Abstract
The lateral line system, formed of both superficial (pit organs) and canal neuromasts, is one of the major mechanosensory systems in fish. It has always been assumed that this system depends on neurotrophins and their cognate Trk receptors for development and maintenance, as has been shown in other mechanosensitive systems of vertebrates. However, until now this issue has not been specifically addressed. In this study we used immunohistochemistry to investigate the occurrence and localization both of neurotrophins (NGF-, BDNF- and NT-3-like) and of Trk-like proteins (TrkA-, TrkB-, TrkC-like) in alevins of Salmo salar and S. trutta. All cells in the pit organs of S. salar displayed strong immunoreactivity for TrkB-like and BDNF-like, whereas they were restricted to the hair cells in S. trutta. The hair, supporting and mantle cells of S. salar, and the mantle cells of S. trutta, also expressed TrkA-like immunoreactivity. In the canal neuromasts BDNF-, TrkA- and TrkB-like proteins were present in all cells, without differences between species. NGF-, NT-3- and TrkC-like immunoreactivity were never detected. The present results suggest that mechanoreceptive hair cells, as well as supporting cells, in the lateral line system are under the control of the BDNF–TrkB-like complex, and probably of ligands of TrkA-like receptors.
Keywords: hair cells, lateral line system, neuromast, neurotrophins, teleosts
Introduction
The lateral line system (LLS) represents one of the major sensory systems in bony and cartilaginous fish, as well as in aquatic amphibians and the aquatic larvae of terrestrial amphibians. Its localization varies among species, but is usually in the lateral parts of the body and in the head (see Webb, 1989; Rouse & Pickles, 1991). Two main parts can be considered: one situated in the internal cephalic and trunk canals, and the other one in the body surface forming the so-called pit organs (for a review see Coombs et al. 1989).
The sense organs of the LLS are called neuromasts. They consist of sensory hair, supporting and mantle cells. Hair cells bear one kinocilium and up to 40-stereocilia encapsed in a more or less developed gelatinous cap, i.e. the cupula (Cernuda-Cernuda & García-Fernández, 1996; Alexandre & Ghysen, 1999). The neuromasts are supplied by the peripheral processes of the bipolar sensory neurones of two cranial ganglia, located anterior to or behind the ear, which innervates the head and trunk-tail neuromasts, respectively (Alexandre & Ghysen, 1999). Functionally, the neuromasts of the LLS are regarded as mechanoreceptive, detecting water displacement, sounds at frequencies higher than 500 Hz, magnetic fields and the location of obstacles (for references see Engelman et al. 2000; Northcutt et al. 2000; Popper, 2000).
In higher vertebrates, the sensory systems, including some kinds of mechanoreceptors, are under the control of specific growth factors for development and maintenance. In particular, the muscular (Fan et al. 2000), cutaneous (Ichikawa et al. 2000) and cochleo-vestibular mechanoreceptors (Fritzsch et al. 1997; Vega et al. 1999) depend on specific neurotrophin-Trk receptors (Lewin & Barde, 1996; Huang & Reichardt, 2001). Both common (Hashimoto & Heinrich, 1997; Hallbook et al. 1998) and specific (Gotz et al. 1994; Lai et al. 1998; Nilsson et al. 1998; Caminos et al. 1999) neurotrophins and their parent Trk-like receptors (Martin et al. 1995, 1998; Caminos et al. 1999) have been demonstrated in fishes (see also Heinrich & Lum, 2000). Nevertheless, the occurrence of neurotrophins or Trk-like proteins in the LLS has never been investigated in depth, although neuromasts express mRNA and immunoreactivity for BDNF in developing zebrafish (Hashimoto & Heinrich, 1997; Lum et al. 2001) and Trk-like proteins seem to be absent in Dicentrarchus labrax (Hannestad et al. 2000).
Thus the present study was designed to investigate the immunolocalization of neurotrophin-like (nerve growth factor – NGF-, brain-derived neurotrophic factor – BDNF- and neurotrophin-3 – NT-3-) and Trk-like proteins (TrkA, TrkB an TrkC) in neuromasts of the LLS in alevins of a Salmo trutta and S. salar. On the other hand, because the neuromast of the LLS structurally resembles the inner ear, and the hair cells in this place display S100 protein immunoreactivity (Saidel et al. 1990; Foster et al. 1993), we investigated in parallel the localization of this protein, as well as the innervation of the neuromast cells.
Materials and methods
Materials
Alevins of a S. trutta (n = 10) and S. salar (n = 10), aged 1–2 months, were obtained from the aquarium of the Department of Functional Biology, Section of Genetics (Prof E. García Vázquez) of the University of Oviedo. Specimens were anaesthetized with MS222 (ethyl-m-amino benzoate; 0.4 g L−1), killed and fixed in toto in Bouin’s fixative for 24 h and then routinely processed for paraffin embedding. The pieces were cut in serial frontal, horizontal and sagital sections 10 µm thick, and collected on gelatine-coated microscope slides.
Immunohistochemistry
The sections were processed for indirect peroxidase immunohistochemistry as described elsewhere (Hannestad et al. 2000), Briefly, deparaffinated and rehydrated sections were rinsed in Tris-HCl buffer (0.05 m, pH 7.5) containing 0.1% bovine serum albumin and 0.2% Triton-X 100. Endogenous peroxidase activity and non-specific binding were blocked (3% H2O2 and 25% fetal calf serum, respectively), and sections were incubated overnight with the primary antibodies (see Table 1). Then sections were washed in the same buffer and incubated for 1.5 h at room temperature with peroxidase-labelled sheep antirabbit IgG (Amersham, UK), diluted 1 : 100. The immunoreaction was visualized using 3–3′ diaminobenzidine as a chromogen.
Table 1.
Antibodies used
| Antibody | Raised in | Specificity | Dilution | Supplier |
|---|---|---|---|---|
| anti-TrkA | Rabbit | TrkA (residues 763–777) | 1:100 | Santa Cruz Biotechnology |
| anti-TrkB | Rabbit | TrkB (residues 794–808) | 1:100 | Santa Cruz Biotechnology |
| anti-TrkC | Rabbit | TrkC (residues 798–812) | 1:100 | Santa Cruz Biotechnology |
| anti-NGF | Rabbit | recombinant NGF | 1:1000 | Chemicon, Inc |
| anti-BDNF | Rabbit | recombinant BDNF | 1:1000 | Chemicon, Inc |
| anti-NT-3 | Rabbit | recombinant NT-3 | 1:500 | Chemicon, Inc |
| anti-S100 protein | Rabbit | α and β subunits of S100 protein | 1:1000 | Dako |
| anti-NFP (clone 1592) | Mouse | Medium and high NFP subunits | 1:100 | Boehringer-Manhein |
BDNF: brain-derived neurotrophic factor; NFP: neurofilament proteins; NGF: nerve growth factor; NT-3: neurotrophin 3.
The antibodies against Trk proteins used here were against mammalian Trks, and map within the catalytic domain of the Trk proteins, a region with a highly preserved evolutionary structure (Barde, 1994; Martin et al. 1995, 1998; Hallbook, 1999; Heinrich & Lum, 2000). Moreover, using Western blot analysis in teleosts, these antibodies recognize proteins whose estimated molecular masses are equivalent to those of the mammalian full-length Trks (De Girolamo et al. 1999, 2000; Lucini et al. 1999b, 2001). On the other hand, the antibodies used to detect neurotrophins were raised against mammalian proteins, which are also highly preserved during evolution (see Götz & Schartl, 1994; Hallbook, 1999; Heinrich & Lum, 2000).
Additional experiments following an identical protocol were carried out to localize S100 protein (Saidel et al. 1990; Foster et al. 1993) and neurofilament proteins (Blank et al. 1997; Hall & Yao, 2000; Table 1).
Double immunohistochemistry
To better analyse the innervation of the LLS, some sections were incubated overnight in a humid chamber at 4 °C with a 1 : 1 mixture of the working solution of the antibodies against S100 protein or neurofilaments (see Table 1). After rinsing in the above-mentioned buffer, the sections were first incubated for 1 h at room temperature with Texas red-labelled sheep antirabbit IgG (Amersham, UK) diluted 1:50, then rinsed again and incubated for another hour with FluoroLinkCy2-labelled goat antimouse IgG (Amersham, UK), diluted 1:500. The sections were finally studied and photographed using a Bio-Rad MR-600 confocal-laser scanning microscope (Servicio de Proceso de Imagenes, University of Oviedo).
Controls
The specificity of the immunoreactivity developed was tested by substituting a buffer either for the Trk antibodies, or the antirabbit IgG, in repeated trials. To avoid cross-reactivity of each Trk antisera to another, aliquots of each Trk antiserum were absorbed with an excessive amount of heterologous antigen (for details see Hannestad et al. 2000). For the other proteins investigated, controls were performed using a non-immune rabbit serum instead of the primary antibodies. Under these conditions no specific immunoreactivity was observed.
Results
In both species analysed, the neuromasts were found superficially in the pit organs, and deeply in the cephalic and trunk canals. The structure of these sensory systems was almost identical in Salmo trutta and S. salar, and they were composed of hair cells, supporting cells and mantle cells. As predicted, the hair cells in the neuromasts, independently of location, displayed immunoreactivity for S100 protein (see Figs 1A, D, 2A and 3A), whereas supporting and mantle cells were unreactive.
Fig. 1.
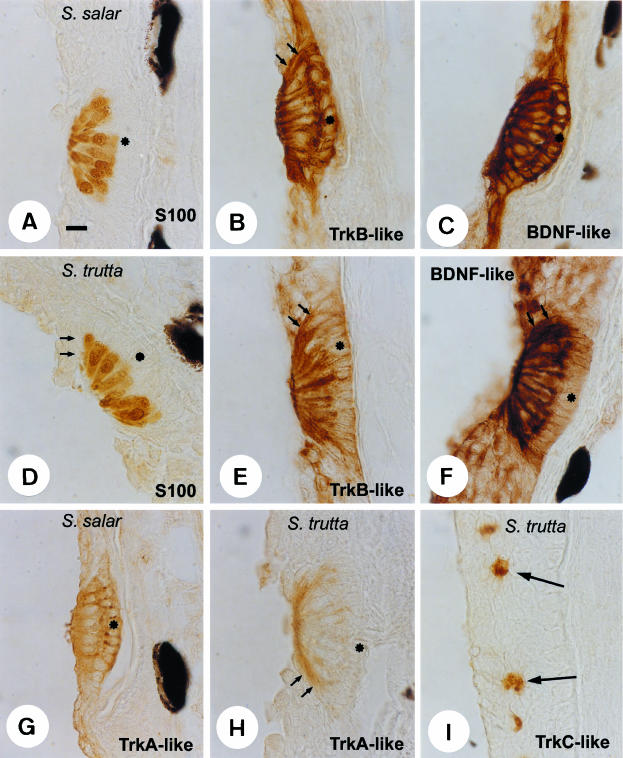
Immunohistochemical localization of S100 (A,D), TrkB-like (B,E), BDNF-like (C,F) and TrkA-like (G,H) proteins in the trunk pit organs of Salmo salar and S. trutta. S100 protein was an excellent and selective marker for hair cells. TrkB-like and TrkA-like proteins were detected in all cells of neuromast in S. salar whereas in S. trutta TrkB-like protein was restricted to hair cells, and TrkA-like protein was found in mantle cells. TrkC-like protein was exclusively detected in unidentified epidermic cells (arrows in I) of S. trutta. Small arrows indicate mantle cells. Asterisks show the supporting cells. Scale bar = 10 μm.
Fig. 2.
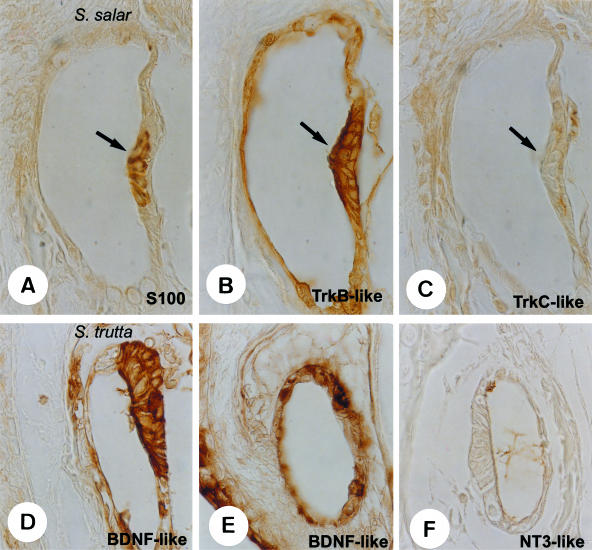
Immunohistochemical detection of S100 (A), TrkB-like (B), TrkC-like (C), BDNF-like (D,E) and NT-3-like (F) proteins in neuromast of the trunk canals (arrows) of Salmo salar and S. trutta. S100 protein immunoreactivity was restricted to hair cells, whereas all cells displayed TrkB-like and DBDN-like proteins. TrkC-like and NT-3 proteins were regularly absent. TrkB-like and BDNF-like proteins were also found in non-sensory cells of the canals. Scale bar = 10 μm.
Fig. 3.
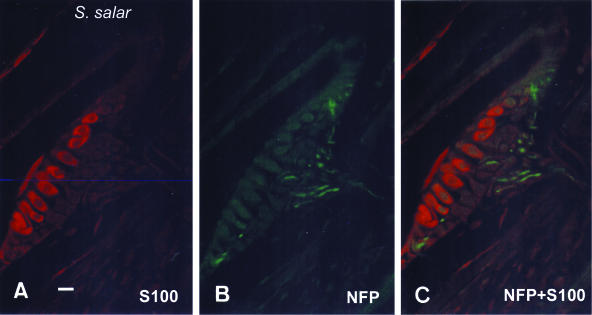
Single (A,B) and double (C) immunohistochemistry for S100 protein (A) and neurofilament proteins (B) in neuromasts of the trunk canals of Salmo salar. Sensory axons innervate hair cells but also supporting cells. Scale bar = 16 μm.
Pit organs
The patterns of expression and cell localization for both neurotrophin-like and Trk-like receptors in the species examined were similar, although slight differences were observed. In S. salar all cells in the neuromast exhibited a strong immunoreactivity for TrkB-like protein (Fig. 1B), whereas in S. trutta it was apparently restricted to the hair cells (Fig. 1E). Furthermore, neuromasts also showed TrkA-like protein immunoreactivity localized in all cells in S. salar (Fig. 1G), and in the mantle cells in S. trutta (Fig. 1H). Regarding the TrkC-like receptor, it was always absent in the neuromast (data not shown), although it was detected in scattered unidentified cells placed on the skin of the head of S. trutta (Fig. 1I).
The only neurotrophin localized in the pit organs was BDNF-like protein, and its distribution matched that of TrkB-like receptor. It was observed in all cells of the neuromasts in S. salar (Fig. 1C), and in the hair cells of S. trutta (Fig. 1F), but the occurrence of BDNF-like protein immunoreactivity in mantle cells cannot be ruled out in this species.
Outside the neuromasts, some basal and suprabasal epidermic cells were immunoreactive for TrkB- and BDNF-like proteins in S. salar (Fig. 1B, C), and a fine and diffuse immunoreactivity for BDNF-like protein was observed in all epidermic layers of S. trutta (Fig. 1F).
Canal neuromasts
Although the distribution and organization of hair cells into the trunk and cephalic channels differs between S. salar and S. trutta, the cell localization of Trk-like and neurotrophin-like proteins was identical. The hair and supporting cells of the canal neuromasts displayed TrkA-like (not shown) and TrkB-like (Fig. 2B) receptors, but not TrkC-like (Fig. 2C) receptor immunoreactivity. Furthermore, faint but specific immunoreactivity was also observed in the non-sensory walls of the canals (Fig. 2B).
On the other hand, and as for the pit organs, the only neurotrophin detected in the canal neuromasts was BDNF-like protein, which was also present in the non-sensory cells of the walls of canals (Fig. 2D,E); NGF-like (not shown) and NT-3 (Fig. 2F) proteins were regularly absent.
Innervation of the LLS
Neurofilament-immunorective nerve fibres reached neuromasts of the pit organs and canals from large subcutaneous nerves (data not shown). Within the sensory organs, nerve fibres are in contact with the basal pole of hair cells, but also with supporting and mantle cells (Fig. 3). Regarding innervation, no differences were noted between S. trutta and S. salar.
The results on the distribution of Trk- and neurotrophin-like proteins in pit organs and neuromasts of canals are summarized in Table 2.
Table 2.
Distribution of neurotrophin-like and Trk-like proteins in the lateral line system of alevins of S. salar and S. trutta
| NGF | BDNF | NT-3 | TrkA | TrkB | TrkC | |
|---|---|---|---|---|---|---|
| Salmo salar | ||||||
| Pit organs | ||||||
| Hair cells | – | +++ | – | + | +++ | – |
| Supporting cells | – | +++ | – | ++ | +++ | – |
| Mantle cells | – | +++ | – | ++ | +++ | – |
| Canal neuromasts | ||||||
| Hair cells | – | +++ | – | ++ | +++ | – |
| Supporting cells | – | ++ | – | ++ | ++ | – |
| Mantle cells | – | ++ | – | ++ | ++ | – |
| Salmo trutta | ||||||
| Pit organs | ||||||
| Hair cells | – | +++ | – | – | +++ | – |
| Supporting cells | – | – | – | – | – | – |
| Mantle cells | – | – | – | ++ | – | – |
| Canal neuromasts | ||||||
| Hair cells | – | +++ | – | + | +++ | – |
| Supporting cells | – | ++ | – | ++ | ++ | – |
| Mantle cells | – | ++ | – | ++ | ++ | – |
The intensity of immunostaining in neuromasts was evaluated as strong (+++), moderate (++), faint (+) or absent (−).
Discussion
The sensory systems of vertebrates are under the control of different families of growth factors for development and maintenance, one of the most important being the family of neurotrophins (see Fariñas, 1999; Huang & Reichardt, 2001). Neurotrophins act on cells through signal-transducing tyrosine-kinase receptors called Trk proteins (Lewin & Barde, 1996). Both neurotrophins and their parent Trk receptors are highly preserved during evolution, and they have been isolated in all vertebrates (see Hallbook, 1999), including fish (Heinrich & Sam, 2000). Trk-like receptors, but not neurotrophin ligands, have been found also in some invertebrates (Van Kestener et al. 1998; Lucini et al. 1999a; see also Jaaro et al. 2001). However, the tissue distribution of neurotrophins and/or Trk receptors in vertebrates other than mammals and birds is poorly known. Recently, Trk-like proteins have been detected in neuronal and non-neuronal cells of fish and reptiles, but attention was not focused on the LLS (De Girolamo et al. 1999, 2000; Lucini et al. 1999b, 2001; Hannestad et al. 2000).
This paper reports for the first time the occurrence and cell distribution of BDNF-like and TrkB-like proteins, and to a lesser extent TrkA-like protein, in mechanoreceptors of LLS in fish. Previous studies have demonstrated the presence of BDNF mRNA in the neuromasts of developing zebrafish (Hashimoto & Heinrich, 1997; Lum et al. 2001), and the absence of Trk-like protein in LLS of Dicentrarchus labrax (Hannestad et al. 2000), although we have recently found TrkB-like protein immunoreactivity in hair cells of neuromasts of alevins of this species (Catania et al. unpubl. obs.). Conversely, NGF-like, NT-3-like and TrkC-like proteins were regularly absent from neuromasts although all were detected in other tissues such as the central nervous system, gills and skin (not shown; see also Hannestad et al. 2000; Lum et al. 2001). BDNF-like protein was strongly expressed in LLS organs (see also Hashimoto & Heinrich, 1997; Lum et al. 2001), mainly in the hair cells but also in the supporting cells in both the pit organs and neuromasts of the canals. Consistently with the expression of BDNF-like protein, the main Trk-like receptor detected in neuromasts was the TrkB-like receptor, regarded as its physiological receptor (Lewin & Barde, 1996). Furthermore, the cellular distribution of TrkB-like receptor basically matched that of BDNF-like protein, thus suggesting autocrinia and/or paracrinia in the neuromasts of LLS.
In addition to TrkB-like protein, TrkA-like receptor was detected in all cells of pit organs in S. salar, and apparently restricted to the mantle cells in S. trutta. These species-specific differences in the expression of TrkA-like protein were inexistent in the neuromasts of the canals, and could be related to sensory modalities (see Engelmann et al. 2000).
The antibodies against Trk used here map within the catalytic domain of the Trk proteins, a region with a high homology in all vertebrates (Barde, 1994; Hallbook, 1999) being higher than 90% in the zebrafish and rat (Martin et al. 1995, 1998). Furthermore, all the key elements found in mammalian Trks, including those involved in signal transmission, are identical in zebrafish and mammals (Heinrich & Lum, 2000). They recognize full-length Trk proteins in teleosts (De Girolamo et al. 1999, 2000; Lucini et al. 1999b). The neurotrophin sequences are also highly preserved during evolution in vertebrates (see Götz & Schartl, 1994; Hallbook, 1999; Heinrich & Lum, 2000). Nevertheless, only BDNF-like protein was detected in the LLS whereas NGF-like and NT-3-like molecules were always absent. It remains to be established if these negative results are due to poor cross-reactivity of the antibodies employed or these molecules are really absent in the LLS.
It is accepted that LLS neuromasts, especially those of canals, are closely related to the inner ear organ, and represent an adaptation in fishes of the vestibular system. Supporting this view they share a placodal origin, types of sensory cells, presence of supporting cells, localization of the sensory ganglia and central projections (see Northcutt et al. 2000). Interestingly, the sensory vestibular epithelium, especially hair cells, of mammals (Montcouquiol et al. 1998; Popper et al. 1999; Qun et al. 1999), birds (see Vazquez et al. 1994; Pirvola et al. 1997) and amphibians (Don et al. 1997) express BDNF mRNA, as well as TrkB mRNA (Don et al. 1997). As speculated in this paper regarding neuromasts, autocrine/paracrine signalling was also thought to be present in the mammalian inner ear (Knipper et al. 1996; Robinson et al. 1996). The data on the TrkA-like receptor must be further investigated since this receptor is expressed only transiently, and is not necessary for development, in the inner ear of higher vertebrates (see Schimmang et al. 1997; Vega et al. 1999).
Our data regarding the innervation of the LLS are in good agreement with previous studies in other teleost species (for references see Northcutt et al. 2000). Sensory axons contact hair cells, as well as supporting cells and mantle cells (Alexandre & Ghysen, 1999; see also Smith, 2000). Although further studies are necessary, the occurrence of nerve fibres in this latter localization could be explained by the formation of new hair cells in this area (Jorgensen, 1991; Fritzsch et al. 1998; Witte et al. 2001) The relationship between innervation and the pattern of expression of Trk-like or neurotrophin-like proteins remains to be analysed in future studies.
The function of Trk-like and neurotrophin-like proteins in neuromasts, and in fish in general, is essentially unknown, but it could be related to the cell turnover (Balak et al. 1990; Williams & Holder, 2000) and probably to the development or maintenance of synaptogenesis (Montcouquiol et al. 1998).
Finally, it must be noted that our results demonstrate that S100 protein is an excellent marker for hair cells in neuromasts of the LLS, as it occurs in their parent cells in the inner ear (Saidel et al. 1990; Foster et al. 1993).
Acknowledgments
Drs Germana and Catania contributed equally to this paper. We thank Professor E. García-Vazquez, Department of Functional Biology, for providing animals used in the present study. This study was supported by a grant from the University of Messina-Young Researchers Project 2000. The paper is dedicated to Professor Giovanni Germanà on his 65th birthday.
References
- Alexandre D, Ghysen A. Somatotopy of the lateral line projection in larval zebrafish. Proc. Natl Acad. Sci. USA. 1999;96:7558–7562. doi: 10.1073/pnas.96.13.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balak KJ, Corwin JT, Jones JE. Regenerated hair cells can originate from supporting cells progeny: evidence from phototoxicity and laser ablation experiments in the lateral lines system. J. NeuroSci. 1990;10:2502–2512. doi: 10.1523/JNEUROSCI.10-08-02502.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde YA. Neurotrophins: a family of proteins supporting survival of neurons. Prog. Clin. Biol. Res. 1994;390:45–56. [PubMed] [Google Scholar]
- Blank H, Muller B, Korf H. Comparative investigations of the neuronal apparatus in the pineal organ and retina of the rainbow trout: Immunocytochemical demonstration of neurofilament 200-kDa and neuropeptide Y, and tracing with DiI. Cell Tissue Res. 1997;288:417–425. doi: 10.1007/s004410050828. [DOI] [PubMed] [Google Scholar]
- Caminos E, Becker E, Martin-Zanca D, Vecino E. Neurotrophins and their receptors in tench retina during optic nerve regeneration. J.Comparative Neurol. 1999;404:321–331. doi: 10.1002/(sici)1096-9861(19990215)404:3<321::aid-cne4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Cernuda-Cernuda R, García-Fernández JM. Structural diversity of the ordinary and specialized lateral line organs. Microsc. Res. Technique. 1996;34:302–312. doi: 10.1002/(SICI)1097-0029(19960701)34:4<302::AID-JEMT3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Coombs S, Gömer P, Münz H, editors. The Mechanosensory Lateral Line Neurobiology and Evolution. New York: Springer; 1989. [Google Scholar]
- De Girolamo P, Lucini C, Andreozzi G, Coppola L, Vega JA, Castaldo L. Co-localization of Trk neurotrophin receptors and regulator peptides in the endocrine cells of the teleostean stomach. Anat. Rec. 1999;256:219–226. doi: 10.1002/(SICI)1097-0185(19991101)256:3<219::AID-AR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- De Girolamo P, Arcamone N, Lucini C, Castaldo L, Vega JA, Gargiulo G. The teleost kidney express Trk neurotrophin receptor-like proteins. Anat. Embryol. 2000;201:429–433. doi: 10.1007/s004290050330. [DOI] [PubMed] [Google Scholar]
- Don DM, Newman AN, Micevych PE, Popper P. Expression of brain-derived neurotrophic factor and its receptor mRNA in the vestibuloauditory system of the bullfrog. Hearing Res. 1997;114:10–20. doi: 10.1016/s0378-5955(97)00113-5. [DOI] [PubMed] [Google Scholar]
- Engelmann J, Hanke W, Mogdans J, Bleckmann H. Hydrodinamic stimuli and the fish lateral line. Nature. 2000;408:51–52. doi: 10.1038/35040706. [DOI] [PubMed] [Google Scholar]
- Fan G, Copray S, Huang EJ, Jones K, Yan Q, Walro J, Jaenisch R, Kucera J. Formation of a full complement of cranial proprioceptors requires multiple neurotrophins. Dev. Dynamics. 2000;218:359–370. doi: 10.1002/(SICI)1097-0177(200006)218:2<359::AID-DVDY9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Fariñas I. Neurotrophin actions during the development of the peripheral nervous system. Microsc. Res. Technique. 1999;45:233–242. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<233::AID-JEMT7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Foster JD, Drescher MJ, Khan KM, Drescher DG. Immunohistochemical localization of S-100 protein in the saccule of the rainbow trout (Salmo gairdnerii R.) Hearing Res. 1993;68:180–188. doi: 10.1016/0378-5955(93)90122-h. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Silos-Santiago I, Bianchi LM, Fariñas I. The role of neurotrophic factors in regulating the development of inner ear innervation. Trends NeuroSci. 1997;20:159–164. doi: 10.1016/s0166-2236(96)01007-7. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Barbacid M, Silos-Santigo I. Nerve dependency of developing and mature sensory receptor cells. Ann. NY Acad. Sci. 1998;30:14–27. doi: 10.1111/j.1749-6632.1998.tb10543.x. [DOI] [PubMed] [Google Scholar]
- Götz R, Koster R, Winkler C, Raulf F, Lottpeich F, Schartl M, Tho H. Neurotrophin-6 is a novel member of the nerve growth factor family. Nature. 1994;372:266–269. doi: 10.1038/372266a0. [DOI] [PubMed] [Google Scholar]
- Götz R, Schartl M. The conservation of neurotrophic factors during vertebrate evolution. Comparative Biochem. Physiol. Pharmacol. Toxicol. Endocrinol. 1994;108:1–10. doi: 10.1016/1367-8280(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Hall GF, Yao J. Neuronal morphology, axonal integrity, and axonal regeneration in situ are regulated by cytoskeletal phosphorylation in identified lamprey central neurons. Microsc. Res. Technique. 2000;48:32–46. doi: 10.1002/(SICI)1097-0029(20000101)48:1<32::AID-JEMT5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Hallbook F, Ibañez CF, Persson H. Evolutionary studies of the nerve growth factor family revealed a novel member abundantly expressed in Xenopus ovary. Neuron. 1991;6:845–858. doi: 10.1016/0896-6273(91)90180-8. [DOI] [PubMed] [Google Scholar]
- Hallbook F. Evolution of the vertebrate neurotrophin and Trk receptor gene families. Curr. Opinion NeuroBiol. 1999;9:616–621. doi: 10.1016/S0959-4388(99)00011-2. [DOI] [PubMed] [Google Scholar]
- Hallbook F, Lundin LG, Kullander K. Lampetra fluvitiles neurotrophin homolog, descendant of a neurotrophin ancestor, discloses the early molecular evolution of neurotrophins in the vertebrate subphylum. J. NeuroSci. 1998;18:8700–8711. doi: 10.1523/JNEUROSCI.18-21-08700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, Marino F, Germana A, Catania S, Abbate F, Ciriaco E, Vega JA. Trk neurotrophin receptor-like proteins in the teleost Dicentarchus labrax. Cell Tissue Res. 2000;300:1–9. doi: 10.1007/s004410000181. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Heinrich G. Brain-derived neurotrophic factor gene expression in the developing zebrafish. Int. J. Dev. NeuroSci. 1997;15:983–997. doi: 10.1016/s0736-5748(97)00017-8. [DOI] [PubMed] [Google Scholar]
- Heinrich G, Lum T. Fish neurotrophins and Trk receptors. Int. J. Dev. NeuroSci. 2000;18:1–27. doi: 10.1016/s0736-5748(99)00071-4. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LJ. Neurotrophins: roles in neuronal development and function. Annu. Rev. NeuroSci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaaro H, Beck G, Conticello SG, Fainzilber M. Evolving better brains: a need for neurotrophins? Trends NeuroSci. 2001;24:79–85. doi: 10.1016/s0166-2236(00)01690-8. [DOI] [PubMed] [Google Scholar]
- Jorgensen JM. Regeneration of lateral line and inner ear vestibular cells. Ciba Foundation Symp. 1991;160:151–170. doi: 10.1002/9780470514122.ch8. [DOI] [PubMed] [Google Scholar]
- Knipper M, Zimmerman U, Rohbock K, Kopschall I, Zenner HP. Expression of neurotrophin receptor trkB in rat cochlear hair cells at time of rearrangement of innervation. Cell Tissue Res. 1996;283:339–353. doi: 10.1007/s004410050545. [DOI] [PubMed] [Google Scholar]
- Lai KO, Fu WY, Ip FC IPNY. Cloning and expression of a novel neurotrophin, NT-7, from carp. Mol. Cell. NeuroSci. 1998;11:64–76. doi: 10.1006/mcne.1998.0666. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the Neurotrophins. Annu. Rev. NeuroSci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Lucini C, Castaldo L, Lamanna C, Maruccio L, Vega JA, Gargiulo G. Neuronal and non-neuronal trk neurotrophin receptor-like proteins in Eisenia foetida (Annelida Oligochaeta) Neurosci. Lett. 1999a;261:163–166. doi: 10.1016/s0304-3940(99)00011-7. [DOI] [PubMed] [Google Scholar]
- Lucini C, De Girolamo P, Maruccio L, Lamanna C, Castaldo L, Vega JA. Trk-neurotrophin receptor-like immunoreactivity in the gut of teleost species. Cell Tissue Res. 1999b;296:323–330. doi: 10.1007/s004410051292. [DOI] [PubMed] [Google Scholar]
- Lucini C, De Girolamo P, Lamanna C, Botte V, Vega JA, Castaldo L. TrkA and TrkC neurotrophin receptor-like proteins in the lizard gut. Cell Tissue Res. 2001;303:345–350. doi: 10.1007/s004410000342. [DOI] [PubMed] [Google Scholar]
- Lum T, Huyn H, Heinrich Brain-derived neurotrophic factor and TrkB tyrosine kinase receptor gene expression in zebrafish embryo and larva. Int. J. Dev. Neuroscience. 2001;19:569–587. doi: 10.1016/s0736-5748(01)00041-7. [DOI] [PubMed] [Google Scholar]
- Martin C, Marazzi G, Sandell JH, Heinrich G. Five Trk receptors in zebrafish. Dev. Biol. 1995;169:745–758. doi: 10.1006/dbio.1995.1184. [DOI] [PubMed] [Google Scholar]
- Martin SC, Sandell JH, Heinrich G. Zebrafish TrkC1 and TrkC2 receptors define two different cell populations in the nervous system during the period of axonogenesis. Dev. Biol. 1998;195:114–130. doi: 10.1006/dbio.1997.8839. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Valat J, Travo C, Sans A. A role of BDNF in early postnatal rat vestibular epithelia maturation: implication of supporting cells. Eur. J. NeuroSci. 1998;10:598–606. doi: 10.1046/j.1460-9568.1998.00070.x. [DOI] [PubMed] [Google Scholar]
- Montgomery J, Carton G, Voigt R, Baker C, Diebel C. Sensory processing of water currents by fishes. Phil. Trans. Roy. Soc. London. Series B. Biol. Sci. 2000;355:1325–1327. doi: 10.1098/rstb.2000.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson AS, Fainzilber M, Falck P, Ibáñez CF. Neurotrophin-7: a novel member of the neurotrophin family from the zebrafish. FEBS Lett. 1998;424:285–290. doi: 10.1016/s0014-5793(98)00192-6. [DOI] [PubMed] [Google Scholar]
- Northcutt RG, Holmes PH, Albert JS. Distribution and innervation of lateral line organs in the channel catfish. J. Comparative Neurol. 2000;421:570–592. [PubMed] [Google Scholar]
- Ogata Y, Slepecky NB. Immunocytochemical localization of calmodulin in the vestibular end-organs of the gerbil. J. Vestibular Res. 1998;8:209–216. [PubMed] [Google Scholar]
- Pirvola U, Hallbook F, Xing-Qun L, Virkkala J, Saarma M, Ylikosky M. Expresión of Neurotrophins and Trk receptors in the developing, adult and regenerating avian cochlea. J. NeuroBiol. 1997;33:1019–1033. doi: 10.1002/(sici)1097-4695(199712)33:7<1019::aid-neu11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Popper AN. Hair cells heterogeneity and ultrasonic hearing: recent advances in understanding fish hearing. Trans. Roy. Soc. London. Series B. Biol. Sci. 2000;355:1277–1280. doi: 10.1098/rstb.2000.0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper P, Lopez I, Beizai P, Li G, Kim J, Micevych PE, Honrubia V. Expression of BDNF and TrkB mRNA in the crista neurosensoy epithelium and vestibular ganglia following ototoxic damage. Brain Res. 1999;846:40–51. doi: 10.1016/s0006-8993(99)01941-1. [DOI] [PubMed] [Google Scholar]
- Qun LX, Pirvola U, Saarma M, Ylikoski J. Neurotrophic factors in auditory periphery. Ann. New York Acad. Sci. 1999;884:292–304. doi: 10.1111/j.1749-6632.1999.tb08649.x. [DOI] [PubMed] [Google Scholar]
- Robinson M, Adu J, Davies AM. Timing and regulation of trkB and BDNF mRNA expression in placode-derived sensory neurons and their targets. Eur. J. NeuroSci. 1996;8:2399–2406. doi: 10.1111/j.1460-9568.1996.tb01203.x. [DOI] [PubMed] [Google Scholar]
- Rouse GW, Pickles JO. Paired development of hair cells in neuromasts of the teleost lateral line. Philosophical. Trans. Roy. Soc. London. Series B. Biol. Sci. 1991;246:123–128. doi: 10.1098/rspb.1991.0133. [DOI] [PubMed] [Google Scholar]
- Saidel WM, Presson JC, Chang JS. S-100 immunoreactivity identifies a subset of hair cells in the utricule and saccule of fish. Hearing Res. 1990;47:139–146. doi: 10.1016/0378-5955(90)90171-k. [DOI] [PubMed] [Google Scholar]
- Schimmang T, Alvarez-Bolado G, Minichello L, Vazquez E, Giraldez F, Klein R, Represa J. Survival of inner ear sensory neurons in trk mutant mice. Mechanisms Dev. 1997;64:77–85. doi: 10.1016/s0925-4773(97)00047-6. [DOI] [PubMed] [Google Scholar]
- Smith CUM. Biology of the Sensory Systems. Chichester: John Wiley & Sons Ltd; 2000. Equilibrium and hearing: the use of hair cells. pp. 93–113. [Google Scholar]
- Van Kestener RE, Fainzilber M, Hauser G, Van Miner J, Vreugden Smit AB, Ibáñez CF, et al. Early evolutionary origin of neurotrophin receptor family. EMBO J. 1998;17:2534–2542. doi: 10.1093/emboj/17.9.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez E, Van De Water TR, Del Valle ME, Vega JA, Staecker H, Giradles F, et al. Pattern of TrkB protein-like immunoreactivity in vivo and the in vitro effects of brain-derived neurotrophic factor (BDNF) on developing cochlear and vestibular neurons. Anat. Embryol. 1994;189:157–167. doi: 10.1007/BF00185774. [DOI] [PubMed] [Google Scholar]
- Vega JA, San Jose I, Cabo R, Rodriguez S, Represa J. Trks and p75 genes are differentially expressed in the inner ear of human embryos. What may Trks and p75 null mutant mice suggest on human development? Neurosci. Lett. 1999;272:103–106. doi: 10.1016/s0304-3940(99)00577-7. [DOI] [PubMed] [Google Scholar]
- Webb JF. Gross morphology and evolution of the mechanoreceptive lateral-line system in teleost fishes. Brain Behav. Evol. 1989;33:34–53. doi: 10.1159/000115896. [DOI] [PubMed] [Google Scholar]
- Williams JA, Holder N. Cell turnover in neuromasts of zebrafish larvae. Hearing Res. 2000;143:171–181. doi: 10.1016/s0378-5955(00)00039-3. [DOI] [PubMed] [Google Scholar]
- Witte MC, Montcouquiol M, Corwin JT. Regeneration in avian hair cell epithelia: identification of intracellular signals required for S-phase entry. Eur. J. NeuroSci. 2001;14:829–838. doi: 10.1046/j.0953-816x.2001.01695.x. [DOI] [PubMed] [Google Scholar]