Abstract
The blood–brain barrier (BBB) is formed by brain endothelial cells lining the cerebral microvasculature, and is an important mechanism for protecting the brain from fluctuations in plasma composition, and from circulating agents such as neurotransmitters and xenobiotics capable of disturbing neural function. The barrier also plays an important role in the homeostatic regulation of the brain microenvironment necessary for the stable and co-ordinated activity of neurones. The BBB phenotype develops under the influence of associated brain cells, especially astrocytic glia, and consists of more complex tight junctions than in other capillary endothelia, and a number of specific transport and enzyme systems which regulate molecular traffic across the endothelial cells. Transporters characteristic of the BBB phenotype include both uptake mechanisms (e.g. GLUT-1 glucose carrier, L1 amino acid transporter) and efflux transporters (e.g. P-glycoprotein). In addition to a role in long-term barrier induction and maintenance, astrocytes and other cells can release chemical factors that modulate endothelial permeability over a time-scale of seconds to minutes. Cell culture models, both primary and cell lines, have been used to investigate aspects of barrier induction and modulation. Conditioned medium taken from growing glial cells can reproduce some of the inductive effects, evidence for involvement of diffusible factors. However, for some features of endothelial differentiation and induction, the extracellular matrix plays an important role. Several candidate molecules have been identified, capable of mimicking aspects of glial-mediated barrier induction of brain endothelium; these include TGFβ, GDNF, bFGF, IL-6 and steroids. In addition, factors secreted by brain endothelial cells including leukaemia inhibitory factor (LIF) have been shown to induce astrocytic differentiation. Thus endothelium and astrocytes are involved in two-way induction. Short-term modulation of brain endothelial permeability has been shown for a number of small chemical mediators produced by astrocytes and other nearby cell types. It is clear that endothelial cells are involved in both long- and short-term chemical communication with neighbouring cells, with the perivascular end feet of astrocytes being of particular importance. The role of barrier induction and modulation in normal physiology and in pathology is discussed.
Keywords: astrocytes, blood–brain barrier, brain endothelium, modulation, permeability
Introduction: anatomy and physiology of the blood–brain barrier
The blood–brain barrier (BBB) is formed by brain endothelial cells lining the cerebral vasculature, and is an important mechanism for protecting the brain from fluctuations in plasma composition, e.g. during exercise and following meals, and from circulating agents such as neurotransmitters and xenobiotics capable of disturbing neural function (Abbott & Romero, 1996). The barrier also plays an important role in the homeostatic regulation of the brain microenvironment necessary for the healthy function of the CNS. The brain capillaries are ∼50–100 times tighter than peripheral microvessels as a result of complex tight junctions (zonulae occludentes) that cause severe restriction of the paracellular (tight junctional) pathway for diffusion of hydrophilic solutes, so that penetration across the brain endothelium is effectively confined to transcellular mechanisms. Small lipophilic molecules such as oxygen, CO2 and ethanol can freely diffuse across the lipid membranes of the endothelium. Small polar solutes needed for brain function are transported by a number of specific carriers (e.g. GLUT-1 for glucose, L-system carrier L1 for large neutral amino acids such as leucine) and specific carriers meditate the efflux from the CNS of potentially toxic metabolites (e.g. glutamate). P-glycoprotein is an energy-dependent efflux carrier with broad specificity that keeps out more hydrophobic molecules; it has been localized to the luminal brain endothelial membrane, and plays a major role in protecting the brain from xenobiotics. The brain endothelium has lower levels of endocytosis/transcytosis than peripheral capillaries, but has specific systems for receptor-mediated and adsorptive endocytosis, that can transfer certain peptides and lipoproteins to the brain. The brain endothelium contains a number of enzyme systems that support the protective and detoxifying roles of the BBB, including enzymes such as monoamine oxidase that ensure that central synaptic function is not adversely affected by circulating neuroactive agents. Thus the term ‘BBB’ covers a number of static and dynamic properties that enable the endothelium to protect and regulate the brain microenvironment (Abbott & Romero, 1996).
Induction of the BBB phenotype: role of astrocytes
Although a number of the properties listed are expressed to some degree in peripheral capillary endothelium, most of them are up-regulated in brain endothelium to such an extent that they can be identified as ‘markers’ of BBB phenotype and function. There is great interest in the mechanism(s) for this up-regulation (Bauer & Bauer, 2000), to understand more completely the normal physiological function of the BBB, and to gain insights into pathological conditions causing loss or breakdown of aspects of BBB, as an important first step in designing effective therapies and treatment strategies.
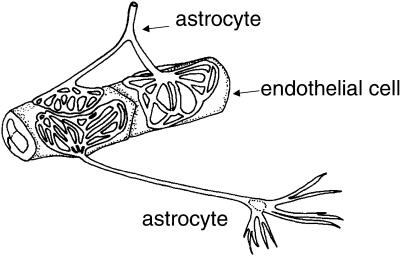
Anatomical examination of the brain microvasculature shows that the endfeet of astrocytic glia form a lacework of fine lamellae closely apposed to the outer surface of the endothelium (Kacem et al. 1998) (Fig. 1). This close anatomical apposition led to the suggestion that inductive influences from astrocytic glia could be responsible for the development of the specialized BBB phenotype of the brain endothelium (Davson & Oldendorf, 1967). Early evidence in support of this hypothesis came from grafting experiments in which brain vessels growing into grafts of peripheral tissue became less tight to intravascular tracers, while the relatively leaky vessels of peripheral tissues became tighter on growing into grafts of brain tissue (reviewed in Bauer & Bauer, 2000). Later studies showed that cultured astrocytes implanted into areas with normally leaky vessels were able to induce tightening of the endothelium, indicating that astrocytes were a major source of the inductive influence from neural tissue (Janzer & Raff, 1997). However, some grafts of embryonic brain into host brain do not show tight endothelia, and destruction of glia and neurones does not always cause barrier breakdown (Krum et al. 1997). These studies suggest that successful barrier induction and maintenance depends critically on the local conditions and maturational state, with important implications for therapeutic grafting for repair of the human brain. The situation in brain tumours is complex. Some glioma cells (e.g. C6) implanted into rat brain become vascularized by leaky vessels (Bauer & Bauer, 2000), suggesting either a deficit in production of inductive factors by the proliferating glioma cells, or enhanced production of ‘permeability factors’ that counteract the inductive effects. However, there is no simple way of predicting BBB permeability from the neuropathological classification of human tumour type.
Fig. 1.
Schematic drawing showing some features of the perivascular astrocytic end feet, which form ‘rosette’-like structures on the brain capillary surface. This arrangement would be expected to be optimal for two-way induction and communication between the astrocytes and endothelium, while not forming a physical barrier, so preserving free diffusion between the endothelium and the brain parenchyma. Based on Kacem et al. (1998).
More recent studies in animal models have confirmed the progressive appearance of the full BBB phenotype in blood vessels growing into the developing CNS. There are some species differences in the time-course of barrier tightening, and the barrier becomes relatively impermeable to large proteins such as albumin and horseradish peroxidase before it can effectively exclude smaller solutes such as mannitol and ions (Butt et al. 1990; Bauer & Bauer, 2000). There is also evidence for gradual maturation of BBB transporters, as seen by differences between the embyronic, neonatal and adult transporter phenotype (Braun et al. 1980).
In vitro cell culture models have provided a great deal of information about the induction of the BBB phenotype in brain endothelium, and have generally confirmed the key inductive role of astrocytes (Reinhart & Gloor, 1997; Bauer & Bauer, 2000). Freshly isolated brain endothelial cells and some immortalized brain endothelial cell lines will grow as a flat monolayer on plastic or on porous filter inserts, and will retain aspects of a BBB phenotype, but generally with some loss of full barrier expression (e.g. leakier tight junctions, down-regulation of enzyme and transport systems) (Krämer et al. 2001). Some of these properties can be up-regulated by co-culture with cells of glial origin, such as primary astrocytes, astrocytic cell lines, or glioma cells. Thus up-regulation of tight junctional proteins and tightness has been clearly demonstrated (Dehouck et al. 1990; Rubin et al. 1991; Rist et al. 1997; Sobue et al. 1999). Gamma-glutamyl transpeptidase (γ-GTP) is highly expressed in the brain endothelium in situ, and appears to play a role in amino acid transport; expression is lower in primary cultured brain endothelium, but is partly restored in brain endothelium co-cultured with glia (El Hafny et al. 1996). Several specific transport systems are up-regulated in brain endothelial models exposed to glial influence; this has been documented for GLUT-1, the L-system and A-system amino acid carriers, and P-glycoprotein (El Hafny et al. 1997; Bauer & Bauer, 2000). Interestingly, the multidrug-resistance-related protein MRP1, which shows low expression in brain endothelium in situ, shows greater expression in cultured brain endothelium. This could indicate that neighbouring glial cells cause suppression of endothelial MRP1 expression under normal conditions (Regina et al. 1998). Glial-mediated suppression of some clotting factors (tissue plasminogen activator and anticoagulant thrombomodulin) in brain endothelium has also been shown (Tran et al. 1999). Expression of transferrin receptor, and transcytotic mechanisms for low-density lipoprotein (LDL) are also up-regulated by astrocytic influence (Dehouck et al. 1994).
Where tested on other model systems, including endothelial cells from non-brain sources such as human umbilical vein and bovine aorta, and the ECV304 cell line used as a model with a strong endothelial phenotype, induction such as junctional tightening and up-regulation of BBB markers can be seen (Hurst & Fritz, 1996; Dolman et al. 1998; Kuchler-Bopp et al. 1999). This suggests that the ability to respond to the glial inductive signals is not confined to brain endothelial cells.
Nature of inductive signals from glia to endothelium
The nature of the glial influence responsible for induction of BBB features has been a subject of debate and experimentation. Some but not all of the inductive effects reported above can be produced by application of conditioned medium taken from growing glial cells, evidence for action of a soluble factor or factors (Hurst & Fritz, 1996; Sobue et al. 1999). However, induction is generally more effective in co-cultures where glial cells are grown on the undersurface of porous filters, with endothelium on the upper surface, especially when the filter pore-size is large enough to allow glial processes to contact the basal surface of the endothelium or its basal lamina. This suggests that induction depends on the correct apical/basal polarity between endothelium and glia, and either direct glial–endothelial contact, extracellular matrix-associated chemical signalling, or a high local concentration of (labile) glial-secreted signal acting on the abluminal endothelial membrane.
The chemical nature of the glial-produced inductive signal(s) is currently unclear, although several candidate molecules have been identified, with evidence suggesting that different agents may regulate different aspects of the BBB phenotype. Thus TGF-β (Tran et al. 1999), GDNF (Igarashi et al. 1999; Utsumi et al. 2000), bFGF (Sobue et al. 1999), IL-6 and hydrocortisone (Hoheisel et al. 1998) can each mimic particular aspects of the inductive up-regulation or suppression caused by glial cells. Attempts to isolate the inductive influence(s) from glial conditioned medium have been only partially successful. Early studies found induction was associated with large-molecular-weight peptides/proteins, while recent work using ECV304 cells as assay system found that the inductive influence from C6 glioma behaved like a non-proteinaceous agent of < 1 kDa molecular weight (Ramsohoye & Fritz, 1998). Many of the implicated chemicals have potential as differentiating agents, suggesting that the BBB phenotype represents an enhanced state of differentiation, which can be triggered and maintained by a number of influences, some of them derived from glia. This view is supported by the observation that the differentiating effects of intraluminal flow, raised intracellular cAMP or application of retinoic acid are more effective in inducing the BBB phenotype in endothelial cells co-cultured with glia (Rubin et al. 1991; El Hafny et al. 1996; Pekny et al. 1998).
In some places in the nervous system, a blood–tissue barrier is present in the endothelium in the absence of contact by astrocytes. Thus the microvessels on the pial surface lacking astrocytic ensheathment show at least some BBB features, likely to be due to soluble factors acting from the glia limitans or the subarachnoid CSF (Allt & Lawrenson, 1997). In peripheral nerves, there is a moderately well-developed blood–nerve barrier at the level of the endoneurial capillary endothelium. Since peripheral nerves lack astrocytes but contain Schwann cells, it is possible that Schwann cells have equivalent inductive potential in this case (Allt & Lawrenson, 2000). Although BBB induction by mixed neuronal/glial populations (as in brain slices) and by isolated neuronal membrane fractions has been observed (Tontsch & Bauer, 1991), most studies indicate that the predominant influence maintaining the mature BBB is astrocytic.
Inductive influence of brain endothelium on astrocytes
Given the complexity of BBB induction by glial cells, it is clear that close communication between endothelium and glial cells must occur. It is therefore not surprising to find evidence that the endothelial cells have a reciprocal inductive influence on astrocytes (Estrada et al. 1990; Sperri et al. 1997; Wagner & Gardner, 2000). Thus square (orthogonal) arrays of particles on astrocytic end feet recently identified as sites of aquaporin-4 localization (Rash et al. 1998) are up-regulated in co-culture with endothelium. The observed up-regulation of γ-GTP in endothelial cells by glia involves a two-way exchange of signals and an extracellular matrix-mediated effect, while the up-regulation of alkaline phosphatase in the same system involves only a diffusive signal (Mizuguchi et al. 1997). When endothelial and glial cells are grown together there is a mutual up-regulation of antioxidant enzymes so that the endothelial and glial partnership is better able to deal with oxidative stress, e.g. in hypoxia/reperfusion injury (Schroeter et al. 1999). Co-culturing also leads to reciprocal effects on receptor phenotype. Recently, leukaemia inhibitory factor (LIF) released by endothelial cells of the optic nerve has been shown to induce astrocytic differentiation (Mi et al. 2001). Thus maintenance of the adult BBB appears to depend on continuing exchange of inductive signals between glia and endothelium, and disturbance of this induction may be instrumental in several neuropathologies involving BBB dysfunction, such as tumours and multiple sclerosis.
Short–term interaction between glia and endothelium
In addition to the long-term processes involved in induction via altered gene expression, glial–endothelial interactions also occur over a shorter time-scale (seconds to minutes), involving receptor-mediated signalling. So far this has been most clearly documented by monitoring intracellular calcium waves, with evidence for a role for ATP as a glial-endothelial signalling molecule (Paemeleire et al. 1999; Paemeleire & Leybaert, 2000). Such studies have given support to the idea that astrocytes may play a key role in modulating the energy supply to neurones, including the possibility of regulating endothelial transport in a way that supports neuronal function (Magistretti et al. 1999)
Humoral modulation of brain endothelial permeability
A number of chemical agents have been shown to modulate the permeability of the blood–brain barrier (Table 1) (for reviews see Abbott & Revest, 1991; Abbott, 1998, 2000), and at least some of these may be released by astrocytic glial cells (asterisked in Table 1). The list of agents includes several families of inflammatory mediators, consistent with studies showing increased permeability of the BBB in CNS inflammation (Abbott, 2000). Where the increase in permeability is transient and does not involve cell death, opening of the paracellular (tight junctional) pathway has generally been found to be responsible. Attention has thus focused on the cellular mechanisms controlling the tight junction and the associated cytoskeleton.
Table 1.
Humoral agents reported to increase blood-brain barrier permeability. Those asterisked have been shown to be released by astrocytic glia
| Bradykinin, serotonin (5HT), histamine, thrombin |
| Purine and pyrimidine nucleotides: ATP*, UTP, ADP, AMP |
| Endothelin-1 (ET-1)* |
| Substance P |
| Glutamate*, Quinolenic acid |
| Platelet activating factor (PAF) |
| Arachidonic acid, prostaglandins, leukotrienes |
| Cytokines: IL-1α, IL-1β*, IL-2, IL-6*, TNFα* |
| Macrophage inflammatory proteins: MIP-1, MIP-2* |
| Complement-derived polypeptide C3a-desArg |
| Nitric oxide*, free radicals |
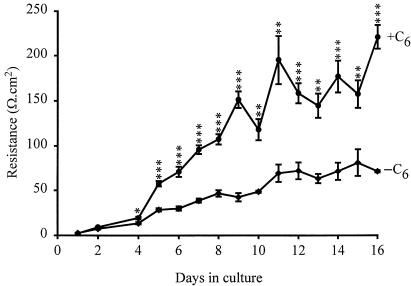
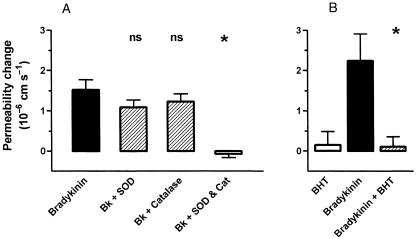
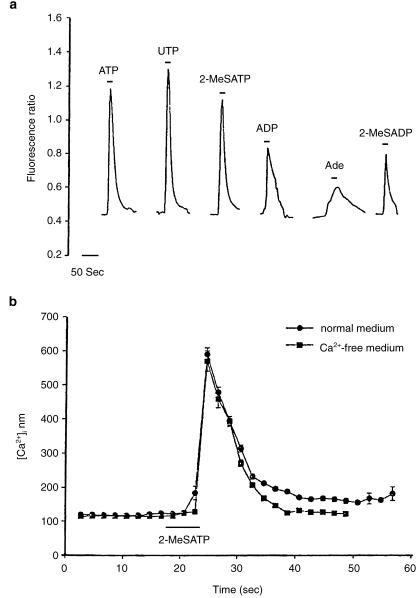
Several of the molecules causing barrier opening (Table 1) have been shown to act via receptor-mediated processes, activating signal transduction pathways within the endothelium. Many of these involve elevation of intracellular calcium concentration ([Ca2+]i), leading to the suggestion that raised [Ca2+]i is a common factor in tight junction opening (Olesen, 1989; Abbott & Revest, 1991; Abbott, 1998). Although relevant calcium-dependent changes in endothelial cells have been reported (e.g. Ca2+-calmodulin-dependent phosphorylation of cytoskeletal proteins, Joó, 1993) the details of the molecular cascade are not clear. It is also not known whether all agents act through a final common path, or whether different agonists can act through different elements of cytoskeletal control. Examination both in situ and in vitro is beginning to reveal the details of the signal transduction pathways involving particular receptors and their interactions, for example for histamine (Easton et al. 1997; Sarker et al. 1998), bradykinin and nucleotides. The ECV304/C6 co-culture model gives a sufficiently tight monolayer with brain endothelial phenotype for studies of both intracellular signals and tight junction modulation (Easton & Abbott, 1997) (Fig. 2); modulation by bradykinin appears to involve B2 receptors, elevation of [Ca2+]i and activation of phospholipase A2 (Abbott, 2000). In pial microvessels studied in situ, Sarker et al. (2000) showed that B2 receptor activation resulted in a permeability increase involving generation of free radicals (Fig. 3). Activation of cultured brain endothelium by ATP and related nucleotides caused elevation of [Ca2+]i predominantly via P2U (= P2Y2) receptors (Nobles et al. 1995). Interestingly, P2Y1 receptor activation could also be detected in cells grown on a biological matrix (Sipos et al. 2000) (Fig. 4), evidence that the endothelial receptor phenotype was influenced by its local environment.
Fig. 2.
Development of tightness of the BBB model formed by culture of ECV304 cells, which show a robust endothelial phenotype. When grown without C6 glioma cells (–C6), the transendothelial electrical resistance (TEER, a measure of tight junctional restriction of the paracellular pathway) develops slowly over 15 days. TEER is enhanced when the cells are co-cultured, grown on filters with C6 glioma cells in the base of the wells (+C6). The model has been used to examine the mechanisms by which bradykinin modulates [Ca2+]i and TEER. From Easton & Abbott (1997).
Fig. 3.
Evidence for a role of free radicals in bradykinin-mediated increase in permeability of rat pial microvessels in situ, measured with a fluorescence technique. A, the free radical scavengers superoxide dismutase (SOD) and catalase (CAT) (each 100 U mL−1) applied separately to the brain surface had little effect on the permeability response to 5 μM bradykinin, but completely blocked it when used in combination. B, the lipid peroxidation chain blocker butylated-hydroxytoluene (BHT; 1 mM) also inhibited the permeability response to 5 μM bradykinin. (n.s., not significant; **P < 0.01). From Sarker et al. (2000), by permission.
Fig. 4.
Evidence for nucleotide receptors coupled to elevation of [Ca2+]i on primary cultured rat brain endothelial cells grown on a biological matrix. (a) Calcium responses measured with fura2 (fluorescence ratio) were elicited by 100 μM ATP and several related agonists; evidence for presence of different receptors includes the observed response to UTP (P2Y2) and to 2-MeSATP/ 2-MeSADP/ADP (P2Y1). (b) The response to 2-MeSATP was similar in calcium-free and normal medium, evidence for mediation by a metabotropic rather than ionotropic receptor. From Sipos et al. (2000), by permission.
Both in situ and in vitro, the receptor-mediated barrier opening to low doses of agonists can be short-lived, reversing on withdrawal of the agent or even before (Butt, 1995; Easton & Abbott, 1997). This raises the interesting possibility that barrier opening can occur as a well-regulated process under normal physiological conditions, in response to agents released locally. A number of fine nerve terminals end close to the capillary endothelium, capable of releasing histamine, 5HT, substance P and other specific neurotransmitters. Given the role of the BBB in protecting the brain from noxious agents and maintaining CNS homeostasis, such transient barrier opening would generally be expected to be deleterious. However, there may be situations in which modest and reversible barrier opening could bring physiological advantage. Thus the plasma is a rich source of factors required for the normal repair processes of the brain, including growth factors supporting neurite sprouting and outgrowth in regions of neuronal damage and death. In addition, transient barrier opening could be a good way to maintain immunological surveillance of the CNS, and for neurones to ‘sample’ plasma composition as part of the brain's key function in the normal regulatory control of the body.
Quite small stresses to the CNS such as exposure of the meninges and minor head injury have been shown to cause measurable and transient increases in the permeability of the BBB (Easton et al. 1997; Ingebrigtsen et al. 1999). Where these sites have been examined morphologically, they typically appear as focal leaks affecting only a proportion of microvessels in any anatomical region, and only a proportion of junctions in individual microvessel segments. Since we lack non-invasive ways of pin-pointing small leaks in the BBB, it is possible that cyclical opening and closing of the barrier occurs in a small percentage of brain microvessels under normal physiological conditions. Such cyclical activity would have a negligible effect on the general homeostasis of the brain microenvironment, but could be sufficient to satisfy the local requirements that triggered the barrier opening. This idea prompts a closer examination of the cellular mechanisms that could underlie controlled BBB modulation.
Source of BBB permeability modulating agents
Since the mechanisms controlling the tight junctions of the brain endothelium are the ‘effectors’ of BBB permeability modulation, and the chemical agents listed in Table 1 are the substances capable of exerting the modulation, the possible sources of these molecules are of special interest. In some cases, the endothelium is both able to release the agent and respond to it, e.g. endothelin (ET-1), acting on ETA receptors (Chen et al. 2000), and ATP acting on nucleotide receptors (Sipos et al. 2000). Under pathological conditions, mast cells and perivascular microglia (resident macrophages of the CNS) may release inflammatory agents close to the endothelium. The cell types capable of mediating physiological responses include the fine nerve terminals of a number of neuronal populations which run close to microvessels and release agents able to influence endothelial function, such as histamine, substance P and glutamate. As indicated above, astrocytes are able to release several humoral agents including glutamate, aspartate, taurine, ATP, ET-1, NO, TNFα, MIP2 and IL-1β (Chen et al. 2000; Ostrow et al. 2000; Wang et al. 2000; Zhang et al. 2000; Kim & Shin, 2001), although the regulation of this release is not well understood. Furthermore, in response to some of the agents able to open the BBB, astrocytes can up-regulate and release modulating factors, e.g. bradykinin causes up-regulation of astrocytic expression and release of the cytokine interleukin-6 (IL-6) (Schwaninger et al. 1999), which has a potentiating action on bradykinin-mediated BBB opening. Thus in addition to a role in barrier induction and maintenance, astrocytes may play active roles in modulating BBB permeability over shorter time scales. The presence of such potentiating mechanisms also means that agents present at concentrations too low to open the barrier alone are able to exert an effect in the presence of low concentrations of the potentiating agents.
Conclusions
In summary, the development and maintenance of the BBB formed by brain endothelium, and the specializations of the perivascular astrocytes that enable them to act in partnership with the endothelium, involve complex cell–cell exchange of chemical signals, inducing BBB features in the longer term, and modulating cellular physiology in the shorter term. Investigation of this mutual interaction is important for understanding the biological basis of neuropathies in which BBB dysfunction occurs, and in development of effective therapeutic strategies.
Acknowledgments
Supported by The Wellcome Trust, MRC, and the KCL Blood–Brain Barrier Consortium with Industry (Lilly, Astra Zeneca, Aventis, Mindset and GlaxoSmithKline).
References
- Abbott NJ, Revest PA. Control of brain endothelial permeability. Cerebrovasc. Brain Metab. Rev. 1991;3:39–72. [PubMed] [Google Scholar]
- Abbott NJ, Romero IA. Transporting therapeutics across the blood–brain barrier. Mol. Med. Today. 1996;2:106–113. doi: 10.1016/1357-4310(96)88720-x. [DOI] [PubMed] [Google Scholar]
- Abbott NJ. Role of intracellular calcium in regulation of brain endothelial permeability. In: Pardridge WM, editor. Introduction to the Blood–Brain Barrier: Methodology and Biology. Cambridge, UK: Cambridge University Press; 1998. pp. 345–353. [Google Scholar]
- Abbott NJ. Inflammatory mediators and modulation of blood–brain barrier permeability. Cellular Mol. Neurobiol. 2000;20:131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allt G, Lawrenson JG. Is the pial microvessel a good model for blood–brain barrier studies? Brain Res. Rev. 1997;24:67–76. doi: 10.1016/s0165-0173(97)00011-8. [DOI] [PubMed] [Google Scholar]
- Allt G, Lawrenson JG. The blood–nerve barrier: enzymes, transporters and receptors – a comparison with the blood–brain barrier. Brain Res. Bull. 2000;52:1–12. doi: 10.1016/s0361-9230(00)00230-6. [DOI] [PubMed] [Google Scholar]
- Annunziata P, Cioni C, Toneatto S, Paccagnini E. HIV-1 gp120 increases the permeability of rat brain endothelium cultures by a mechanism involving substance P. AIDS. 1998;12:2377–2388. doi: 10.1097/00002030-199818000-00006. [DOI] [PubMed] [Google Scholar]
- Banks WA. Characterization of interleukin-1α binding to mouse brain endothelial cells. J. Pharmacol. Exp. Therapeutics. 1999;291:665–670. [PubMed] [Google Scholar]
- Bauer HC, Bauer H. Neural induction of the blood–brain barrier: still an enigma. Cellular Mol. Neurobiol. 2000;20:13–28. doi: 10.1023/A:1006939825857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun LD, Cornford EM, Oldendorf WH. Newborn rabbit blood–brain barrier is selectively permeable and differs substantially from the adult. J. Neurochem. 1980;34:147–152. doi: 10.1111/j.1471-4159.1980.tb04633.x. [DOI] [PubMed] [Google Scholar]
- Butt AM. Effect of inflammatory agents on electrical resistance across the blood–brain barrier in pial microvessels of anaesthetized rats. Brain Res. 1995;696:145–150. doi: 10.1016/0006-8993(95)00811-4. [DOI] [PubMed] [Google Scholar]
- Butt AM, Jones HC, Abbott NJ. Electrical resistance across the blood–brain barrier in anaesthetized rats: a developmental study. J. Physiol. 1990;429:47–62. doi: 10.1113/jphysiol.1990.sp018243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Matsumura K, Shirakawa N, Maeda M, Jikihara I, Kobayashi S, et al. Pyrogenic cytokines injected into the rat cerebral ventricle induce cyclooxygenase-2 in brain endothelial cells and also upregulate their receptors. Eur. J. Neurosci. 2001;13:1781–1790. doi: 10.1046/j.0953-816x.2001.01551.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, McCarron RM, Azzam N, Bembry J, Reutzler C, Lenz FA, et al. Endothelin-1 and nitric oxide affect human cerebromicrovascular endothelial responses and signal transduction. Acta Neurochirurgica Suppl. 2000;76:131–135. doi: 10.1007/978-3-7091-6346-7_27. [DOI] [PubMed] [Google Scholar]
- Davson H, Oldendorf WH. Transport in the central nervous system. Proc. Royal Soc. Med. 1967;60:326–328. doi: 10.1177/003591576706000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehouck B, Dehouck M-P, Fruchart J-C, Cecchelli R. Upregulation of the low density lipoprotein receptor at the blood–brain barrier: intercommunications between brain capillary endothelial cells and astrocytes. J. Cell Biol. 1994;126:465–473. doi: 10.1083/jcb.126.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehouck M-P, Meresse S, Delorme P, Fruchart J-C, Cecchelli R. An easier, reproducible, and mass-production method to study the blood–brain barrier in vitro. J. Neurochem. 1990;54:1798–1801. doi: 10.1111/j.1471-4159.1990.tb01236.x. [DOI] [PubMed] [Google Scholar]
- Dolman DEM, Anderson P, Rollinson C, Abbott NJ. Characterisation of a new in vitro model of the blood–brain barrier (BBB) J. Physiol. 1998;505P:56–57P. [Google Scholar]
- Easton AS, Abbott NJ. The effects of bradykinin on a cell culture model of the blood–brain barrier. J. Physiol. 1997;505:49–50P. [Google Scholar]
- Easton AS, Sarker MH, Fraser PA. Two components of blood–brain barrier disruption in the rat. J. Physiol. 1997;503:613–623. doi: 10.1111/j.1469-7793.1997.613bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Hafny B, Bourre J-M, Roux F. Synergistic stimulation of gamma-glutamyl transpeptidase and alkaline phosphatase activities by retinoic acid and astroglial factors in immortalized rat brain microvessel endothelial cells. J. Cellular Physiol. 1996;167:451–460. doi: 10.1002/(SICI)1097-4652(199606)167:3<451::AID-JCP9>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- El Hafny B, Chappey O, Piciotti M, Debray M, Boval B, Roux F. Modulation of P-glycoprotein activity by glial factors and retinoic acid in an immortalized rat brain microvessel endothelial cell line. Neurosci. Lett. 1997;236:107–111. doi: 10.1016/s0304-3940(97)00679-4. [DOI] [PubMed] [Google Scholar]
- Estrada C, Bready JV, Berliner JA, Pardridge WM, Cancilla PA. Astrocyte growth stimulation by a soluble factor produced by cerebral endothelial cells in vitro. J. Neuropathol. Exp. Neurol. 1990;49:539–549. doi: 10.1097/00005072-199011000-00001. [DOI] [PubMed] [Google Scholar]
- Hoheisel D, Nitz T, Franke H, Wagner J, Hakvoort A, Tilling T, et al. Hydrocortisone reinforces the blood–brain barrier properties in a serum free cell culture system. Biochem. Biophys. Res. Comm. 1998;247:312–315. doi: 10.1006/bbrc.1997.8051. [DOI] [PubMed] [Google Scholar]
- Hurst RD, Fritz IB. Properties of an immortalised vascular endothelial/glioma cell co-culture model of the blood–brain barrier. J. Cellular Physiol. 1996;167:81–88. doi: 10.1002/(SICI)1097-4652(199604)167:1<81::AID-JCP9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Igarashi Y, Utsumi H, Chiba H, Yamada-Sasamori Y, Tobioka H, Kamimura Y, et al. Glial cell line-derived neurotrophic factor induces barrier function of endothelial cells forming the blood–brain barrier. Biochem. Biophys. Res. Comm. 1999;261:108–112. doi: 10.1006/bbrc.1999.0992. [DOI] [PubMed] [Google Scholar]
- Ingebrigtsen T, Waterloo K, Jacobsen EA, Langbakk B, Romner B. Traumatic brain damage in minor head injury: relation of serum S-100 protein measurements to magnetic resonance imaging and neurobehavioural outcome. Neurosurgery. 1999;45:468–475. doi: 10.1097/00006123-199909000-00010. [DOI] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood–brain barrier properties in endothelial cells. Nature. 1997;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Joó F. Brain microvascular cyclic nucleotides and protein phosphorylation. In: Pardridge WM, editor. The Blood–Brain Barrier: Cellular and Molecular Biology. New York: Raven Press; 1993. pp. 267–287. [Google Scholar]
- Kacem K, Lacombe P, Seylaz J, Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia. 1998;23:1–10. [PubMed] [Google Scholar]
- Kim HM, Shin TY. Inhibitory effect of tumor necrosis factor-alpha secretion from rat astrocytes by Chilbokeum. Immunopharmacol. Immunotoxicol. 2001;23:97–106. doi: 10.1081/iph-100102571. [DOI] [PubMed] [Google Scholar]
- Krämer SD, Abbott NJ, Begley DJ. Biological models to study blood–brain barrier permeation. In: Testa B, van de Waterbeemd H, Folkers G, Guy R, editors. Pharmacokinetic Optimization in Drug Research: Biological, Physicochemical and Computational Strategies. Weinheim: Wiley-VCH; 2001. pp. 127–153. [Google Scholar]
- Krum JM, Kenyon KL, Rosenstein JM. Expression of blood–brain barrier characteristics following neuronal loss and astroglial damage after administration of anti-Thy-1 immunotoxin. Exp. Neurol. 1997;146:33–45. doi: 10.1006/exnr.1997.6528. [DOI] [PubMed] [Google Scholar]
- Kuchler-Bopp S, Delanoy JP, Artault JC, Zaepfel M, Dietrich JR. Astrocytes induce several blood–brain barrier properties in non-neural endothelial cells. Neuroreport. 1999;10:1347–1353. doi: 10.1097/00001756-199904260-00035. [DOI] [PubMed] [Google Scholar]
- Kustova Y, Grinberg A, Basile AS. Increased blood–brain barrier permeability to LP-BM5 infected mice is mediated by neuroexcitatory mechanisms. Brain Res. 1999;839:153–163. doi: 10.1016/s0006-8993(99)01734-5. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L, Rothman DL, Shulman RG. Energy on demand. Science. 1999;283:496–497. doi: 10.1126/science.283.5401.496. [DOI] [PubMed] [Google Scholar]
- Makic JB, Stins M, Jovanovic S, Kim KS, Bartus RT, Zlokovic BV. Cereport (RMP-7) increases the permeability of human brain microvascular endothelial cell monolayers. Pharmaceut. Res. 1999;16:1360–1365. doi: 10.1023/a:1018938722768. [DOI] [PubMed] [Google Scholar]
- Matsuo Y, Mihara S-I, Ninomiya M, Fujimoto M. Protective effect of endothelin type A receptor antagonist on brain edema and injury after transient middle cerebral artery occlusion in rats. Stroke. 2001;32:2143–2148. doi: 10.1161/hs0901.94259. [DOI] [PubMed] [Google Scholar]
- Mi H, Haeberle H, Barres BA. Induction of astrocyte differentiation by endothelial cells. J. Neurosci. 2001;21:1538–1547. doi: 10.1523/JNEUROSCI.21-05-01538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi H, Utoguchi N, Mayumi T. Preparation of glial extracellular matrix: a novel method to analyze glial–endothelial interaction. Brain Res. Protocols. 1997;1:339–343. doi: 10.1016/s1385-299x(97)00008-1. [DOI] [PubMed] [Google Scholar]
- Narushima I, Kita T, Kubo K, Yonetani Y, Momochi C, Yoshikawa I, et al. Contribution of endothelin-1 to disruption of blood–brain barrier permeability in dogs. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;360:639–645. doi: 10.1007/s002109900137. [DOI] [PubMed] [Google Scholar]
- Nobles M, Revest PA, Couraud P-O, Abbott NJ. Characteristics of nucleotide receptors that cause elevation of cytoplasmic calcium in immortalized rat brain endothelial cells and primary cultures. Br. J. Pharmacol. 1995;115:1245–1252. doi: 10.1111/j.1476-5381.1995.tb15032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen S-P. An electrophysiological study of microvascular permeability and its modulation by chemical mediators. Acta Physiol. Scand. 1989;136(Suppl. 579):1–28. [PubMed] [Google Scholar]
- Ostrow LW, Langan TJ, Sachs F. Stretch-induced endothelin-1 production by astrocytes. J. Cardiovasc. Pharmacol. 2000;36(Suppl. 1):S274–S277. doi: 10.1097/00005344-200036051-00081. [DOI] [PubMed] [Google Scholar]
- Paemeleire K, de Hemptinne A, Leybaert L. Chemically, mechanically, and hyperosmolarity-induced calcium responses of rat cortical capillary endothelial cells in culture. Exp. Brain Res. 1999;126:473–481. doi: 10.1007/s002210050755. [DOI] [PubMed] [Google Scholar]
- Paemeleire K, Leybaert L. ATP-dependent astrocyte-endothelial calcium signalling following mechanical damage to a single astrocyte in astrocyte-endothelial co-cultures. J. Neurotrauma. 2000;17:345–358. doi: 10.1089/neu.2000.17.345. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin WJ, Gera L, Stewart JM. Bradykinin antagonist decreases early disruption of the blood–spinal cord barrier after spinal cord injury in mice. Neurosci. Lett. 2001;307:25–28. doi: 10.1016/s0304-3940(01)01904-8. [DOI] [PubMed] [Google Scholar]
- Pekny M, Stanness KA, Eliasson C, Betsholtz C, Janigro D. Impaired induction of blood–brain barrier properties in aortic endothelial cells by astrocytes from GFAP-deficient mice. Glia. 1998;22:390–400. [PubMed] [Google Scholar]
- Prat A, Biernacki K, Pouly S, Nalbantoglu J, Couture R, Antel JP. Kinin B1 receptor expression and function in human brain endothelial cells. J. Neuropathol. Exp. Neurol. 2000;59:896–906. doi: 10.1093/jnen/59.10.896. [DOI] [PubMed] [Google Scholar]
- Ramsohoye PV, Fritz IB. Preliminary characterization of glial-secreted factors responsible for the induction of high electrical resistances across endothelial monolayers in a blood–brain barrier model. Neurochem. Res. 1998;23:1545–1551. doi: 10.1023/a:1020932121378. [DOI] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Hudson CS, Agre P, Nielsen S. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membrane in rat brain and spinal cord. Proc. Natl Acad. Sci. USA. 1998;95:11981–11986. doi: 10.1073/pnas.95.20.11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regina A, Koman A, Piciotti M, El Hafny B, Center MS, Bergmann R, et al. Mrp1 multidrug resistance-associated protein and P-glycoprotein expression in rat brain microvessel endothelial cells. J. Neurochem. 1998;71:705–715. doi: 10.1046/j.1471-4159.1998.71020705.x. [DOI] [PubMed] [Google Scholar]
- Reinhart CA, Gloor SM. Co-culture blood–brain barrier models and their use for pharmatoxicological screening. Toxicol. Vitro. 1997;11:513–518. doi: 10.1016/s0887-2333(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Rist RJ, Romero IA, Chan MW, Couraud PO, Roux F, Abbott NJ. F-actin cytoskeleton and sucrose permeability of immortalised rat brain microvascular endothelial cell monolayers: effects of cyclic AMP and astrocytic factors. Brain Res. 1997;768:10–18. doi: 10.1016/s0006-8993(97)00586-6. [DOI] [PubMed] [Google Scholar]
- Rubin LL, Hall DE, Porter S, Barbu K, Cannon C, Horner HC, et al. A cell culture model of the blood–brain barrier. J. Cell Biol. 1991;115:1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker MH, Easton AS, Fraser PA. Regulation of cerebral microvascular permeability by histamine in the anaesthetized rat. J. Physiol. 1998;528:177–187. doi: 10.1111/j.1469-7793.1998.909bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker MH, Hu DE, Fraser PA. Acute effects of bradykinin on cerebral microvascular permeability in the anaesthetized rat. J. Physiol. 2000;528:177–187. doi: 10.1111/j.1469-7793.2000.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter ML, Mertsch K, Giese H, Muller S, Sporbert A, Hickel B, et al. Astrocytes enhance radical defence in capillary endothelial cells constituting the blood–brain barrier. FEBS Lett. 1999;449:241–244. doi: 10.1016/s0014-5793(99)00451-2. [DOI] [PubMed] [Google Scholar]
- Schulz J, Plesnila N, Eriskat J, Stoffel M, Pruneau D, Baethmann A. LIF16-0687 a novel non-peptide bradykinin B2 receptor antagonist reduces vasogenic brain edema from a focal lesion in rats. Acta Neurochirurgica Suppl. 2000;76:137–139. doi: 10.1007/978-3-7091-6346-7_28. [DOI] [PubMed] [Google Scholar]
- Schwaninger M, Sallmann S, Petersen N, Schneider A, Prinz S, Libermann TA, et al. Bradykinin induces interleukin-6 expression in astrocytes through activation of nuclear factor-κB. J. Neurochem. 1999;73:1461–1466. doi: 10.1046/j.1471-4159.1999.0731461.x. [DOI] [PubMed] [Google Scholar]
- Sipos I, Dömötör E, Abbott NJ, Adam-Vizi V. The pharmacology of nucleotide receptors on primary rat brain endothelial cells grown on a biological extracellular matrix: effects on intracellular calcium concentration. Br. J. Pharmacol. 2000;131:1195–1203. doi: 10.1038/sj.bjp.0703675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue K, Yamamoto N, Yoneda K, Hodgson ME, Yamashiro K, Tsuruoka N, et al. Induction of blood–brain barrier properties in immortalized bovine brain endothelial cells by astrocytic factors. Neurosci. Res. 1999;35:155–164. doi: 10.1016/s0168-0102(99)00079-6. [DOI] [PubMed] [Google Scholar]
- Sperri PE, Grant MB, Gomez J, Vernadakis A. Endothelial cell conditioned media mediated regulation of glutamine synthase activity in glial cells. Dev. Brain Res. 1997;104:205–208. doi: 10.1016/s0165-3806(97)00173-9. [DOI] [PubMed] [Google Scholar]
- St'astny F, Skultetyova I, Pliss L, Jezova D. Quinolinic acid enhances permeability of rat brain microvessels to plasma albumin. Brain Res. Bull. 2000;53:415–420. doi: 10.1016/s0361-9230(00)00368-3. [DOI] [PubMed] [Google Scholar]
- Tontsch U, Bauer H-C. Glial cells and neurons induce blood–brain barrier related enzymes in cultured cerebral endothelial cells. Brain Res. 1991;539:247–253. doi: 10.1016/0006-8993(91)91628-e. [DOI] [PubMed] [Google Scholar]
- Tran ND, Correale J, Schreiber SS, Fisher M. Transforming growth factor-beta mediates astrocyte-specific regulation of brain endothelial anticoagulant factors. Stroke. 1999;30:1671–1678. doi: 10.1161/01.str.30.8.1671. [DOI] [PubMed] [Google Scholar]
- Utsumi H, Chiba H, Kamimura Y, Osanai M, Igarashi Y, Tobioka H, et al. Expression of GFRα-1, receptor for GDNF, in rat brain capillary during postnatal development of the BBB. Am. J. Physiol., Cell Physiol. 2000;279:C361–C368. doi: 10.1152/ajpcell.2000.279.2.C361. [DOI] [PubMed] [Google Scholar]
- Wagner S, Gardner H. Modes of regulation of laminin-5 production by rat astrocytes. Neurosci. Lett. 2000;284:105–108. doi: 10.1016/s0304-3940(00)00987-3. [DOI] [PubMed] [Google Scholar]
- Wang JY, Shum AY, Chao CC, Kuo JS, Wang JY. Production of macrophage inflammatory protein-2 following hypoxia/reoxygenation in glial cells. Glia. 2000;32:155–164. [PubMed] [Google Scholar]
- Zhang W, Smith C, Howlett C, Stanimirovic D. Inflammatory activation of human brain endothelial cells by hypoxic astrocytes in vitro is mediated by IL-1beta. J. Cerebral Blood Flow Metabolism. 2000;20:967–978. doi: 10.1097/00004647-200006000-00009. [DOI] [PubMed] [Google Scholar]