Abstract
The caudal neurosecretory system of the flounder (Platichthys flesus) has been examined by immunocytochemistry and in situ hybridization for the expression of parathyroid hormone-related protein (PTHrP) and calcium-sensing receptors (CaSR). The N-terminus nucleotide and deduced amino acid sequences of flounder PTHrP were determined and used to prepare oligonucleotide probes and homologous antiserum. The Dahlgren cells of the posterior spinal cord and their axons contained PTHrP protein which was also detected around the capillaries of the urophysis. PTHrP gene expression was abundant in the Dahlgren perikarya and axons in the spinal cord, but it was absent from nerve endings in the urophysis. Calcium-sensing receptor protein was present in the Dahlgren perikarya and axons, also with abundant gene expression, but there was neither protein nor mRNA in the urophysis. There were no apparent differences between freshwater- and seawater-adapted fish in either CaSR or PTHrP expression in the caudal neurosecretory system. These observations suggest that Dahlgren cells produce PTHrP which may be released from axons abutting capillaries in the urophysis. However, the sensing of ionic calcium appears to be confined to the perikarya of the Dahlgren cells in the spinal cord neuropil, suggesting that they are responsive to calcium in the central nervous system rather than the general circulation.
Keywords: calcium-sensing receptors, flounder (Platichthys flesus), immunocytochemistry, in situ hydbrization, parathyroid hormone-related protein (PTHrP)
Introduction
The caudal neurosecretory system of the flounder (Platichthys flesus) consists of large neurones, Dahlgren cells, located in the spinal cord associated with the last eight preterminal vertebrae, and the urophysis which is a neurohaemal organ at the posterior end of the nerve cord Arnold-Reed et al. 1991). Axons of the Dahlgren cells extend down the nerve cord to the urophysis where they contact basement membranes or cells of the dense capillary network of the urophysial neurohaemal structure. The capillaries of the urophysis unite to form a vein entering the kidney where it divides into a secondary renal portal capillary system. Neurosecretory factors from the Dahlgren cells reach the general circulation via the kidney where they may interact with kidney cells as well as interrenal tissue and the Corpuscles of Stannius.
So far two bioactive neurosecretory factors, urotensins I and II (UI and UII), have been identified in flounder Dahlgren cells (Arnold-Reed et al. 1991; Winter et al. 1999). These peptides are common to Dahlgren cells of other fish species (Lederis et al. 1981) and they are chemically related to hypothalamic factors; UI shares homology with corticotrophin-releasing factor and UII has limited similarity to somatostatin. The urotensins have been shown to have cardiovascular and possible osmoregulatory functions in fish (Loretz et al. 1982; Mimassi et al. 2000). Though originally identified in fish, UI and UII have now been detected in mammals where they have biological effects (Conlon, 2000). However, the urotensins may not be the only factors produced by Dahlgren cells and processed via the urophysis. The caudal neurosecretory system occupies an important position in the nervous system receiving inputs from other nerve cells locally, from higher centres via descending fibres and from the spinal fluid via dendritic processes. Anatomically the kidney and its associated tissues, the Corpuscles of Stannius and interrenal tissue, are the first recipients of venous drainage from the urophysis and may be expected to have functional associations which involve secretions from the Dahlgren cells.
In a previous study the presence of immunoreactive parathyroid hormone-related protein (irPTHrP) was demonstrated in Dahlgren cells using antiserum to human 1–16 PTHrP (Danks et al. 1995). PTHrP was originally identified as the agent produced by human tumour cells which caused humoral hypercalcaemia of malignancy (HHM) (Moseley et al. 1987) by interactions in bone and kidney. Parathyroid hormone (PTH), the principal hypercalcaemic factor in amniote vertebrates, and PTHrP share limited amino acid identity at the N-terminus of the molecule which is the region through which both peptides interact with the common receptor in bone and kidney (Abou-Samra et al. 1992; Jüppner, 1999). Fish do not have parathyroid glands and there is no unequivocal evidence that they are able to produce PTH, although this remains a possibility. Nevertheless, they are able to maintain internal circulating content of ionic calcium within strict limits independent of variations in the external calcium concentrations. Cloning of the PTHrP genes of sea bream (Flanagan et al. 2000) and puffer fish (Power et al. 2000) has allowed investigation of the function of the native peptide as a potential hypercalcaemic factor. It has now been shown that the N-terminal 1–34 amino acid peptide of puffer fish PTHrP increases calcium-uptake in sea bream larvae (Guerreiro et al. 1999, 2001), suggesting that it may have a role in raising internal calcium concentrations in teleosts. The principal hypocalcaemic factor in fish is stanniocalcin produced by the Corpuscles of Stannius and which are located in the kidney (Wendelaar Bonga & Pang, 1986). It is possible that the caudal neurosecretory system may be involved in calcium ion control via PTHrP secretion and should be included amongst the neural tissues of the central nervous system producing calcitropic factors (Hull et al. 1998).
Secretion of the hypercalcaemic hormone PTH by the parathyroid gland in mammals is controlled by feedback interaction of ionic calcium which binds to a membrane-located calcium-sensing receptor (CaSR) (Brown et al. 1993). Secretion of PTHrP by cells of human tumours derived from astrocytes and glial cells can also be regulated by calcium via a membrane-located CaSR (Chattopadhyay et al. 2000). PTH has been detected in brain tissues of mice (Hull et al. 1998) and PTHrP has been found in several regions of rat brain including hypothalamic nuclei (Weir et al. 1990; Weaver et al. 1995) and epithelial cells of the meninges (Struckhoff & Turzynski, 1995).
These several observations suggest that nervous and associated tissues are responsive to ionic calcium and may play a part in calcium homeostasis, perhaps locally as well as systemically. In fishes, CaSR, which are structurally related to pheromone receptors, have already been identified in olfactory organs (Naito et al. 1998); moreover, the olfactory epithelium of sea bream appears to be sensitive to calcium ions so that changes in external Ca2+ can change the firing rate of olfactory nerves (Hubbard et al. 2000). Therefore, in this study we have looked for expression of PTHrP, using homologous oligonucleotide probes and antiserum to N-terminus flounder PTHrP, and CaSR gene expression in the caudal neurosecretory system of the euryhaline flounder. The CaSR genes have been cloned in the puffer fish and sea bream (Flanagan et al. 2002) so that oligonucleotide probes from these sequences have been used to detect CaSR gene expression in the caudal neurosecretory system of the euryhaline flounder (Platichthys flesus) by in situ hybridization. CaSR protein has been detected by specific antiserum to an epitope of puffer fish CaSR.
Materials and methods
Isolation and sequencing of flounder cDNA for PTHrP
Flounder kidney RNA was separated using TRI-reagent according to the manufacturer's instructions and as previously reported for sea bream kidney (Flanagan et al. 2000). cDNA of flounder kidney was subject to RT-PCR using primers chosen from sea bream and puffer fish PTHrP sequences (Power et al. 2000). The PCR reactions were performed in a final volume of 50μL, containing 0.1ng of cDNA, 0.2mm of each primer, 3mm MgCl2, 10mM dNTPs, 1.25U of HotStart Polymerase (Qiagen) and the supplied reaction buffer. The cycling conditions were as follows: (i) initial denaturation for 15min at 95°C, (ii) 30 cycles of 1-min duration at 94°C, 54°C and 72°C and (iii) a final extension step of 10 min at 72°C. The PCR product obtained was subcloned into pGEM-T (Promega) and sequenced.
The coding region was identified by similarity searches using BLAST v 2.0 (Altschul et al. 1997).
Preparation of antiserum to flounder N-terminus 1–34 PTHrP
A peptide was synthesized (Sheffield University Biomolecular Synthesis Service) from the deduced amino acid sequence of flounder N-terminus PTHrP. This peptide was used to raise antibodies in rabbits by first injecting a solution of peptide in saline, emulsified in Freund's Complete Adjuvant, into four subcutaneous sites then after 2 months via a similar route using Freund's Incomplete Adjuvant followed by three subsequent similar injections at approximately 1-month intervals.
Antiserum to other PTHrP peptides
Antiserum to oligopeptides of other amino acid (aa) sequences of sea bream PTHrP (Flanagan et al. 2000) were also prepared and used in the immunocytochemistry studies. The peptides of sea bream PTHrP, aa 10–20 and the nuclear transporter region aa 80–95 differed from flounder PTHrP by only a single amino acid. Antisera to these peptides were raised by conjugation of the peptides to bovine thyroglobulin (Nevalainen et al. 1996) and injected into rabbits, R184 for N-terminus 10–20 and R186 for the nuclear receptor region, as described above.
Preparation of antiserum to an oligopeptide of puffer fish calcium-sensing receptor
An oligopeptide, aa 106–115, from the extracellular region of the Fugu calcium-sensing receptor (CaSR) (GenBank accession number AB008857) was conjugated to bovine thyroglobulin and used to prepare antisera in rabbits as previously described (Nevalainen et al. 1996).
Immunocytochemistry of flounder caudal neurosecretory system
The terminal region of the spinal cord, approximately the region of the final eight vertebrae, with the urophysis attached was dissected out from flounder vertebral column and fixed in sublimated Bouin-Hollande (Kraicer et al. 1967). Tissue was collected from fish killed by decapitation without anaesthetic after being maintained in either full sea water or adapted for 2weeks to fresh water (Carrick & Balment, 1983; Arnold-Reed et al. 1991). Tissues were dehydrated through graded concentrations of ethanol and embedded in paraffin wax. Longitudinal 4-μm-thick sections were cut and mounted on APES-coated slides. Immunocytochemistry was carried out as previously described (Santos et al. 2001), based on the methods of Sternberger (1974), using swine antirabbit serum as the linking reagent and diaminobenzidine as the chromogen.
Control reactions used normal rabbit serum instead of the specific primary antiserum.
In situ hybridization
Preparation of labelled probes
Gene expression in tissue sections was detected by the digoxygenin (DIG)-labelled oligonucleotide probe system as previously described (Flanagan et al. 2000). For PTHrP, two probes (27-mers) were chosen from the cDNA sequence of flounder PTHrP between amino acids 6 and 14 (Probe 1) and 31 and 39 (Probe 2). Specificity of the sequences was checked using the BLAST program (Altschul et al. 1997). Probes were end-labelled with DIG using terminal transferase in a 20-μL reaction volume.
Labelled probes were purified on a 2-cm column of Sephadex G25 soaked in elution buffer (0.1× SSC [sodium chloride/sodium citrate, pH 7.0] + 0.1% SDS), by sequential addition of 10× 200-µL aliquots of elution buffer. The presence of labelled probe was detected in the fractions by adsorption of 1-µL aliquots onto nitrocellulose paper and reaction with alkaline-phosphatase-labelled antiserum to DIG. The fractions containing labelled probe were pooled, divided into 10-µL aliquots, lyophilized and stored at −20 °C.
Hybridization procedure
The method for in situ hybridization of flounder tissues was the same as that used for sea bream tissues (Flanagan et al. 2000). Sections were dewaxed and rehydrated through a graded series of ethanol, slides were immersed in 25% deionized formamide in 3× SSC for 60 min. Twenty microlitres of hybridization mix containing 1 µL of labelled probe was prepared and applied to each section. Control reactions either omitted the labelled probe or included a sense probe instead of the antisense sequence. Sections were covered with glass coverslips and incubated overnight at room temperature. At the end of the incubation period coverslips were removed by soaking in 4×SSC, sections were then washed twice in 3×SSC, each time for 15 min. More stringent washes followed: 1× SSC and then 0.1× SSC for 5 min each. Sections were incubated in blocking solution (2% dried milk in buffer 1) for 30 min then the hybridized probe was detected by incubation for 2 h with anti-DIG/AP conjugated serum at 1 : 500 dilution in blocking solution. After washing in buffer 1 (Tris/NaCl, pH 7.5) then buffer 2 (Tris/NaCl/MgCl2, pH 9.5) colour was developed by incubation in a solution of NBT/BCIP and levamisole in buffer 2 overnight in the dark. After washing in deionized water and running tap water, sections were mounted in glycerogel.
Results
cDNA of flounder PTHrP
The first 34 amino acids of flounder PTHrP were deduced from the isolated nucleotide sequence and compared with those of sea bream and puffer fish. (Fig. 1). It is evident that all three sequences are very similar with 31 of 34 amino acids identical in all three.
Fig. 1.
Flounder, sea bream and puffer fish N-terminus 1–34 parathyroid hormonerelated protein amino acid sequences; 31 of the 34 amino acids are identical.
PTHrP in the caudal neurosecretory system
The antiserum to N-terminus 1–34 flounder PTHrP detected peptide in all the Dahlgren cells of the posterior spinal cord which were examined by ICC and there were no apparent differences between those of FW- and SW-adapted fish. Figure 2(A) shows PTHrP in the cytoplasm of a large Dahlgren perikaryon and in the nearby nerve axon; Fig. 2(B) shows a negative reaction in the same neurone using normal rabbit serum as the primary antibody. Antiserum to the nuclear transporter region of sea bream PTHrP (Flanagan et al. 2000) also detected the epitope in Dahlgren cells; there appears to be a concentration around the nuclear envelope, and epithelial cells lining the central canal of the spinal cord also reacted with the antibody (Fig. 2C). Axons in the spinal cord also contained PTHrP (Fig. 2A) and some of these appeared to originate from Dahlgren cells (Fig. 2C). PTHrP was also apparent in the nerve endings around the capillaries of the urophysis (Fig. 2D). Both large and small Dahlgren cells contained PTHrP and colocalized with urotensin I (Fig. 3A, B) and urotensin II (Fig. 3C, D) but the content of urotensin II appeared to be variable in different neurones.
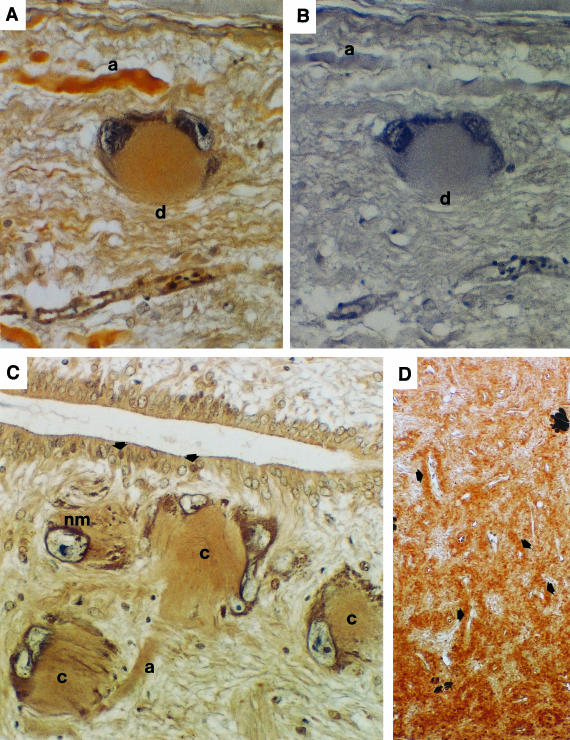
FIGURE 2.
(A) Immunocytochemistry of flounder spinal cord with antiserum to flounder 1–34 PTHrP showing PTHrP in the cytoplasm of a Dahlgren cell (d) and in a nerve axon (a) nearby. (B) The same cells did not react with normal rabbit serum which replaced the specific primary antiserum used in (A). (C) A group of Dahlgren cells close to the central canal of the spinal cord showing immunoreaction with antiserum to sea bream PTHrP nuclear transporter region; both cytoplasm (c) and axons (a) of the Dahlgren cells contained PTHrP, with an apparent concentration around the nuclear membrane (nm). Ependymal cells of the central canal also reacted with the antiserum and in some of them reaction was concentrated in the nucleus (arrows). (D) The antisera to PTHrP detected peptide in the nerve endings in the urophysis around the capillaries (arrows) as illustrated by reaction with antiserum to the nuclear transporter region of sea bream PTHrP.
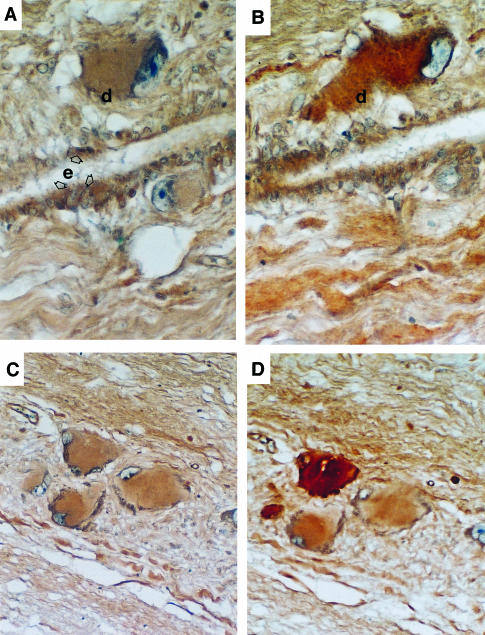
FIGURE 3.
(A) PTHrP in a Dahlgren cell (d) and ependymal cells (e) of the central canal. (B) The same Dahlgren cell also contains urotensin I, detected by specific antiserum to flounder urotensin I. (C) A group of Dahlgren cells showing reaction with antiserum to flounder 1–34 PTHrP. (D) Immunocytochemistry of the same cells using antiserum to flounder urotensin II (UII) showing the presence of UII in the neurones but which is not evenly distributed in all the cells.
In situ hybridization of PTHrP
The two homologous PTHrP probes both hybridized similarly in tissues of the caudal neurosecretory system, demonstrating the distribution of PTHrP gene expression. Perikarya of the Dahlgren cells contained abundant gene expression, and nerve axons at the urophysis interface showed hybridization (Fig. 4A). Ependymal cells of the central canal (Fig. 4A) also contained gene expression, but there was none obvious in the urophysis; Fig. 4(B) shows a control in situ hybridization reaction (no probe) in which no non-specific signal was detected in the perikarya of Dahlgren cells, in nerve axons or in the urophysis.
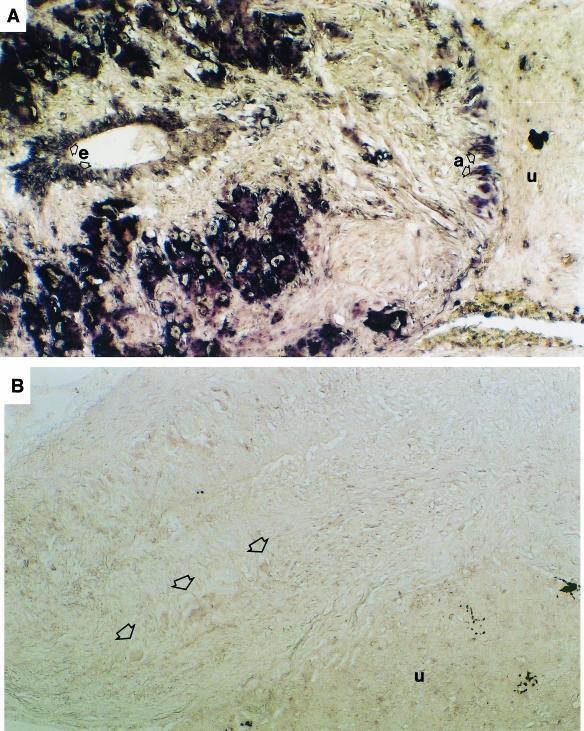
FIGURE 4.
(A) In situ hybridization of PTHrP with an homologous oligonucleotide probe showing abundant gene expression in the cytoplasm of the Dahlgren cells. These are located in the posterior region adjacent to the urophysis (u) in which there is no hybridization. Axons at the interface between the nerve cord and the urophysis show hybridization (a), and ependymal cells (e) of the central canal also show gene expression. (B) A negative in situ hybridization reaction showing part of the spinal cord with Dahlgren cells (arrows) and the urophysis (u).
Calcium-sensing receptors
Antiserum to an epitope of puffer fish CaSR detected receptors in the perikarya of Dahlgren cells (Fig. 5A) and in axons of the spinal cord. However, receptor peptide was scarcely detectable in the urophysis, and the axons of the spinal cord with CaSR appeared not to penetrate into the urophysis. There was abundant gene expression in the Dahlgren perikarya (Fig. 5B), as demonstrated by in situ hybridization using an oligonucletide probe to sea bream CaSR and in motor neurones of the spinal cord (Fig. 5C).
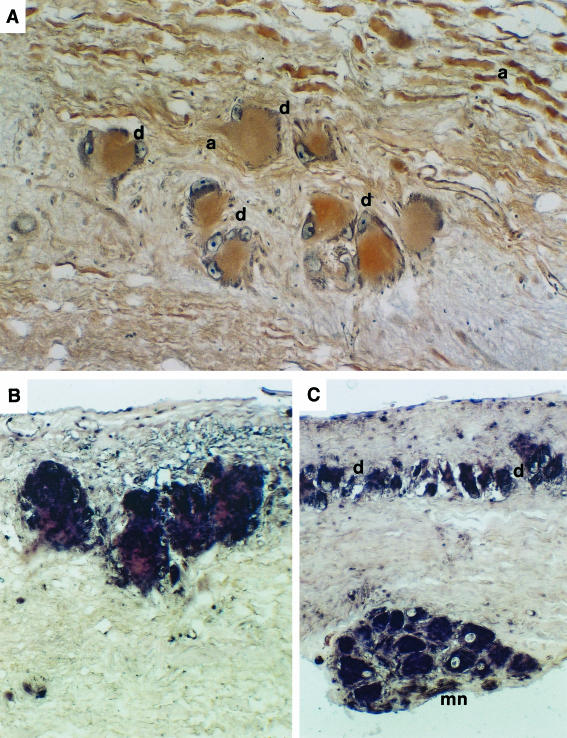
FIGURE 5.
(A) Immunocytochemistry of calcium-sensing receptors showing receptors in Dahlgren cells (d) and nerve axons (a) in the spinal cord. (B) Abundant gene expression of the CaSR in large Dahlgren cells demonstrated by in situ hybridization using an= oligonucleotide probe from puffer fish CaSR. (C) In situ hybridization of CaSR in small Dahlgren cells (d) and in motor neurones (mn) of the flounder spinal cord.
There were no apparent differences in abundance or distribution of either PTHrP or CaSR gene expression between SW- and FW-adapted fish.
Discussion
Cloning of a partial sequence of flounder PTHrP has shown that there is considerable sequence identity between the N-terminus of PTHrP peptides of sea bream, puffer fish and flounder, all three of which are considered to be advanced teleosts. The close identity of these sequences suggests that similar peptides were present in ancestors of teleost fishes and that the sequence has a long-established vital function.
PTHrP peptide occurred in all the Dahlgren cells, suggesting a fundamental function common to all Dahlgren cells; in contrast, urotensins I and II are not distributed uniformly in all Dahlgren cells. Axons of the Dahlgren cells also contained PTHrP and the presence of PTHrP in the urophysis, with concentrations around the capillaries, suggests that PTHrP reaches the urophysis by axonal transport. The presence of neurosecretory granules in the axons of the flounder urophysis have been reported (Arnold-Reed et al. 1991) and it would be interesting to see if PTHrP is associated with or contained in any of these. Although PTHrP peptide was present in the axons, both in the spinal cord and in the urophysis, gene expression could only be detected by in situ hybrdization in the spinal cord sections. A low level of hybridization did occur in the urophysis but this was in the endothelial cells lining the capillaries. In the frog pituitary abundant PTHrP gene expression was detected in the pars intermedia but the protein was present in the pars distalis cells (Danks et al. 1997), suggesting that PTHrP proteins are transported away from the site of translation and possibly into neighbouring cells. It is interesting that mammalian astrocytes and glial cells contain PTHrP (Chattopadhyay et al. 2000); moreover cellular processes of astrocytes cluster around capillaries and appear to take part in exchange of material between the capillary and astrocyte. This function appears to be similar to that of the Dahlgren cells in the fish caudal neurosecretory system. Astrocytes of the mammalian central nervous system are considered to be supporting cells and do not have neurotransmitter function, but Dahlgren cells of the flounder have been characterized electrophysiologically (Hubbard et al. 1996a, 1996b) as well as having been shown to produce bioactive peptides UI and UII. The electrophysiological properties have indicated two populations of Dahlgren cells located remote from or close to the terminus of the spinal cord (Hubbard et al. 1996b); however, our current observations do not indicate that either CaSR or PTHrP gene expression differed significantly in these populations.
The colocalization of PTHrP and the calcium-sensing receptor suggests that synthesis and/or secretion of PTHrP by Dahlgren cells may be partially controlled by calcium ions as it is in mammalian astrocytes (Chattopadhyay et al. 2000). Although the Dahlgren perikarya and nerve axons had CaSR they did not appear to occur on these axons in the urophysis, suggesting that the calcium ion control operated within the central nervous system rather than via the plasma in the capillaries of the urophysis. Such a system is more likely to be connected to the exterior via sensory inputs from organs such as the olfactory epithelium and possibly the gills. The olfactory glands of fish have been shown to possess calcium-sensing receptors related to pheromone receptors (Naito et al. 1998) and reductions in environmental Ca2+ cause large increases in olfactory nerve firing rate in the sea bream (Hubbard et al. 2000). Thus the central nervous system is a potential route between tissues, such as the olfactory epithelium with sensory neurones, which are sensitive to environmental ion changes and internal tissues involved in responses to correct internal ion concentrations. This potential influence of the central nervous system (CNS) in control of Dahlgren cell function supports other evidence of the caudal neurosecretory system as a co-ordinating centre for changes in reproductive, osmoregulatory and nutritional activity in the euryhaline flounder (Winter et al. 2000).
There were no obvious differences in the expression of either PTHrP or CaSR in the Dahlgren cells of fish adapted to either FW or SW, which could indicate that functions in the adapted status were equally important. PTHrP is also a multifunctional protein with different regions of the molecule having specific functions, such as nuclear transport (Henderson et al. 1995) and RNA binding (Aarts et al. 1999). It was notable that there was a concentration of PTHrP in the nuclear envelop of at least some Dahlgren cells suggesting intracrine functions. There are numerous potential cleavage sites within PTHrP molecules, including teleost PTHrPs (Flanagan et al. 2000; Power et al. 2000), so that there may be specific active peptides produced by post-translational cleavage from the original transcription product.
The drainage of urophysial veins into the kidney suggests a potential interaction between PTHrP and tissues in the kidney, which include inter-renal, kidney tubule and corpuscles of Stannius. Recent observations suggest that PTHrP may have several effects in mammalian kidneys, including modulating renal blood flow and glomerular filtration rate as well as actions in renal injury and repair (Esbrit et al. 2001). Moreover, PTHrP infusion has been shown to increase renal tubular calcium reabsorption in normal human volunteers (Syed et al. 2001), suggesting that it may have a similar function in flounder, a function of particular importance in adaptation to low calcium-containing fresh water. In the winter flounder isolated proximal kidney tubules in primary culture have been shown to respond to heterologous PTHrP by increasing inorganic phosphate excretion (Renfro, 1999), which suggests that the endogenous peptide may have a similar effect. The urophysial peptide UII influences calcium handling by rat aorta (Gibson et al. 1988) again indicating a possible role of the CaSR of Dahlgren cells and the multifactorial nature of calcium physiology in the renal/urophysial system of the flounder. More experimental studies are needed to determine the specific functions of PTHrP and calcium ions in the caudal neurosecretory and renal systems.
Acknowledgments
This work was funded by BBSRC grant number 34/SO9873.
References
- Aarts MM, Levy D, He B, Stregger S, Chen T, Richsrds S, Henderson JE. Parthyroid hormone-related protein interacts with RNA. J. Biol. Chem. 1999;274:4832–4838. doi: 10.1074/jbc.274.8.4832. [DOI] [PubMed] [Google Scholar]
- Abou-Samra A-B, Jüppner H, Force T, Freeman MW, Kong F-X, Schipani E, et al. Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. ProcNatl AcadSciUSA. 1992;89:2732–2736. doi: 10.1073/pnas.89.7.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller TL, et al. Gapped blast and PSI-blast: a new generation of protein database search programs. NuclAcid Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold-Reed DE, Balment RJ, McCrohan CR, Hackney CM. The caudal neurosecretory system of Platichthys flesus: general morphology and responses to altered salinity. Comparative BiochemPhysiol. 1991;99A:137–143. [Google Scholar]
- Brown EM, Gamba G, Riccardi D, Lombardio M, Butters R, Kifor O, et al. Cloning and characterisation of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- Carrick S, Balment RJ. The renin-angiotensin system and drinking in the euryhaline flounder, Platichthys flesus. General Comparative Endocrinol. 1983;51:423–433. doi: 10.1016/0016-6480(83)90059-x. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay N, Evliyaoglu C, Heese O, Carroll R, Sanders J, Black P, et al. Regulation of secretion of PTHrP by Ca2+-sensing receptor in human astrocytes, astrocytomas and meningiomas. AmJPhysiol. 2000;279:C691–C699. doi: 10.1152/ajpcell.2000.279.3.C691. [DOI] [PubMed] [Google Scholar]
- Conlon JM. Singular contribution of fish neuroendocrinology to mammalian regulatory peptide research. Regulatory Peptides. 2000;93:3–12. doi: 10.1016/s0167-0115(00)00172-5. [DOI] [PubMed] [Google Scholar]
- Danks JA, Balment RJ, Hubbard PC, Ingleton PM. Parathyroid hormone-related protein in the urophysis of the flounder (Platichthys flesus) JEndocrinol. 1995;147(Suppl):P61. [Google Scholar]
- Danks JA, McHale JC, Martin TJ, Ingleton PM. Parathyroid hormone-related protein in tissues of the emerging frog (Rana temporaria): immunohistochemistry and in situ hybridisation. J. Anat. 1997;190:229–238. doi: 10.1046/j.1469-7580.1997.19020229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbrit P, Santos S, Ortega A, Fernandez-Agullo T, Velez E, Troyam S, et al. Parathyroid hormone-related protein as a renal regulating factor – From vessels to glomeruli and tubular epithelium. AmJNephrol. 2001;21:179–184. doi: 10.1159/000046244. [DOI] [PubMed] [Google Scholar]
- Flanagan JA, Power DM, Bendell LA, Guerreiro PM, Fuentes J, Clark MS, et al. Cloning of the cDNA for sea bream (Sparus aurata) parathyroid hormone-related protein. General Comparative Endocrinol. 2000;118:373–382. doi: 10.1006/gcen.2000.7481. [DOI] [PubMed] [Google Scholar]
- Flanagan JA, Bendell LA, Guerreiro PM, Clark MS, Power DM, Canario AVM, Brown BL, Ingleton PM. Cloning of the cDNA for the putative calcium-sensing receptor and its tissue distribution in sea bream (Sparus aurata) Gen. Comp. Endocrinol. 2002 doi: 10.1016/s0016-6480(02)00035-7. in press. [DOI] [PubMed] [Google Scholar]
- Gibson A, Conyers S, Bern HA. The influence of urotensin II on calcium flux in rat aorta. J. Pharmacy Pharmacol. 1988;40:893–895. doi: 10.1111/j.2042-7158.1988.tb06298.x. [DOI] [PubMed] [Google Scholar]
- Guerreiro PM, Fuentes J, Canario AVM, Power DM, Ingleton PM, Flik G. Parathyroid hormone-related protein: a calcium regulating factor in a marine teleost, Sparus aurata. JEndocrinol. 1999;163(Suppl):P4. [Google Scholar]
- Guerreiro PM, Fuentes J, Power DM, Ingleton PM, Flik G, Canario AVM. Parathyroid hormone-related protein: a calcium regulatory factor in sea bream (Sparus aurata) AmJPhysiol. 2001;281:R855–R860. doi: 10.1152/ajpregu.2001.281.3.R855. [DOI] [PubMed] [Google Scholar]
- Henderson JE, Amizuka N, Warshawsky H, Diasotto D, Lanske BMK, Goltzman D, Karaplis AC. Nucleolar localisation of parathyroid hormone-related protein enhances survival of chondrocytes under conditions that promote apoptotic cell death. Mol. Cell. Biol. 1995;15:4064–4075. doi: 10.1128/mcb.15.8.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard PC, Balment RJ, McCrohan CR. Adrenergic receptor activation hyperpolarizes the caudal neurosecretory cells of the flounder, Platichthys flesus. J. Neuroendocrinol. 1996a;8:153–159. doi: 10.1111/j.1365-2826.1996.tb00836.x. [DOI] [PubMed] [Google Scholar]
- Hubbard PC, McCrohan CR, Banks JR, Balment RJ. Electrophysiological characterisation of cells of the caudal neurosecretory system in the teleost, Platichthys flesus. Comparative Biochem. Physiol. A. 1996b;115:293–301. [Google Scholar]
- Hubbard PC, Barata EN, Canario AVM. Olfactory sensitivity to changes in environmental [Ca2+] in the marine teleost Sparus aurata. J. Exp. Biol. 2000;203:3821–3829. doi: 10.1242/jeb.203.24.3821. [DOI] [PubMed] [Google Scholar]
- Hull KL, Fathimani K, Sharma P, Harvey S. Calcitropic peptides: neural aspects. Comparative BiochemPhysiolC. 1998;119:389–410. doi: 10.1016/s0742-8413(98)00010-3. [DOI] [PubMed] [Google Scholar]
- Jüppner H. Receptors for parathyroid hormone and parathyroid hormone-related peptide: Exploration of their biological importance. Bone. 1999;25:87–90. doi: 10.1016/s8756-3282(99)00110-6. [DOI] [PubMed] [Google Scholar]
- Kraicer J, Herlant M, Duclos P. Changes in adenohypophyseal cytology and nucleic acid content in the rat 32 days after bilateral adrenalectomy and the chronic injection of cortisol. Can. J. Physiol. Pharmacol. 1967;45:947. doi: 10.1139/y67-112. 956. [DOI] [PubMed] [Google Scholar]
- Lederis K, Ichikawa M, Mcmaster D. Urophysial peptides and proteins. In: In: Farner DS, Lederis K, editors. Neurosecretion, Molecules, Cells, Systems. New York: Plenum Press; 1981. pp. 403–412. [Google Scholar]
- Loretz CA, Bern HA, Foskett K, Mainoya JR. The caudal neurosecretory system and osmoregulation in fish. In: In: Farner DS, Lederis K, editors. Neurosecretion: Molecules, Cells, Systems. New York: Plenum Press; 1982. pp. 319–328. [Google Scholar]
- Mimassi N, Shahbazi F, Jensen J, Mabin D, Conlon MJ, Le Mevel J-C. Cardiovascular actions of centrally and peripherally administered trout urotensin-I in the trout. Am. J. Physiol. 2000;279:R484–R491. doi: 10.1152/ajpregu.2000.279.2.R484. [DOI] [PubMed] [Google Scholar]
- Moseley JM, Kubota M, Diefenbach-Jagger H, Wettenhall REH, Kemp BE, Suva LJ, Rodda CP, Ebeling PR, Zajac JD, Martin TJ. Parathyroid hormone-related protein purified from a human lung cancer cell line. Proc. Natl. Acad. Sci. USA. 1987;84:5048–5052. doi: 10.1073/pnas.84.14.5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito T, Saito Y, Yamamoto Y, Nozaki Y, Tomura K, Hazama M, et al. Putative pheromone receptors related to the Ca2+-sensing receptor in Fugu. ProcNatl AcadSciUSA. 1998;95:5178–5181. doi: 10.1073/pnas.95.9.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevalainen MT, Valve EM, Ingleton PM, Haarkonen PL. Expression and hormone regulation of prolactin receptors in rat dorsal and lateral prostate. Endocrinology. 1996;137:3078–3088. doi: 10.1210/endo.137.7.8770934. [DOI] [PubMed] [Google Scholar]
- Power DM, Ingleton PM, Flanagan J, Canario AVM, Danks JA, Elgar G, et al. Genomic structure and expression of parathyroid hormone-related protein gene (PTHrP) in a teleost, Fugu rubripes. Gene. 2000;250:67. doi: 10.1016/s0378-1119(00)00167-0. –. 76. [DOI] [PubMed] [Google Scholar]
- Renfro JL. The use of teleost renal proximal tubule primary monolayer cultures for assessment of tissue-level kidney functions. Comparative Biochem. Physiol. 1999;124(Suppl):S36. [Google Scholar]
- Santos CRA, Ingleton PM, Cavaco JEB, Kelly PA, Edery M, Power DM. Cloning, characterisation and tissue distribution of prolactin receptor in the sea bream (Sparus aurata) Gen. Comp. Endocrinol. 2001;121:32–47. doi: 10.1006/gcen.2000.7553. [DOI] [PubMed] [Google Scholar]
- Struckhoff G, Turzynski A. Demonstration of parathyroid hormone-related protein in meninges and its receptor in astrocytes: evidence for a paracrine meningo-astrocytic loop. Brain Res. 1995;676:1. doi: 10.1016/0006-8993(95)00088-8. –. 9. [DOI] [PubMed] [Google Scholar]
- Sternberger LA. Immunocytochemistry. Prentice-Hall: Englewood Cliffs, NJ; 1974. [Google Scholar]
- Syed MA, Horwitz MJ, Tedesco MB, Garcia-Ocana A, Wisnieswski SR, Stewart AF. Parathyroid hormone-related protein-(1–36) stimulates renal tubular calcium reabsorption in normal volunteers: Implications for the pathogenesis of human humoral hypercalcaemia of malignancy. J. Clin. Endocrinol. Metabolism. 2001;86:1525–1531. doi: 10.1210/jcem.86.4.7406. [DOI] [PubMed] [Google Scholar]
- Weaver DR, Deeds JD, Lee K, Segre GV. Localisation of parathyroid hormone-related peptide (PTHrP) and PTH/PTHrP receptor mRNAs in rat brain. Mol. Brain Res. 1995;28:296–307. doi: 10.1016/0169-328x(94)00222-z. [DOI] [PubMed] [Google Scholar]
- Weir EC, Brines ML, Ikeda K, Burtis WJ, Broadus AE, Robbins RJ. Parathyroid hormone-related gene is expressed in the mammalian central nervous system. Proc. Natl Acad. Sci. USA. 1990;87:108–112. doi: 10.1073/pnas.87.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendelaar Bonga S, Pang PKT. Stannius corpuscles. In: Pang PKT, Schreibmann MP, editors. Vertebrate Endocrinology: Fundamentals and Biomedical Implications. Vol. 1. Orlando, FL: Academic Press; 1986. pp. 439–464. Morphological Considerations. [Google Scholar]
- Winter MJ, Hubbard PC, McCrohan CR, Balment RJ. A homologous radioimmunoassay for the measurement of urotensin II in the euryhaline flounder, Platichthys flesus. General Comparative Endocrinol. 1999;114:249–256. doi: 10.1006/gcen.1998.7245. [DOI] [PubMed] [Google Scholar]
- Winter MJ, Ashworth A, Bond H, Brierley MJ, McCrohan CR, Balment RJ. The caudal neurosecretory system: control and function of a novel neuroendocrine system in fish. Biochem. Cell Biol. 2000;78:193–203. [PubMed] [Google Scholar]