Abstract
The endothelium dynamically regulates the extravasation of hormones, macromolecules and other solutes. In pathological conditions, endothelial hyperpermeability can be induced by vasoactive agents, which induce tiny leakage sites between the cells, and by cytokines, in particular vascular endothelial growth factor, which increase the exchange of plasma proteins by vesicles and intracellular pores. It is generally believed that the interaction of actin and non-muscle myosin in the periphery of the endothelial cell, and the destabilization of endothelial junctions, are required for endothelial hyperpermeability induced by vasoactive agents. Transient short-term hyperpermeability induced by histamine involves Ca2+/calmodulin-dependent activation of the myosin light chain (MLC) kinase. Prolonged elevated permeability induced by thrombin in addition involves activation of the small GTPase RhoA and Rho kinase, which inhibits dephosphorylation of MLC. It also involves the action of other protein kinases. Several mechanisms can increase endothelial barrier function, depending on the tissue affected and the cause of hyperpermeability. They include blockage of specific receptors, and elevation of cyclic AMP by agents such as β2-adrenergic agents. Depending on the vascular bed, nitric oxide and cyclic GMP can counteract or aggravate endothelial hyperpermeability. Finally, inhibitors of RhoA activation and Rho kinase represent a potentially valuable group of agents with endothelial hyperpermeability-reducing properties.
Keywords: endothelial barrier function, intracellular signalling
Introduction
Circulating blood provides oxygen, nutrients and hormones to the tissues and removes carbon dioxide and waste products. The exchange of these factors is regulated by the endothelium, which forms the interface between blood and tissues. The endothelium controls the extravasation of solutes, macromolecules and white blood cells, and maintains a continued flow of blood by preventing loss of blood constituents and volume. With the exception of a few tissues, in which the endothelium has pores, the endothelium forms a closed barrier for macromolecules. Exchange of these macromolecules in a healthy continuous endothelium is thought to occur largely by receptors or transporters or via vesicular shuttling (Predescu & Palade, 1993; see also J. A. Firth's contribution in this issue of the Journal of Anatomy). However, in many pathological conditions, associated in particular with inflammation and angiogenesis, the endothelium becomes locally hyperpermeable for macromolecules during a short or prolonged period of time by the formation of small gaps between or through the cells. The accompanying leakage provides enhanced delivery of plasma proteins, including complement factors, immunoglobulins and coagulation factors, to the interstitium. These proteins are involved in counteracting infectious agents or assisting tissue repair and angiogenesis, e.g. by providing a fibrin/vitronectin-matrix.
Endothelial hyperpermeability can be encountered in various forms: transient leakage of post-capillary venules upon exposure to vasoactive agents (Michel & Curry, 1999); prolonged leakage by vasoactive agents, such as thrombin (Malik & Horgan, 1987); prolonged leakage accompanied by leucocyte extravasation (Rampart & Williams, 1986; Shanley et al. 1995; Malik & Lo, 1996) or injury (Cuénoud et al. 1987); leakage of the endothelium at sites of angiogenesis (Joris et al. 1990; Dvorak et al. 1995; Hashizume et al. 2000); and focal leakage in conduit vessels because of single defective junctions or cell division (Chuang et al. 1990; Huang & Chien, 1992). When a healthy post-capillary venule is exposed to a vasoactive agent, small pericellular gaps are transiently formed. It is generally thought that an increase in contractile forces at the cell margins and a reduction of the tethering forces between adjacent endothelial cells cause these gaps (Fig. 1). The minute gaps formed between the endothelial cells allow the extravasation of macromolecules. Similarly, during the extravasation of leucocytes small gaps may occur either by the extravasation of the leucocyte itself or by the local delivery of vasoactive agents by the activated white blood cell. Alternatively, it has been suggested that vasoactive agents may form intracellular gaps that connect the luminal and basolateral side of the cell. Upon serial sectioning of stimulated frog microvessels, Michel & Neal (1999; see also Neal & Michel, 1996) observed the occurrence of transcellular gaps close to the cellular junctions. Intracellular gap formation has also been encountered in microvascular endothelial cells that had been exposed to the angiogenic growth factor VEGF (Robert & Palade, 1995; Esser et al. 1998; Dvorak et al. 1999). VEGF-induced gaps, together with defective endothelial junctions, contribute to the leakiness of tumour vessels (Feng et al. 1999; Hashizume et al. 2000). It is likely that the formation of such transcellular gaps requires reorganization of the cellular cytoskeleton and membrane fusion.
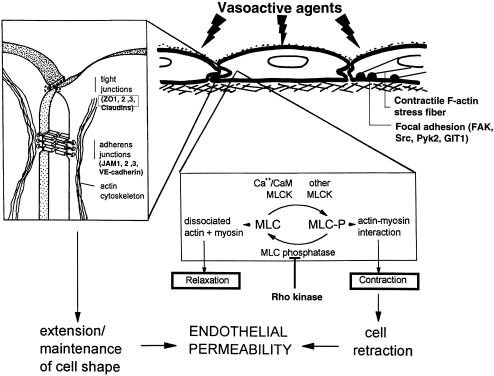
Fig. 1.
Endothelial permeability is regulated by MLC phosphorylation and reorganization of the actin cytoskeleton and by tethering forces in the intercellular junctions of endothelial cells. Actin non-muscle–myosin interaction is regulated by myosin light chain (MLC) phosphorylation, which is controlled by Ca2+/calmodulin-dependent activation of MLC kinase (MLCK) and regulation of the MLC phosphatase activity. Both tight junctions (containing occludin, claudins, ZO-1, ZO-2 and ZO-3) and adherens junctions (V,E-cadherin connected via α- and β-catenins and plakoglobin connected to the actin cytoskeleton) contribute to junctional stability. (Adapted from van Hinsbergh, 1997.)
In this survey we shall mainly focus on the role of actin–myosin interaction in the regulation of enhanced endothelial permeability and on mechanisms that can counteract increased endothelial permeability. Other contributions in this issue will deal with cellular junctions (Alexander & Elrod, 2002) and VEGF-induced vascular leakage (Bates et al. 1999, 2002)
Transient response to a vasoactive agent: histamine
Vasoactive agents, such as histamine and substance P, induce a rapid (< 20 s) and transient (5–10 min) increase in permeability. This can be visualized by intravital microscopy of the extravasation of fluorescently labelled macromolecules, and occurs only in the post-capillary venules. By measuring the extravasation of Monstral blue–albumin complex, silver staining of endothelial junctions and subsequent electron microscopy, McDonald and colleagues showed that these agents induce the formation of tiny gaps (about 1.5 µm) between the cells (Hirata et al. 1995; Baluk et al. 1997). The gaps are divided into multiple pores by cellular protrusions that connect the two adjacent cells.
This process can be mimicked in cultured endothelial cells. When endothelial cells are cultured on a porous filter, the exchange of macromolecules and the transendothelial electrical resistance can be determined. Such monolayers display many physiological properties of the endothelial barrier including the presence of tight junctions between the cells; a physiologically electrical resistance; repulsion of negatively charged molecules; selective passage of molecules depending on their Stokes radius; endothelial polarity; and a response of the cells to vasoactive agents (Langeler & van Hinsbergh, 1988; Langeler et al. 1989).
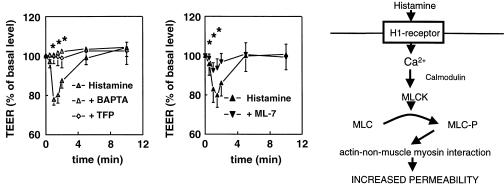
When such monolayers are stimulated by histamine in the absence of serum, the transendothelial electrical resistance rapidly drops and returns to normal values in 5 min (Fig. 2). The response is completely blocked by an H1-antagonist. The histamine-induced increase in permeability depends on an increase in cytoplasmic calcium ions, and is completely blocked by the calcium chelator BAPTA. The increase in cytoplasmic Ca2+ concentration causes a Ca2+/calmodulin-dependent activation of the myosin light chain kinase (MLCK), an enzyme that phosphorylates myosin light chains (MLCs), small regulatory proteins that activate the myosin molecule and promote actin–myosin interaction. Indeed, the calmodulin inhibitor trifluoperazine (TFP) and the MLCK inhibitor ML-7 each completely prevent the histamine-induced change in transendothelial electrical resistance (van Nieuw Amerongen et al. 1998). Parallel to the drop in electrical resistance, a transient increase in the phosphorylation status of the myosin light chains (MLCs) is observed, which further strengthens the idea that histamine affects endothelial permeability by stimulating actin non-muscle–myosin interaction in the periphery of the cell (Fig. 2). Whether the accompanying local contraction is the only force that causes the minute gaps or whether the tethering forces of junctional proteins are also affected by histamine within the 5-min period of hyperpermeability is still under investigation. Histamine-induced phosphorylation of V,E-cadherin (Winter et al. 1999) and occludin has been reported (Hirase et al. 2001). However, the latter process required the involvement of RhoA and Rho kinase, and probably reflects a type of prolonged endothelial permeability (see below). Indeed, when the effect of histamine was evaluated in the presence of serum, a prolonged increase in endothelial permeability was seen (van Nieuw Amerongen et al. 1998), which much resembles the increase in permeability induced by thrombin.
Fig. 2.
Histamine increases endothelial permeability and decreases the transendothelial electrical resistance (TEER). The decrease in TEER is inhibited by the Ca2+ chelator BAPTA, the calmodulin inhibitor trifluoperazine (TFP), and the MLCK inhibitor ML-7 (van Nieuw Amerongen et al. 1998). The right panel gives a schematic representation of the mechanism by which histamine increases MLC phosphorylation and actin-non-muscle–myosin interaction. An additional effect of histamine on junctional stability remains possible.
Prolonged endothelial permeability induced by thrombin
Thrombin and histamine induce an identical rise in the cytoplasmic Ca2+ concentration of endothelial cells in vitro (Shasby et al. 1997). However, thrombin induces a prolonged increase of endothelial permeability lasting for 1–1.5 h, while histamine only induces a transient increase for 5 min. This suggests that additional mechanisms are involved in the prolonged decrease in endothelial barrier function. Prolonged endothelial hyperpermeability is a serious and life-threatening clinical complication, and better understanding of the underlying mechanisms may help to develop new treatment strategies. It should be noted that thrombin was used in the in vitro experiments as a model compound to induce prolonged barrier dysfunction. Thrombin can induce endothelial hyperpermeability in lungs and brain in vivo (Johnson et al. 1983; Malik & Horgan, 1987; Minnear et al. 1987). Furthermore, it has been reported that administration of a high dose of antithrombin III can reduce endotoxin-induced lung hyperpermeability in animals (Dickneite & Kroez, 2001). However, the in vitro observations on the mechanism of thrombin-induced hyperpermeability in vitro and the in vivo observations on thrombin-induced vascular leakage have not yet been linked.
The thrombin-induced hyperpermeability was accompanied by a 30-min increase in MLC phosphorylation, the formation of F-actin stress fibres and an isometric tension in the cell, while MLC phosphorylation by histamine was transient within 5 min and did not induce an isometric tension (Garcia & Schaphorst, 1995; Garcia et al. 1995; Goeckeler & Wysolmerski, 1995; Moy et al. 1996). Cytochalasin D and an MLCK inhibitor inhibited the effect of thrombin, which indicates that actin and MLC phosphorylation play a role in the thrombin-induced isometric contraction and prolonged endothelial permeability. Chelation of the cytoplasmic Ca2+ ions in cultured endothelial cells by BAPTA reduced the thrombin-induced endothelial permeability only by 50%, in contrast to the complete effect of this chelator on histamine-induced permeability (Draijer et al. 1995a; Garcia et al. 1995; van Nieuw Amerongen et al. 1998). Thus, other mechanisms than the Ca2+/calmodulin-dependent activation of MLC kinase must contribute to the sustained MLC phosphorylation and prolonged endothelial permeability (Fig. 3). In bovine endothelial cells protein kinase C has been reported to enhance endothelial permeability by increasing MLC phosphorylation or by acting on actin fibres by phosphorylation of caldesmon (Lynch et al. 1990; Stasek et al. 1992; Vuong et al. 1998). However, in human endothelial cells we and other investigators could not demonstrate a role for protein kinase C either in MLC phosphorylation or in increased permeability (Yamada et al. 1990; Garcia et al. 1995; Yamada & Yokota, 1996; van Nieuw Amerongen et al. 1998).
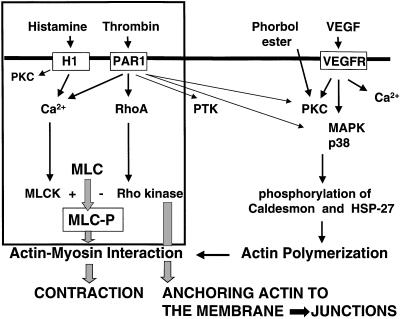
Fig. 3.
Schematic representation of the effects of thrombin on the endothelial actin cytoskeleton that contribute to endothelial permeability. The Ca2+/calmodulin-dependent activation of MLCK acts together with the RhoA/Rho kinase-dependent inhibition of MLC phosphatase (Ca2+ sensitization). Thrombin can also activate MAP kinase and protein kinase C (PKC), thereby influencing actin polymerization, and on protein tyrosine kinase (PTK) activity, which may affect junctional integrity.
In a search for additional kinases that affected the phosphorylation of the MLC our attention was drawn to Rho kinase, a multifunctional kinase that is activated by RhoA. Although initial studies by Amano et al. (1996) suggested that Rho kinase may act as MLC kinase, subsequent studies showed that Rho kinase primarily increases the phosphorylation of MLC by an inhibitory phosphorylation of the regulatory subunit of the MLC phosphatase (PP1M) (Kimura et al. 1996; Essler et al. 1998; Kawano et al. 1999). Inhibition of either RhoA or Rho kinase reduced thrombin-induced permeability by 50% and this inhibitory effect was additive to that of Ca2+ chelation (Essler et al. 1998; van Nieuw Amerongen et al. 1998, 2000a; Carbajal & Schaeffer, 1999; Carbajal et al. 2000; Garcia et al. 1999). Furthermore, thrombin-induced permeability was partly reduced by the tyrosine kinase inhibitors genistein and herbimycin A. It is likely that phosphorylation of tethering proteins in the junctional complexes, such as V,E-cadherin and occludin, affects the stability and restoration of cell-to-cell contacts (Rabiet et al. 1996; Andriopoulou et al. 1999; Cohen et al. 1999; Corada et al. 1999; Ukropec et al. 2000; Hirase et al. 2001).
Calcium sensitization by RhoA and Rho kinase
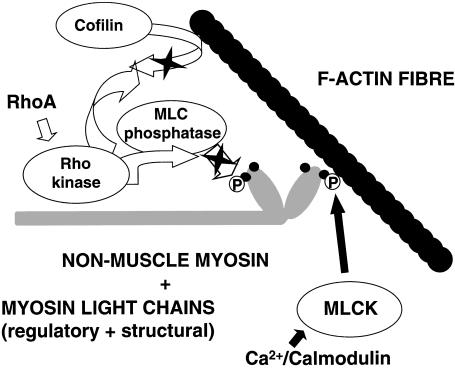
Rho GTPases are a family of small GTPases with profound actions on the actin cytoskeleton of cells. With respect to the functioning of the vascular system they are involved in the regulation of cell shape, cell contraction, cell motility and cell adhesion (Table 1). The three most prominent family members of the Rho GTPases are RhoA, Rac and cdc42. Activation of RhoA induces the formation of f-actin stress fibres in the cell, while Rac and cdc42 affect the actin cytoskeleton by inducing membrane ruffles and microspikes, respectively (Hall, 1998). While Rac and cdc42 can affect MLCK activity to a limited extent via activation of protein PAK (Chew et al. 1998; Goeckeler et al. 2000), RhoA has a prominent stimulatory effect on actin–myosin interaction by its ability to stabilize the phosphorylated state of MLC (Amano et al. 1996; Kimura et al. 1996; Kawano et al. 1999; Katoh et al. 2001). This occurs by activation of Rho kinase that in its turn inhibits the phosphatase PP1M that hydrolyses phosphorylated MLC (Fig. 4). In addition, Rho kinase inhibits the actin-severing action of cofilin and thus stabilizes f-actin fibres (Sumi et al. 1999; Toshima et al. 2001). Furthermore, Rho kinase can also be involved in anchoring the actin cytoskeleton to proteins in the plasma membrane and thus may potentially act on the interaction between junctional proteins and the actin cytoskeleton (Fukata et al. 1999; Takaishi et al. 2000).
Table 1.
Processes in the vascular system in which Rho GTPases are involved
| Cell contraction | |
| SMC contraction – blood pressure, vasospasm | |
| EC retraction – vascular leakage | |
| Platelets – activation/aggregation | |
| Cell motility | |
| Healing of an endothelial monolayer | |
| Angiogenesis | |
| SMC – atherosclerosis, restenosis | |
| Cell adhesion | |
| Platelet adhesion | |
| Leukocyte transmigration – inflammation | |
| Cell shape | |
| Myocyte – cardiac hypertrophy | |
| EC – atherosclerosis, restenosis (shear stress) |
Details are given by van Nieuw Amerongen & van Hinsbergh, 2001.
Fig. 4.
Schematic picture of the effects of Ca2+/calmodulin-dependent MLCK activity and RhoA/Rho kinase on actin–non–muscle–myosin interaction in endothelial cells. It should be noted that Rho kinase may also have additional effects on the anchoring of the actin cytoskeleton to proteins in the plasma membrane.
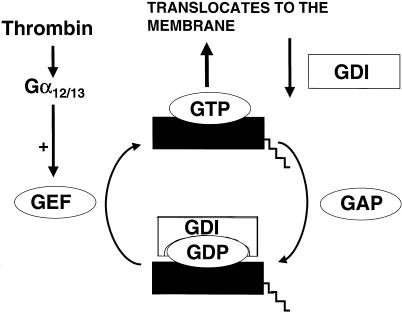
Thrombin can activate RhoA via Gα12/13 and a so-called guanine nucleotide exchange factor (GEF) (Seasholtz et al. 1999). The GEF exchanges RhoA-bound GDP for GTP, by which RhoA becomes active (Fig. 5). By this activation RhoA is translocated to the membrane, where it binds by its lipophilic geranyl-geranyl-anchor. RhoA can be activated by a number of vasoactive agents, including lysophosphatidic acid, thrombin and endothelin. Different GEFs are involved in the activation of RhoA, some of which are regulated by protein phosphorylation (Mehta et al. 2001). The membrane-bound RhoA is dissociated from the membrane by the action of a guanine dissociation inhibitor (GDI) or after the action of a GTPase-activating protein (GAP). The guanine dissociation inhibitors (GDIs) are regulatory proteins that bind to the carboxyl terminus of RhoA. GDIs inhibit the activity of RhoA by retarding the dissociation of GDP and detaching active RhoA from the plasma membrane.
Fig. 5.
Schematic representation of the activation of the RhoA. The GTP binding form of RhoA is translocated to the membrane where it is active. Guanine exchange factors (GEFs) activate RhoA, while GTPase-activating proteins (GAPs) bring RhoA back to its inactive form. The guanine dissociation factors (GDIs) dissociate active RhoA from the membrane, facilitate its inactivation, and inhibit its activation by binding to the carboxyl-terminal part of RhoA.
Thrombin directly activates RhoA in human endothelial cells and induces translocation of RhoA to the plasma membrane (van Nieuw Amerongen et al. 2000c). Under the same conditions the related GTPase Rac was not activated. Specific inhibition of RhoA by C3 transferase from Clostridium botulinum reduced the thrombin-induced increase in endothelial MLC phosphorylation and permeability (Essler et al. 1998; van Nieuw Amerongen et al. 1998; Carbajal et al. 1999), but did not affect the transient histamine-dependent increase in permeability (van Nieuw Amerongen et al. 1998). The effect of RhoA appears to be mediated via Rho kinase, because the specific Rho kinase inhibitor Y27632 similarly reduced thrombin-induced endothelial permeability. The effects of Y27632 and the Ca2+ chelator BAPTA on endothelial permeability were additive, suggesting two separate pathways that act on MLC phosphorylation and endothelial permeability (van Nieuw Amerongen et al. 2000a). Indeed, the Ca2+/calmodulin- and MLCK-dependent MLC phosphorylation is sensitized by the RhoA- and Rho kinase-dependent pathway that reduces dephosphorylation of MLC (Fig. 3).
To see whether activation of RhoA per se was sufficient to induce increased permeability, we used LPA that in our experimental conditions activated RhoA but did not affect the cytoplasmic Ca2+ concentration (Schulze et al. 1997; van Nieuw Amerongen et al. 2000c). Indeed, LPA increased endothelial permeability by a Rho kinase-dependent mechanism. However, even without an increase in cytoplasmic Ca2+ concentration a low degree of MLCK activity occurred in the cells as the effect was inhibitable by the MLCK inhibitor KT 5926 (van Nieuw Amerongen et al. 2000c). Furthermore, it should be noted that other investigators reported a decrease in endothelial permeability under their experimental conditions (English et al. 1999; Garcia et al. 2001; Minnear et al. 2001). This suggests that the physiological interactions of LPA with the endothelium in vivo may be complex.
Improving the endothelial barrier function
Understanding the mechanisms of how endothelial permeability proceeds only becomes clinically meaningful if it contributes to an understanding of how to prevent vascular leakage and how to improve the endothelial barrier function in disease states, in which vascular leakage is already present. As shown above, actin–myosin interaction and loss of junctional integrity are pivotal processes in endothelial permeability. Therefore, attempts to stabilize the endothelial barrier function have mainly focused on these mechanisms (Table 2). Early studies by Rippe & Grega (1978) showed that β-adrenergic stimuli were able to reduce a general histamine-induced leakage in animals (reviewed in Grega et al. 1988). This appeared related to their ability to enhance the endothelial cyclic AMP concentration. Indeed, several agents that counteract contraction in smooth muscle cells (Somlyo & Somlyo, 2000) can also reduce endothelial permeability. This holds true for agents that increase the cyclic AMP or cyclic GMP concentration and for RhoA- or Rho kinase-inhibiting agents. However, considerable regional variation exists between the responsiveness of various vascular beds, and the response of the endothelium can be sensitive to desensitization. In the following paragraphs we shall discuss various targets for improving endothelial barrier function.
Table 2.
Mechanisms reducing endothelial permeability
| • Receptor blockade (H1 antagonists) |
| • Cyclic AMP elevation |
| • Cyclic GMP/NO (lung, large vessels) |
| • PKC inhibition (bovine, porcine EC) |
| • Rho and Rho kinase inhibition |
| • Inhibition of leucocyte adhesion and activation (anti-LFA-1, anti-ICAM-1) |
| • Inhibition of VEGF induced leakage (anti-VEGF antibodies, VEGF receptor tyrosine kinase inhibitor SU5416, Angiopoietin) (tumour vascular bed) |
Receptor blockade
Direct interference of the interaction of vasoactive agents or permeability-enhancing factors with the endothelium by receptor blockade is a first approach to preventing vascular leakage. The efficacy of antagonists against the H1-type histamine receptor is well known, but only effective when histamine plays a pivotal role in leakage. Similarly, it can be anticipated that antagonists of the two VEGF receptors may be helpful for reducing VEGF-induced leakage. At present VEGF receptor-blocking antibodies or agents that specifically inhibit the tyrosine kinase activity of VEGF receptor-2, such as SU5416, may be effective. The recent data showing that angiopoietin-1 can prevent VEGF-induced leakage are challenging. Finally, reducing receptors and ligands involved in leucocyte–endothelium interaction can be effective. In animal studies, simultaneous blocking of LFA1 and ICAM-1 has been shown to reduce TNFα- and inflammation-induced pulmonary hyperpermeability (Lo et al. 1992; Shanley et al. 1995; Malik & Lo, 1996; Horgan et al. 1991).
Cyclic AMP
Cyclic AMP (cAMP) is one of the signalling molecules that in general improves endothelial barrier function. Elevation of endothelial cyclic AMP appeared effective in reducing the permeability of endothelial cells in vitro (Stelzner et al. 1989; Langeler & van Hinsbergh, 1991; Moy et al. 1993), in reducing histamine-induced general oedema in experimental animals (Rippe & Grega, 1978) and in preventing the formation of small intercellular gaps induced by substance P (Baluk & McDonald, 1994). The effect is fast and occurs both in endothelial cells under basal conditions and after exposure to vasoactive agents. Elevation of cAMP has many effects on endothelial cells. With respect to its effect on barrier function, cAMP stabilizes endothelial junctions, inhibits MLC kinase, reduces actin–non-muscle interaction and the formation of stress fibres, and prevents agonist-induced endothelial gap formation (He & Curry, 1993; Moy et al. 1993, 1998; Siflinger-Birnboim et al. 1993; Adamson et al. 1998). Its efficacy is independent on whether cAMP is elevated by activation of adenylate cyclase or by the inhibition of cAMP-degrading phosphodiesterases (Stelzner et al. 1989; Langeler & van Hinsbergh, 1991; Suttorp et al. 1993), which broadens possible therapeutic approaches. β2-adrenergic agents and serotonin increase cAMP levels in endothelial cells and were shown to improve endothelial barrier function in vitro and in animals. Indeed, catecholamines are used to reduce brain oedema over a period of several hours. On the other hand, therapeutic use of β2-adrenergic agonists in capillary leakage syndrome is still limited, even in combination with a phosphodiesterase inhibitor, because desensitization of endothelial cells for these agents occurs, which shortens the time these compounds are effective in reducing vascular leakage (Doorenbos et al. 1988; Droder et al. 1992). A second point of attention should be that certain blood vessels do not respond with a reduction of permeability, but on the contrary with an increase (Warren et al. 1993). In particular, the permeability increasing effect of cyclic AMP in cultured heart microvascular endothelial cells (Hempel et al. 1996) should be further evaluated for its consequences in the situation in vivo.
Cyclic GMP and nitric oxide (NO)
Another signalling molecule that was shown to counteract contraction in smooth muscle cells and which may reduce endothelial permeability, in particular in the lung, is cyclic GMP. In contrast to cyclic AMP, cyclic GMP does not affect basal endothelial barrier function, but reduces thrombin- or hydrogen-peroxide-induced enhanced endothelial permeability in vitro (Baron et al. 1989; Westendorp et al. 1994; Draijer et al. 1995a; Suttorp et al. 1996; Holschermann et al. 1997). The effects of cyclic GMP on in vivo barrier properties, however, are controversial. Cyclic GMP may promote or impair the endothelial barrier function depending on the vascular bed involved and the local condition of the endothelium (Zimmerman et al. 1990; Lofton et al. 1991; Meyer & Huxley, 1992; Yuan et al. 1993). While it may increase endothelial permeability in peripheral vessels, it may have beneficial effects in the lung and in large arteries.
Cyclic GMP is generated by a Ca2+/nitric oxide (NO)-dependent pathway or by cGMP-increasing agonists (Murad, 1994, 1998; Hobbs & Ignarro, 1996). Several mechanisms have been identified by which cGMP could improve endothelial barrier function in vitro. The first is a feedback mechanism by activating cGMP-dependent protein kinase I (Draijer et al. 1995a, 1995b). In the endothelial cells of large arteries and veins, but not in those of the umbilical veins and kidney glomeruli where cGMP-dependent protein kinase is absent, activation of cGMP-dependent kinase I reduces the agonist-induced rise in [Ca2+]i responsible for the increase in permeability. A second mechanism by which cGMP can act is inhibition of a cAMP-degrading phosphodiesterase and subsequent elevation of cyclic AMP. This mechanism accounts for the reduction in endothelial permeability by cGMP in endothelial cells of the umbilical vein (Draijer et al. 1995a). Inhalation of nitric oxide has attracted attention as an agent that may counteract pulmonary oedema. It may affect oedema both by vascular relaxation and by effects on endothelial barrier function. Although the effects of NO on pulmonary oedema have been reported in animal studies (Bloomfield et al. 1997; Garat et al. 1997; McElroy et al. 1997), no standard therapeutic application of NO/cGMP-elevating agents with regard to the improvement of endothelial barrier function in man is currently available.
RhoA and cholesterol-lowering statins
The finding that RhoA contributes to prolonged endothelial permeability provides a new potential target for pharmacological intervention in vascular leakage syndromes. The fact that RhoA activation is also involved in leucocyte extravasation further adds to this potential, because leucocyte extravasation and the accompanying activation often contributes to vascular leakage. RhoA has a geranylated lipid anchor required for binding to the membrane, to which it translocates during its activation. Simvastatin and other 3-hydroxy-3-methyl-glutaryl co-enzyme A reductase inhibitors prevent the formation of this lipid anchor as they inhibit isoprenylation of proteins in general. The thrombin-induced translocation of RhoA to the membrane of cultured endothelial cells is inhibited by the pre-incubation of the cells with simvastatin (van Nieuw Amerongen et al. 2000b). This is paralleled by a reduction in the permeability induced by thrombin.
Several reports indicate that statins have a beneficial effect on endothelial barrier dysfunction. At a regular dose, simvastatin reduced the activation and P-selectin-mediated extravasation of leucocytes in the microcirculation (Pruefer et al. 1999). Also in hyperlipidaemic mice the extravasation of leucocytes was attenuated by simvastatin (Scalia et al. 2001). Furthermore, treatment of 16-month-old Watenabe heritable hyperlipidaemic rabbits for 1 month with 15 mg kg−1 simvastatin reduced vascular leakage in both the thoracic and the abdominal part of the aorta, as revealed by the Evans blue dye exclusion test. In these animals the decreased permeability was not accompanied by a reduction of oil red O-positive atherosclerotic lesions. Finally, Dell’Omo et al. (2000) reported that simvastatin reduced microvascular permeability in hypercholesterolaemic man. Whether these latter findings completely depend on an effect on the endothelium or whether other factors, such as reduced influx of monocytes or other leucocytes, also contribute is not yet known. A direct effect of statins on leucocyte adhesion via inhibition of LFA1 has been suggested (Weitz-Schmidt et al. 2001). This may reduce leucocyte extravasation and the accompanying leakage. On the other hand a role of RhoA and Rho kinase as a target for therapeutic intervention of vascular leakage becomes a real option after the finding by Chiba et al. (2001) that the Rho kinase inhibitor Y27632 reduces vascular leakage in the rabbit lung. While the statins may not act rapidly enough in acute vascular leakage syndromes because of a delayed accumulation in the cell, Rho kinase inhibitors, such as Y27632 (Uehata et al. 1997; Ishizaki et al. 2000) and fasudil-derived compounds (Shimokawa et al. 1999), may provide a new approach and should be further evaluated for their potential to counteract specific forms of vascular leakage in vivo.
Perspective
In this survey we have focused on the role of intracellular signalling in the regulation of endothelial hyperpermeability, in particular related to MLC phosphorylation and actin non-muscle–myosin interaction. The data on histamine-induced hyperpermeability reflect closely those observed induced by a single vasoactive agent in a healthy microvessel as observed by investigators of the microcirculation in vivo (Curry, 1992; He & Curry, 1993; Michel & Curry, 1999). However, such models do not explain the prolonged leakage as is often seen in intensive care patients. Apparently additional factors are involved that are not yet adequately recognized. We believe that further insight in the regulatory mechanisms that contribute to endothelial permeability helps to understand how this prolonged leakage is generated and how it can be treated. Elucidation of how and in which precise conditions the activation of RhoA and Rho kinase, the regulation of endothelial junctional proteins and the contribution of angiogenesis-related factors affect endothelial permeability will be a challenge for the coming years and provide a perspective on focused treatment.
Acknowledgments
The financial support of the Universiteits Stimulerings Fonds van de Vrije Universiteit, Amsterdam, is gratefully acknowledged.
References
- Adamson RH, Liu B, Fry GN, Rubin LL, Curry FE. Microvascular permeability and number of tight junctions are modulated by cAMP. Am. J. Physiol. 1998;274:H1885–H1894. doi: 10.1152/ajpheart.1998.274.6.H1885. [DOI] [PubMed] [Google Scholar]
- Alexander JS, Elrod JW. Extracellular matrix, junctional integrity and matrix metalloproteinase interactions in barrier regulation. J. Anat. 2002 doi: 10.1046/j.1469-7580.2002.00057.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Andriopoulou P, Navarro P, Zanetti A, Lampugnani MG, Dejana E. Histamine induces tyrosine phosphorylation of endothelial cell-to-cell adherens junctions. Arterioscler. Thromb. Vasc Biol. 1999;19:2286–2297. doi: 10.1161/01.atv.19.10.2286. [DOI] [PubMed] [Google Scholar]
- Baluk P, Hirata A, Thurston G, Fujiwara T, Neal CR, Michel CC, et al. Endothelial gaps: time course of formation and closure in inflamed venules of rats. Am. J. Physiol. 1997;272:L155–L170. doi: 10.1152/ajplung.1997.272.1.L155. [DOI] [PubMed] [Google Scholar]
- Baluk P, McDonald DM. The beta 2-adrenergic receptor agonist formoterol reduces microvascular leakage by inhibiting endothelial gap formation. Am. J. Physiol. 1994;266:L461–L468. doi: 10.1152/ajplung.1994.266.4.L461. [DOI] [PubMed] [Google Scholar]
- Baron DA, Lofton CE, Newman WH, Currie MG. Atriopeptin inhibition of thrombin-mediated changes in the morphology and permeability of endothelial monolayers. Proc. Natl Acad. Sci. USA. 1989;274:H1776–H1784. doi: 10.1073/pnas.86.9.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates DO, Hillman NJ, Pocock TM, Neal CR, Williams B. Regulation of microvascular permeability by vascular endothelial growth factors. J. Anat. 2002 doi: 10.1046/j.1469-7580.2002.00066.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates DO, Lodwick D, Williams B. Vascular endothelial growth factor and microvascular permeability. Microcirculation. 1999;6:83–96. [PubMed] [Google Scholar]
- Bloomfield GL, Holloway S, Ridings PC, Fisher BJ, Blocher CR, Sholley M, et al. Pretreatment with inhaled nitric oxide inhibits neutrophil migration and oxidative activity resulting in attenuated sepsis-induced acute lung injury. Crit. Care Med. 1997;25:584–593. doi: 10.1097/00003246-199704000-00006. [DOI] [PubMed] [Google Scholar]
- Carbajal JM, Schaeffer RC., Jr RhoA inactivation enhances endothelial barrier function. Am. J. Physiol. 1999;277:C955–C964. doi: 10.1152/ajpcell.1999.277.5.C955. [DOI] [PubMed] [Google Scholar]
- Carbajal JM, Gratrix ML, Yu CH, Schaeffer RC., Jr ROCK mediates thrombin's endothelial barrier dysfunction. Am. J. Physiol Cell Physiol. 2000;279:C195–C204. doi: 10.1152/ajpcell.2000.279.1.C195. [DOI] [PubMed] [Google Scholar]
- Chew TL, Masaracchia RA, Goeckeler ZM, Wysolmerski RB. Phosphorylation of non-muscle myosin II regulatory light chain by p21-activated kinase (gamma-PAK) J. Muscle Res. Cell Motil. 1998;19:839–854. doi: 10.1023/a:1005417926585. [DOI] [PubMed] [Google Scholar]
- Chiba Y, Ishii Y, Kitamura S, Sugiyama Y. Activation of rho is involved in the mechanism of hydrogen-peroxide-induced lung edema in isolated perfused rabbit lung. Microvasc. Res. 2001;62:164–71. doi: 10.1006/mvre.2001.2329. [DOI] [PubMed] [Google Scholar]
- Chuang PT, Cheng HJ, Lins SJ, Jan KM, Lee MML, Chien S. Macromolecular transport across arterial and venous endothelium in rats: studies with Evans blue-albumin and horseradish peroxidase. Arteriosclerosis. 1990;10:188–197. doi: 10.1161/01.atv.10.2.188. [DOI] [PubMed] [Google Scholar]
- Cohen AW, Carbajal JM, Schaeffer RC., Jr VEGF stimulates tyrosine phosphorylation of beta-catenin and small–pore endothelial barrier dysfunction. Am. J. Physiol. 1999;277:H2038–H2049. doi: 10.1152/ajpheart.1999.277.5.H2038. [DOI] [PubMed] [Google Scholar]
- Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, et al. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc. Natl Acad. Sci. USA. 1999;96:9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuénoud HF, Joris I, Langer RS, Majno G. Focal arteriolar insudation: a response of arterioles to chronic nonspecific irritation. Am. J. Pathol. 1987;127:592–604. [PMC free article] [PubMed] [Google Scholar]
- Curry FE. Modulation of venular microvessel permeability by calcium influx into endothelial cells. FASEB J. 1992;6:2456–2466. doi: 10.1096/fasebj.6.7.1563597. [DOI] [PubMed] [Google Scholar]
- Dell’Omo G, Bandinelli S, Penno G, Pedrinelli R, Mariani M. Simvastatin, capillary permeability, and acetylcholine-mediated vasomotion in atherosclerotic, hypercholesterolemic men. Clin. Pharmacol. Ther. 2000;68:427–434. doi: 10.1067/mcp.2000.109787. [DOI] [PubMed] [Google Scholar]
- Dickneite G, Kroez M. Treatment of porcine sepsis with high-dose antithrombin III reduces tissue edema and effusion but does not increase risk for bleeding. Blood Coagul. Fibrinolysis. 2001;12:459–467. doi: 10.1097/00001721-200109000-00006. [DOI] [PubMed] [Google Scholar]
- Doorenbos CJ, van Es A, Valentijn RM, van Es LA. Systemic capillary leak syndrome. Preventive treatment with terbutaline. Neth. J. Med. 1988;32:178–184. [PubMed] [Google Scholar]
- Draijer R, Atsma DE, van der Laarse A, van Hinsbergh VW. cGMP and nitric oxide modulate thrombin-induced endothelial permeability. Regulation via different pathways in human aortic and umbilical vein endothelial cells. Circ Res. 1995a;76:199–208. doi: 10.1161/01.res.76.2.199. [DOI] [PubMed] [Google Scholar]
- Draijer R, Vaandrager AB, Nolte C, de Jonge HR, Walter U, van Hinsbergh VW. Expression of cGMP-dependent protein kinase I and phosphorylation of its substrate, vasodilator-stimulated phosphoprotein, in human endothelial cells of different origin. Circ. Res. 1995b;77:897–905. doi: 10.1161/01.res.77.5.897. [DOI] [PubMed] [Google Scholar]
- Droder RM, Kyle RA, Greipp PR. Control of systemic capillary leak syndrome with aminophylline and terbutaline. Am. J. Med. 1992;92:523–526. doi: 10.1016/0002-9343(92)90749-2. [DOI] [PubMed] [Google Scholar]
- Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular Permeability Factor/Vascular Endothelial Growth Factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF, Nagy JA, Feng D, Brown LF, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor and the significance of microvascular hyperpermeability in angiogenesis. In: Claesson Welsh L, editor. Vascular Growth Factors and Angiogenesis. Heidelberg: Springer-Verlag; 1999. pp. 98–132. [DOI] [PubMed] [Google Scholar]
- English D, Kovala AT, Welch Z, Harvey KA, Siddiqui RA, Brindley DN, et al. Induction of endothelial cell chemotaxis by sphingosine 1-phosphate and stabilization of endothelial monolayer barrier function by lysophosphatidic acid, potential mediators of hematopoietic angiogenesis. J. Hematother. Stem Cell Res. 1999;8:627–634. doi: 10.1089/152581699319795. [DOI] [PubMed] [Google Scholar]
- Esser S, Wolburg K, Wolburg H, Breier G, Kurzchalia T, Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J. Cell Biol. 1998;140:947–959. doi: 10.1083/jcb.140.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essler M, Amano M, Kruse HJ, Kaibuchi K, Weber PC, Aepfelbacher M. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho kinase in human endothelial cells. J. Biol. Chem. 1998;273:21867–21874. doi: 10.1074/jbc.273.34.21867. [DOI] [PubMed] [Google Scholar]
- Feng D, Nagy JA, Pyne K, Hammel I, Dvorak HF, Dvorak AM. Pathways of macromolecular extravasation across microvascular endothelium in response to VPF/VEGF and other vasoactive mediators. Microcirculation. 1999;6:23–44. [PubMed] [Google Scholar]
- Fukata Y, Oshiro N, Kinoshita N, Kawano Y, Matsuoka Y, Bennett V, et al. Phosphorylation of adducin by Rho-kinase plays a crucial role in cell motility. J. Cell Biol. 1999;145:347–361. doi: 10.1083/jcb.145.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garat C, Jayr C, Eddahibi S, Laffon M, Meignan M, Adnot S. Effects of inhaled nitric oxide or inhibition of endogenous H2O2 formation on hyperoxic lung injury. Am. J. Respir. Crit Care Med. 1997;155:1957–1964. doi: 10.1164/ajrccm.155.6.9196102. [DOI] [PubMed] [Google Scholar]
- Garcia JGN, Davis HW, Patterson CE. Regulation of endothelial gap formation and barrier dysfunction: role of myosin light chain phosphorylation. J. Cell Physiol. 1995;163:510–522. doi: 10.1002/jcp.1041630311. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JG, Schaphorst KL. Regulation of endothelial cell gap formation and paracellular permeability. J. Invest. Med. 1995;43:117–126. [PubMed] [Google Scholar]
- Garcia JG, Verin AD, Schaphorst K, Siddiqui R, Patterson CE, Csortos C, Natarajan V. Regulation of endothelial cell myosin light chain kinase by Rho, cortactin, and p60 (src) Am. J. Physiol. 1999;276:L989–L998. doi: 10.1152/ajplung.1999.276.6.L989. [DOI] [PubMed] [Google Scholar]
- Goeckeler ZM, Wysolmerski RB. Myosin light chain kinase-regulated endothelial cell contraction: the relationship between isometric tension, actin polymerization, and myosin phosphorylation. J. Cell Biol. 1995;130:613–627. doi: 10.1083/jcb.130.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeckeler ZM, Masaracchia RA, Zeng Q, Chew TL, Gallagher P, Wysolmerski RB. Phosphorylation of myosin light chain kinase by p21-activated kinase PAK2. J. Biol. Chem. 2000;275:18366–18374. doi: 10.1074/jbc.M001339200. [DOI] [PubMed] [Google Scholar]
- Grega GJ, Persson CGA, Svensjo E. Endothelial cell reactions to inflammatory mediators assessed in vivo by fluid and solute flux analysis. In: Ryan US, editor. Endothelial Cells. Boca Raton: CRC Press Inc; 1988. pp. 103–119. [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hashizume H, Baluk P, Morikawa S, McLean JW, Thurston G, Roberge S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol. 2000;156:1363–1380. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Curry FE. Differential actions of cAMP on endothelial [Ca2+]i and permeability in microvessels exposed to ATP. Am. J. Physiol. 1993;265:H1019–H1023. doi: 10.1152/ajpheart.1993.265.3.H1019. [DOI] [PubMed] [Google Scholar]
- Hempel A, Noll T, Muhs A, Piper HM. Functional antagonism between cAMP and cGMP on permeability of coronary endothelial monolayers. Am. J. Physiol. 1996;270:H1264–H1271. doi: 10.1152/ajpheart.1996.270.4.H1264. [DOI] [PubMed] [Google Scholar]
- Hirase T, Kawashima S, Wong EY, Ueyama T, Rikitake Y, Tsukita S, et al. Regulation of tight junction permeability and occludin phosphorylation by Rhoa-p160ROCK-dependent and – independent mechanisms. J. Biol. Chem. 2001;276:10423–10431. doi: 10.1074/jbc.M007136200. [DOI] [PubMed] [Google Scholar]
- Hirata A, Baluk P, Fujiwara T, McDonald DM. Location of focal silver staining at endothelial gaps in inflamed venules examined by scanning electron microscopy. Am. J. Physiol. 1995;269:L403–L418. doi: 10.1152/ajplung.1995.269.3.L403. [DOI] [PubMed] [Google Scholar]
- Hobbs AJ, Ignarro LJ. Nitric oxide-cyclic GMP signal transduction system. Meth. Enzymol. 1996;269:134–148. doi: 10.1016/s0076-6879(96)69016-8. [DOI] [PubMed] [Google Scholar]
- Holschermann H, Noll T, Hempel A, Piper M. Dual role of cGMP in modulation of macromolecule permeability of aortic endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 1997;272:H91–H98. doi: 10.1152/ajpheart.1997.272.1.H91. [DOI] [PubMed] [Google Scholar]
- Horgan MJ, Ge M, Gu J, Rothlein R, Malik AB. Role of ICAM-1 in neutrophil-mediated lung vascular injury after occlusion and reperfusion. Am. J. Physiol. 1991;261:H1578–H1584. doi: 10.1152/ajpheart.1991.261.5.H1578. [DOI] [PubMed] [Google Scholar]
- Huang A-L, Jan K-M, Chien S. Role of intercellular junctions in the passage of horseradish peroxidase across aortic endothelium. Lab. Invest. 1992;67:201–210. [PubMed] [Google Scholar]
- Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M, et al. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol. 2000;57:976–983. [PubMed] [Google Scholar]
- Johnson A, Tahamont MV, Malik AB. Thrombin-induced lung vascular injury. Roles of fibrinogen and fibrinolysis. Am. Rev. Respir. Dis. 1983;128:38–44. doi: 10.1164/arrd.1983.128.1.38. [DOI] [PubMed] [Google Scholar]
- Joris I, Cuenoud HF, Doern GV, Underwood JM, Majno G. Capillary leakage in inflammation. A study by vascular labeling. Am. J. Pathol. 1990;137:1353–1363. [PMC free article] [PubMed] [Google Scholar]
- Katoh K, Kano Y, Amano M, Onishi H, Kaibuchi K, Fujiwara K. Rho-kinase – mediated contraction of isolated stress fibers. J. Cell Biol. 2001;153:569–584. doi: 10.1083/jcb.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M, et al. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J. Cell Biol. 1999;147:1023–1038. doi: 10.1083/jcb.147.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Langeler EG, Snelting-Havinga I, van Hinsbergh VW. Passage of low density lipoproteins through monolayers of human arterial endothelial cells. Effects of vasoactive substances in an in vitro model. Arteriosclerosis. 1989;9:550–559. doi: 10.1161/01.atv.9.4.550. [DOI] [PubMed] [Google Scholar]
- Langeler EG, van Hinsbergh VW. Characterization of an in vitro model to study the permeability of human arterial endothelial cell monolayers. Thromb. Haemost. 1988;60:240–246. [PubMed] [Google Scholar]
- Langeler EG, van Hinsbergh VW. Norepinephrine and iloprost improve barrier function of human endothelial cell monolayers: role of cAMP. Am. J. Physiol. 1991;260:C1052–C1059. doi: 10.1152/ajpcell.1991.260.5.C1052. [DOI] [PubMed] [Google Scholar]
- Lo SK, Everitt J, Gu J, Malik AB. Tumor necrosis factor mediates experimental pulmonary edema by ICAM-1 and CD18-dependent mechanisms. J. Clin. Invest. 1992;89:981–988. doi: 10.1172/JCI115681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofton CE, Baron DA, Heffner JE, Currie MG, Newman WH. Atrial natriuretic peptide inhibits oxidant-induced increases in endothelial permeability. J. Mol. Cell Cardiol. 1991;23:919–927. doi: 10.1016/0022-2828(91)90134-8. [DOI] [PubMed] [Google Scholar]
- Lynch JJ, Ferro TJ, Blumenstock FA, Brockenauer AM, Malik AB. Increased endothelial albumin permeability mediated by protein kinase C activation. J. Clin. Invest. 1990;85:1991–1998. doi: 10.1172/JCI114663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik AB, Horgan MJ. Mechanisms of thrombin-induced lung vascular injury and edema. Am. Rev. Respir. Dis. 1987;136:467–470. doi: 10.1164/ajrccm/136.2.467. [DOI] [PubMed] [Google Scholar]
- Malik AB, Lo SK. Vascular endothelial adhesion molecules and tissue inflammation. Pharmacol. Rev. 1996;48:213–229. [PubMed] [Google Scholar]
- McElroy M, Wiener-Kronish J, Myazaki H, Sawa T, Modelska K, Dobbs L, et al. Nitric oxide attenueates lung endothelial injury caused by sublethal hyperoxia in rats. Am. J. Physiol. Lung Cell Mol. Physiol. 1997;272:L631–L638. doi: 10.1152/ajplung.1997.272.4.L631. [DOI] [PubMed] [Google Scholar]
- Mehta D, Rahman A, Malik AB. Protein kinase C-alpha signals rho-guanine nucleotide dissociation inhibitor phosphorylation and rho activation and regulates the endothelial cell barrier function. J. Biol. Chem. 2001;276:22614–22620. doi: 10.1074/jbc.M101927200. [DOI] [PubMed] [Google Scholar]
- Meyer DL, Huxley VH. Capillary hydraulic conductivity is elevated by cGMP-dependent vasodilators. Circ. Res. 1992;70:382–391. doi: 10.1161/01.res.70.2.382. [DOI] [PubMed] [Google Scholar]
- Michel CC, Curry FE. Microvascular permeability. Physiol. Rev. 1999;79:703–761. doi: 10.1152/physrev.1999.79.3.703. [DOI] [PubMed] [Google Scholar]
- Michel CC, Neal CR. Openings through endothelial cells associated with increased microvascular permeability. Microcirculation. 1999;6:45–54. [PubMed] [Google Scholar]
- Minnear FL, Martin D, Hill L, Taylor AE, Malik AB. Lung morphological and permeability changes induced by intravascular coagulation in dogs. Am. J. Physiol. 1987;253:H634–H644. doi: 10.1152/ajpheart.1987.253.3.H634. [DOI] [PubMed] [Google Scholar]
- Minnear FL, Patil S, Bell D, Gainor JP, Morton CA. Platelet lipid (s) bound to albumin increases endothelial electrical resistance: mimicked by LPA. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;281:L1337–L1344. doi: 10.1152/ajplung.2001.281.6.L1337. [DOI] [PubMed] [Google Scholar]
- Moy AB, Bodmer JE, Blackwell K, Shasby S, Shasby DM. cAMP protects endothelial barrier function independent of inhibiting MLC20-dependent tension development. Am. J. Physiol. 1998;274:L1024–L1029. doi: 10.1152/ajplung.1998.274.6.L1024. [DOI] [PubMed] [Google Scholar]
- Moy AB, Shasby SS, Scott BD, Shasby DM. The effect of histamine and cyclic adenosine monophosphate on myosin light chain phosphorylation in human umbilical vein endothelial cells. J. Clin. Invest. 1993;92:1198–1206. doi: 10.1172/JCI116690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy AB, Van Engelenhoven J, Bodmer J, Kamath J, Keese C, Giaever I, et al. Histamine and thrombin modulate endothelial focal adhesion through centripetal and centrifugal forces. J. Clin. Invest. 1996;97:1020–1027. doi: 10.1172/JCI118493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad F. Regulation of cytosolic guanylyl cyclase by nitric oxide: the NO-cyclic GMP signal transduction system. Adv. Pharmacol. 1994;26:19–33. doi: 10.1016/s1054-3589(08)60049-6. [DOI] [PubMed] [Google Scholar]
- Murad F. Nitric oxide signaling: would you believe that a simple free radical could be a second messenger, autacoid, paracrine substance, neurotransmitter, and hormone? Recent Prog. Horm Res. 1998;53:43–59. [PubMed] [Google Scholar]
- Neal CR, Michel CC. Openings in frog microvascular endothelium induced by high intravascular pressures. J. Physiol. 1996;492:39–52. doi: 10.1113/jphysiol.1996.sp021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, Draijer R, Vermeer MA, van Hinsbergh VWM. Transient and prolonged increase in endothelial permeability induced by histamine and thrombin: role of protein kinases, calcium, and RhoA. Circ. Res. 1998;83:1115–1123. doi: 10.1161/01.res.83.11.1115. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VWM. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ. Res. 2000a;87:335–340. doi: 10.1161/01.res.87.4.335. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, Vermeer MA, Negre-Aminou P, Lankelma J, Emeis JJ, van Hinsbergh VWM. Simvastatin improves disturbed endothelial barrier function. Circulation. 2000b;102:2803–2809. doi: 10.1161/01.cir.102.23.2803. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, Vermeer MA, van Hinsbergh VWM. Role of RhoA and rho kinase in lysophosphatidic acid–induced endothelial barrier dysfunction. Arterioscler Thromb Vasc Biol. 2000c;20:E127–E133. doi: 10.1161/01.atv.20.12.e127. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, van Hinsbergh VWM. Cytoskeletal effects of rho-like small guanine nucleotide-binding proteins in the vascular system. Arterioscler. Thromb. Vasc. Biol. 2001;21:300–311. doi: 10.1161/01.atv.21.3.300. [DOI] [PubMed] [Google Scholar]
- Predescu D, Palade GE. Plasmalemmal vesicles represent the large pore system of continuous microvascular endothelium. Am. J. Physiol. 1993;265:H725–H733. doi: 10.1152/ajpheart.1993.265.2.H725. [DOI] [PubMed] [Google Scholar]
- Pruefer D, Scalia R, Lefer AM. Simvastatin inhibits leukocyte–endothelial cell interactions and protects against inflammatory processes in normocholesterolemic rats. Arterioscler Thromb. Vasc. Biol. 1999;19:2894–2900. doi: 10.1161/01.atv.19.12.2894. [DOI] [PubMed] [Google Scholar]
- Rabiet MJ, Plantier JL, Rival Y, Genoux Y, Lampugnani MG, Dejana E. Thrombin-induced increase in endothelial permeability is associated with changes in cell-to-cell junction organization. Arterioscler Thromb. Vasc. Biol. 1996;16:488–496. doi: 10.1161/01.atv.16.3.488. [DOI] [PubMed] [Google Scholar]
- Rampart M, Williams TJ. Polymorphonuclear leukocyte-dependent plasma leakage in the rabbit skin is enhanced or inhibited by prostacyclin, depending on the route of administration. Am. J. Pathol. 1986;124:66–73. [PMC free article] [PubMed] [Google Scholar]
- Rippe B, Grega GJ. Effects of isoprenaline and cooling on histamine induced changes of capillary permeability in the rat hindquarter vascular bed. Acta Physiol. Scand. 1978;103:252–262. doi: 10.1111/j.1748-1716.1978.tb06212.x. [DOI] [PubMed] [Google Scholar]
- Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J. Cell Sci. 1995;108:2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- Scalia R, Gooszen ME, Jones SP, Hoffmeyer M, Rimmer DM, 3rd, Trocha SD, et al. Simvastatin exerts both anti-inflammatory and cardioprotective effects in apolipoprotein E-deficient mice. Circulation. 2001;103:2598–2603. doi: 10.1161/01.cir.103.21.2598. [DOI] [PubMed] [Google Scholar]
- Schulze C, Smales C, Rubin LL, Staddon JM. Lysophosphatidic acid increases tight junction permeability in cultured brain endothelial cells. J. Neurochem. 1997;68:991–1000. doi: 10.1046/j.1471-4159.1997.68030991.x. [DOI] [PubMed] [Google Scholar]
- Seasholtz TM, Majumdar M, Brown JH. Rho as a mediator of G protein-coupled receptor signaling. Mol. Pharmacol. 1999;55:949–956. doi: 10.1124/mol.55.6.949. [DOI] [PubMed] [Google Scholar]
- Shanley TP, Warner RL, Ward PA. The role of cytokines and adhesion molecules in the development of inflammatory injury. Mol Med. Today. 1995;1:40–45. doi: 10.1016/1357-4310(95)80019-0. [DOI] [PubMed] [Google Scholar]
- Shasby DM, Stevens T, Ries D, Moy AB, Kamath JM, Kamath AM, et al. Thrombin inhibits myosin light chain dephosphorylation in endothelial cells. Am. J. Physiol. 1997;272:L311–L319. doi: 10.1152/ajplung.1997.272.2.L311. [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Seto M, Katsumata N, Amano M, Kozai T, et al. Rho-kinase-mediated pathway induces enhanced myosin light chain phosphorylations in a swine model of coronary artery spasm. Cardiovasc. Res. 1999;43:1029–1039. doi: 10.1016/s0008-6363(99)00144-3. [DOI] [PubMed] [Google Scholar]
- Siflinger-Birnboim A, Bode DC, Malik AB. Adenosine 3′,5′-cyclic monophosphate attenuates neutrophil-mediated increase in endothelial permeability. Am. J. Physiol. 1993;264:H370–H375. doi: 10.1152/ajpheart.1993.264.2.H370. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J. Physiol. 2000;522:177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasek J, Patterson CE, Garcia JGN. Protein kinase C phosphorylates caldesmon77 and vimentin and enhances albumin permeability across bovine pulmonary artery endothelial cell monolayers. J. Cell Physiol. 1992;153:62–75. doi: 10.1002/jcp.1041530110. [DOI] [PubMed] [Google Scholar]
- Stelzner TJ, Weil JV, O'Brien RF. Role of cyclic adenosine monophosphate in the induction of endothelial barrier properties. J. Cell Physiol. 1989;139:157–166. doi: 10.1002/jcp.1041390122. [DOI] [PubMed] [Google Scholar]
- Sumi T, Matsumoto K, Takai Y, Nakamura T. Cofilin phosphorylation and actin cytoskeletal dynamics regulated by rho- and Cdc42-activated LIM-kinase 2. J. Cell Biol. 1999;147:1519–1532. doi: 10.1083/jcb.147.7.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttorp N, Hippenstiel S, Fuhrmann M, Krull M, Podzuweit T. Role of nitric oxide and phosphodiesterase isoenzyme II for reduction of endothelial hyperpermeability. Am. J. Physiol. 1996;270:C778–C785. doi: 10.1152/ajpcell.1996.270.3.C778. [DOI] [PubMed] [Google Scholar]
- Suttorp N, Weber U, Welsch T, Schudt C. Role of phosphodiesterases in the regulation of endothelial permeability in vitro. J. Clin. Invest. 1993;91:1421–1428. doi: 10.1172/JCI116346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi K, Matozaki T, Nakano K, Takai Y. Multiple downstream signalling pathways from ROCK, a target molecule of Rho small G protein, in reorganization of the actin cytoskeleton in Madin-Darby canine kidney cells. Genes Cell. 2000;5:929–936. doi: 10.1046/j.1365-2443.2000.00377.x. [DOI] [PubMed] [Google Scholar]
- Toshima J, Toshima JY, Amano T, Yang N, Narumiya S, Mizuno K. Cofilin phosphorylation by protein kinase testicular protein kinase 1 and its role in integrin-mediated actin reorganization and focal adhesion formation. Mol. Biol. Cell. 2001;12:1131–1145. doi: 10.1091/mbc.12.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- Ukropec JA, Hollinger MK, Salva SM, Woolkalis MJ. SHP2 association with VE-cadherin complexes in human endothelial cells is regulated by thrombin. J. Biol. Chem. 2000;275:5983–5986. doi: 10.1074/jbc.275.8.5983. [DOI] [PubMed] [Google Scholar]
- Van Hinsbergh VWM. Endothelial permeability for macromolecules – mechanistic aspects of pathophysiological modulation. Arterioscler Thromb Vasc. Biol. 1997;17:1018–1023. doi: 10.1161/01.atv.17.6.1018. [DOI] [PubMed] [Google Scholar]
- Vuong PT, Malik AB, Nagpala PG, Lum H. Protein kinase C beta modulates thrombin-induced Ca2+ signaling and endothelial permeability increase. J. Cell Physiol. 1998;175:379–387. doi: 10.1002/(SICI)1097-4652(199806)175:3<379::AID-JCP16>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Warren JB, Wilson AJ, Loi RK, Coughlan ML. Opposing roles of cyclic AMP in the vascular control of edema formation. FASEB J. 1993;7:1394–1400. doi: 10.1096/fasebj.7.14.7693536. [DOI] [PubMed] [Google Scholar]
- Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, Cottens S, Takada Y, Hommel U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat. Med. 2001;7:687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- Westendorp RG, Draijer R, Meinders AE, van Hinsbergh VW. Cyclic-GMP-mediated decrease in permeability of human umbilical and pulmonary artery endothelial cell monolayers. J. Vasc Res. 1994;31:42–51. doi: 10.1159/000159030. [DOI] [PubMed] [Google Scholar]
- Winter MC, Kamath AM, Ries DR, Shasby SS, Chen YT, Shasby DM. Histamine alters cadherin-mediated sites of endothelial adhesion. Am. J. Physiol. 1999;277:L988–L995. doi: 10.1152/ajplung.1999.277.5.L988. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Furumichi T, Furui H, Yokoi T, Ito T, Yamauchi K, et al. Roles of calcium, cyclic nucleotides, and protein kinase C in regulation of endothelial permeability. Arteriosclerosis. 1990;10:410–420. doi: 10.1161/01.atv.10.3.410. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Yokota M. Enhancement of barrier function of human aortic endothelial cells by activators of protein kinase C. Biochem. Mol Biol. Int. 1996;39:69–76. doi: 10.1080/15216549600201071. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Granger HJ, Zawieja DC, DeFily DV, Chilian WM. Histamine increases venular permeability via a phospholipase C-NO synthase-guanylate cyclase cascade. Am. J. Physiol. Heart Circ. Physiol. 1993;264:H1734–H1739. doi: 10.1152/ajpheart.1993.264.5.H1734. [DOI] [PubMed] [Google Scholar]
- Zimmerman RS, Trippodo NC, MacPhee AA, Martinez AJ, Barbee RW. High-dose atrial natriuretic factor enhances albumin escape from the systemic but not the pulmonary circulations. Circ. Res. 1990;67:461–468. doi: 10.1161/01.res.67.2.461. [DOI] [PubMed] [Google Scholar]