Abstract
Vascular endothelial permeability is maintained by the regulated apposition of adherens and tight junctional proteins whose organization is controlled by several pharmacological and physiological mediators. Endothelial permeability changes are associated with: (1) the spatial redistribution of surface cadherins and occludin, (2) stabilization of focal adhesive bonds and (3) the progressive activation of matrix metalloproteinases (MMPs). In response to peroxide, histamine and EDTA, endothelial cells sequester VE-cadherin and alter its cytoskeletal binding. Simultaneously, these mediators enhance focal adhesion to the substratum. Oxidants, cytokines and pharmacological mediators also trigger the activation of matrix metalloproteinases (MMPs) in a cytoskeleton and tyrosine phosphorylation dependent manner to degrade occludin, a well-characterized tight junction element. These related in vitro phenomena appear to co-operate during inflammation, to increase endothelial permeability, structurally stabilize cells while also remodelling cell junctions and substratum.
Keywords: ERK1/2, focal adhesion, integrin, p38 MAP kinase
Introduction
Increased microvascular permeability is a central hallmark of inflammation, and is the basis of oedematous tissue injury in many acute and chronic disease states, including: ischemia-reperfusion (I/R), sepsis and acute respiratory distress. While increased microvascular solute permeability is an important index of inflammation, the subcellular mechanisms responsible for increased solute exchange in inflammation are still not completely understood, and remain a topic of debate. The filtration characteristics of microvessels under normal conditions and during inflammation were initially described by several multiple pore-based solute exchange systems originally proposed by Pappenheimer et al. (Pappenheimer et al. 1951) and others (Simionescu et al. 1975; Palade et al. 1979; Wissig, 1979; Siflinger-Birnboim et al. 1987). While the structural basis for pore-based models remains controversial, recently these models have been revived to explain some forms of microvascular permeability. Vesico-vestibular organelles (VVOs) (Dvorak et al. 1996; Feng et al. 1996) have been currently described as a possible structures that would provide the basis for a transcytotic channel/pore system as originally proposed. In this manner, VVOs and perhaps other structures could fulfil some or all of the characteristics of the classical, theoretical pore system. Evidence for this type of system remains largely based on microscopic studies, and more information on the molecular structure(s) and the mechanisms regulating these pores is still needed to document a role for them in inflammation.
Inflammatory changes in microvascular permeability are correlated with the reorganization and widening of interendothelial junctions that are the main barriers in the paracellular solute exchange pathway (Firth et al. 1983). Formation of gaps between adjacent endothelial cells in response to inflammatory mediators is well documented (Majno & Palade 1961; Kevil et al. 2000), and the molecular and biochemical basis of junctional remodelling and its relation to permeability is rapidly becoming better understood.
Many groups now support a model where inflammatory permeability represents a highly coordinated loss of junctional integrity, that allows recoil or active retraction of cell borders to alter the width of the endothelial ‘clefts’ and increase junctional solute exchange (Haselton et al. 1989; Dejana, 1997; Nieuw Amerongen et al. 2000a; Baldwin & Thurston, 2001). This mechanism is completely reversible and can restore or enhance organization of tight and adherens junctions to affect endothelial solute permeability properties.
The proteins that form tight junctions in endothelial cells include: occludin, claudin family members, junctional adhesion molecules (JAMs) 1–3, cingulin, 7H6, spectrin and linker proteins like the ZO-family members, which bind or link these proteins to each other and the cytoskeleton (Furuse et al. 1993; Hirase et al. 1997). Adherens junctions contain the transmembrane proteins, cadherins, and their linker proteins, catenins, that fasten them to the cytoskeleton. Adherens junctions also associate with signalling proteins which include kinases, phosphatases, G-proteins (Brady-Kalnay et al. 1995; Daniel & Reynolds, 1997; Kaplan et al. 2001) and adaptors (e.g. Shc) (Xu et al. 1997). PECAM-1 (CD31) is also found within junctions, but is not exclusively distributed to junctions. PECAM-1 is located over the entire surface, and interacts with tight and adherens proteins (Albelda et al. 1991; Muller & Randolph, 1999). Ilan et al. (2000) have shown that PECAM-1 can act as a scaffold to recruit β and γ-catenins to cell contacts in a PKC-dependent manner (Ilan et al. 2000). In general, junctions in endothelial cells contain many of the same elements of epithelia, but show a less distinct spatial separation between tight and adherens junctional components than epithelia. The integrity of junctions is regulated by cytoskeletal tension, alterations in junctional protein binding and linkage between junctional proteins and the cytoskeleton, all of which help govern cleft size and hence solute permeability.
The organization of endothelial junctional complexes is regulated by hormones, cytokines and drugs, as well as by oxidants and nitric oxide that are secreted by adventitial cells like astrocytes and pericytes (Sobue et al. 1999). Some of these factors will also affect the net expression of junctional proteins including cadherins, ZO-1 and occludin (Gardner et al. 1996; Abbruscato & Davis, 1999; Oshima et al. 2001). Thus, the composition, organization and solute permeability of tight and adherens junctions can vary immensely depending on different environmental and humoral factors.
During inflammation, changes in endothelial junction dissociation probably reflect both active contraction and passive recoil of cell–cell borders, which occur without a loss of cell adhesion to the underlying substratum. Since common cytoskeletal elements are shared between these systems, complex relationships probably exist which link permeability, cell–matrix adhesion and the spatial redistribution of tight and adherens junctional proteins during junctional opening and restoration. This has not been evaluated. While endothelial junctions and focal adhesions are being remodelled, matrix metalloproteinases (MMPs) also appear to be activated. This activation of MMPs may be another substrate-focal adhesion regulated event that is crucial in regulating solute permeability under some circumstances. Junctional and focal adhesion plaque reorganization and MMP activation may be two linked events in microvascular permeability. Altering endothelial bonds to the substratum may organize multiple signal modules found at focal adhesion plaques to control junctional structure (and permeability). The MMPs activated by these modules appear to decrease the restrictive filtration properties of the subcellular matrix (Partridge et al. 1993), but also proteolytically modify junctional components (Wachtel et al. 1999). Together, these events likely contribute to acute and chronic forms of inflammation.
Mechanisms for permeability alteration
The reversible changes in endothelial permeability triggered by diverse pharmacological mediators (thrombin, histamine, bradykinin), oxidants, prostanoids and other factors probably occur by a limited number of mechanisms (Haselton et al. 1989; Lum & Malik, 1996). Many initial studies showed that increased permeability involves activation of PKC isoforms and elevation of cell Ca2+ in what was initially conceived as a ‘contractile’ model, where junctions are actively retracted to increase cleft width. More recently, tyrosine kinases and p38, p42/44 MAP kinases have been shown to play important roles in permeability, both in regulating cell tension and the structural remodelling of endothelial junctions and focal adhesion structure (Carbajal & Schaeffer, 1998; Kevil et al. 1998a; Yuan et al. 1998). Permeability can also be decreased by agents that elevate cAMP, lower Ca2+, or block the MAPK and tyrosine kinases (Minnear et al. 1986; Haselton et al. 1993; Adamson et al. 1998). At least some of the important targets of these signal pathways lie in the cytoskeletal junctional complexes. Therefore, endothelial cleft space is controlled through cytoskeletal tension, cytoskeleton-junction binding and factors governing junctional bond strength.
Leukocyte proteases may disintegrate endothelial junctions in some types of chronic inflammation (Carden et al. 1998). In addition to pharmacological regulation of the barrier, leucocyte (neutrophil) proteases such as elastase may proteolyse cadherins and other junctional elements (catenins) (Allport et al. 2000) with destructive effects on barrier restriction. In addition to elastase, MMPs that are found within leucocytes, e.g. MMP-9, might similarly disintegrate junctions in some phases of leucocyte extravasation, and may contribute to leucocyte-dependent injury (Wachtel et al. 1999). Thus, in addition to the pharmacological regulation of barrier structure, inflammatory permeability during injury in vivo probably incorporates hormonal, cytokine and protease effects on junctional integrity.
Role of focal adhesions in inflammation
Focal adhesion plaques organize endothelial monolayers, and probably help to transduce signals in response to several mediators in inflammation. The association of integrins with the cytoskeleton and cytoskeleton-associated proteins allows cell binding to the substratum, and maintains and regulates endothelial permeability. β1 and β3 integrins are often spatially associated with cell junctions (Alexander et al. 2001; Voura et al. 2001), and may co-operate with junctions to modulate endothelial permeability. In support of this, it has been shown that antibodies to integrin subunits, and synthetic integrin-blocking increase endothelial solute permeability. Tsukada et al. (1995) and Qiao et al. (1995) have also shown in vivo that interruption of focal adhesions in the lung microvasculature increases capillary permeability. Lampugnani et al. (1991) have also reported that human endothelium treated with cAMP analogues show a diffuse distribution of β1 and β3 integrins, actin and vinculin, which correlated with an apparent enhancement of adhesion to the substrate. Therefore, maintenance and perhaps regulation of the endothelial barrier require some minimum level of integrin to substrate binding, since interruption of integrin-dependent contacts dysregulates permeability maintenance. How focal adhesion plaque structure is altered during inflammation, and whether focal adhesion plaques interact with adherens and/or tight junctions to regulate permeability has not been thoroughly investigated.
Direct measurement of endothelial–substrate adhesion has been investigated in only a few studies (Hoying & Williams, 1994). In a H2O2 model of oxidant-mediated cell injury, we found that endothelial cells exhibited a time- and dose-dependent increase in substrate binding (focal adhesion), which correlated with increases in endothelial permeability (Alexander et al. 2001). These events were paralleled by a large increase in the tyrosine phosphorylation of several proteins, consistent with phosphorylation of focal adhesion kinase (FAK), and perhaps catenins and paxillin, as has been previously reported under similar conditions (Vepa et al. 1997, 1999). Vascular endothelial growth factor (VEGF) also promotes tyrosine phosphorylation of FAK and several other plaque proteins (Abedi & Zachary, 1997). Tyrosine phosphorylation of FAK and other substrate adhesive proteins appears therefore to be a common motif which occurs in response to several other inflammatory mediators and may affect several other processes including cell tension and junction stability.
Although tyrosine phosphorylation is induced by oxidants, such as H2O2, the net tyrosine phosphorylation might not only reflect de novo activation of tyrosine kinases, but also the ability of oxidants to potently inhibit multiple cell phosphatases, particularly tyrosine phosphatases. Combined, these two effects can initiate, sustain and magnify tyrosine phosphorylation-mediated responses induced by mediators other than oxidants. Oxidants (particularly H2O2) will also directly activate the mitogen-activated protein kinases (MAPKs) that contribute to oxidant-dependent permeability changes (Guyton et al. 1996), and may be downstream targets of tyrosine kinases. The increased adhesion of endothelial cells to the extracellular matrix produced by activation of these kinases may help stabilize monolayers against detachment (by lateral tensile forces) that occurs during vasodilatation, like that induced by inflammatory mediators, e.g. histamine or bradykinin. In doing so, increased endothelial adhesion to the extracellular matrix could compensate endothelial cells for the diminished cell–cell binding (junctional cleft widening) seen in inflammation.
Altered cell–substrate adhesion in inflammation has not been thoroughly investigated and exactly how it might participate in the regulation of permeability is still unclear. One possible other function that focal adhesion plaques could serve in permeability regulation is the assembly of complexes containing protein kinases and other proteins necessary to initiate the downstream signalling events that reorganize junction and regulate permeability. As stated above, tyrosine kinases now appear to be central regulators in inflammatory signalling. Focal adhesion plaques associate several proteins, especially the focal adhesion kinase (FAK), which participates in permeability regulation through tyrosine kinase signalling in response to several stimuli. Yuan et al. (1998) have demonstrated that tyrosine phosphatase inhibitors, including phenylarsine oxide and sodium orthovanadate, which increase total tyrosine phosphorylation, also increase vascular permeability. In vitro, endothelial tyrosine phosphorylation of several proteins, including that of paxillin and focal adhesion kinase (pp125FAK), is increased by tyrosine phosphatase blockers. Histamine and phorbol myristate acetate (PMA) treatment similarly enhance tyrosine phosphorylation of paxillin and pp125FAK, and are associated with increased permeability. These effects were blocked by damnacanthal, a tyrosine kinase inhibitor. VEGF also promotes a similar phosphorylation of FAK (Abedi & Zachary, 1997), consistent with this model; VEGF-mediated permeability is blocked by AG126, another tyrosine kinase inhibitor (Kevil et al. 1998a).
Paxillin appears to be necessary for the activation of FAK and the establishment of cell contacts and spreading (Wade et al. 2002). Therefore, paxillin and FAK may help to regulate substrate adhesion by nucleating focal adhesions. Increased paxillin and FAK phosphorylation are associated with increased cell–substrate adhesion, but it is not clear how their activity is associated with permeability changes. Cohen et al. (1999) have also demonstrated that the phosphorylation of beta catenin in response to VEGF is also PKC dependent. Therefore, FAKs in these complexes may control the assembly of junctions and the downstream activity of other signal complexes that affect junctions.
G-proteins also play important roles in permeability regulation through these complexes (Alexander, 2000; Nieuw Amerongen et al. 2000b; Nieuw Amerongen & van Hinsbergh, 2001). Rho and Cdc42 associate with cell junctions and may be controlled through the action of several inflammatory mediators. With respect to endothelial permeability, the inactivation of Rho by the Rho-associated protein kinase (ROCK) inhibitor, Y-27632, with no other stimulation leads to decreased permeability (Carbajal & Schaeffer, 1998). However, that study reported that following stimulation, endothelial permeability and structural changes were only partially mediated by Rho and ROCK. Thrombin-induced phosphorylation of paxillin, FAK, myosin light chains and F-actin stress fibre formation (Carbajal & Schaeffer, 1999) were reduced by the ROCK inhibitor Y-27632 in endothelial cells. Despite these changes, permeability is still significantly increased through formation of cell gaps, consistent with an actomyosin contraction-independent reorganization of junctions in permeability. Similarly, Wojciak-Stothard et al. (2001) report that Rho A controls endothelial permeability, junction assembly and contractility, but not stress fibres; Cdc42 did not appear to play a role in permeability. Consequently, actomyosin and junctional responses may make independent contributions in responses to different inflammatory mediators.
In epithelial systems, G-proteins regulate junctional contacts through Cdc42 and Rac-1 that may function antagonistically with Rho. It has been shown that Cdc42 and Rac-1 are bound at cell contacts, and appear to promote the stability of these contacts. These proteins unbind from junctions when cell contacts are experimentally dissociated (Takaishi et al. 1997). The over-expression of a constitutively activated form of Rac-1 has also been shown to stabilize cadherin/catenin/actin complexes, while a negative mutant will dissociate these complexes; a similar role has been suggested for Cdc42 (Kuroda et al. 1997). Cadherin engagement in epithelia can control the activity of G-proteins as well. In confluent monolayers forming contacts, Rac-1 and Cdc42 activity was high, while Rho A activity was low compared to low-density cultures (Noren et al. 2001). In that report it was demonstrated that cells exposed to the extracellular domain of cadherins blocked Rho A and increased Rac-1 activity. Therefore there probably is crosstalk between junctions and G-proteins in a bi-directional manner. Kaibuchi et al. (1999) have suggested that these interactions govern binding interactions within catenin complexes and the cytoskeleton to regulate junction integrity.
We found that H2O2 promotes the association of paxillin and p130 CAS with the triton cytoskeleton, while the association of FAK with the triton cytoskeleton remained constant. Importantly, while cell borders have been observed to retract in response to H2O2 (Kevil et al. 2000, 2001a), we found that the overall size of H2O2-treated cells in the trypsinized monolayers was relatively maintained in relation to trypsinized control cells. This was observed as a conservation of total cell surface area and perimeter following exposure to trypsin. This oxidant-mediated adhesion of cells to the substratum also visibly reorganizes the spatial distribution of several components of focal adhesion plaques, including β1 integrins, paxillin, vinculin, p125 FAK and p130 CAS. Following peroxide exposure, some paxillin, FAK and p130 CAS appears at junctions at many points of cell contact. While not yet proven, it is possible that these proteins are deliberately distributed to junctions to transduce signals between focal contacts and the junction (Turner, 2000).
The substrate binding that occurs in response to H2O2 takes place rapidly (< 15 min), and begins to reverse within 30 min. Since this substrate binding effect begins to diminish in some cases while permeability can continue to increase, it may have developed as a means to temporarily preserve matrix adhesion in ‘acute’ (< 1 h) permeability responses. Mechanistically, the substrate binding effect of oxidants seems to involve p42/44 MAP kinase and cell calcium elevation, since only the antagonists for calcium and ERK 1, 2 partly reduced oxidant-induced cell substrate adhesion. The adhesion was not prevented by a classical protein kinase C (PKC) antagonist, or a protein kinase G (PKG) blocker. Recently, we also found that focal adhesions in endothelium increased in response to treatment with PAO (30 µm), a tyrosine phosphatase inhibitor, which provides further support for protein tyrosine phosphorylation in this response.
It is still unclear as to whether increased substrate adhesion precedes, follows or is contemporary with kinase signalling at the junction. Studies by Williams et al. (1996) suggest that tyrosine kinase activity is necessary for cell motility and mobilization of paxillin to focal adhesions. Tu et al. (2001) reported that the adhesion of cells to the substratum is a requirement for the tyrosine phosphorylation of FAK in response to PKC activation. However, under these circumstances p42/44 MAP kinase could still be activated (Tu et al. 2001). In that report the PKC-dependent phosphorylation of FAK (in response to phorbol ester) was cytoskeleton and anchorage dependent (Tu et al. 2001). Therefore, while FAK and MAPK both appear to be cytoskeleton and anchorage dependent, they may be parallel systems.
With respect to MAPKs, Aplin et al. (1999) have argued that integrin-mediated organization of the cytoskeleton can bring activated MAP kinase into close approach with some targets within an adhesion/cytoskeletal signalling module. The effects of MAP kinases appear to involve MAPK binding to the cytoskeleton, or a physical approach to target molecules (Ingram et al. 2000). Under conditions of cell detachment or treatment with an actin filament toxin (cytochalasin D) the ability of extracellular signal-regulated protein kinases (ERK) to phosphorylate at least one target, Elk-1, is impaired. The binding of MAP kinases to the assembled cytoskeleton also appears to be regulated by their level of activation (McNicol et al. 2001), and is not affected by activation of Rho (Matrougui et al. 2001). Therefore, the binding and targeting of kinases like FAK and ERK depends on cell anchorage and cytoskeletal stability. It is interesting that in some cases, the actin microfilament toxin cytochalasin D increases tyrosine phosphatase activity (Osdoit & Rosa, 2001), and prevents the activation of p42/44MAPK (Takeuchi et al. 1997; Tsakiridis et al. 1997). Therefore, the assembly of the stress fibres/focal adhesions might create a scaffold for the assembly of several kinases and phosphatases into a signalling module that in turn drives downstream events in junction regulation while also controlling cell tension.
Lastly, an important but largely unexplored possibility is that focal adhesive complexes also appear to bind the p47 subunit of NADPH oxidase in endothelial cells (Gu et al. 2002). In that study, p47 phox-derived oxidant formation was blocked by cytoskeletal disruption, and suggests a novel source of signal oxidants which could activate oxidant-regulated kinases like FAK and ERK.
Therefore, mediators which promote tyrosine phosphorylation-dependent changes in barrier may also alter cell substrate adhesion. Monolayers of endothelial cells which have been exposed to several non-oxidant, pro-inflammatory mediators including: histamine, thrombin and bradykinin also caused endothelial cells to become relatively resistant to trypsin, as was observed for H2O2, consistent with idea that these mediators promote cell–substrate adhesion. Substrate adhesion dependent signals triggered by these pharmacological mediators may recruit and activate MAPKs in a simlar manner as that seen in permeability responses produced by oxidants (Alexander et al. 2001). Consistent with this model, we have previously reported that oxidant-mediated permeability changes critically require participation of the p42/44 MAPK and p38 MAPK. These kinases are both activated by H2O2, and their blockade prevented oxidant-mediated changes in endothelial solute permeability (Kevil et al. 2000, 2001b) and may also be significant in responses to other mediators.
How the assembly or disassembly of the actin cytoskeleton is related to increased microvascular permeability is controversial. On the one hand, oxidants will activate p38 MAPK to phosphorylate HSP27 which organizes F-actin assembly into stress fibres in some permeability responses (Huot et al. 1997). The appearance of actin stress fibres is frequently observed in endothelial cells following exposure to inflammatory mediators (Welles et al. 1985a, 1985b; Haselton et al. 1989). However, agents that maintain and stabilize actin filaments, like phalloidin, decrease permeability (Alexander et al. 1988; Kurose et al. 1993). While actin filament contraction has also been linked to increased permeability, dynamic changes in actin filaments may be necessary to promote cell border retraction, or to signalling changes in endothelial permeability (Nieuw Amerongen et al. 2000a; Murphy et al. 2001).
Mechanistically, at least one important structural event in permeability regulation that is controlled by the activity of MAPKs may be the dissociation of occludin (a component of the tight junction), from ZO-1, the protein that links occludin to the cytoskeleton (Kevil et al. 2000, 2001b). In response to H2O2, occludin appears to dissociate spatially from ZO-1, while ZO-1 retains its position close to points of cell–cell contact. This result is consistent with a MAPK-dependent interruption between ZO-1 and occludin. If MAPKs are inhibited in the presence of H2O2, occludin remains associated with the cell junction, supporting MAPKs as regulators of tight junctional protein docking with the cytoskeleton. Therefore, one possible mechanism for this change in occludin docking may be the observed increase in MAPK-dependent occludin serine-phosphorylation seen following exposure to H2O2 (Kevil et al. 2000). Although so far we have only observed this in response to oxidants, this may occur following exposure to other mediators as well.
Junction protein accessibility and binding
In addition to the alterations in occludin-ZO-1 docking seen with oxidants like H2O2, we have also observed that both endothelial classical cadherins (pan-reactive) and vascular endothelial (VE-) cadherins are redistributed in response to several inflammatory mediators. Since the bonds created by pan-reactive and VE-cadherins help establish, maintain and regulate endothelial solute permeability (Lampugnani et al. 1992; Alexander et al. 1998), then changes in cadherin binding probably also contribute to endothelial permeability changes. These changes could reflect alterations in cadherin binding affinity, or redistribution of cadherins away from junctions, or both events. Since it is difficult to measure cadherin-binding affinity, we considered whether cells might sequester cadherins during inflammation as a means of reducing the cadherin homotypic bonding. Using H2O2 as a model of oxidant stress, endothelial cells promoted significant internalization of both pan- and VE-cadherins within 60 min. The amount of cadherins internalized was analysed by two different methods (trypsin protection and an ELISA method). Both methods suggest that approximately 50% of the cadherins normally accessible for binding to adjacent cells become inaccessible (i.e. to antibody binding and trypsinization). Calcium chelation (using EDTA, a treatment commonly used to promote cadherin endocytosis) (Kartenbeck et al. 1991) produced a similar effect, but induced a quantitatively greater internalization of cadherins.
While extracellular calcium depletion-mediated internalization of VE-cadherin was seen to decrease the association of VE-cadherin with the cytoskeleton, both histamine and H2O2 seem to transiently increase VE-cadherin association with the cytoskeleton. While calcium chelation promotes disintegration of cadherin dimers, and dramatic internalization of cadherins, it may induce internalization by a different mechanism than that seen with inflammatory mediators, and the decreased cytoskeletal binding with calcium depletion may reflect this. Decreased cadherin access, measured by ELISA, could reflect blocking of a recognized cadherin epitope (similar to that in trypsin studies) since immunostaining for cadherins under conditions which decrease cadherin access do not show a dramatic redistribution of cadherin staining to endocytic vesicles. Staining for cadherins under these conditions still remains associated mostly with cell borders.
Junctional organization regulated through cadherin endocytosis and recycling may depend on the activity of Rac1 and other small g-proteins (Kartenbeck et al. 1991; Nieuw Amerongen et al. 2000b; Akhtar & Hotchin, 2001; Nieuw Amerongen & van Hinsbergh, 2001). Cadherin endocytosis may also co-internalize other surface proteins like c-Met, the HGF-receptor, and is regulated by the g-proteins Rho, Rac and Rab (Kamei et al. 1999), as well as PI3 kinase. Active Rac1 appears to restrict clathrin-mediated endocytosis, but if sufficient active Rac1 is microinjected into cells, cadherins are removed from junctions, suggesting that this may occur through a clathrin-independent pathway (Braga et al. 2000). Activated Rho A will inhibit clathrin-dependent endocytosis, and enhance clathrin-independent endocytosis (Lamaze et al. 1996). Therefore, endocytosis of endothelial cadherins in inflammation is likely to be clathrin independent. With respect to cadherin endocytosis in endothelial cells, it is worth noting that inflammation-induced cadherin sequestration and substrate adhesion both appear to be PKC-independent events (Alexander et al. 1998, 2000; Kevil et al. 1998b). However, since PKCs have been shown to play roles in permeability, it is possible that PKC activity occurs parallel or downstream of the internalization/substrate adhesion events. MT-MMP1 may also be regulated through endocytosis in a clathrin-dynamin dependent manner (Jiang et al. 2001).
Although endocytosis and changes in membrane protein docking are two potentially important mechanisms for the regulation of junction proteins, it is also possible that cadherin-dependent cell adhesion might involve steric ‘shielding’ by other surface molecules, as has been suggested for the membrane-associated mucin episialin/MUC1 (Wesseling et al. 1996). Since our model suggests that there may be a reciprocal regulation of permeability and focal adhesion/focal signalling that is linked with the sequestering of these junctional proteins, it is interesting to speculate that during inflammation, cadherins and focal adhesion plaque proteins might occupy the same microdomain, and are related or linked phenomena. This is possible since both cadherins and integrins on the cell surface are apparently protected from trypsin (and binding) and initially are located at or near cell–cell borders. These two possibly related phenomena may co-ordinate two different classes of adhesive molecules in permeability regulation.
Matrix metalloproteinases (MMPs)
Several tissue and leucocyte proteases have gained attention as mediators of I/R-mediated endothelial permeability because of their effects on interendothelial junctions. We have reported that neutrophil elastase proteolyses VE-cadherin, but apparently not pan-reactive cadherins, and that VE-cadherin proteolysis in respiratory distress is correlated with increased endothelial permeability (Carden et al. 1998). In a model of sepsis, Bannerman et al. (1998) have shown that adherens junctions can be proteolysed by caspases leading to permeability alterations. Based on recent inhibitor and activity studies, there is also growing evidence that several Zn2+-dependent proteases, the matrix metalloproteinases (MMPs), also contribute to post-ischaemic tissue injury in the brain, heart and mesentery (Smith & Gabler, 1994a, 1994b; Danielsen et al. 1998; Asahi et al. 2001; Etoh et al. 2001) and also to inflammatory agonist-driven forms of permeability (Ehringer et al. 2000).
MMP synthesis is known to be oxidant-dependent (Rajagopalan et al. 1996; Qin et al. 1999) and, similar to the expression of endothelial cell adhesion molecules, is triggered by the activation of redox-sensitive transcription factors like NF-κB and AP-1 (Yoshida et al. 2001; Bond et al. 2001). The MMP-2 promoter also contains AP-1, AP-2, PEA3, C/EBP, Sp1 and Sp3 binding sites, of which the Sp1, Sp3 and AP-2 have been shown to help regulate MMP-2 expression (Qin et al. 1999). Oxidants, like hydrogen peroxide, can enhance the transcription and translation of MMP-1, 2 and 9, and may account for at least part of the increased permeability caused by forms of oxidant stress, particularly reperfusion injury (Rajagopalan et al. 1996; Brenneisen et al. 1997a, 1997b; Cheung et al. 2000).
MMPs have long been regarded solely as proteinases that degrade extracellular matrix (ECM) components, hence their original description as ‘collagenases’ or ‘gelatinases’. MMPs have been implicated in some forms of increased microvascular permeability; however, these MMP-dependent permeability changes have been ascribed to effects on matrix integrity and filtration properties of the ECM (Partridge et al. 1993).
Recently, MMPs have been reported to proteolyse both VE-cadherin and occludin, two junctional, non-matrix targets (Herren et al. 1998; Wachtel et al. 1999). Given that intercellular junctions represent a second significant target for MMPs, the activation/synthesis of MMPs during inflammation may possibly lead to increased microvascular permeability through destruction of the tight, and perhaps adherens, junctional proteins that maintain the normal endothelial barrier to solute exchange. The destruction of these elements could lead to sustained changes in permeability that would require new synthesis of these proteins to restore normal barrier. MMPs can be activated by oxidants (Rajagopalan et al. 1996) and by membrane-type MMPs. Thrombin-dependent changes in permeability may involve the activation of MMP-2 through activation of MT-MMP1 in human endothelium (Lafleur et al. 2001), an event that also appears to depend upon tyrosine phosphorylation (Fernandez-Patron et al. 1999). TGF-b-mediated permeability responses have also been linked with the synthesis, secretion and activation of MMP-9 (Behzadian et al. 2001). While cytokine-dependent permeability changes have been ascribed to alterations in matrix filtration properties (Partridge et al. 1993), or decreased expression of junctions (Mankertz et al. 2000; Wachtel et al. 2001), cytokine induction of MMPs leading to junctional reorganization may be an important mechanism for increased permeability during chronic inflammation (Wachtel et al. 1999).
It has also been reported that the MAP kinase pathway (p38 MAP kinase, MEK-1), as well as PKC, also mediate the activation of MMP-9 (Yao et al. 2001); this is also independent of PI3 kinase activity. There may also be reciprocal regulation of these kinases by MMPs, as MT-MMP1 seems to modulate the activation of ERK (Gingras et al. 2001). One very interesting novel finding that may link many of these events is the observation that claudins can form a tripartite complex with pro-MMP2 and MT-MMP1 yielding active MMP-2 (Miyamori et al. 2000). G-proteins also participate in the activation of membrane metalloproteinases and also MMP-2. The activation of the MT-MMPs is Rac1 and Cdc42 dependent, but is not influenced by Rho A (Okamoto et al. 1999; Kawano et al. 2000). MMP-dependent cadherin proteolysis has also been reported in some cell types, and also appears to require activation by Rac1 (Ito et al. 1999).
Several MMPs exist which could contribute to permeability changes. The particular MMP isoforms expressed by endothelium and their activity vary enormously, and are controlled by cell cycle, cytokines and other humoral mediators, oxidants and the abundance of their endogenous inhibitors, the tissue inhibitors of metalloproteinases (TIMPs). It is worth noting that without cytokine treatment, MMP-9 is only very weakly expressed by endothelial cells. Therefore, acute (i.e. protein synthesis independent) permeability changes that can be linked to MMPs are more likely to be mediated by MMP-2, which is constitiutively expressed by endothelial cells.
Mechanistically, MMP-2 at the cell surface is activated post-translationally through the formation of a trimolecular complex of TIMP-2, the membrane type MMP-1 (MT-MMP1) and pro-MMP-2 that cleaves and activates pro-MMP-2 to MMP-2 (Gingras et al. 2000). Since MT-MMP1 is localized to caveolae, MT–MMP interactions within caveolae may contribute to their activation (Annabi et al. 2001). The signals which control this activation are not presently understood, but may include regulated secretion of MT-MMP-1 or structural redistribution of proteins which bind MMPs, e.g. integrins (Deryugina et al. 2001) to control the pool of these trimolecular complexes, and the total amount of activated MMP.
MMP-2 may also be regulated through Rho. In smooth muscle cells, Clostridium difficile toxin B promotes MMP-2 activation, possibly in an MT-MMP1-dependent mechanism (Koike et al. 2000); this may also be true for endothelium. Tyrosine phosphorylation-dependent increases in solute permeability may also be MMP dependent in some cases, since permeability induced by the tyrosine phosphatase inhibitor phenylarsine oxide can be blocked by MMP inhibitors (Wachtel et al. 1999). Conversely, activation of MT-MMP1 may promote focal adhesion by acting as a convertase for the integrins like αvβ3 integrin, which enhances their binding (Deryugina et al. 2001).
MMPs have been demonstrated to associate with focal adhesions (Partridge et al. 1993). The distribution of MMP-2 in non-treated HUVECs is pericellular and may correspond to focal adhesive plaques. MMP-2 is distributed near, but not at, interendothelial junctions, spatially close to occludin, now considered a potential target for MMPs. Further, we have observed that this distribution of MMP-2 is altered by several inflammatory mediators (unpublished observations).
In addition to oxidant-induced transcription/translation of MMPs, oxidants also potently activate latent ‘pro’-forms of MMPs, and could rapidly mobilize MMPs early (< 1 h) following reperfusion. Following reperfusion, oxidants as well as cytokines and growth factors may co-operatively control MMPs by many different mechanisms. This control could be influenced by many factors: (1) activation of latent MMPs ‘pro-forms’ by limited proteolysis involving changes in cell shape, (2) oxidation of regulatory MMP thiols and (3) inactivation of endogenous inhibitors of MMPs, such as TIMPs. FAK phosphorylation and focal adhesion signalling may participate in the regulation of MMP-dependent permeability through regulation of MMP-2 and -9 secretion that is known to be substrate adhesion-dependent (Sein et al. 2000). It is also worth noting that oxidants and peroxynitrite will promote net MMP activity through the inactivation of the MMP inhibitors (tissue inhibitor of metalloproteinases, ‘TIMPs’) and will therefore augment MMP-dependent responses (Stricklin & Hoidal, 1992; Frears et al. 1996; Shabani et al. 1998).
Summary
Oxidants and other inflammatory mediators increase endothelial monolayer permeability through several co-operative events that include: (1) enhanced cell–substrate adhesion, (2) junctional protein redistribution and (3) MMP activation (Fig. 1). The increased substrate adhesion may stabilize endothelial cells against detachment, but probably also provides a structural scaffold on which signal modules are activated, controlling other events in permeability. Two possible downstream events that might be regulated by this type of signalling include junctional protein redistribution and activation of MMPs. These two events contribute to diminish cell adhesion and widen the intercellular cleft, the major pathway for paracellular solute exchange during inflammation.
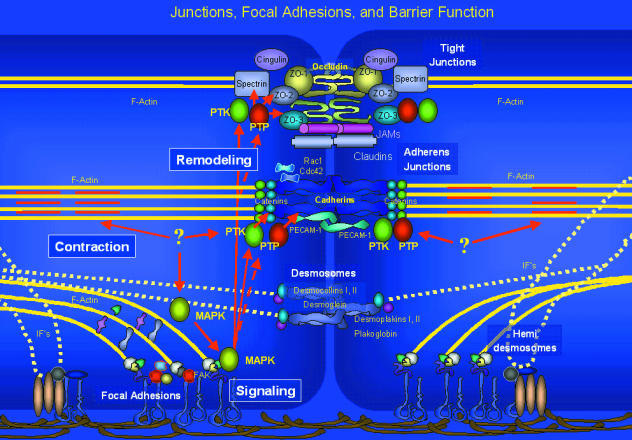
Fig. 1.
Junctions, focal adhesions and permeability regulation. The model proposed in this review suggests that permeability is regulated by events that include the focal adhesion plaque and cytoskeleton-dependent activation of kinases, particularly FAK and MAPKs. The activation of MAPKs triggers alterations in the assembly of tight junctions, but probably also influences adherens junctional stability. The signals transduced by focal adhesive signalling scaffolds may further affect the presentation of cadherins. Protein tyrosine kinases (PTK) and protein tyrosine phosphatases (PTP) activated through these scaffolds likely govern adherens and tight junction organization via enhanced kinase activity (solid red lines) and phosphatase inhibition (broken red lines).
The permeability dysfunction produced by oxidants (and other inflammatory mediators) is blocked by tyrosine kinase inhibitors, and can be reproduced by inhibition of tyrosine phosphatases. This supports tyrosine phosphorylation as a common event in many forms of increased endothelial permeability (Kevil et al. 2001a; Abedi & Zachary, 1997; Schaphorst et al. 1997; Carbajal & Schaeffer, 1998; Esser et al. 1998; Yuan et al. 1998; Andriopoulou et al. 1999). There may be multiple mechanisms through which tyrosine phosphorylation is increased, including: receptor tyrosine kinases, inhibition of tyrosine phosphatases and PKC-dependent tyrosine kinase activation (Tu et al. 2001). While the exact protein targets that need to be tyrosine phosphorylated to alter permeability are not clear, suggested targets include components of endothelial junctions (Esser et al. 1998; Andriopoulou et al. 1999) and focal adhesions (Yuan et al. 1998). Tyrosine phosphorylation of catenins, cadherins and other linker/adaptor molecules likely control junctional organization. In fact, cadherin complexes are known to directly bind to tyrosine kinases, e.g. EGF receptors, Src and Fer (Daniel & Reynolds, 1997). These complexes also bind tyrosine phosphatases SHP-1, 2 and PTP-u. Since there is considerable cross-talk between the systems and the Rho GTPases, it has been suggested that cadherin–catenin tyrosine phosphorylation could regulate the association of these complexes, providing an additional level of signalling complexes (Fig. 2) (Daniel & Reynolds, 1997).
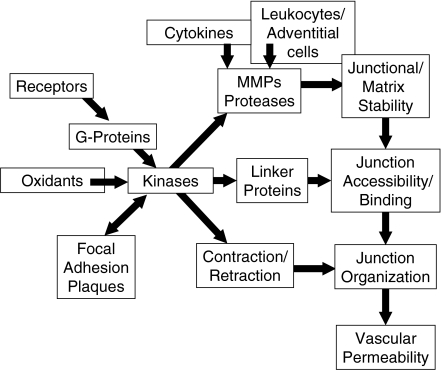
Fig. 2.
Signalling and structural interactions in inflammation. Inflammatory microvascular permeability is induced acutely by activation of receptor, oxidants, and chronically by proteases like MMPs and elastase which are derived from leucocytes, adventitial cells and endothelial cells. Cytokines can also induce endothelial MMP synthesis and activation. Acutely, receptor-mediated, g-protein-linked activation of multiple kinases promote alterations in junction-linker protein-cytoskeletal binding and also stimulate cytoskeletal retraction. Active MMPs and other proteases will degrade components of both extracellular matrix and junctions, decreasing both junctional bonds and restrictive properties of the matrix. Activation of kinases may also affect junction bonds by altering the spatial distribution of junctional proteins. Together these changes in junctional organization help to promote the increased vascular permeability seen during inflammation.
The scaffold that includes the actin cytoskeleton plays a dual role in permeability alterations. First, the actomyosin cytoskeleton provides the basis for cell contraction and also forms an essential part of the assembly onto which the junctional and focal adhesion signal modules are organized, bringing multiple kinases and g-proteins into contact with their targets.
Although the model we have proposed here mostly reflect in vitro changes produced by oxidants, these events may also occur following exposure to other mediators, and might represent part of a general model for alterations in endothelial solute permeability. Future studies will be needed to investigate the precise molecular events in endothelial permeability regulation and maintenance.
Acknowledgments
This work was funded by NIH grants HL47615 (NHLBI) and PO1 DK43785 (NIDDK).
References
- Abbruscato TJ, Davis TP. Protein expression of brain endothelial cell E-cadherin after hypoxia/aglycemia: influence of astrocyte contact. Brain Res. 1999;842:277–286. doi: 10.1016/s0006-8993(99)01778-3. [DOI] [PubMed] [Google Scholar]
- Abedi H, Zachary I. Vascular endothelial growth factor stimulates tyrosine phosphorylation and recruitment to new focal adhesions of focal adhesion kinase and paxillin in endothelial cells. J. Biol. Chem. 1997;272:15442–15451. doi: 10.1074/jbc.272.24.15442. [DOI] [PubMed] [Google Scholar]
- Adamson RH, Liu B, Fry GN, Rubin LL, Curry FE. Microvascular permeability and number of tight junctions are modulated by cAMP. Am. J. Physiol. 1998;274:H1885–H1894. doi: 10.1152/ajpheart.1998.274.6.H1885. [DOI] [PubMed] [Google Scholar]
- Akhtar N, Hotchin NA. RAC1 regulates adherens junctions through endocytosis of E-cadherin. Mol. Biol. Cell. 2001;12:847–862. doi: 10.1091/mbc.12.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albelda SM, Muller WA, Buck CA, Newman PJ. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J. Cell Biol. 1991;114:1059–1068. doi: 10.1083/jcb.114.5.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JS, Hechtman HB, Shepro D. Phalloidin enhances endothelial barrier function and reduces inflammatory permeability in vitro. Microvasc. Res. 1988;35:308–315. doi: 10.1016/0026-2862(88)90085-4. [DOI] [PubMed] [Google Scholar]
- Alexander JS, Jackson SA, Chaney E, Kevil CG, Haselton FR. The role of cadherin endocytosis in endothelial barrier regulation: involvement of protein kinase C and actin–cadherin interactions. Inflammation. 1998;22:419–433. doi: 10.1023/a:1022325017013. [DOI] [PubMed] [Google Scholar]
- Alexander JS. Rho, tyrosine kinase, Ca (2+), and junctions in endothelial hyperpermeability. Circ. Res. 2000;87:268–271. doi: 10.1161/01.res.87.4.268. [DOI] [PubMed] [Google Scholar]
- Alexander JS, Alexander BC, Eppihimer LA, Goodyear N, Haque R, Davis CP, et al. Inflammatory mediators induce sequestration of VE-cadherin in cultured human endothelial cells. Inflammation. 2000;24:99–113. doi: 10.1023/a:1007025325451. [DOI] [PubMed] [Google Scholar]
- Alexander JS, Zhu Y, Elrod JW, Alexander B, Coe L, Kalogeris TJ, et al. Reciprocal regulation of endothelial substrate adhesion and barrier function. Microcirculation. 2001;8:389–401. doi: 10.1038/sj/mn/7800111. [DOI] [PubMed] [Google Scholar]
- Allport JR, Muller WA, Luscinskas FW. Monocytes induce reversible focal changes in vascular endothelial cadherin complex during transendothelial migration under flow. J. Cell Biol. 2000;148:203–216. doi: 10.1083/jcb.148.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriopoulou P, Navarro P, Zanetti A, Lampugnani MG, Dejana E. Histamine induces tyrosine phosphorylation of endothelial cell-to-cell adherens junctions. Arterioscler. Thromb. Vasc. Biol. 1999;19:2286–2297. doi: 10.1161/01.atv.19.10.2286. [DOI] [PubMed] [Google Scholar]
- Annabi B, Lachambre M, Bousquet-Gagnon N, Page M, Gingras D, Beliveau R. Localization of membrane-type 1 matrix metalloproteinase in caveolae membrane domains. Biochem. J. 2001;353:547–553. doi: 10.1042/0264-6021:3530547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin AE, Short SM, Juliano RL. Anchorage-dependent regulation of the mitogen-activated protein kinase cascade by growth factors is supported by a variety of integrin alpha chains. J. Biol. Chem. 1999;274:31223–31228. doi: 10.1074/jbc.274.44.31223. [DOI] [PubMed] [Google Scholar]
- Asahi M, Wang X, Mori T, Sumii T, Jung JC, Moskowitz MA, Fini ME, Lo EH. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood–brain barrier and white matter components after cerebral ischemia. J. Neurosci. 2001;21:7724–7732. doi: 10.1523/JNEUROSCI.21-19-07724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin AL, Thurston G. Mechanics of endothelial cell architecture and vascular permeability. Crit. Rev. Biomed. Eng. 2001;29:247–278. doi: 10.1615/critrevbiomedeng.v29.i2.20. [DOI] [PubMed] [Google Scholar]
- Bannerman DD, Sathyamoorthy M, Goldblum SE. Bacterial lipopolysaccharide disrupts endothelial monolayer integrity and survival signaling events through caspase cleavage of adherens junction proteins. J. Biol. Chem. 1998;273:35371–35380. doi: 10.1074/jbc.273.52.35371. [DOI] [PubMed] [Google Scholar]
- Behzadian MA, Wang XL, Windsor LJ, Ghaly N, Caldwell RB. TGF-beta increases retinal endothelial cell permeability by increasing MMP-9: possible role of glial cells in endothelial barrier function. Invest. Ophthalmol. Vis. Sci. 2001;42:853–859. [PubMed] [Google Scholar]
- Bond M, Chase AJ, Baker AH, Newby AC. Inhibition of transcription factor NF-kappaB reduces matrix metalloproteinase-1-3 and -9 production by vascular smooth muscle cells. Cardiovasc. Res. 2001;50:556–565. doi: 10.1016/s0008-6363(01)00220-6. [DOI] [PubMed] [Google Scholar]
- Brady-Kalnay SM, Rimm DL, Tonks NK. Receptor protein tyrosine phosphatase PTPmu associates with cadherins and catenins in vivo. J. Cell. Biol. 1995;130(4):977–986. doi: 10.1083/jcb.130.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VM, Betson M, Li X, Lamarche-Vane N. Activation of the small GTPase Rac is sufficient to disrupt cadherin- dependent cell-cell adhesion in normal human keratinocytes. Mol. Biol. Cell. 2000;11:3703–3721. doi: 10.1091/mbc.11.11.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneisen P, Briviba K, Wlaschek M, Wenk J, Scharffetter-Kochanek K. Hydrogen peroxide (H2O2) increases the steady-state mRNA levels of collagenase/MMP-1 in human dermal fibroblasts. Free Radic. Biol. Med. 1997a;22:515–524. doi: 10.1016/s0891-5849(96)00404-2. [DOI] [PubMed] [Google Scholar]
- Brenneisen P, Briviba K, Wlaschek M, Wenk J, Scharffetter-Kochanek K. Hydrogen peroxide (H2O2) increases the steady-state mRNA levels of collagenase/MMP-1 in human dermal fibroblasts. Free Radic. Biol. Med. 1997b;22:515–524. doi: 10.1016/s0891-5849(96)00404-2. [DOI] [PubMed] [Google Scholar]
- Carbajal JM, Schaeffer RC. H2O2 and genistein differentially modulate protein tyrosine phosphorylation, endothelial morphology, and monolayer barrier function. Biochem. Biophys. Res. Commun. 1998;249:461–466. doi: 10.1006/bbrc.1998.9172. [DOI] [PubMed] [Google Scholar]
- Carbajal JM, Schaeffer RC. RhoA inactivation enhances endothelial barrier function. Am. J. Physiol. 1999;277:C955–C964. doi: 10.1152/ajpcell.1999.277.5.C955. [DOI] [PubMed] [Google Scholar]
- Carden D, Xiao F, Moak C, Willis BH, Robinson-Jackson S, Alexander S. Neutrophil elastase promotes lung microvascular injury and proteolysis of endothelial cadherins. Am. J. Physiol. 1998;275:H385–H392. doi: 10.1152/ajpheart.1998.275.2.H385. [DOI] [PubMed] [Google Scholar]
- Cheung PY, Sawicki G, Wozniak M, Wang W, Radomski MW, Schulz R. Matrix metalloproteinase-2 contributes to ischemia-reperfusion injury in the heart. Circulation. 2000;101:1833–1839. doi: 10.1161/01.cir.101.15.1833. [DOI] [PubMed] [Google Scholar]
- Cohen AW, Carbajal JM, Schaeffer RC. VEGF stimulates tyrosine phosphorylation of beta-catenin and small–pore endothelial barrier dysfunction. Am. J. Physiol. 1999;277:H2038–H2049. doi: 10.1152/ajpheart.1999.277.5.H2038. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Reynolds AB. Tyrosine phosphorylation and cadherin/catenin function. Bioessays. 1997;19:883–891. doi: 10.1002/bies.950191008. [DOI] [PubMed] [Google Scholar]
- Danielsen CC, Wiggers H, Andersen HR. Increased amounts of collagenase and gelatinase in porcine myocardium following ischemia and reperfusion. J. Mol. Cell Cardiol. 1998;30:1431–1442. doi: 10.1006/jmcc.1998.0711. [DOI] [PubMed] [Google Scholar]
- Dejana E. Endothelial adherens junctions: implications in the control of vascular permeability and angiogenesis. J. Clin. Invest. 1997;100:S7–S10. [PubMed] [Google Scholar]
- Deryugina EI, Strongin AY. An alternative processing of integrin alpha(v) subunit in tumor cells by membrane type-1 matrix metalloproteinase. J. Biol. Chem. 2002;277:7377–7385. doi: 10.1074/jbc.M109580200. [DOI] [PubMed] [Google Scholar]
- Dvorak AM, Kohn S, Morgan ES, Fox P, Nagy JA, Dvorak HF. The vesiculo-vacuolar organelle (VVO): a distinct endothelial cell structure that provides a transcellular pathway for macromolecular extravasation. J. Leukoc. Biol. 1996;59:100–115. [PubMed] [Google Scholar]
- Ehringer WD, Wang OL, Haq A, Miller FN. Bradykinin and alpha-thrombin increase human umbilical vein endothelial macromolecular permeability by different mechanisms. Inflammation. 2000;24:175–193. doi: 10.1023/a:1007037711339. [DOI] [PubMed] [Google Scholar]
- Esser S, Lampugnani MG, Corada M, Dejana E, Risau W. Vascular endothelial growth factor induces VE-cadherin tyrosine phosphorylation in endothelial cells. J. Cell Sci. 1998;111(13):1853–1865. doi: 10.1242/jcs.111.13.1853. [DOI] [PubMed] [Google Scholar]
- Etoh T, Joffs C, Deschamps AM, Davis J, Dowdy K, Hendrick J, et al. Myocardial and interstitial matrix metalloproteinase activity after acute myocardial infarction in pigs. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H987–H994. doi: 10.1152/ajpheart.2001.281.3.H987. [DOI] [PubMed] [Google Scholar]
- Feng D, Nagy JA, Hipp J, Dvorak HF, Dvorak AM. Vesiculo-vacuolar organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine, and serotonin. J. Exp. Med. 1996;183:1981–1986. doi: 10.1084/jem.183.5.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Patron C, Zhang Y, Radomski MW, Hollenberg MD, Davidge ST. Rapid release of matrix metalloproteinase (MMP)-2 by thrombin in the rat aorta: modulation by protein tyrosine kinase/phosphatase. Thromb. Haemost. 1999;82:1353–1357. [PubMed] [Google Scholar]
- Firth JA, Bauman KF, Sibley CP. The intercellular junctions of guinea-pig placental capillaries: a possible structural basis for endothelial solute permeability. J. Ultrastruct. Res. 1983;85:45–57. doi: 10.1016/s0022-5320(83)90115-6. [DOI] [PubMed] [Google Scholar]
- Frears ER, Zhang Z, Blake DR, O'Connell JP, Winyard PG. Inactivation of tissue inhibitor of metalloproteinase-1 by peroxynitrite. FEBS Lett. 1996;381:21–24. doi: 10.1016/0014-5793(96)00065-8. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner TW, Lesher T, Khin S, Vu C, Barber AJ, Brennan WA. Jr Histamine reduces ZO-1 tight-junction protein expression in cultured retinal microvascular endothelial cells. Biochem. J. 1996;320:717–721. doi: 10.1042/bj3200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras D, Page M, Annabi B, Beliveau R. Rapid activation of matrix metalloproteinase-2 by glioma cells occurs through a posttranslational MT1-MMP-dependent mechanism. Biochim. Biophys. Acta. 2000;1497:341–350. doi: 10.1016/s0167-4889(00)00071-9. [DOI] [PubMed] [Google Scholar]
- Gingras D, Bousquet-Gagnon N, Langlois S, Lachambre MP, Annabi B, Beliveau R. Activation of the extracellular signal-regulated protein kinase (ERK) cascade by membrane-type-1 matrix metalloproteinase (MT1-MMP) FEBS Lett. 2001;507:231–236. doi: 10.1016/s0014-5793(01)02985-4. [DOI] [PubMed] [Google Scholar]
- Gu Y, Xu YC, Wu RF, Souza RF, Nwariaku FE, Terada LS. TNFalpha activates c-Jun amino terminal kinase through p47 (phox) Exp. Cell Res. 2002;272:62–74. doi: 10.1006/excr.2001.5404. [DOI] [PubMed] [Google Scholar]
- Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J. Biol. Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- Haselton FR, Alexander JS, Mueller SN. Adenosine decreases permeability of in vitro endothelial monolayers. J. Appl. Physiol. 1993;74:1581–1590. doi: 10.1152/jappl.1993.74.4.1581. [DOI] [PubMed] [Google Scholar]
- Haselton FR, Mueller SN, Howell RE, Levine EM, Fishman AP. Chromatographic demonstration of reversible changes in endothelial permeability. J. Appl. Physiol. 1989;67:2032–2048. doi: 10.1152/jappl.1989.67.5.2032. [DOI] [PubMed] [Google Scholar]
- Herren B, Levkau B, Raines EW, Ross R. Cleavage of beta-catenin and plakoglobin and shedding of VE-cadherin during endothelial apoptosis: evidence for a role for caspases and metalloproteinases. Mol. Biol. Cell. 1998;9:1589–1601. doi: 10.1091/mbc.9.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, et al. Occludin as a possible determinant of tight junction permeability in endothelial cells. J. Cell Sci. 1997;110:1603–1613. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- Hoying JB, Williams SK. Endothelial cell monolayers viewed by TIRF microscopy. In Vitro Cell Dev. Biol. Anim. 1994;30A:1–3. doi: 10.1007/BF02631406. [DOI] [PubMed] [Google Scholar]
- Huot J, Houle F, Marceau F, Landry J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ. Res. 1997;80:383–392. doi: 10.1161/01.res.80.3.383. [DOI] [PubMed] [Google Scholar]
- Ilan N, Cheung L, Pinter E, Madri JA. Platelet-endothelial cell adhesion molecule-1 (CD31), a scaffolding molecule for selected catenin family members whose binding is mediated by different tyrosine and serine/threonine phosphorylation. J. Biol. Chem. 2000;275:21435–21443. doi: 10.1074/jbc.M001857200. [DOI] [PubMed] [Google Scholar]
- Ingram AJ, James L, Cai L, Thai K, Ly H, Scholey JW. NO inhibits stretch-induced MAPK activity by cytoskeletal disruption. J. Biol. Chem. 2000;275:40301–40306. doi: 10.1074/jbc.M007018200. [DOI] [PubMed] [Google Scholar]
- Ito K, Okamoto I, Araki N, Kawano Y, Nakao M, Fujiyama S, et al. Calcium influx triggers the sequential proteolysis of extracellular and cytoplasmic domains of E-cadherin, leading to loss of beta-catenin from cell-cell contacts. Oncogene. 1999;18:7080–7090. doi: 10.1038/sj.onc.1203191. [DOI] [PubMed] [Google Scholar]
- Jiang A, Lehti K, Wang X, Weiss SJ, Keski-Oja J, Pei D. Regulation of membrane-type matrix metalloproteinase 1 activity by dynamin-mediated endocytosis. Proc. Natl. Acad. Sci. USA. 2001;98:13693–13698. doi: 10.1073/pnas.241293698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi K, Kuroda S, Fukata M, Nakagawa M. Regulation of cadherin-mediated cell-cell adhesion by the Rho family GTPases. Curr. Opin. Cell Biol. 1999;11:591–596. doi: 10.1016/s0955-0674(99)00014-9. [DOI] [PubMed] [Google Scholar]
- Kamei T, Matozaki T, Sakisaka T, Kodama A, Yokoyama S, Peng YF, et al. Coendocytosis of cadherin and c-Met coupled to disruption of cell-cell adhesion in MDCK cells – regulation by Rho, Rac and Rab small G proteins. Oncogene. 1999;18:6776–6784. doi: 10.1038/sj.onc.1203114. [DOI] [PubMed] [Google Scholar]
- Kaplan DD, Meigs TE, Casey PJ. Distinct regions of the cadherin cytoplasmic domain are essential for functional interaction with Ga12 and b-catenin. J. Biol. Chem. 2001;276:44037–44043. doi: 10.1074/jbc.M106121200. [DOI] [PubMed] [Google Scholar]
- Kartenbeck J, Schmelz M, Franke WW, Geiger B. Endocytosis of junctional cadherins in bovine kidney epithelial (MDBK) cells cultured in low Ca2+ ion medium. J. Cell Biol. 1991;113:881–892. doi: 10.1083/jcb.113.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Okamoto I, Murakami D, Itoh H, Yoshida M, Ueda S, et al. Ras oncoprotein induces CD44 cleavage through phosphoinositide 3-OH kinase and the rho family of small G proteins. J. Biol. Chem. 2000;275:29628–29635. doi: 10.1074/jbc.M002440200. [DOI] [PubMed] [Google Scholar]
- Kevil CG, Payne DK, Mire E, Alexander JS. Vascular permeability factor/vascular endothelial cell growth factor- mediated permeability occurs through disorganization of endothelial junctional proteins. J. Biol. Chem. 1998a;273:15099–15103. doi: 10.1074/jbc.273.24.15099. [DOI] [PubMed] [Google Scholar]
- Kevil CG, Ohno N, Gute DC, Okayama N, Robinson SA, Chaney E, et al. Role of cadherin internalization in hydrogen peroxide-mediated endothelial permeability. Free Radic. Biol. Med. 1998b;24:1015–1022. doi: 10.1016/s0891-5849(97)00433-4. [DOI] [PubMed] [Google Scholar]
- Kevil CG, Oshima T, Alexander B, Coe LL, Alexander JS. H2O2-mediated permeability: role of MAPK and occludin. Am. J. Physiol. Cell Physiol. 2000;279:C21–C30. doi: 10.1152/ajpcell.2000.279.1.C21. [DOI] [PubMed] [Google Scholar]
- Kevil CG, Okayama N, Alexander JS. H2O2-mediated permeability II: importance of tyrosine phosphatase and kinase activity. Am. J. Physiol. Cell Physiol. 2001a;281:C1940–C1947. doi: 10.1152/ajpcell.2001.281.6.C1940. [DOI] [PubMed] [Google Scholar]
- Kevil CG, Oshima T, Alexander JS. The role of p38 MAP kinase in hydrogen peroxide mediated endothelial solute permeability. Endothelium. 2001b;8:107–116. doi: 10.3109/10623320109165320. [DOI] [PubMed] [Google Scholar]
- Koike T, Kuzuya M, Asai T, Kanda S, Cheng XW, Watanabe K, et al. Activation of MMP-2 by Clostridium difficile toxin B in bovine smooth muscle cells. Biochem. Biophys. Res. Commun. 2000;277:43–46. doi: 10.1006/bbrc.2000.3630. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Fukata M, Fujii K, Nakamura T, Izawa I, Kaibuchi K. Regulation of cell-cell adhesion of MDCK cells by Cdc42 and Rac1 small GTPases. Biochem. Biophys. Res. Commun. 1997;240:430–435. doi: 10.1006/bbrc.1997.7675. [DOI] [PubMed] [Google Scholar]
- Kurose I, Kubes P, Wolf R, Anderson DC, Paulson J, Miyasaka M, Granger DN. Inhibition of nitric oxide production. Mechanisms of vascular albumin leakage. Circ. Res. 1993;73:164–171. doi: 10.1161/01.res.73.1.164. [DOI] [PubMed] [Google Scholar]
- Lafleur MA, Hollenberg MD, Atkinson SJ, Knauper V, Murphy G, Edwards DR. Activation of pro-(matrix metalloproteinase-2) (pro-MMP-2) by thrombin is membrane-type-MMP-dependent in human umbilical vein endothelial cells and generates a distinct 63 kDa active species. Biochem. J. 2001;357:107–115. doi: 10.1042/0264-6021:3570107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamaze C, Chuang TH, Terlecky LJ, Bokoch GM, Schmid SL. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature. 1996;382:177–179. doi: 10.1038/382177a0. [DOI] [PubMed] [Google Scholar]
- Lampugnani MG, Resnati M, Dejana E, Marchisio PC. The role of integrins in the maintenance of endothelial monolayer integrity. J. Cell Biol. 1991;112:479–490. doi: 10.1083/jcb.112.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Resnati M, Raiteri M, Pigott R, Pisacane A, Houen G, et al. A novel endothelial-specific membrane protein is a marker of cell-cell contacts. J. Cell Biol. 1992;118:1511–1522. doi: 10.1083/jcb.118.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lum H, Malik AB. Mechanisms of increased endothelial permeability. Can. J. Physiol. Pharmacol. 1996;74:787–800. doi: 10.1139/y96-081. [DOI] [PubMed] [Google Scholar]
- Majno G, Palade GE. Studies on inflammation. I. The effect of serotonin and histamine on vascular permeability: an electron microscopic study. J. Biophys. Biochem. Cytol. 1961;11:607–626. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankertz J, Tavalali S, Schmitz H, Mankertz A, Riecken EO, Fromm M, et al. Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J. Cell Sci. 2000;113:2085–2090. doi: 10.1242/jcs.113.11.2085. [DOI] [PubMed] [Google Scholar]
- Matrougui K, Tanko LB, Loufrani L, Gorny D, Levy BI, Tedgui A, et al. Involvement of Rho-kinase and the actin filament network in angiotensin II-induced contraction and extracellular signal-regulated kinase activity in intact rat mesenteric resistance arteries. Arterioscler. Thromb. Vasc. Biol. 2001;21:1288–1293. doi: 10.1161/hq0801.093653. [DOI] [PubMed] [Google Scholar]
- McNicol A, Shibou TS, Pampolina C, Israels SJ. Incorporation of map kinases into the platelet cytoskeleton. Thromb. Res. 2001;103:25–34. doi: 10.1016/s0049-3848(01)00271-7. [DOI] [PubMed] [Google Scholar]
- Minnear FL, Johnson A, Malik AB. Beta-adrenergic modulation of pulmonary transvascular fluid and protein exchange. J. Appl. Physiol. 1986;60:266–274. doi: 10.1152/jappl.1986.60.1.266. [DOI] [PubMed] [Google Scholar]
- Miyamori H, Hasegawa K, Kim KR, Sato H. Expression of metastasis-associated mts1 gene is co-induced with membrane type-1 matrix metalloproteinase (MT1-MMP) during oncogenic transformation and tubular formation of Madin Darby canine kidney (MDCK) epithelial cells. Clin. Exp. Metastasis. 2000;18:51–56. doi: 10.1023/a:1026523418456. [DOI] [PubMed] [Google Scholar]
- Muller WA, Randolph GJ. Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J. Leukoc. Biol. 1999;66:698–704. doi: 10.1002/jlb.66.5.698. [DOI] [PubMed] [Google Scholar]
- Murphy JT, Duffy SL, Hybki DL, Kamm K. Thrombin-mediated permeability of human microvascular pulmonary endothelial cells is calcium dependent. J. Trauma. 2001;50:213–222. doi: 10.1097/00005373-200102000-00005. [DOI] [PubMed] [Google Scholar]
- Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ. Res. 2000a;87:335–340. doi: 10.1161/01.res.87.4.335. [DOI] [PubMed] [Google Scholar]
- Nieuw Amerongen GP, Vermeer MA, van Hinsbergh VW. Role of RhoA and Rho kinase in lysophosphatidic acid–induced endothelial barrier dysfunction. Arterioscler. Thromb. Vasc. Biol. 2000b;20:E127–E133. doi: 10.1161/01.atv.20.12.e127. [DOI] [PubMed] [Google Scholar]
- Nieuw Amerongen GP, van Hinsbergh VW. Cytoskeletal effects of rho-like small guanine nucleotide-binding proteins in the vascular system. Arterioscler. Thromb. Vasc. Biol. 2001;21:300–311. doi: 10.1161/01.atv.21.3.300. [DOI] [PubMed] [Google Scholar]
- Noren NK, Niessen CM, Gumbiner BM, Burridge K. Cadherin engagement regulates Rho family GTPases. J. Biol. Chem. 2001;276:33305–33308. doi: 10.1074/jbc.C100306200. [DOI] [PubMed] [Google Scholar]
- Okamoto I, Kawano Y, Matsumoto M, Suga M, Kaibuchi K, Ando M, et al. Regulated CD44 cleavage under the control of protein kinase C, calcium influx, and the Rho family of small G proteins. J. Biol. Chem. 1999;274:25525–25534. doi: 10.1074/jbc.274.36.25525. [DOI] [PubMed] [Google Scholar]
- Osdoit S, Rosa JP. Fibrin clot retraction by human platelets correlates with alpha (IIb) beta (3) integrin-dependent protein tyrosine dephosphorylation. J. Biol. Chem. 2001;276:6703–6710. doi: 10.1074/jbc.M008945200. [DOI] [PubMed] [Google Scholar]
- Oshima T, Laroux FS, Coe LL, Morise Z, Kawachi S, Bauer P, et al. Interferon-gamma and interleukin-10 reciprocally regulate endothelial junction integrity and barrier function. Microvasc. Res. 2001;61:130–143. doi: 10.1006/mvre.2000.2288. [DOI] [PubMed] [Google Scholar]
- Palade GE, Simionescu M, Simionescu N. Structural aspects of the permeability of the microvascular endothelium. Acta Physiol. Scand. Suppl. 1979;463:11–32. [PubMed] [Google Scholar]
- Pappenheimer JR, Renkin EM, Borrero LM. Filtration diffusion and molecular sieving through peripheral capillary membranes. Am. J. Physiol. 1951;167:13–46. doi: 10.1152/ajplegacy.1951.167.1.13. [DOI] [PubMed] [Google Scholar]
- Partridge CA, Jeffrey JJ, Malik AB. A 96-kDa gelatinase induced by TNF-alpha contributes to increased microvascular endothelial permeability. Am. J. Physiol. 1993;265:L438–L447. doi: 10.1152/ajplung.1993.265.5.L438. [DOI] [PubMed] [Google Scholar]
- Qiao RL, Yan W, Lum H, Malik AB. Arg-Gly-Asp peptide increases endothelial hydraulic conductivity: comparison with thrombin response. Am. J. Physiol. 1995;269:C110–C117. doi: 10.1152/ajpcell.1995.269.1.C110. [DOI] [PubMed] [Google Scholar]
- Qin H, Sun Y, Benveniste EN. The transcription factors Sp1, Sp3, and AP-2 are required for constitutive matrix metalloproteinase-2 gene expression in astroglioma cells. J. Biol. Chem. 1999;274:29130–29137. doi: 10.1074/jbc.274.41.29130. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J. Clin. Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaphorst KL, Pavalko FM, Patterson CE, Garcia JG. Thrombin-mediated focal adhesion plaque reorganization in endothelium: role of protein phosphorylation. Am. J. Respir. Cell Mol. Biol. 1997;17:443–455. doi: 10.1165/ajrcmb.17.4.2502. [DOI] [PubMed] [Google Scholar]
- Sein TT, Thant AA, Hiraiwa Y, Amin AR, Sohara Y, Liu Y, et al. A role for FAK in the Concanavalin A-dependent secretion of matrix metalloproteinase-2 and -9. Oncogene. 2000;19:5539–5542. doi: 10.1038/sj.onc.1203932. [DOI] [PubMed] [Google Scholar]
- Shabani F, McNeil J, Tippett L. The oxidative inactivation of tissue inhibitor of metalloproteinase-1 (TIMP-1) by hypochlorous acid (HOCI) is suppressed by anti-rheumatic drugs. Free Radic. Res. 1998;28:115–123. doi: 10.3109/10715769809065797. [DOI] [PubMed] [Google Scholar]
- Siflinger-Birnboim A, Del Vecchio PJ, Cooper JA, Blumenstock FA, Shepard JM, Malik AB. Molecular sieving characteristics of the cultured endothelial monolayer. J. Cell Physiol. 1987;132:111–117. doi: 10.1002/jcp.1041320115. [DOI] [PubMed] [Google Scholar]
- Simionescu N, Siminoescu M, Palade GE. Permeability of muscle capillaries to small heme-peptides. Evidence for the existence of patent transendothelial channels. J. Cell Biol. 1975;64:586–607. doi: 10.1083/jcb.64.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR, Gabler WL. Doxycycline suppression of ischemia-reperfusion-induced hepatic injury. Inflammation. 1994a;18:193–201. doi: 10.1007/BF01534560. [DOI] [PubMed] [Google Scholar]
- Smith JR, Gabler WL. Effects of doxycycline in two rat models of ischemia/reperfusion injury. Proc. West Pharmacol. Soc. 1994b;37:3–4. [PubMed] [Google Scholar]
- Sobue K, Yamamoto N, Yoneda K, Hodgson ME, Yamashiro K, Tsuruoka N, et al. Induction of blood–brain barrier properties in immortalized bovine brain endothelial cells by astrocytic factors. Neurosci. Res. 1999;35:155–164. doi: 10.1016/s0168-0102(99)00079-6. [DOI] [PubMed] [Google Scholar]
- Stricklin GP, Hoidal JR. Oxidant-mediated inactivation of TIMP. Matrix Supplement. 1992;1:325. [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. J. Cell Biol. 1997;139:1047–1059. doi: 10.1083/jcb.139.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y, Suzawa M, Kikuchi T, Nishida E, Fujita T, Matsumoto T. Differentiation and transforming growth factor-beta receptor down-regulation by collagen–alpha2beta1 integrin interaction is mediated by focal adhesion kinase and its downstream signals in murine osteoblastic cells. J. Biol. Chem. 1997;272:29309–29316. doi: 10.1074/jbc.272.46.29309. [DOI] [PubMed] [Google Scholar]
- Tsakiridis T, Wang Q, Taha C, Grinstein S, Downey G, Klip A. Involvement of the actin network in insulin signalling. Soc. Generalphysiol. Series. 1997;52:257–271. [PubMed] [Google Scholar]
- Tsukada H, Ying X, Fu C, Ishikawa S, McKeown-Longo P, Albelda S, et al. Ligation of endothelial alpha v beta 3 integrin increases capillary hydraulic conductivity of rat lung. Circ. Res. 1995;77:651–659. doi: 10.1161/01.res.77.4.651. [DOI] [PubMed] [Google Scholar]
- Tu LC, Chou CK, Chen HC, Yeh SF. Protein kinase C-mediated tyrosine phosphorylation of paxillin and focal adhesion kinase requires cytoskeletal integrity and is uncoupled to mitogen-activated protein kinase activation in human hepatoma cells. J. Biomed. Sci. 2001;8:184–190. doi: 10.1007/BF02256411. [DOI] [PubMed] [Google Scholar]
- Turner CE. Paxillin interactions. J. Cell Sci. 2000;113(Part 23):4139–4140. doi: 10.1242/jcs.113.23.4139. [DOI] [PubMed] [Google Scholar]
- Vepa S, Scribner WM, Natarajan V. Activation of protein phosphorylation by oxidants in vascular endothelial cells: identification of tyrosine phosphorylation of caveolin. Free Radic. Biol. Med. 1997;22:25–35. doi: 10.1016/s0891-5849(96)00241-9. [DOI] [PubMed] [Google Scholar]
- Vepa S, Scribner WM, Parinandi NL, English D, Garcia JG, Natarajan V. Hydrogen peroxide stimulates tyrosine phosphorylation of focal adhesion kinase in vascular endothelial cells. Am. J. Physiol. 1999;277:L150–L158. doi: 10.1152/ajplung.1999.277.1.L150. [DOI] [PubMed] [Google Scholar]
- Voura EB, Ramjeesingh RA, Montgomery AM, Siu CH. Involvement of integrin alpha (v) beta (3) and cell adhesion molecule L1 in transendothelial migration of melanoma cells. Mol Biol Cell. 2001;12:2699–2710. doi: 10.1091/mbc.12.9.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtel M, Frei K, Ehler E, Fontana A, Winterhalter K, Gloor SM. Occludin proteolysis and increased permeability in endothelial cells through tyrosine phosphatase inhibition. J. Cell Sci. 1999;112(Part 23):4347–4356. doi: 10.1242/jcs.112.23.4347. [DOI] [PubMed] [Google Scholar]
- Wachtel M, Bolliger MF, Ishihara H, Frei K, Bluethmann H, Gloor SM. Down-regulation of occludin expression in astrocytes by tumour necrosis factor (TNF) is mediated via TNF type-1 receptor and nuclear factor-kappaB activation. J. Neurochem. 2001;78:155–162. doi: 10.1046/j.1471-4159.2001.00399.x. [DOI] [PubMed] [Google Scholar]
- Wade R, Bohl J, Vande PS. Paxillin null embryonic stem cells are impaired in cell spreading and tyrosine phosphorylation of focal adhesion kinase. Oncogene. 2002;21:96–107. doi: 10.1038/sj.onc.1205013. [DOI] [PubMed] [Google Scholar]
- Welles SL, Shepro D, Hechtman HB. Eicosanoid modulation of stress fibers in cultured bovine aortic endothelial cells. Inflammation. 1985a;9:439–450. doi: 10.1007/BF00916343. [DOI] [PubMed] [Google Scholar]
- Welles SL, Shepro D, Hechtman HB. Vasoactive amines modulate actin cables (stress fibers) and surface area in cultured bovine endothelium. J. Cell Physiol. 1985b;123:337–342. doi: 10.1002/jcp.1041230307. [DOI] [PubMed] [Google Scholar]
- Wesseling J, van der Valk SW, Hilkens J. A mechanism for inhibition of E-cadherin-mediated cell-cell adhesion by the membrane-associated mucin episialin/MUC1. Mol. Biol. Cell. 1996;7:565–577. doi: 10.1091/mbc.7.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GM, Kemp SJ, Brindle NP. Involvement of protein tyrosine kinases in regulation of endothelial cell organization by basement membrane proteins. Biochem. Biophys. Res. Commun. 1996;229:375–380. doi: 10.1006/bbrc.1996.1813. [DOI] [PubMed] [Google Scholar]
- Wissig SL. Identification of the small pore in muscle capillaries. Acta Physiol. Scand. Suppl. 1979;463:33–44. [PubMed] [Google Scholar]
- Wojciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J. Cell Sci. 2001;114:1343–1355. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- Xu Y, Guo DF, Davidson M, Inagami T, Carpenter G. Interaction of the adaptor protein Shc and the adhesion molecule cadherin. J. Biol. Chem. 1997;272:13463–13466. doi: 10.1074/jbc.272.21.13463. [DOI] [PubMed] [Google Scholar]
- Yao J, Xiong S, Klos K, Nguyen N, Grijalva R, Li P, et al. Multiple signaling pathways involved in activation of matrix metalloproteinase-9 (MMP-9) by heregulin-beta1 in human breast cancer cells. Oncogene. 2001;20:8066–8074. doi: 10.1038/sj.onc.1204944. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Korfhagen TR, Whitsett JA. Surfactant protein D regulates NF-kappa B and matrix metalloproteinase production in alveolar macrophages via oxidant-sensitive pathways. J. Immunol. 2001;166:7514–7519. doi: 10.4049/jimmunol.166.12.7514. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Meng FY, Huang Q, Hawker J, Wu HM. Tyrosine phosphorylation of paxillin/pp125FAK and microvascular endothelial barrier function. Am. J. Physiol. 1998;275:H84–H93. doi: 10.1152/ajpheart.1998.275.1.H84. [DOI] [PubMed] [Google Scholar]