Abstract
The proximal region of the superficial digital flexor tendon of pigs passes under the tibiotarsal joint, where it is subjected to compressional and tensional forces. This region was divided into a surface portion (sp), which is in direct contact with the bone and into a deep portion (dp), which is the layer opposite the articulating surface. The purpose of this work was to analyse the distribution and organisation of the collagen bundles and proteoglycans in the extracellular matrix in sp and dp. Toluidine-blue-stained sections were analysed under a polarising microscope. Strong basophilia and metachromasia were observed in sp, demonstrating accumulation of proteoglycan in a region bearing compression, but the intensity was reduced the further layers were from the bone. Linear dichroism confirmed that the glycosaminoglycan molecules were disposed predominantly parallel to the longest axis of the collagen fibrils. Birefringence analysis showed a higher molecular order and aggregation of the collagen bundles in areas where the tension was more prominent. The crimp pattern was more regular in dp than in sp, probably as a requirement for tendon stretching. The optical anisotropy exhibited by the collagen bundles also confirmed the helical organisation of the collagen bundles in the tendon. Hyaluronidase digestion caused a decrease in the basophilia, but this was not eliminated, supporting the idea that in the matrix, proteoglycans are not completely available to the enzyme action.
Keywords: biomechanics, collagen, extracellular matrix, proteoglycan
Introduction
Tendons have the role of transmitting tension forces from the muscle to the bone (Vogel & Koob, 1989; Vidal & Carvalho, 1990; Cribb & Scott, 1995; Birch et al. 1997; Milz et al. 1998). Any tendons that wrap around a bony pulley are subject to both tensional and compressional forces. Tendons are distinguished by an extensive extracellular matrix (ECM) rich mainly in type I collagen fibrils orientated along the length of the tendon. Collagen makes up about 90% of the dry mass of this tissue (Nimni & Harkness, 1988). These collagen bundles act as transductors, transforming mechanical energy into signals that stimulate the fibroblast to modulate the molecular arrangement of the extracellular environment. Production of signals in the ECM is dependent on the levels of macromolecular organisation of their components (Vidal, 1966, 1969).
Proteoglycans (PGs) constitute about 1% of the tendon and are represented mostly by the small PGs decorin and fibromodulin (Vogel & Heinegård, 1985). Non-collagenous glycoproteins are also present, but in a lesser amount. Large PGs are present especially in compressed regions (Vogel et al. 1994).
Studies based on the anisotropic properties of collagen fibres showed that the glycanic chains of the PGs were aligned parallel to the collagen fibrils and displayed a helical structure under certain experimental conditions (Vidal, 1963, 1964). Later it was proposed that the polypeptide core was tilted with respect to the collagen fibrils, and the glycanic chains, with various degrees of helical arrangement, were parallel to the collagen fibrils (Vidal & Mello, 1984). For a good account of optical anisotropy see Módis (1991).
The collagen fibres are distributed in different patterns. In tissue or tendon regions where tension is exerted in all directions, the collagen bundles are interwoven without regular orientation, while in regions where the tension is only in one direction, the fibres exhibit an orderly parallel arrangement (O'Brien, 1997).
Some of the collagen fibres in tendons have a wavy form known as crimp (Gathercole & Keller, 1991). It is detected by polarisation microscopy since it is revealed when one observes the birefringence resulting from the macromolecular orientation of collagen fibres (Vidal, 1995). The variations in crimp in the different regions of the same tendon, as well as the distribution of the fibres and their bundles, could reveal details of the fibre organisation in tendons that might explain the crimp organisation (Vidal, 1995).
Studies carried out with tendons from rabbits (Merrilees & Flint, 1980), canines (Okuda et al. 1987), bovines (Vogel et al. 1986; Koob & Vogel, 1987; Robbins et al. 1997) and amphibians (Carvalho & Vidal, 1994; Carvalho & Felisbino, 1999; Felisbino & Carvalho, 1999) showed that areas under compression have an increased glycosaminoglycan (GAG) content. Part of these PGs aggregate with hyaluronan (Vogel & Heinegård, 1985; Evanko & Vogel, 1990). These authors have also demonstrated that the composition and organisation of the extracellular matrix is not the same for the different regions of the tissue, depending on the presence of biomechanical forces.
Fibrocartilage has been found in areas of tendon subjected to compressive and frictional load regimes, in addition to tension (Benjamin et al. 1995). These areas exhibit not only a weave basket-like distribution of collagen fibres and rounded cells usually in lacunae, but also an increased amount of large PGs (Felisbino & Carvalho, 1999).
In swines, the superficial digital flexor tendon (SDFT) experiences different mechanical forces throughout, and our aim was therefore to analyse the effect of compressive forces on the organisation of the extracellular matrix in the proximal region of the SDFT of pigs. This region passes under the tibiotarsal joint and experiences compressive and frictional forces in addition to tensional forces.
Materials and methods
Biological material
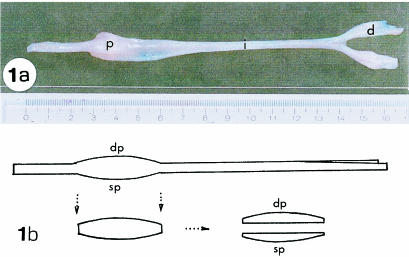
Five male 45-day-old pigs, of the large White lineage, obtained from the Experimental Surgical Nucleus of the University Medical School – UNICAMP, were used. The hind limbs were dissected to obtain the superficial digital flexor tendon (SDFT) (Fig. 1a). Only the proximal region, which passes under the tibiotarsal joint and is subjected to expressive compressive forces, was employed here. This region was divided into sp, which was near the bone, and dp, which was the layer opposite the articulating surface (Fig. 1b).
Fig. 1.
Dorsal view of the superficial digital flexor tendon (SDFT). (a) The SDFT was divided into a proximal region (p), which is under the tibiotarsal joint and is subject to compression in addition to tension, an intermediate region (i) where only tension forces are present, and a distal region (d) region, which also withstands compressive force. (b) A diagrammatic representation of a lateral view of the tendon, showing the sp portion, which remains in contact with the bones of the tibiotarsal joint, and the dp portion, the layer opposite the articulating layer.
Polarised-light microscopy
The tendon was fixed in 10% paraformaldehyde in Milloning's buffer, pH 7.4, for 24 h, washed in distilled water several times, dehydrated in ethanol, embedded in Paraplast Plus so that the segments were orientated to obtain histological sections parallel to the longest axis of the tendon, and then cut into 6-µm sections. The preparations were stained with a 0.025% toluidine blue solution in McIlvaine buffer at pH 4.0 (Mello & Vidal, 1980). The toluidine-blue-stained sections were studied visually to detect basophilia and metachromasia and to determine their distribution. Selective absorption of polarised light may be observed when toluidine blue planar molecules bind to orientated macromolecules of the ECM. The difference in the absorbance of polarised light when the material is parallel and perpendicular to the plane of polarized light is known as linear dichroism (LD). LD observations were carried out by alternating the orientation of the major axis of fibres, parallel (A∥), and perpendicular (A⊥) to the azimuth of the electric vector of the polarised light (EVPL). The Zeiss Pol Fotomicroscope was used for this purpose.
Birefringence and morphometric analyses
The birefringence and area measurements were performed under monochromatic (λ = 546 nm) light and using software from the Image-Global Laboratory Data Translation (USA). Areas of crimp were accurately segmented and were expressed in square micrometres after calibration with a micrometric slide using the objective neofluar Zeiss 16× optovar 1.25. The birefringence was expressed as transmittance after transformation of the grey values into transmittance values for the same segmented areas (B. C. Vidal, 2000, pers. obs.). Statistic analysis was performed using the Mann–Whitney test (Minitab-Release 11).
Enzymatic treatment
Some sections of the proximal region of the SDFT were treated with hyaluronidase (1 mg enzyme per mL of 0.9% NaCl solution) for 2 h at 37 °C (Kiernan, 1981). The sections were then washed in distilled water and stained with 0.025% toluidine blue for 15 min, washed again, clarified in xylene and mounted in Entelan (Merck).
Results
Sections of sp and dp of the pig tendon were stained with toluidine blue and observed under polarised light. They exhibited LD with a stronger metachromasy, especially in sp, which is the portion nearest to the bone (Fig. 2).
Fig. 2.
Linear dichroism of the toluidine-blue-stained sections. (a) and (b) correspond to the absorbance when the longest axis of the tendon is perpendicular and parallel, respectively, to the azimuth of the polariser. Comparison of images (a) with (b) reveals higher absorption when the fibres are perpendicular to the plane of the polarised light. Note the stronger metachromasy in sp, which is near the bone. In the deep layer (dp), the staining and even metachromasy are noticeably reduced. (b) Same section, but with fibres positioned parallel to the polarised light plane. Scale bars = 238 µm.
In this work, sections of the proximal region of the SDFT were analysed, staining with the cationic dye, toluidine blue. A more intense staining may be observed in sp, near the bone, as compared to the opposite layer, dp, which is more distant from the bone. When observations were carried out using a polarising microscope, LD was detected with a stronger metachromasy, especially in sp. When the fibres were perpendicular (Fig. 2a) to the azimuth of the EVPL, greater absorbance was detected. In contrast, in the parallel position (Fig. 2b) less light absorbance was observed. The further the collagen bundles were from the bone, and thus closer to the opposite layer, so the metachromasy and LD were clearly diminished (Fig. 2b).
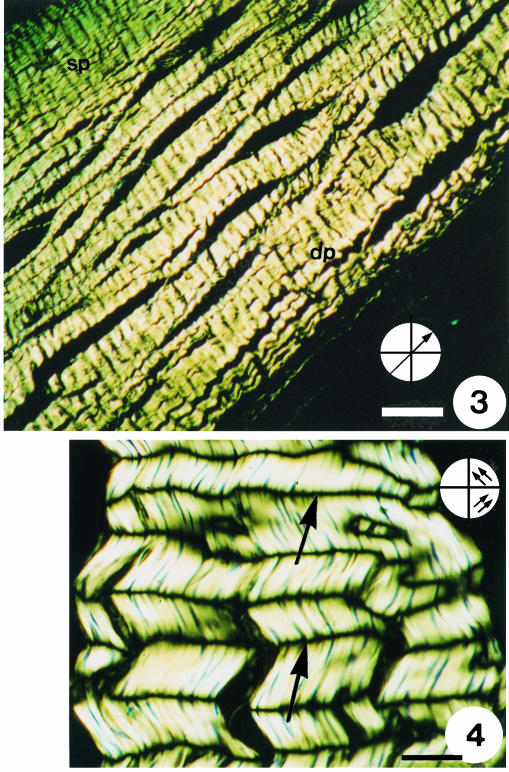
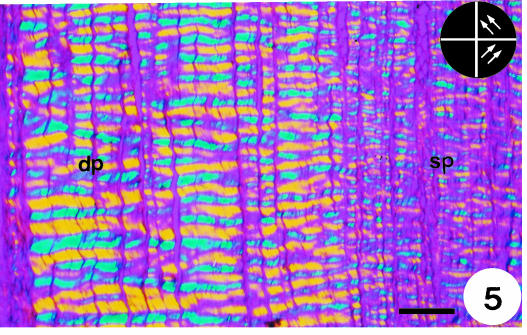
When the toluidine-blue-stained sections were observed with a crossed analyser and polariser, at 45° with respect to the polarisers (⊕), a strong birefringence was detected in the median portion of the proximal region of the SDFT (Fig. 3). A greenish colour was observed in sp, representing the less compacted collagen fibres present in that area. When the major axis of the tendon was positioned parallel to one of the polarisers, the collagen bundles, which were aligned at 45° to the polarisers (Fig. 4), exhibited a striking birefringence and a well-defined wavy-like pattern (crimp). Changes in the crimp were observed in the collagen bundles nearest the bone. When the tendon was positioned parallel to one of the polarisers and a first-order red gypsy compensator was used, birefringent colours could be seen (Fig. 5). The fibres that appear in blue are in the addition position with respect to the compensator direction (γ), which means that the fibre electric oscillation is at 45° to the polariser axis. In contrast, the yellow colour means that the same source of birefringence is in the subtraction position. The regularity of the collagen bundles was evident in the portion distant from the bone, but not in sp, and the crimp patterns seemed to be more regular in dp where tension dominated and compression was reduced. The standard deviations of the measurements (sp standard deviation = 17, dp standard deviation = 14) confirmed this assumption. The size of the crimp as well as the birefringence values were larger in dp than in sp (Table 1).
Fig. 3.
Birefringent images of sections of the proximal region of the SDFT, stained by toluidine blue, pH 4.0. The main axis of the tendon was positioned at 45° to the polarisers (⊕). Stronger birefringence is observed in the layer opposite (dp) the bone. A weaker birefringence and a greenish staining were observed in the superficial portion (sp), near the bone and where compressive forces were present. Scale bar = 238 µm.
Fig. 4.

Birefringent images of sections of the proximal region of the SDFT, stained by toluidine blue, pH 4.0. The main axis of the tendon was positioned at 45° to the polarisers (⊕). Detail of the crimp aspect of the dp layer. In this case the tendon was positioned parallel to the plane of polarisation. Dark regions (→) indicate areas of extinction where the fibres are positioned parallel to one of the planes of polarisation. Scale bar = 37 µm.
Fig. 5.
Similar image to those in Figs 3 and 4, but with no staining and using a first-order red compensator. The tendon is positioned parallel to one of the polarisers. The blue colour represents the fibres orientated in an additive position with respect to the compensator, and the yellow colour to the subtractive position. The different crimp patterns in the sp and dp portions are clearly visible. Inset to the right is a diagram representing the direction of the crimps. Scale bar = 238 µm.
Table 1.
Non-parametric test (Mann–Whitney) of the birefringence (transmittance) and area (µm2) measurements for the dp and sp portions. η1 and η2 correspond to the median values. W represents the rank sum (Bhattacharyya & Johnson, 1977) obtained for the sp and dp portions. The test is significant at 0.0001 (adjusted for ties) (Farthofer & Lee, 1995)
| η1-dp | η2-sp | η1 − η2 | W | |
|---|---|---|---|---|
| Areas | 92.57 | 59.09 | 23 | 136 229.0 |
| Birefringence | 41.23 | 35.8 | 6.11 | 139 156.0 |
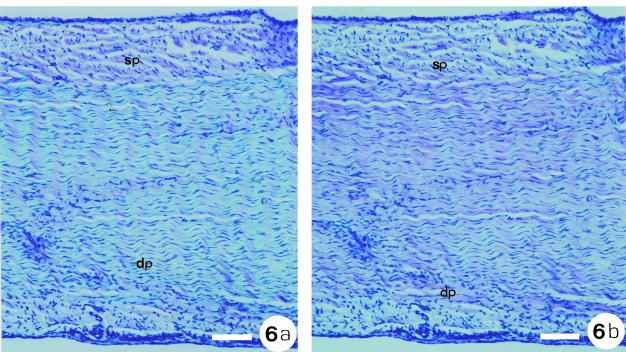
The sections treated with hyaluronidase, stained by toluidine blue, and observed under polarised light (Fig. 6), showed some metachromasy when the tendon was perpendicular (Fig. 6b) to the polariser, in the region far from the bone. In sp no difference with respect to the dichroism was observed.
Fig. 6.
Distal portion of the sp and dp regions treated with testicular hyaluronidase and stained with toluidine blue, pH 4.0. Observations were made under polarised light. (a) The fibres were positioned parallel to the plane of polarised light. (b) The fibres were perpendicular to the plane of polarised light. Note that even with enzymatic digestion, glycosaminoglycans were not completely removed, indicating that they are not totally available to the enzyme attack in situ, probably due to their interactions with other components of the extracellular matrix. The linear dichroism (A⊥ > A∥) reinforces the idea that proteoglycans are orientated along the axis of the tendon. Scale bar = 238 µm.
Discussion
Tendons which pass under a joint are an excellent biological model for studying the biomechanical and morphological adaptations as a function of the presence of different mechanical forces.
The process of evolution has selected a special composition and organised structure for the ECM, to attain certain biomechanical and functional advantages (Vidal & Carvalho, 1990). The hypothesis of this work was that for the two different portions of the tendon, respectively under pressure and tension, there were different assemblies of macromolecules to supra-organize those regions in order to respond to their biomechanical exigencies.
The collagen molecules are the most abundant component of the tendon, arranged in fibrils, forming fibres that are assembled into bundles of collagen fibres (Jozsa et al. 1991). The mechanical properties of tendons depend on the orientation of the fibrils and collagen bundles, on the diameter of the fibrils and on the level of organisation, which plays an important role in the mechanical properties of this tissue (Birk et al. 1989; Vidal & Carvalho, 1990).
Studies based on the birefringence analysis in tendon sections stained with toluidine blue have shown that the protein core of the PG are tilted with respect to the long axis of the collagen fibrils, while the glycosaminoglycans (GAGs) are statistically parallel (Vidal & Vilarta, 1988). Similar results were found in the articular cartilage (Vidal & Vilarta, 1988; Vilarta & Vidal, 1989).
Here the dichroism observed in sections of the compressive portion of the swine tendon, besides exhibiting greater light absorption when the tendon was perpendicular to the plane of the polarised light, once again confirming that the GAGs are disposed parallel to the long axis of the tendon (Vidal & Mello, 1984), also showed a stronger metachromasy in the sp portion, where the compressive forces were more intense than in the dp. This result is similar to results obtained with rabbit (Merrilees & Flint, 1980), bovine (Vogel et al. 1994) and frog (Carvalho & Vidal, 1994) tendons.
Topological differences with respect to the organisation of the ECM inside a region under compression were observed in rabbits (Merrilees & Flint, 1980) and bovines (Koob & Vogel, 1987), in agreement with the histochemical and histophysiological data obtained in the present research.
The crimp patterns in the sp and dp portions were quite different, and denote the importance of the arrangement and organisation of the collagen fibres for the biomechanical requirements of the tissue. The greater degree of compactness of the collagen fibres observed in the dp portion as compared to the sp portion was indicative of the differential organisation of the ECM in these two portions, which experience tension and compressive forces, respectively.
Studies using segments of tendon (Viidik & Ekholm, 1968) and ligaments (Yahia et al. 1990) demonstrated that the fibres of tendons have a wavy course in relaxed preparations, but not when the tissue was placed in a device to keep them in a stretched state, demonstrating the importance of the wavy aspect of the collagen bundles in reducing the strain caused by the tensional forces.
The different patterns of crimp organisation observed here are represented by a greater birefringence and larger areas of crimp in the dp region compared with the sp region. The stress and strain to which the collagen bundles are submitted require larger dimensions of crimp areas, giving more opportunity for the collagen bundles to adapt to tension. Collagen fibres and bundles in the dp portion exhibited molecular order and a three-dimensional helical superstructure that satisfy the thermodynamic requirements created by the biomechanical demands of that area. In evolutionary terms, the physicochemical characteristics of the tendon macromolecules would favour a specific self-assembly process to generate the tendon supra-organization (Vidal, 1995). Natural selection has determined a special composition and an organized structure in the tendon.
In conclusion, our results have shown that inside a region withstanding tensional and compressional forces, the ECM organisation depends on the dominant type of biomechanical forces present. So near the bone surface, where stronger compressive forces are expected, the crimp morphology is not as well defined as in the opposite portion, i.e. further from the bone, which experiences weaker compressive forces. Also it was clear that PG is much more prominent in the portion bearing more compressive forces. The more abundant PG and its interaction with collagen fibrils may have influence on the crimp morphology observed in areas under more compression.
Acknowledgments
We are grateful to the Experimental Surgical Nucleus of the University Medical School – UNICAMP/Campinas, for supplying the pigs and to Mr E. D. Rodrigues for the drawing of Fig. 1(B). V. L. C. Feitosa was the recipient of a CAPES-PICDT fellowship.
References
- Benjamin M, Qin S, Ralphs JR. Fibrocartilage associated with human tendons and their pulleys. J. Anat. 1995;187:625–633. [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya GK, Johnson RA. Statistical concepts and methods. In: Bhattacharyya GK, Johnson RA, editors. Nonparametric Inference. New York: John Wiley, Sons; 1977. pp. 505–539. [Google Scholar]
- Birch HL, Wilson AM, Goodship AE. The effect of exercise-induced localized hyperthermia on tendon cell survival. J. Exp. Biol. 1997;200:1703–1708. doi: 10.1242/jeb.200.11.1703. [DOI] [PubMed] [Google Scholar]
- Birk DE, Southern JF, Zycband EI, Fallon JT, Trelstad RL. Collagen fibril bundles: a branching assembly unit in tendon morphogenesis. Development. 1989;107:437–443. doi: 10.1242/dev.107.3.437. [DOI] [PubMed] [Google Scholar]
- Carvalho HF, Vidal BC. Cell types and evidence for traumatic cell death in a pressure-bearing tendon of Rana catesbeiana. Tissue Cell. 1994;26:841–848. doi: 10.1016/0040-8166(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Carvalho HF, Felisbino SL. The development of the pressure-bearing tendon of the bullfrog Rana catesbeiana. Anat. Embryol. 1999;200:55–64. doi: 10.1007/s004290050259. 10.1007/s004290050259. [DOI] [PubMed] [Google Scholar]
- Cribb AM, Scott JE. Tendon response to tensile stress: an ultrastructural investigation of collagen: proteoglycan interactions in stressed tendon. J. Anat. 1995;187:423–428. [PMC free article] [PubMed] [Google Scholar]
- Evanko SP, Vogel KG. Ultrastructure and proteoglycan composition in the developing fibrocartilaginous region of bovine tendon. Matrix. 1990;10:420–436. doi: 10.1016/s0934-8832(11)80150-2. [DOI] [PubMed] [Google Scholar]
- Farthofer RN, Lee ES. Introduction to Biostatistics. New York: Academic Press; 1995. pp. 281–282. [Google Scholar]
- Felisbino SL, Carvalho HF. Identification and distribution of type VI collagen in tendon fibrocartilages. J. Submicroscopic Cytol. Pathol. 1999;31:187–195. [PubMed] [Google Scholar]
- Gathercole LJ, Keller A. Crimp morphology in the fibre-forming collagens. Matrix. 1991;11:214–234. doi: 10.1016/s0934-8832(11)80161-7. [DOI] [PubMed] [Google Scholar]
- Jozsa L, Kannus P, Balint JB, Reffy A. Three-Dimensional ultrastructure of human tendon. Acta Anat. 1991;142:306–312. doi: 10.1159/000147207. [DOI] [PubMed] [Google Scholar]
- Kiernan JA. Histological and Histochemical Methods. Oxford: Pergamon Press; 1981. p. 166. [Google Scholar]
- Koob TJ, Vogel KB. Proteoglycan synthesis in organ cultures from regions of bovine tendon subjected to different mechanical forces. Biochem. J. 1987;246:589–598. doi: 10.1042/bj2460589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello ML, Vidal BC. Práticas de Biologia Celular. 1. São Paulo: S, Edigard Blücher; 1980. p. 71. [Google Scholar]
- Merrilees MJ, Flint MH. Ultrastructural study of tension and pressure zones in a rabbit flexor tendon. Am. J. Anat. 1980;157:87–106. doi: 10.1002/aja.1001570109. [DOI] [PubMed] [Google Scholar]
- Milz S, McNeilly C, Putz R, Ralphs J, Benjamin M. Fibrocartilages in the extensor tendons of the interphalangeal joints of human toes. Anat. Record. 1998;252:264–270. doi: 10.1002/(SICI)1097-0185(199810)252:2<264::AID-AR11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Módis L. Organization of the extracellular matrix: a polarization microscopic approach. In: Módis L, editor. Physical Backgrounds of Polarization Microscopy. Boca Raton, Florida: CRC Press; 1991. pp. 10–29. [Google Scholar]
- Nimni ME, Harkness RD. Molecular structure and function of collagen. In: Nimni ME, editor. Collagen, Vol. I: Biochemistry. Boca Raton: CRC Press; 1988. [Google Scholar]
- O'Brien M. Structure and metabolism of tendon. Scand. J. Med. Sci. Sports. 1997;7:55–61. doi: 10.1111/j.1600-0838.1997.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Okuda Y, Gorski KN, Amadio PC. Biomechanical, histological and biomechanical analyses of canine tendon. J. Orthopaedic Res. 1987;5:60–68. doi: 10.1002/jor.1100050109. [DOI] [PubMed] [Google Scholar]
- Robbins RJ, Evanko SP, Vogel KG. Mechanical loading and TGF-β regulate proteoglycan synthesis in tendon. Arch. Biochem. Biophysics. 1997;240:682–688. doi: 10.1006/abbi.1997.0102. [DOI] [PubMed] [Google Scholar]
- Vidal BC. Pleochroism in tendon and its bearing to acid mucopolysaccharides. Protoplasma. 1963;56:529–536. [Google Scholar]
- Vidal BC. The part played by the mucopolysaccharides in the from birefringence of the collagen. Protoplasma. 1964;59:472–479. [Google Scholar]
- Vidal BC. Macromolecular disorientation in detached tendons. Protoplasma. 1966;62:121–132. doi: 10.1007/BF01248078. [DOI] [PubMed] [Google Scholar]
- Vidal BC. Collagen bundle regulation and control. Revista Brasileira Pesquisas Médicas E Biológicas. 1969;2:356–359. [Google Scholar]
- Vidal BC, Mello ML. Proteoglycan arrangement in tendon collagen bundles. Cellular Mol. Biol. 1984;30:195–204. [PubMed] [Google Scholar]
- Vidal BC, Vilarta R. Articular cartilage: collagen II-proteoglycan interactions. Availability of reactive groups. Variation in birefringence and differences as compared to collagen I. Acta Histochem. 1988;83:189–205. doi: 10.1016/s0065-1281(88)80056-4. [DOI] [PubMed] [Google Scholar]
- Vidal BC, Carvalho HF. Aggregational state and molecular order of tendons as a function of age. Matrix. 1990;10:48–57. doi: 10.1016/s0934-8832(11)80137-x. [DOI] [PubMed] [Google Scholar]
- Vidal BC. Crimp as part of a helice structure. C. R. Acad. Sci. Paris, Sci. la Vie/Life Sci. 1995;318:173–178. [PubMed] [Google Scholar]
- Viidik A, Ekholm R. Light and electron microscopic studies of collagen fibers under strain. Z. Anat. Entwicklungsgeschichte. 1968;127:154–164. doi: 10.1007/BF00521981. [DOI] [PubMed] [Google Scholar]
- Vilarta R, Vidal BC. Anisotropic and biomechanical properties of tendons modified by exercise and denervation: aggregation and macromolecular order in collagen bundles. Matrix. 1989;9:55–61. doi: 10.1016/s0934-8832(89)80019-8. [DOI] [PubMed] [Google Scholar]
- Vogel KG, Heinegård D. Characterization of proteoglycans from adult bovine tendon. J. Biol. Chem. 1985;260:298–306. [PubMed] [Google Scholar]
- Vogel KG, Keller EJ, Lenhoff RJ, Campell K, Koob TJ. Proteoglycan synthesis by fibroblast cultures initiated from regions of adult bovine tendon subjected to different mechanical forces. Eur. J. Cellular Biol. 1986;41:102–112. [PubMed] [Google Scholar]
- Vogel KG, Koob TJ. Structural specialization in tendons under compression. Int. Rev. Cytol. 1989;115:267–293. doi: 10.1016/s0074-7696(08)60632-4. [DOI] [PubMed] [Google Scholar]
- Vogel KG, Sandy JD, Pogány G, Robbins JR. Aggrecan in bovine tendon. Matrix Biol. 1994;14:171–179. doi: 10.1016/0945-053x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Yahia LH, Drouin G, Newman N. Structure-function relationship of human spinal ligaments. Z. Mikrosk. Anat. Forsch. 1990;104:33–45. [PubMed] [Google Scholar]