Abstract
Septic encephalopathy is associated with breakdown of the blood–brain barrier and cerebral oedema. These features are also common properties of brain tumours. Perimicrovessel oedema, disruption of associated astrocyte end feet and neuronal injury occur in a porcine model of acute septic encephalopathy. The adrenergic system has been implicated in the inflammatory response to sepsis and may play a role in controlling blood–brain barrier permeability, since the β2-adrenoceptor agonist dopexamine inhibits perimicrovessel oedema formation whereas the α1-adrenoceptor agonist methoxamine provokes it. Electron microscopy revealed tight junction opening in high-grade astrocytoma microvessels. Expression of the tight junction protein occludin is reduced in these microvessels and this reduction is inversely correlated with the degree of cerebral oedema. Normal astrocytes secrete factors that induce barrier properties in endothelial cells, whereas high-grade astrocytomas secrete vascular endothelial growth factor, which stimulates angiogenesis, down regulates occludin and increases endothelial cell permeability. The water channel protein aquaporin-4 is normally expressed in astrocyte foot processes around cerebral microvessels. Its expression is massively up-regulated in high-grade astrocytoma and around metastatic adenocarcinoma. There is a significant correlation between aquaporin-4 expression and the degree of cerebral oedema, but it is not clear whether increased aquaporin-4 expression enhances oedema formation or clearance. These results suggest that the pathophysiology of brain oedema is multifactorial, but that there may be common processes operating regardless of the aetiology.
Keywords: aquaporin-4, astrocytes, endothelial cells, occludin, tight junctions
Blood–brain barrier breakdown and oedema formation in septic encephalopathy
Systemic sepsis and its consequences (septic shock, sepsis syndrome and acute respiratory distress syndrome) are the commonest causes of death in intensive care units (Pine et al. 1983; Parillo et al. 1990; Tran et al. 1990). It is associated with widespread endothelial cell damage from uncontrolled inflammation (Bone, 1992; Thijs et al. 1993; Bradley et al. 1994) and it progressively causes multiorgan failure (Knaus et al. 1985). There is a considerable amount of information about the deleterious effects of systemic sepsis on the lung, liver kidney and heart (Coalson et al. 1975; Lang et al. 1984; Tighe et al. 1989, 1990; Deng et al. 1995; Tighe et al. 1995, 1998). However, despite the facts that an acutely altered sensorium is a frequent sequel to sepsis and is associated with a significantly increased mortality (Jeppsson et al. 1981), it is only relatively recently that the effects of sepsis on the brain have begun to receive attention.
Septic encephalopathy is the commonest form of encephalopathy presenting in intensive care units (Bleck et al. 1993). It is a symmetric diffuse brain dysfunction that varies in its presentation from impaired attention/concentration, to delirium and coma. The blood–brain barrier appears to be compromised in septic encephalopathy, since the cerebrospinal fluid of patients contains elevated protein levels (Young et al. 1992). Moreover, colloidal iron oxide (Clawson et al. 1966), 14C-amino acids (Jeppsson et al. 1981) and 125I-albumin (Deng et al. 1995) have been shown to enter the brain parenchyma from the circulation in rodents with systemic sepsis.
The cellular pathology underlying the blood–brain barrier dysfunction in systemic sepsis is beginning to be revealed. In an early study, Clawson et al. (1966) reported increased pinocytosis in cerebral microvessel endothelia and swelling of astrocytes in the brains of rabbits with endotoxaemia. More recently, the effect of systemic sepsis on porcine frontal cortex was investigated 8 h after the induction of caecal peritonitis (Papadopoulos et al. 1999). In comparison with sham-operated controls (Fig. 1A), severe perimicrovessel oedema was present in septic pigs (Fig. 1B) and astrocyte endfeet surrounding cortical microvessels were frequently grossly swollen and their membranes ruptured and detached from the microvessel walls. This perimicrovessel oedema is likely to impair the movement of oxygen, nutrients and metabolites across the microvessel wall and swelling of astrocyte endfeet is known to be a consequence of blood–brain barrier breakdown (Norenberg, 1994). In addition, astrocytes are an important source of factors that ‘tighten’ endothelia (Janzer & Raff, 1997) and so damage to their endfeet may further impair blood–brain barrier function in sepsis.
Fig. 1.
Electron micrographs of porcine frontal cortex showing: (A) A normal microvessel from a sham-operated control pig, with negligible perimicrovessel oedema. Magnification = 3500×. (B) A microvessel from a pig 8 h after the induction of caecal peritonitis, with severe perimicrovessel oedema. Magnification = 4400×. (C) A microvessel from a pig treated with the β2-adrenoceptor agonist dopexamine, 8 h after the induction of caecal peritonitis. Less oedema is present than in (B). Magnification = 3500×. ecn = endothelial cell nucleus, rbc = red blood cell.
Dark, shrunken neurones were present in the oedematous cerebral cortex of pigs 8 h after the induction of peritonitis and the cytoplasm of the remaining neurones appeared ‘dilute’ (Papadopoulos et al. 1999). These observations suggest that neurodegenerative change occurs in the septic brain, but the mediators/mechanisms involved are unknown. Cerebral blood flow is decreased in sepsis (Bowton et al. 1989; Maekawa et al. 1991), but this decrease is unlikely to be sufficient to cause neuronal degeneration (Astrup et al. 1981; Hossman, 1994). However, circulating inflammatory mediators (Goris, 1990) could gain access to the brain parenchyma in sepsis through the impaired blood–brain barrier and act directly on neurones, possibly initiating apoptosis. On the other hand, neuronal damage could result from sepsis-damaged astrocytes being unable to fulfil their important homeostatic function (Landis, 1996) within the brain.
The ultrastructure of cortical microvessel endothelial cells from septic pigs appeared normal and was indistinguishable from that of non-septic controls. The intercellular tight junctions appeared morphologically intact and sepsis did not result in a change in the mean cross-sectional area of microvessel endothelial cells or lumina (Papadopoulos et al. 1999). This is perhaps surprising in view of the other evidence (perimicrovessel oedema, swelling and rupture of astrocyte endfeet, neuronal degeneration) suggesting blood–brain barrier breakdown in these animals. However, it is possible that the relatively short time period (8 h) between the induction of sepsis and harvesting brain tissue was insufficient to cause gross structural alterations in cerebral microvessel endothelial cells. Nevertheless, the lack of such morphological change does not preclude impairment of microvessel endothelial cell function. Increased pinocytosis such as that reported to result from endotoxaemia (Clawson et al. 1966) might occur in sepsis, or the interendothelial cell junctional proteins could be disrupted, leading to leakage across the normally tight junctions and the development of oedema and tissue damage. Both of these possibilities require investigation in order to understand the development of the neuropathology associated with septic encephalopathy.
The adrenergic system has been implicated in the inflammatory response to sepsis (Tighe et al. 1996). β2-adrenoceptors have been shown to have anti-inflammatory properties and β2-adrenoceptor stimulation in vitro decreases vascular endothelial cell permeability (Zink et al. 1993). In the porcine caecal peritonitis model of systemic sepsis, intravenous treatment with the β2-adrenoceptor agonist dopexamine (0.6 mg kg−1 h−1) protected against sepsis-induced perimicrovessel oedema (Fig. 1C) in the cerebral cortex (Davies et al. 2001). In contrast, intravenous administration of the α1-adrenoceptor agonist methoxamine (80 mg h−1) caused perimicrovessel oedema in non-septic pigs and β2-adrenoceptor blockade with ICI 118 551 (a 20-µg kg−1 bolus given intravenously every hour) did not prevent the formation of perimicrovessel oedema in septic pigs. Methoxamine treatment also caused swelling of microvessel endothelial cells in both septic and non-septic pigs. Conjoint dopexamine and methoxamine treatment did not prevent this endothelial cell swelling (Davies et al. 2001). Therefore, β2-adrenoceptor stimulation appears to inhibit the inflammatory response in sepsis and blood–brain barrier permeability. On the other hand, α1-adrenoceptor stimulation appears to provoke the inflammatory cascade and open the blood–brain barrier, even in the absence of sepsis. Although the results of these studies have implications for the development of therapeutic strategies to control opening of the blood–brain barrier, it is still important to determine which substances normally act through the adrenoceptors, how these actions are triggered and whether their activation leads to modulation of tight junction permeability or transcellular permeability.
Tight junction proteins and blood–brain barrier opening in brain tumours
Cerebral oedema is a consequence of the two commonest types of human brain tumour, high-grade astrocytoma and metastatic adenocarcinoma and it contributes to morbididty and mortality (Thapar et al. 1995). The microvessels of brain tumours characteristically lose their blood–brain barrier properties and leak fluid into the brain (Seitz & Wechsler, 1987; Groothuis et al. 1991). Investigation of the ultrastructure of human gliomas has revealed opening of intermicrovessel endothelial cell tight junctions (Long, 1970; Bar-Sella et al. 1979; Nir et al. 1986; Shibata, 1989). The microvessels of metastatic adenocarcinomas are also ‘leaky’. They exhibit the characteristics of the tissue from which they are derived and hence fail to form tight junctions (Long, 1979). Thus, the open tight junctions between microvessel endothelial cells in astrocytomas and metastatic adenocarcinomas are likely to underlie the associated oedema formation.
The molecular composition of tight junctions comprises the proteins occludin (Fig. 2A; Furuse et al. 1993; Hirase et al. 1997), claudins (Furuse et al. 1998; Morita et al. 1999a, 1999b, 1999c) and junctional adhesion molecule (Martin-Padura et al. 1998). These transmembrane proteins bind the adjacent endothelial cell membranes together. They also bind to the intracellular proteins ZO-1 (Stevenson et al. 1986; Watson et al. 1991), ZO-2 (Gumbiner et al. 1991), ZO-3 (Haskins et al. 1998), cingulin (Citi et al. 1988), 7H6 (Zhong et al. 1993) and symplekin (Keon et al. 1996) that couple the tight junctions to the actin cytoskeleton of endothelial cells.
Fig. 2.
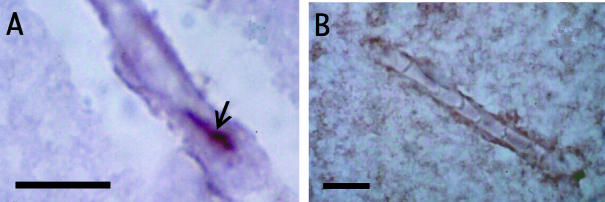
Photomicrographs of cerebral microvessels in longitudinal section from: (A) non-neoplastic human brain showing a tight junction (arrow) immunolabelled with the monoclonal occludin antibody MOC37 (Saitou et al. 1997); (B) occludin immunonegative grade IV astrocytoma. The sections are counterstained with cresyl violet. Scale bars = 10 μm.
Liebner et al. (2000) reported that claudin-1 expression was lost from nearly all microvessels in high-grade astrocytomas. In contrast, claudin-5 and occludin expression were only down regulated in hyperplastic vessels and ZO-1 expression was largely unaffected. Papadopoulos et al. (2001) investigated occludin expression by microvessels in non-neoplastic cerebral cortex, low-grade astrocytomas and high-grade astrocytomas. There was a significant inverse correlation between occludin expression and increasing malignancy. In high-grade astroctomas more than two-thirds of the microvessels did not show any occludin expression (Fig. 2B) and in agreement with the results of Liebner et al. (2000), hyperplastic microvessels did not show any occludin expression. Microvessels in metastatic adenocarcinomas to brain did not show occludin expression (Papadopoulos et al. 2001), as they are derived from tissues whose microvessels do not exhibit tight junctions. These results reveal that the molecular structure of microvessel tight junctions is altered in high-grade astrocytomas as well as their ultrastructure (Liebner et al. 2000; Papadopoulos et al. 2001). Moreover, the presence of a significant inverse correlation between brain CT enhancement (a measure of oedema) and occludin expression (Papadopoulos et al. 2001) suggests that loss of junctional proteins is a central event in opening of the blood brain barrier.
The fact that loss of claudin-1 expression appears to be more severe than loss of occludin expression in high-grade astrocytoma and occludin expression decreases with increasing malignancy suggests that down-regulation of claudin-1 expression is the earlier event in tight junction opening. Liebner et al. (2000) provided freeze-fracture evidence that tight junction particles that are normally located on the P-face (Kniesel et al. 1996) are shifted to the E-face in high-grade astrocytoma. In view of the fact that an increase in the E- face : P-face ratio of tight junction particles correlates with an increase in transendothelial permeability (Wolburg et al. 1994; Tsukita & Furuse, 1999), Liebner et al. (2000) suggested that the loss of claudin-1 from tight junctions correlates with the switch of junction particles from the P- to E-face. Papadopoulos et al. (2001) reported the occurrence of two forms of human brain endothelial occludin. The 55-kDa form was present in non-neoplastic brain and its expression decreases with increasing malignancy. In contrast, the 60-kDa form was absent from non-neoplastic brain, but weakly expressed by all low-grade and some high-grade astrocytomas. Thus, although the loss of occludin expression occurs late in the series of events at the tight junction that lead to opening of the blood–brain barrier, the switch from the 55-kDa to 60-kDa form occurs relatively early. The fact that claudin-5 and ZO-1 expression is not down-regulated in the majority of high-grade astrocytoma microvessels (Liebner et al. 2000) suggests that these junctional proteins are not directly involved in opening of tight junctions in high-grade astrocytoma. This is supported by the fact that the expression and localization of ZO-1 in tight junctions do not correlate with transepithelial resistance in canine kidney cell cultures (Stevenson et al. 1988). The role that junctional adhesion molecule and the intracellular proteins ZO-2, ZO-3, cingulin, 7H6 and symplekin play in opening of the blood–brain barrier in high-grade astrocytomas remains to be investigated.
The sequence of events leading to the disruption of microvessel endothelial cell tight junctions and blood–brain barrier opening in astrocytomas is poorly understood. Given that normal astrocytes play a major role in the induction of the blood–brain barrier (Janzer & Raff, 1997), it is possible that undifferentiated neoplastic astrocytes in high-grade astrocytomas do not produce the factors necessary to maintain the blood–brain barrier in the region of the tumour. However, the chemical nature of any astrocyte-derived inductive signal is not known, although several candidate molecules have been suggested (see Abbott, 2002). A considerable number of humoral factors have been demonstrated to modulate blood–brain barrier permeability (Abbott & Revest, 1991; Abbott, 2000); some of these may be released by neoplastic astrocytes. A third possibility arises from the fact that increased vascularization is a major feature of high-grade astrocytoma. Vascular endothelial growth factor (VEGF) is produced by astrocytomas (Machein et al. 1999) and VEGF receptor expression is up-regulated on astrocytoma microvessels with increasing malignancy (Plate et al. 1994; Valter et al. 1999). VEGF promotes angiogenesis and increases endothelial permeability (Connolly, 1991; Bates et al. 1999; Machein & Plate, 2000). It causes down regulation of occludin expression (Kevil et al. 1998) and an increase in its molecular weight by phosphorylation (Antonetti et al. 1999). Scatter factor/hepatocyte growth factor (SF/HGF) is also expressed by astrocytomas and its expression is up-regulated with increasing malignancy (Lamszus et al. 1999). This cytokine also decreases occludin expression and increases endothelial cell permeability (Jiang et al. 1999). It is therefore possible that VEGF/SF/HGF expression by neoplastic astrocytes causes the phosphorylation of occludin and its subsequent down-regulation, leading to tight junction opening, increased endothelial permeability and cerebral oedema. Current evidence suggests that hypoxia is the driving force for VEGF production in high-grade astrocytomas and thus hypoxia is the most important trigger for angiogenesis and cerebral oedema formation (Plate & Warnke, 1997).
Aquaporin-4 expression and fluid balance in brain tumours
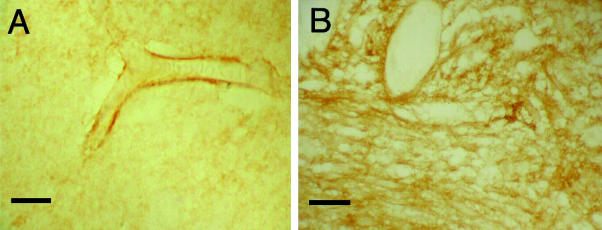
The amount of oedema fluid present in the brain in septic encephalopathy and tumours must reflect a balance between its production and clearance. Evidence has been presented above for the opening of interendothelial cell tight junctions in these conditions, but little is known about the passage of water across cell membranes in the brain. The relatively recent discovery of a family of homologous water channel proteins, the aquaporins (see Borgnia et al. 1999; Ishibashi et al. 2000; Verkman & Mitra, 2000; Verkman, 2002), has stimulated renewed interest in trans-membrane water transport. Ten aquaporins have been characterized in mammals to date. In normal brain, aquaporin-4 expression is restricted to perimicrovessel astrocyte foot processes (Fig. 3A) and the astrocyte foot processes forming the glial limiting membranes (Nielsen et al. 1997; Saadoun et al. 2002). Aquaporin-4 is assembled in endfeet membranes in square (orthogonal) arrays of particles (Rash et al. 1998).
Fig. 3.
Fig. 3 (A) Photomicrograph of a cerebral microvessel from histologically normal human cerebral cortex, showing aquaporin-4 immunolabelled (AB3068, Chemicon) astrocyte end feet surrounding the endothelial cell walls. (B) A photomicrograph showing up-regulation of aquaporin-4 immunoreactivity in astrocyes from a grade IV astrocytoma. Scale bars = 10 μm.
In high-grade astrocytoma, there is massive up-regulation of aquaporin-4 expression throughout the cytoplasm of neoplastic astrocytes (Fig. 3B) and that of reactive atrocytes at the periphery of the tumour (Saadoun et al. 2002). Although metastatic adencarcinoma cells do not express aquaporin-4, there is strong up-regulation of aquaporin-4 expression by reactive astrocytes associated with the tumours (Saadoun et al. 2002). Furthermore, for both types of tumour there was a significant correlation between aquaporin-4 expression and the amount of cerebral oedema present as indicated by the degree of contrast enhancement on CT scans (Saadoun et al. 2002). Therefore, in oedematous brain tumours in which down-regulation of occludin expression indicates open tight junctions (Papadopoulos et al. 2001), there is also increased aquaporin-4 expression. It is unclear whether aquaporin-4 associated with brain tumours functions to enhance oedema fluid formation or its clearance. However, evidence from aquaporin-4 null mice suggests that it is the former, since these mice show less cerebral oedema and a better clinical outcome than controls, following middle cerebral artery occlusion (Manley et al. 2000).
The up-regulation of aquaporin-4 expression is not restricted to brain tumours, since chemical and physical injury (Vizuete et al. 1999) and focal ischaemia (Taniguchi et al. 2000) also lead to aquaporin-4 mRNA up-regulation in regions where the blood–brain barrier is disrupted. These results suggest that it is the disruption of the blood–brain barrier itself and not the cause of the disruption that stimulates aquaporin-4 up-regulation. Astrocytes have a close physical relationship with microvessel endothelial cells (Kacem et al. 1998) and release factors that affect endothelial permeability (see above). There is also evidence that endothelial cells can have a trophic influence on astrocytes (Estrada et al. 1990). It is therefore possible that opening of the blood–brain barrier changes the influence of endothelial cells over neigbouring astrocytes, such that they up-regulate aquaporin-4 expression.
Conclusion
Septic encephalopathy and brain tumours involve disruption of the blood–brain barrier and cerebral oedema formation. Although the initial insults to the brain are quite different, the end-results of increased endothelial permeability and oedema formation are similar. It is therefore possible that a common final pathological pathway exists and if this could be elucidated, it would be an important target for the development of therapeutic strategies. Understanding such a common final pathway may prove useful for the treatment of other conditions that involve breakdown of the blood–brain barrier such as trauma and toxic encephalopathy and allow the selective opening of the barrier for the delivery of pharmacological agents to the brain.
Acknowledgments
I would like to thank Mr R. F. Moss for invaluable help with microscopy and photography.
References
- Abbott NJ, Revest PA. Control of brain endothelial permeability. Cerebrovasc. Brain Metab. Rev. 1991;3:39–72. [PubMed] [Google Scholar]
- Abbott NJ. Inflammatory mediators and modulation of blood–brain barrier permeability. Cell. Mol. Neurobiol. 2000;20:131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott NJ. Astrocyte–endothelial interactions and blood–brain barrier permeability. J. Anat. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J. Biol. Chem. 1999;274:23463–23467. doi: 10.1074/jbc.274.33.23463. [DOI] [PubMed] [Google Scholar]
- Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischaemia. The ischaemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.str.12.6.723. [DOI] [PubMed] [Google Scholar]
- Bar-Sella P, Front D, Hardoff R, Peyser E, Borovich B, Nir I. Ultrastructural basis for different pertechnate uptake patterns by various human brain tumors. J. Neurol. Neurosurg. Psychiatry. 1979;42:924–930. doi: 10.1136/jnnp.42.10.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates DO, Lodwick D, Williams B. Vascular endothelial growth factor and microvascular permeability. Microcirculation. 1999;6:83–96. [PubMed] [Google Scholar]
- Bleck TP, Smith MC, Pierre-Louis SJ, Jares JJ, Murray J, Hansen CA. Neurologic complications of critical medical illnesses. Crit. Care Med. 1993;21:98–103. doi: 10.1097/00003246-199301000-00019. [DOI] [PubMed] [Google Scholar]
- Bone RC. Towards and epidemiology and natural history of SIRS (Systemic inflammatory response syndrome) JAMA. 1992;268:3452–3455. [PubMed] [Google Scholar]
- Borgnia M, Nielsen S, Engel A, Agre P. Cellular and molecular biology of the aquaporin water channels. Ann. Rev. BioChem. 1999;68:425–458. doi: 10.1146/annurev.biochem.68.1.425. [DOI] [PubMed] [Google Scholar]
- Bowton DL, Bertels NH, Prough DS, Stump DA. Cerebral blood flow is reduced in patients with sepsis syndrome. Crit. Care Med. 1989;17:399–403. doi: 10.1097/00003246-198905000-00004. [DOI] [PubMed] [Google Scholar]
- Bradley JR, Wilds D, Rubenstein D. The vascular endothelium in septic shock. J. Infect. 1994;28:1–10. doi: 10.1016/s0163-4453(94)93975-6. [DOI] [PubMed] [Google Scholar]
- Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature. 1988;333:272–276. doi: 10.1038/333272a0. [DOI] [PubMed] [Google Scholar]
- Clawson CC, Harmann JF, Vernier RL. Electron microscopy of the effect of gram-negative endotoxin on the blood–brain barrier. J. Comp. Neurol. 1966;127:183–198. doi: 10.1002/cne.901270204. [DOI] [PubMed] [Google Scholar]
- Coalson JJ, Hinshaw LB, Guenter CA, Berrell EL, Greenfield LJ. Pathophysiologic responses of the subhuman primate in experimental septic shock. J. Lab. Invest. 1975;32:561–569. [PubMed] [Google Scholar]
- Connolly DT. Vascular permeability factor: a unique regulator of blood vessel function. J. Cell. BioChem. 1991;47:219–223. doi: 10.1002/jcb.240470306. [DOI] [PubMed] [Google Scholar]
- Davies DC, Parmar NK, Moss R, Tighe D, Bennett ED. The role of the adrenergic system in septic encephalopathy. Crit. Care. 2001;5(Suppl. 1):S83–S84. [Google Scholar]
- Deng X, Wang X, Andersson R. Endothelial barrier resistance in multiple organs after septic and nonseptic challenges in the rat. J. Appl. Physiol. 1995;78:2052–2061. doi: 10.1152/jappl.1995.78.6.2052. [DOI] [PubMed] [Google Scholar]
- Estrada C, Bready JV, Berliner JA, Pardridge WM, Cancilla PA. Astrocyte growth stimulation by a soluble factor produced by cerebral endothelial cells in vitro. J. Neuropath. Exp. Neurol. 1990;49:539–549. doi: 10.1097/00005072-199011000-00001. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, et al. A novel integral membrane protein localizing at tight junctions. J. Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and – 2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goris RJ. Mediators of multiple organ failure. Intensive Care Med. 1990;16:S192–S196. doi: 10.1007/BF01709699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groothuis DR, Vriesendorp FJ, Kupfer B, Warnke PC, Lapin GD, Kuruvill A, et al. Quantitative measurements of capillary transport in human brain tumors by computed tomography. Ann. Neurol. 1991;30:581–588. doi: 10.1002/ana.410300411. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Lowenkopf T, Apatira D. Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc. Natl Acad Sci. USA. 1991;88:3460–3464. doi: 10.1073/pnas.88.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J. Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, et al. Occludin as a possible determinant of tight junction permeability in endothelial cells. J. Cell Sci. 1997;110:1603–1613. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- Hossman KA. Viability thresholds and the penumbra of focal ischemia. Ann. Neurol. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- Ishibashi K, Kuwahara M, Sasaki S. Molecular biology of aquaporins. Rev. Physiol. Biochem. Pharmacol. 2000;141:1–32. doi: 10.1007/BFb0119576. [DOI] [PubMed] [Google Scholar]
- Janzer RC, Raff MC. Astrocytes induce blood–brain barrier properties in endothelial cells. Nature. 1997;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Jeppsson B, Freund HR, Gimmon Z, James JH, von Meyenfeldt MF, Fischer JE. Blood–brain barrier derangement in sepsis: cause of septic encepalopathy? Am. J. Surg. 1981;141:136–141. doi: 10.1016/0002-9610(81)90026-x. [DOI] [PubMed] [Google Scholar]
- Jiang WG, Martin TA, Matsumoto K, Nakamura T, Mansel RE. Hepatocyte growth factor/scatter factor decreases the expression of occludin and transendothelial resistance (TER) and increasesparacellular permeability in human vascular endothelial cells. J. Cell. Physiol. 1999;181:319–329. doi: 10.1002/(SICI)1097-4652(199911)181:2<319::AID-JCP14>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Kacem K, Lacombe P, Seylaz J, Bonvento G. Structural organisation of the perivascular astrocyte endfeet and their relationship with endothelial glucose transporter: a confocal microscopy study. Glia. 1998;23:1–10. [PubMed] [Google Scholar]
- Keon BH, Schafer S, Kuhn C, Grund C, Franke WW. Symplekin, a novel type of tight junction plaque protein. J. Cell. Biol. 1996;134:1003–1018. doi: 10.1083/jcb.134.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kevil CG, Payne DK, Mire E, Alexander JS. Vascular permeability factor/vascular endothelial cell growth factor-mediated permeability occurs through disorganization of endothelial junctional proteins. J. Biol Chem. 1998;273:15099–15103. doi: 10.1074/jbc.273.24.15099. [DOI] [PubMed] [Google Scholar]
- Knaus WA, Draper EA, Wagner DP, Zimmermann JE. Prognosis in acute organ-system failure. Ann. Surg. 1985;202:685–693. doi: 10.1097/00000658-198512000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniesel U, Risau W, Wolburg H. Development of blood–brain barrier tight junctions in the rat cortex. Dev. Brain Res. 1996;96:229–240. doi: 10.1016/0165-3806(96)00117-4. [DOI] [PubMed] [Google Scholar]
- Lamszus K, Laterra J, Westphal M, Rosen EM. Scatter factor/hepatocyte growth factor (SF/HGF) content and function in human gliomas. Int. J. Dev. NeuroSci. 1999;17:517–530. doi: 10.1016/s0736-5748(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Landis DMD. Brain as a tissue: the interacting cell populations of the central nervous system. In: Ransohoff RM, Benveniste EN, editors. Cytokines and the CNS. Boca Raton: CRC Press; 1996. pp. 63–83. [Google Scholar]
- Lang CH, Bagby GJ, Ferguson JL, Spitzer JJ. Cardiac output and redistribution of organ blood flow in hypermetabolic sepsis. Am. J. Physiol. 1984;246:R331–R337. doi: 10.1152/ajpregu.1984.246.3.R331. [DOI] [PubMed] [Google Scholar]
- Liebner S, Fischmann A, Rascher G, Duffner F, Grote E-H, Kalbacher H, et al. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000;100:323–331. doi: 10.1007/s004010000180. [DOI] [PubMed] [Google Scholar]
- Long DM. Capillary ultrastructure and the blood brain barrier in human malignant brain tumors. J. NeuroSurg. 1970;32:127–144. doi: 10.3171/jns.1970.32.2.0127. [DOI] [PubMed] [Google Scholar]
- Long D. Capillary ultrastructure in human metastatic brain tumors. J. NeuroSurg. 1979;51:409–419. doi: 10.3171/jns.1979.51.1.0053. [DOI] [PubMed] [Google Scholar]
- Machein MR, Kullmer J, Fiebich BL, Plate KH, Warnke PC. Vascular endothelial growth factor expression, vascular volume, and, capillary permeability in human brain tumors. Neurosurgery. 1999;44:732–740. doi: 10.1097/00006123-199904000-00022. [DOI] [PubMed] [Google Scholar]
- Machein MR, Plate KH. VEGF in brain tumors. J. Neuro-Oncol. 2000;50:109–120. doi: 10.1023/a:1006416003964. [DOI] [PubMed] [Google Scholar]
- Maekawa T, Fujii y Sadamitsu D, Yokoya K, Soejima T, Ishikawa T, et al. Cerebral circulation and metabolism in patients with septic encephalopathy. Am. J. Emerg. Med. 1991;9:139–143. doi: 10.1016/0735-6757(91)90175-j. [DOI] [PubMed] [Google Scholar]
- Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen A, et al. Aquaporin-4 deletion in mice reduces brain edema following acute water intoxication. Nature Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J. Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components at tight junction strands. Proc. Natl Acad. Sci. USA. 1999a;96:511–516. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Fujimoto K, Furuse M, Tsukita S. Claudin-11/OSP-based tight junctions of myelin sheaths in brain and Sertoli cells in testis. J. Cell Biol. 1999b;145:579–588. doi: 10.1083/jcb.145.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: Claudin 5/TMVCF constitutes tight junction strands in endothelial cells. J. Cell Biol. 1999c;147:185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Nagelhaus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high resolution immunogold cytochemistry of aquaporin-4 in rat brain. J. NeuroSci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir I, Kohn S, Doron Y, Israel O, Front D. Quantitative analysis of tight junctions and the uptake of 99mTc in human gliomas. Cancer Invest. 1986;4:519–524. doi: 10.3109/07357908609039831. [DOI] [PubMed] [Google Scholar]
- Norenberg MD. Astrocyte responses to CNS injury. J. Neuropathol. Exp. Neurol. 1994;53:213–220. doi: 10.1097/00005072-199405000-00001. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Lamb FJ, Moss RF, Davies DC, Tighe D, Bennett ED. Faecal peritonitis causes oedema and neuronal injury in pig cerebral cortex. Clin. Sci. 1999;96:461–466. [PubMed] [Google Scholar]
- Papadopoulos MC, Saadoun S, Woodrow CJ, Davies DC, Costa-Martins P, Moss RF, et al. Occludin expression in microvessels of neoplastic and non-neoplastic human brain. Neuropathol. Appl. Neurobiol. 2001;27:384–395. doi: 10.1046/j.0305-1846.2001.00341.x. [DOI] [PubMed] [Google Scholar]
- Parillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, et al. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction and therapy. Ann. Intern. Med. 1990;113:227–242. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- Pine RW, Wertz MJ, Lennard ES, Dellinger EP, Carrico CJ, Minshew BH. Determinants of organ malfunction or death in patients with intra-abdominal sepsis. A discriminant analysis. Arch. Surg. 1983;118:242–249. doi: 10.1001/archsurg.1983.01390020084014. [DOI] [PubMed] [Google Scholar]
- Plate KH, Brier G, Weich HA, Mennel HD, Risau W. Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int. J. Cancer. 1994;59:520–529. doi: 10.1002/ijc.2910590415. [DOI] [PubMed] [Google Scholar]
- Plate KH, Warnke PC. Vascular endothelial growth factor. J. Neuro-Oncol. 1997;35:365–372. doi: 10.1023/a:1005845307160. [DOI] [PubMed] [Google Scholar]
- Rash JE, Yasumura T, Hudson CS, Agre P, Nielsen S. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and epedymocyte plasma membranes in rat brain and spinal cord. Proc. Natl Acad. Sci. USA. 1998;95:11981–11986. doi: 10.1073/pnas.95.20.11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Davies DC, Krishna S, Bell BA. Aquaporin-4 expression is increased in oedematous human brain tumours. J. Neurol. Neurosurg. Psychiat. 2002;72:262–265. doi: 10.1136/jnnp.72.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Inazawa J, Fujimoto K, et al. Mammalian occludin in epithelial cells: its expression and subcellular distribution. Eur. J. Cell Biol. 1997;73:222–231. [PubMed] [Google Scholar]
- Seitz RJ, Wechsler W. Immunohistochemical demonstration of serum proteins in human cerebral gliomas. Acta Neuropathol. 1987;73:145–152. doi: 10.1007/BF00693780. [DOI] [PubMed] [Google Scholar]
- Shibata T. Ultrastructure of capillary walls in human brain tumors. Acta Neuropathol. 1989;78:561–571. doi: 10.1007/BF00691283. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Anderson JM, Goodenough DA, Mooseker MS. Tight junction structure and ZO-1 content are identical in two strains of Madin-Darby canine kidney cells which differ in transepithelial resistance. J. Cell Biol. 1988;107:2401–2408. doi: 10.1083/jcb.107.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson BR, Siciliano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Yamashita T, Kumura E, Tamatani M, Kobayashi A, Yohawa T, et al. Induction of aquaporin-4 water channel mRNA after focal ischemia in rat. Mol. Brain Res. 2000;78:131–137. doi: 10.1016/s0169-328x(00)00084-x. [DOI] [PubMed] [Google Scholar]
- Thapar K, Rutka JT, Laws ER., Jr . Brain edema, increased intracranial pressure, vascular effects, and other epiphenomena of human brain tumors. In: Kay AH, Laws ER, editors. Brain Tumors: and Encyclopedic Approach. Edinburgh: Churchill Livingstone; 1995. pp. 163–169. [Google Scholar]
- Thijs LG, de Boer JP, de Groot MC, Hack CE. Coagulation disorders in septic shock. Int. Care Med. 1993;19(Suppl. 1):S8–S15. doi: 10.1007/BF01738944. [DOI] [PubMed] [Google Scholar]
- Tighe D, Moss R, Boghossian S, Heath FM, Chessum B, Bennett ED. Multi-organ damage resulting from experimental faecal peritonitis. Clin. Sci. 1989;76:269–276. doi: 10.1042/cs0760269. [DOI] [PubMed] [Google Scholar]
- Tighe D, Moss R, Hynd J, et al. Pretreatment with pentoxyfylline improves the hemodynamic and histologic changes and decreases neutrophil adhesiveness in a pig fecal peritonitis model. Crit. Care Med. 1990;18:184–189. doi: 10.1097/00003246-199002000-00012. [DOI] [PubMed] [Google Scholar]
- Tighe D, Moss R, Heywood G, Al-Saady N, Webb A, Bennett D. Goal-directed therapy with dopexamine, dobutamine and Volume expansion: effects of sytemic oxygen transport on hepatic ultrastructure in porcine sepsis. Crit. Care Med. 1995;23:1997–2007. doi: 10.1097/00003246-199512000-00008. [DOI] [PubMed] [Google Scholar]
- Tighe D, Moss D, Bennett D. Cell surface adrenergic receptor stimulation modifies the endothelial response to SIRS. Systemic Inflammatory Response Syndrome. New Horizons. 1996;4:426–424. [PubMed] [Google Scholar]
- Tighe D, Moss R, Bennett D. Porcine hepatic response to sepsis and its amplification by an adrenergic receptor α1 agonist and a β2 antagonist. Clin. Sci. 1998;95:467–478. [PubMed] [Google Scholar]
- Tran DD, Groenveld AB, van der Meulen J, Nauta JJ, Strack van Schijndel RJ, Thijs LG. Age, chronic disease, sepsis, organ system failure, and mortality in a medical intensive care unit. Crit. Care Med. 1990;18:474–479. doi: 10.1097/00003246-199005000-00002. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M. Occludin and claudins in tight junction strands: leading or supporting players? Trends. Cell Biol. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- Valter MM, Hugel A, Huang HA, Cavenee WK, Wiestler OD, Pietsch T. Expression of the Ets-1 transcription factor in human astrocytomas is associated with Fms-like tyrosine kinase-1 (Flt-1)/vascular endothelial growth factor recepto-1 synthesis and neoangiogenesis. Cancer Res. 1999;59:5608–5614. [PubMed] [Google Scholar]
- Verkman AS. Aquaporin water channels and endothelial cell function. J. Anat. 2002;200:617–627. doi: 10.1046/j.1469-7580.2002.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkman AS, Mitra AK. Structure and function of aquaporin water channels. Am. J. Physiol. 2000;278:F13–F28. doi: 10.1152/ajprenal.2000.278.1.F13. [DOI] [PubMed] [Google Scholar]
- Vizuete ML, Venero JL, Vargas C, Ilundain AA, Echevarria M, Machado A, et al. Differential upregulation of aquaporin-4 mRNA expression in reactive astrocytes after brain injury: potential role in brain edema. Neurobiol. Dis. 1999;6:245–258. doi: 10.1006/nbdi.1999.0246. [DOI] [PubMed] [Google Scholar]
- Watson PM, Anderson JM, van Itallie CM, Doctrow SR. The tight-junction-specific protein ZO-1 is a component of the human and rat blood–brain barriers. Neurosci. Lett. 1991;129:6–10. doi: 10.1016/0304-3940(91)90708-2. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Neuhaus J, Kniesel U, Krauss B, Schmid EM, Ocalan M, et al. Modulation of tight junction structure in blood–brain barrier endothelial cells. Effects of tissue culture, second messengers and co-cultured astrocytes. J. Cell Sci. 1994;107:1347–1357. doi: 10.1242/jcs.107.5.1347. [DOI] [PubMed] [Google Scholar]
- Young CB, Bolton CF, Archibald YM, Austin TW, Wells GA. The electroencephalogram in sepsis-associated encephalopathy. J. Clin. NeuroPhysiol. 1992;9:145–152. doi: 10.1097/00004691-199201000-00016. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Saitoh T, Minase T, Sawada N, Enomoto K, Mori M. Monolonal antibody 7H6 reacts with a novel tight junction-associated protein distinct from ZO-1, cingulin and ZO-2. J. Cell Biol. 1993;120:477–483. doi: 10.1083/jcb.120.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink S, Rosen P, Sackman B, Lemoine H. Regulation of endothelial permeability by β-adrenoceptor agonists: contribution of β1- and β2-adrenoceptor. Biochim. Biophys. Acta. 1993;1178:286–298. doi: 10.1016/0167-4889(93)90206-5. [DOI] [PubMed] [Google Scholar]