833:1–5. doi: 10.1111/j.1749-6632.1999.tb08560.x. [DOI] [PubMed] [Google Scholar]
- Toews AD, Barrett C, Morell P. Monocyte chemoattractant protein 1 is responsible for macrophage recruitment following injury to sciatic nerve. J. Neurosci. Res. 1998;53:260–267. doi: 10.1002/(SICI)1097-4547(19980715)53:2<260::AID-JNR15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Vital A, Vital C, Julien J, Fontan D. Occurence of active demyelinating lesions in children with hereditary motor and sensory neuropathy (HMSN) type I. Acta Neuropathol. 1992;84:433–436. doi: 10.1007/BF00227671. [DOI] [PubMed] [Google Scholar]
- Werner H, Jung M, Klugmann M, Sereda M, Griffiths IR, Nave KA. Mouse Models of Myelin Disease. Brain Pathol. 1998;8:771–793. doi: 10.1111/j.1750-3639.1998.tb00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan WX, Archelos JJ, Hartung HP, Pollard JD. P0 protein is a target antigen in chronic inflammatory demyelinating polyradiculoneuropathy. Ann. Neurol. 2001;50:286–292. doi: 10.1002/ana.1129. [DOI] [PubMed] [Google Scholar]
- Young P, Suter U. Disease mechanisms and potential therapeutic strategies in Charcot-Marie-Tooth disease. Brain Res. Rev. 2001;36:213–221. doi: 10.1016/s0165-0173(01)00097-2. [DOI] [PubMed] [Google Scholar]
- Zhang S-C, Goetz BD, Carre J-L, Duncan ID. Reactive microglia in dysmyelination and demyelination. Glia. 2001;34:101–109. doi: 10.1002/glia.1045. [DOI] [PubMed] [Google Scholar]