Abstract
Evans Blue Dye (EBD) is widely used to study cellular membrane permeability and has recently been utilised in mdx mice to identify permeable skeletal myofibres that have become damaged as a result of muscular dystrophy. EBD has the potential to be a useful vital stain of myofibre permeability in other models of skeletal muscle injury and membrane-associated fragility. The parameters for its use for such purposes were optimised in the present study. Of particular interest is the use of EBD to identify the onset of muscle damage. This study compared intravenous vs. intraperitoneal injection; tissue fixation; volume of EBD; time of availability in tissue; and persistence after injection in mdx mice (with endogenous muscle damage) and control mice. Satisfactory labelling of permeable myofibres was seen in frozen sections viewed with fluorescence microscopy when intraperitoneal injection of a 1% EBD solution injected at 1% volume relative to body mass was administered between 16 and 24 h prior to tissue sampling. EBD labelling was then assessed in three mouse models of experimental injury and repair – cut injury, whole muscle grafts, and exercise-induced muscle damage. These experiments demonstrated that (i) following a cut injury across myofibres, EBD penetrated up to 150 µm from the injury site over a 20-h period; (ii) EBD was present throughout myofibres of avascular whole muscle graft by one day after transplantation; and (iii) damaged myofibres were detected within 20 min after controlled lengthening–contraction exercise. This simple and inexpensive technique has sensitivity for the detection of increased myofibre permeability and/or sublethal damage that has advantages over other traditional histological techniques at the light microscopy level.
Keywords: cell marker, exercise, muscular dystrophy, myofibre damage, skeletal muscle
Introduction
Evans Blue Dye (EBD – FW 960.82 g mol−1) is widely used to study blood vessel and cellular membrane permeability as it is non-toxic, it can be administered as an intravital dye and it binds to serum albumin – using this as its transporter molecule. The EBD–albumin conjugate (EBA) can be: (i) identified macroscopically by the striking blue colour within tissue (Matsuda et al. 1995); (ii) observed by red auto-fluorescence in tissue sections examined by fluorescence microscopy (Matsuda et al. 1995; Brussee et al. 1997; Tidball et al. 1999); and (iii) assessed and quantified by spectrophotometry for serum samples (Brown et al. 1992), or homogenised tissue (Oztas et al. 1992).
Recently, EBD has been used in mdx mice to identify damaged skeletal myofibres that had become permeable owing to muscular dystrophy (Matsuda et al. 1995; Brussee et al. 1997). The mdx mouse is an animal model for human Duchenne's Muscular Dystrophy (DMD), which is an X chromosome-linked disorder characterised by progressive muscle degeneration and weakness. Matsuda et al. (1995) injected EBD into the tail vein of mdx mice and demonstrated that myofibres that contained EBD were identifiable, via haematoxylin and eosin (H&E) staining, as swollen or hypercontracted. Brussee et al. (1997) also injected EBD into the tail vein of mdx and C57Bl mice in their examination of the fragility of the dystrophic muscle during downhill running on a treadmill, and they similarly observed that the auto-fluorescent fibres showed evidence of hypercontraction or degeneration. In marked contrast to the mdx mouse there was little penetration of EBD in other myopathies resulting from defects in the laminin α-2 chain (Straub et al. 1997). The intensity of EBD fluorescence has been used to identify mechanically induced damage to skeletal muscle in a suspension–reloading model of muscle injury (Tidball et al. 1999).
Despite the wide use of EBD as an in vivo marker of plasma membrane permeability, there has been no detailed presentation of standardised guidelines for its use in skeletal muscle. This is addressed in the present study. Of particular interest were (i) the efficacy of delivery of EBD by intraperitoneal compared with intravenous injection of EBD; (ii) what methods of tissue fixation and processing were optimal for the display and retention of the EBD signal; (iii) how long does EBD persist within injured myofibres; (iv) whether the volume of EBD injected influences the strength of signal observed; (v) how rapidly does EBD get into the circulation in order reliably to detect damaged myofibres; and (vi) whether labelling of damaged myofibres with EBD relied on an intact circulation. This paper defines the parameters for the use of EBD and then applies this to three models of experimental skeletal muscle damage: (i) cut injury; (ii) whole muscle graft; and (iii) exercise-induced muscle damage.
Materials and methods
Animals
Adult, 6- to 12-week-old, normal control C57Bl/10ScSn (hereafter referred to as C57Bl), BALB/c, and dystrophic mdx male mice were used in these experiments. Mice were housed in cages, supplied with food and water without restriction, and maintained in a 12-h light/dark air-conditioned (20–25 °C) environment. All animal procedures were approved by the Animal Ethics and Experimentation Committee of the University of Western Australia in accordance with the guidelines of the National Health and Medical Research Council of Australia.
Animal preparation
Mice were anaesthetised by inhalation of a gaseous mixture of halothane (Fluothane, Zeneca) and nitrous oxide (BOC Gases, Perth) in oxygen (0.5 L min−1 halothane, 0.3 L min−1 N2O, 0.4 L min−1 O2).
Mode of injection of Evans Blue Dye
Mice were injected with 1% EBD (Sigma, St Louis, MO, USA) (w/v) in phosphate-buffered saline (PBS, pH 7.5) sterilised by passage through a Millex®-GP 0.22 µm filter (Millipore, Bedford, MA, USA) and stored at 4 °C. Intravenous (i.v.) injections were made to either of the dorsal veins of the tail. Intraperitoneal (i.p.) injections were made into the right side of the peritoneal cavity. After injection, animals were returned to their cage and allowed food and water ad libitum.
Methods of tissue fixation
In experiments in which fresh frozen tissue was required, anaesthetised mice were killed by cervical dislocation, the tibialis anterior (TA) and extensor digitorum longus (EDL) muscles of the hind limbs were removed, and sliced transversely at the maximum diameter, mounted vertically in tragacanth gum (Sigma) on cork blocks and snap frozen in isopentane, cooled by liquid nitrogen, and stored at −80 °C. In some experiments mice were perfused, via a cannula inserted into the left ventricle of the heart, with 50 mL of PBS containing 0.1% heparin (David Bull Laboratories, Melbourne, Australia) followed by 50 mL of freshly prepared 4% (w/v) paraformaldehyde (PFA) (Merck, Dermstadt, Germany) in PBS (pH 7.5). The TA and EDL muscles were removed and frozen as previously described.
In other experiments in which tissues were fixed by immersion, the complete hindlimbs were immersed in freshly prepared 4% PFA (pH 7.5) for 3 h. Subsequently, the TA and EDL muscles were removed from the limbs and processed for paraffin embedding. Comparison of the efficacy of various fixatives was examined in muscle tissue samples that had been immersed in either 10% buffered formal saline (BFS), 4% PFA or cold 95% ethanol for 3 h.
Histochemistry and fluorescent imaging
Frozen sections (10 µm) were cut at −21 °C on a Leica (CM3050) cryostat, dipped in cold acetone (−20 °C) for 1 min and then air-dried at room temperature (20–22 °C) (RT). The sections were then dipped into xylene (Merck) and mounted with DPx (BDH, Poole, UK) or polyvinyl alcohol (PVA) and a glass coverslip. Where possible, 10 serial sections from different locations within each block were collected, and alternate sections stained with H&E or mounted for fluorescence imaging. Sections from paraffin-embedded muscle were cut at 5 µm thickness on a microtome, baked at 60 °C for 1 h on glass slides and cooled at room temperature before being de-paraffinised in Xylene, rehydrated through graded ethanol concentrations and air-dried. All slides were stored in reduced light conditions to minimise photobleaching.
Viewing and imaging
The H&E-stained muscle sections were viewed with bright-field light microscopy, whereas the unstained frozen sections were viewed by fluorescent microscopy (Leica DMRBE) with the use of an N2.1 green wavelength filter set, a band pass filter of 515–560 nm and a low pass filter of 590 nm. Images were acquired with an ORBIS CCD Spectrasource 16 camera. For experiments in which comparisons were made between different parameters for the use of EBD, images were collected at standardised exposure settings. All digital images were stored as raw images (TIFF files) without any processing of the image.
Immunohistochemistry
Direct detection of serum albumin by an antibody would avoid the need for pre-injection of EBD and ideally would be carried out on paraffin sections in which morphology is consistently superior to frozen sections. Subsequently, anti-rat serum albumin (ICN Aurora, OH, USA) conjugated to horseradish peroxidase (HRP) was used to visualise serum albumin in frozen and paraffin sections (McNeil & Khakee, 1992).
Defining parameters for the use of EBD
1. Mode of injection
To compare i.v. with i.p. injection of EBD, four mdx mice (20 ± 2 g) were injected with a solution of 1% EBD at a volume of 1% of body mass (BM) 1 mg EBD/0.1 mL PBS/10 gBM (Matsuda et al. 1995), referred to subsequently as the standard 1% EBD. Two mice were injected via i.v. and two via an i.p. route. One of each pair was killed at 7 h and 24 h after injection, respectively. The TA and EDL muscles were prepared for frozen sections.
2. Fixation
To compare fixed vs. frozen tissues, one mdx mouse (26 g) was injected i.p. with the standard 1% EBD 24 h before being killed. The TA muscles were removed, cut transversely, and 10 serial sections for each condition were fixed in either: 4% PFA, 10% BFS, cold (−20 °C) 95% ethanol, or snap frozen in isopentane.
3. Volume of EBD
The aim of this experiment was to determine whether the volume of EBD injected i.p. would affect the EBD signal present in permeable myofibres of the mdx muscle or would bring about labelling of normal C57Bl myofibres (C57Bl muscles do not show damage, in contrast to dystrophic mdx mice). Three mdx mice (22 ± 2 g) were injected i.p. with a 1% EBD solution, each at different volumes (0.5%, 1%, 2.5%) relative to body mass, 1 day prior to killing the animals. Two C57Bl mice (26 ± 1 g) were also injected with the 1% EBD solution 1 day prior to death, one at 1%, and the other at 2.5% volume EBD relative to body mass. Paraffin-embedded muscle tissue sections were prepared from the right hindlimb of each mouse, and frozen sections from muscles of the left limb.
4. Timing and persistence of EBD within permeable myofibres
To determine the timing and persistence of EBD after i.p. injection, EBD was injected i.p. into eight mdx mice (23 ± 3 g) at 4, 3, 2 and 1 day, and at 16, 8, 4 and 2 h prior to killing the animals. Two control C57Bl mice (26 ± 1 g) were injected at 4 days and 1 day prior to death. Muscles from the right and left leg were prepared for paraffin embedding and frozen sections, respectively. The presence of EBD in frozen sections observed by fluorescence microscopy was scored on a Likert seven-point scale. For the intensity of staining in individual myofibres (not the number of myofibres containing EBD) the scale, from 0 to 6, was defined as ranging from 0 = no signal; 1 = minimal signal; 2 = weak signal; 3 = good signal; 4 = moderate signal; 5 = strong signal; 6 = very strong signal. A score on this scale for the red fluorescent labelling was recorded for the myofibres and the interstitium, viewed at a final magnification of 200×.
Experimental muscle damage
The optimal conditions were defined from the above studies (1% EBD at 1% BM injected i.p. 16–24 h before sampling, henceforth referred to as 1% EBD), and applied to three experimental muscle injuries.
5. Cut injury
This experiment, using a cut injury to ‘normal’ muscle, was designed to demonstrate (i) how rapidly EBD would be detected within damaged myofibres after a known injury; and (ii) the penetration of EBD along the length of damaged myofibres (before resealing occurs). Nine C57Bl mice had a single transverse cut injury made in the mid-region of the left TA, as described by Grounds et al. (1992). Mice were immediately injected i.p. with 1% EBD, and killed at 4, 8, 16, 20 and 24 h post-injury. Frozen sections were prepared from the TA muscles of the left (cut) and right (control) legs.
6. Whole muscle grafts
To test whether EBD could label damaged myofibres in an avascular muscle graft, four BALB/c mice (20 g) received an autograft of intact whole EDL muscle as described by Roberts et al. (1989). Immediately (time 0) and at 1, 2 and 7 days after surgery one mouse was injected i.p. with 1% EBD. Mice were killed 1 day after EBD injection and muscles fixed with different protocols as detailed in Table 1.
Table 1.
Protocols used to optimise fixation and visualisation of EBD after transplantation of whole muscle autografts
| Time of injection of EBD after grafting (days) | Time of sampling and fixation (days) | Method of fixation |
|---|---|---|
| 0 | 1 | Immersion fixation 4% PFA, 15 min, snap-frozen – (fixed-frozen) |
| 1 | 2 | Immersion fixation 4% PFA, 15 min, snap-frozen – (fixed-frozen) |
| 2 | 3 | Perfusion fixation 4% PFA, snap frozen (perfusion-frozen) |
| 7 | 8 | Isopentane quenched (snap-frozen) |
7. Exercise-induced muscle damage
The permeability of muscle fibres as a result of mild exercise-induced damage was investigated following controlled lengthening–contractions of the TA and EDL muscles (Armstrong et al. 1991; Faulkner & Brooks, 1994). Three C57Bl mice (25 g) were assessed for muscle performance on a custom-made servo-controlled mouse dynamometer that controlled angular displacement, velocity and acceleration of the ankle joint (Hamer et al. 1998) while the exposed common peroneal nerve was directly stimulated via needle electrodes connected to a Grass™ S-88 stimulator. Torque generated by the anterior crural muscles (TA, EDL, and Extensor Hallucis Longus) about the ankle joint axis was recorded from an axial torque transducer placed in-line with the axis of the servomotor (Hamer et al. 1998). The neutral position of the ankle, defined as 0°, was with the foot perpendicular to the tibia. The mean peak torque of the final 50 ms of three repeated 150-ms-duration electrical stimuli, with 30 s between stimuli, was used to determine the isometric torque-volt, torque-frequency, and torque-angle relationships of the right anterior crural muscles. These relationships determined the optimal voltage and frequency of stimulation, as well as the optimal angle for torque production. With the use of the determined stimulation conditions, 20 lengthening–contractions, performed at 1000° s−1 with 30 s between each lengthening–contraction, were conducted on the descending limb of the torque-angle relationship from −15° plantarflexion to −55° plantarflexion. Isometric torque-volt and torque-angle relationships were re-established 10 min before the mice were killed.
To test how rapidly damaged myofibres could be detected after exercise, two mice were injected i.p. with 1% EBD 18 h prior to exercise. Mice were sampled at 20 and 60 min after exercise. To test whether permeability of the myofibres, resulting from this controlled exercise-induced damage, was transient (i.e. the local membrane repaired/resealed quickly), or whether it resulted in sustained permeability, a third mouse was injected with EBD 48 h after exercise and sacrificed 24 h later. Frozen sections were prepared from the TA and EDL muscles.
Results
Defining optimal parameters
1. Mode of injection
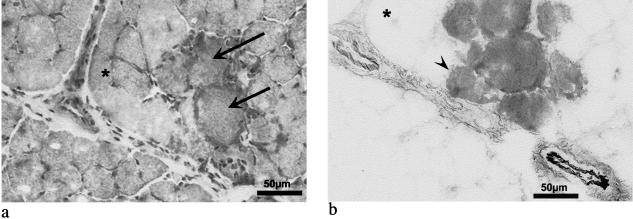
Similar intensity of the red auto-fluorescent EBD signal was seen within myofibres and in the surrounding interstitium in mdx mice in which EBD was injected i.v. and i.p. Figure 1(b) shows the presence of EBD within individual myofibres as well as the presence of EBD in the surrounding interstitium in the transverse section of the muscle from an mdx mouse injected i.p. and the tissue sampled at 24 h. i.p. injection was just as effective and is more convenient than i.v. Therefore, i.p. injection was the method of choice for future experiments. EBD was occasionally seen in myofibres that had a relatively normal histological appearance in H&E-stained sections (compare Fig. 1a and b), indicating that EBD has greater sensitivity than H&E staining for the detection of mildly damaged myofibres.
Fig. 1. Detection of myofibre damage in mdx muscle following i.p. injection of 1% EBD 24h prior to sampling. Transverse frozen sections of the TA stained with H&E (a) shows degeneration of myofibres evident by eosinophilic swollen myofibres (arrows); and (b) EBD in myofibres (dark stained myofibres) shown as an inverted greyscale fluorescent image of the similar field to (a) (see reference asterisk). The interstitium and blood vessel walls also show EBD labelling (see b). Note the fibre containing EBD (arrowhead) in contrast with the relatively normal histological appearance of this fibre in (a).
2. Fixation
Muscles from mdx mice that had been fixed (in 4% PFA, 10% BFS, or cold (−20 °C) ethanol) and paraffin processed showed less EBD signal, compared with fresh-frozen tissue. Macroscopic examination of fresh non-sectioned muscle showed many conspicuous ‘blue’ (EBD) areas, whereas after fixation, the EBD was not as distinct. This was confirmed by viewing frozen and paraffin sections with fluorescence microscopy. Fresh-frozen sections showed strong red auto-fluorescent signal localised in permeable myofibres and the interstitium. In contrast, red fluorescence was weak in all paraffin sections and it appeared that the processing of tissue for paraffin embedding washed out the interstitial EBD as well as that within permeable myofibres. Therefore, fresh-frozen tissue was subsequently used in all other investigations.
A further contrast was seen when frozen and paraffin sections were examined with the use of an antibody to serum albumin. Although paraffin sections were of superior morphology compared with frozen sections, the detection of serum albumin in both was less sensitive than that provided by the EBD technique. When viewed with bright-field microscopy, myofibres that appeared to have a slightly stronger colorimetric signal (brown coloration) for the antibody to serum albumin were distinctively red with fluorescence microscopy of the same section (data not shown).
3. Volume of EBD injected
Injections of different volumes of EBD relative to body mass showed that a 0.5% volume (of a 1% EBD solution) did label permeable myofibres and the interstitium, but the signal strength was greater with a 1% injection volume (i.e. 0.25 mL/25 g mouse). The 2.5% relative volume gave no greater labelling of myofibres, but the interstitium and endothelial walls of blood vessels gave greater signal intensity. In control C57Bl mice injected i.p. with the 2.5% volume of EBD 24 h prior to being killed, strong EBD labelling occurred in the interstitium but no EBD was evident within the myofibres (data not shown). Therefore, injection of a 1% volume of EBD relative to body mass was used in all further studies.
4. Persistence of EBD within permeable myofibres
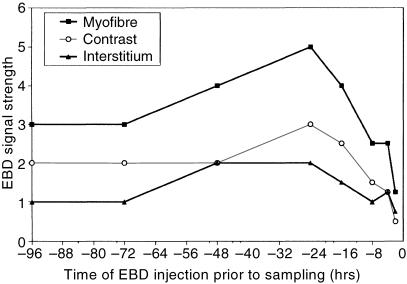
The presence of EBD in frozen sections of muscle from mdx mice that had been injected with EBD at various times prior to sampling was semi-quantified with the use of a Likert seven-point scale (see Methods) in which 6 indicates stronger signal. Graphical representation of the score obtained across these times for the EBD signal in the myofibre and interstitium is presented in Fig. 2. A greater difference in scores represents a greater contrast between the fluorescent signal in myofibres and the interstitium. The greatest contrast occurred when EBD was injected at 24 and 16 h before tissue sampling (Fig. 2). Useful contrast was still evident when EBD was injected up to 4 days and as little as 8 h before sampling (Fig. 2). Sections of two control (uninjured) C57Bl mice that were injected with 1% EBD at 4 and 1 day prior to being killed showed no EBD within myofibres, although signal was evident in the interstitium.
Fig. 2. Strength of EBD fluorescent signal in skeletal myofibres (▪) and interstitium (▴), as well as the resultant contrast (^) between the myofibres and the interstitium of mdx mice given an intraperitoneal injection of 1% EBD at various times before sampling.
Experimental muscle damage
5. Cut injury
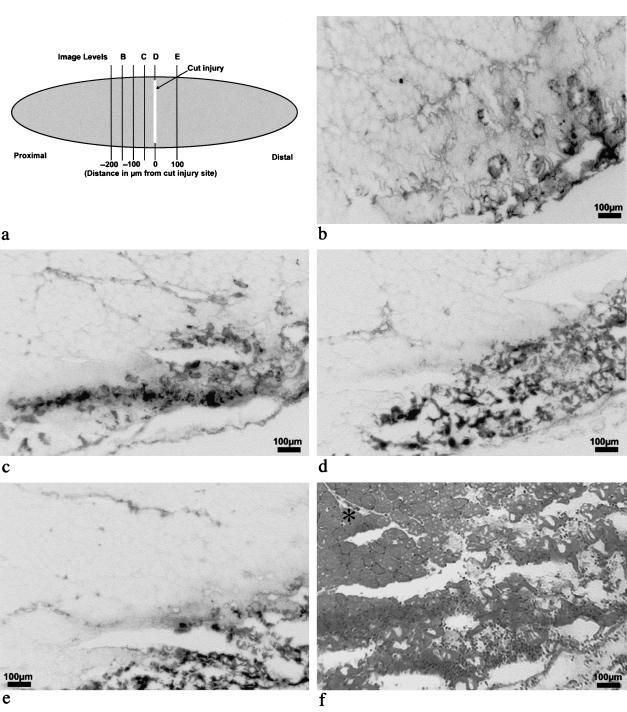
To investigate the penetration of EBD along the length of injured myofibres, an i.p. injection of 1% EBD was given 3–5 min after a transverse cut injury through the belly of the TA of six C57Bl mice with muscle sampled over the following 24 h. Serial transverse sections were taken at multiple 50-µm levels cutting from proximal (level 1, −350 µm) to distal (level 10, +100 µm), through the site of the cut injury (approximately level 8, 0 µm). Figure 3(a) represents the site of the cut injury and the transverse sectioning of the TA muscle. The spread of EBD within damaged myofibres is shown in the transverse sections in Fig. 3(b–f). Some EBD could be detected in myofibres around 150 µm proximal to the cut-injured site (Fig. 3b), but there was little signal beyond this (not shown). EBD increased dramatically at 50 µm proximal (Fig. 3c), and was distinct at 0 µm (Fig. 3d), i.e. closest to the cut-injured site. The EBD signal diminished with distance but was still readily detectable at 100 µm distal to the cut-injured site (Fig. 3e). Figure 3(f) shows the extent of damage within the cut-injured region (close to Level D) and the histological appearance of undamaged muscle.
Fig. 3. Distribution of EBD after cut injury in TA muscle of a C57Bl mouse shown in transverse frozen sections (10µm). The locality of the section levels (b–e) relative to the site of cut injury is depicted in (a). 1% EBD was injected immediately after injury and muscle sampled after 20 h. Inverted greyscale images (b to e) demonstrate the distribution of EBD distant from, and at, the site of damage (dark coloured regions). An H&E-stained section (f), obtained from close to level D (at the injury site), demonstrates inflammatory infiltrate and destruction of tissue with normal morphology in adjacent myofibres (*).
6. Whole muscle grafts
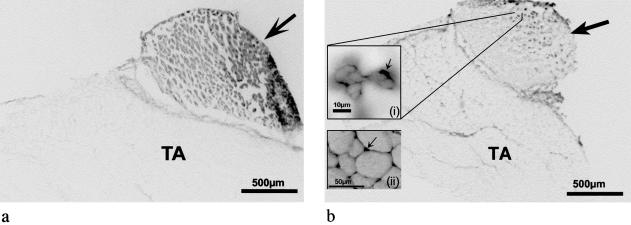
Transplantation of a whole EDL muscle graft results in complete disruption to all vascular supply to the EDL but no direct damage to the structure of the muscle fibres. Permeability to EBD throughout all myofibres of the graft (Fig. 4a) was apparent within 1 day (EBD injected at time 0) and this was maintained for up to a week. To confirm that newly formed myotubes in the graft excluded EBD, one mouse was injected i.p. with 1% EBD at 7 days after grafting (when the necrotic muscle has been replaced by myotubes) and the mouse killed 1 day later (Day 8). The new myotubes were devoid of EBD (Fig. 4b), and EBD was predominantly seen in new blood vessels of the graft and persisting necrotic muscle tissue, as well as some labelling of interstitium and surrounding epimysium.
Fig. 4. EBD staining in whole EDL muscle grafts (arrow) transplanted over TA muscles of BALB/c mice. Mice were injected i.p. with 1% EBD at 1 day (a) or 7 days (b) after grafting, sampled 24 h later and presented as transverse frozen sections (10 µm). A high power inset image of the Day 8 graft (b (i)) shows high EBD signal (black coloured areas) in the immediate area surrounding new myofibres (arrow). A high power inset image (b (ii)) of a representative area of the underlying TA muscle, illustrates the containment of EBD (arrow) to the interstitium in non-damaged skeletal muscle.
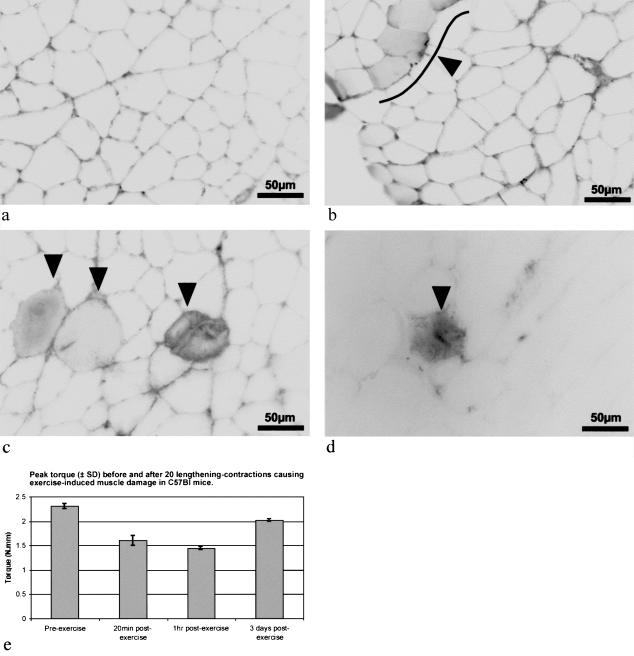
7. Exercise-induced muscle damage
TA muscles of C57Bl mice were subjected to lengthening–contraction exercise 18 h after EBD injection, and were sampled at 20 and 60 min after completion of the exercise. EBD was present in myofibres as early as 20 min after exercise (Fig. 5b). The EBD-positive myofibres at 1 h after exercise also appeared to be swollen (Fig. 5c). The non-exercised control muscle (Fig. 5a), as well as much of the exercised muscle (Fig. 5b–d), showed no evidence of EBD within myofibres. Figure 5(e) shows the mean peak torque achieved during a tetanic isometric contraction at the optimal angle for torque production, for the anterior crural muscles of three mice sampled 20 and 60 min after exercise. These data show that, after the 20 lengthening–contractions, there was a 30% reduction in torque production by 20 min and a further reduction by 1 h to a value of 62% of the pre-exercise torque. By 3 days after exercise, torque production had recovered to 87% of the pre-exercise value (Fig. 5e). Furthermore, at 3 days after exercise, few EBD-positive myofibres were present and the intensity of EBD signal within any myofibres was quite weak (Fig. 5d).
Fig. 5. EBD in exercise-induced muscle damage. Inverted fluorescence greyscale images of transverse frozen sections (10 µm) of the TA muscles of C57Bl mice that have been injected i.p. with 1% EBD at 18 h before exercise (20 lengthening contractions of the anterior crural muscles of the right lower leg). The left leg served as the non-exercised control (a) and showed no EBD within myofibres. In exercised muscles, EBD was seen in some myofibres of muscles sampled at 20 min (b) and 1 h (c) after exercise. EBD was detected by 20 min (arrowhead & line [showing grouped myofibres] in b) and was more pronounced by 1 h after exercise (see arrows in c). (d) Where EBD was injected 2 days after exercise, the muscle, sampled 1 day later, showed EBD staining in a very limited number of myofibres. (e) The mean (± SD) peak torque, recorded from the final 50 ms of three repeated 150-ms maximal isometric contractions (at optimal voltage, angle and frequency) in three C57Bl mice, before and after exercise at various times shows the deficit after exercise, and recovery towards pre-exercise values by 3 days.
Discussion
EBD has been widely used as an in vivo marker for cellular permeability in many different physiological systems (see Introduction). This study has defined the optimal parameters for EBD use in mouse skeletal muscle and then applied the technique in three disparate models of muscle injury. Optimal parameters for the use of EBD, determined for experiments in mouse skeletal muscle, are as follows. A 1% sterile solution (w/v) in PBS (pH 7.5) injected at 1% volume relative to body mass injected by either an i.p. or i.v. route is effective. However, i.p. injection is easier and the method of choice where mice have dark coloured skin (C57Bl and mdx mice), which can make the i.v. injection into a tail vein difficult. The i.p. injection of EBD should be at least 8 h before sampling of the skeletal muscle tissue, being optimally injected 16–24 h before sampling. Useful contrast in signal between the interstitium and myofibres containing EBD, however, is still evident when an i.p. injection is given 4 days before tissue sampling. EBD is seen macroscopically as a blue coloration in tissue; however, this is not readily visible in 10-µm sections with light microscopy. Tissue samples should be snap-frozen by the standard technique of isopentane quenching in liquid N2 and stored at −80 °C. Frozen sections are cut at 10 µm, cold acetone fixed for 2 min, air-dried, quick-dipped in Xylene, and mounted under a coverslip with DPX. Fluorescent microscopy, with the use of a green light filter set (N2.1), a band pass filter of 515–560 nm, and a low pass filter of 590 nm, provides a bright red signal seen in the interstitium, epimysium, perimysium and within permeable myofibres, as well as in the endothelial wall of blood vessels. This allows EBD to be used in quantitative methods, e.g. counting of permeable myofibres or by measurement of fluorescent intensity within myofibres (Tidball et al. 1999). While standard precautions should be taken to prepare and store sections in reduced light conditions to avoid photo-bleaching, this appears not to be critical. Slides stored at room temperature in covered slide trays have been viewed 12 months after initial viewing, with EBD signal still very evident and able to be analysed.
Other models used to examine membrane permeability in damaged muscle have included: Procion Orange as a vital stain for myofibres in mdx mice (Pagel & Partridge, 1999); antibody to serum albumin after mechanical loading injury of the myocardium (Clarke et al. 1995); and lysine fixable fluorescein dextran (∼10 kDa MW) (FdxLys) after lengthening–contractions of skeletal muscle (McNeil & Khakee, 1992). The results of the present study show that EBD provides a more sensitive method of detection than an antibody to serum albumin and, while FdxLys labelling (McNeil & Khakee, 1992) appears to give similar findings, the simplicity and low cost of the EBD method are appealing.
Experimental use of EBD
The use of EBD as an in vivo marker of myofibre permeability facilitates the investigation of the effect of pathology, muscle injury, and exercise on instantaneous and/or sustained permeability of skeletal myofibres. The present study confirms that the EBD technique is highly sensitive and is able to detect permeable myofibres in mouse muscle affected by muscular dystrophy (mdx) that were not detected by standard histological techniques, e.g. H&E staining. Matsuda et al. (1995) and Pagel & Partridge (1999) have also identified large rounded fibres in H&E section as those that were EBD or Procion Orange positive, respectively. Increased permeability of myofibres to large molecular weight proteins, such as serum albumin, would disturb the osmotic balance between a myofibre and the interstitium, resulting in increased fluid component within the myofibre, giving rise to the swollen rounded myofibres. Such increased permeability would also result in the efflux of myo-proteins e.g. myoglobin, and creatine kinase, that are common features of muscular dystrophy (Driessen-Kletter et al. 1990) and exercise-induced muscle damage (Croisier et al. 1996). The use of these sensitive vital dyes has challenged previous concepts on myofibre fragility. For example, Pagel & Partridge (1999), through the use of Procion Orange, demonstrated that myofibre damage is persistent at greater ages (> 15 weeks) in mdx mice than had previously been evident.
In the present study the EBD technique was used in several models of experimental injury. In the cut injury model, EBD labelling was greatest (in the mouse sampled at 20 h) about 50 µm along the length of the myofibres either side of the cut site and was greatly diminished at a distance of 200 µm. This labelling reflects the cumulative movement of EBD (bound to albumin) along the damaged myofibres from the cut injury site during the 20 h following injection. It is known that resealing of damaged myofibres occurs by 12 h after injury although, before this time, hypercontraction of myofibrils may serve to limit movement of molecules into the damaged myofibres (Papadimitriou et al. 1990). Since we have shown that useful EBD signal is detectable within 8 h after i.p. injection, the EBD that penetrated into portions of myofibres prior to sealing (at 12 h after injury) would be readily detectable. However, the strength of signal within the ‘sealed’ portion of myofibres might be lower than the EBD that continues to accumulate in the damaged, ‘excluded’ portion of the myofibre. The kinetics of transport and extent to which EBD actually penetrates through dead or hypercontracted myofibrils at sites of damage is unclear. This situation might be clarified by injecting EBD i.p. 24 h before injury to ensure maximum labelling of permeable myofibres at the time of injury and assessing the extent of labelling by the examination of longitudinal sections.
In the whole muscle grafts, in which the myofibres are not physically damaged, the presence of EBD throughout myofibres of the avascular grafts by 24 h was surprising, and shows that the myofibres of these grafts rapidly become permeable to large MW proteins (such as albumin) that are present in the interstitial fluid. This has implications for the transport of high MW proteins in and out of damaged tissue and the ability potentially to engineer transport of material into damaged or pathological muscle, e.g. in muscular dystrophy.
In exercise-induced muscle damage, the EBD technique provided evidence of increased myofibre permeability within 20 min following 20 lengthening–contractions of the TA muscle in mice. The sublethal damage that may occur during lengthening–contraction exercise at early time points following muscle damage is difficult to detect by conventional histological techniques in tissue sections (Warren et al. 1999). Evidence of damage to sarcomeres has been observed with the use of electron microscopy, within 1 h following lengthening–contraction exercise (Fridén et al. 1983) and immunohistochemistry has shown loss of desmin within 1 h of completion of lengthening–contraction exercise (Fridén & Lieber, 1994). Additional studies are in progress to determine the earliest times when damage can be detected after exercise and what extent of damage is required for the subsequent breakdown (necrosis) of myotubes. The relatively lower numbers of myofibres containing EBD 3 days after the exercise is probably accounted for by resealing of damaged myofibres before the EBD injection. Since resealing of necrotic myofibres occurs by 12 h and is completed by 24 h after injury (Papadimitriou et al. 1990), when EBD was injected at 2 days (and muscle sampled at 3 days), the EBD would have been excluded from the resealed portions of the previously permeable myofibres. Alternatively, it is possible that much of the damage seen at 20 min does not result in necrosis (followed by regeneration) of myofibres. Instead the permeable sarcolemma may be locally repaired, thus preventing breakdown of the myofibre (Grounds, 1998; Togo et al. 1999). Such rapid local membrane repair (McNeil & Terasaki, 2001) would result in reduced damage being detectable in muscles injected after exercise at 2 days with EBD, and sampled at 3 days. Techniques, such as the use of nitroblue tetrazolium (Tang et al. 1998), to assess the viability of the EBD-positive myofibres may be useful to determine whether changes in the in vivo myofibre permeability detected by EBD commit the myofibre to cell death. Detailed analysis of muscle samples, for evidence of muscle cell death and regeneration at various times after exercise, should resolve this situation and such studies are in progress in our laboratory. The apparent improvement in structural integrity at 3 days was also accompanied by a reduced torque deficit when compared with the torque recorded 10 min after the initial damaging exercise. The deficit in torque production immediately following the initial bout of exercise and the return of muscle torque production over time observed in the present experiment are consistent with the published literature (for review see Warren et al. 1999) that report that the decrease in muscle function is greatest immediately after the damaging exercise and progressively returns to normal over a 10-day period.
The value of the EBD technique as a simple and sensitive vital marker of early muscle damage lies in its sensitivity to detect instantaneous and/or sustained changes in permeability of sublethal myofibre injury. The technique is being used in further studies to assess the thresholds of muscle damage in myopathic and normal muscles subjected to various exercise regimes.
References
- Armstrong RB, Warren GL, Warren JA. Mechanisms of exercise-induced muscle fibre injury. Sports Med. 1991;12:184–207. doi: 10.2165/00007256-199112030-00004. [DOI] [PubMed] [Google Scholar]
- Brown MA, Mitar DA, Whitworth JA. Measurement of plasma Volume in pregnancy. Clin. Sci. 1992;83:29–34. doi: 10.1042/cs0830029. [DOI] [PubMed] [Google Scholar]
- Brussee V, Tardif F, Tremblay JP. Muscle fibers of mdx mice are more vulnerable to exercise than those of normal mice. Neuromuscular Disorders. 1997;7:487–492. doi: 10.1016/s0960-8966(97)00115-6. [DOI] [PubMed] [Google Scholar]
- Clarke MS, Caldwell RW, Chiao H, Miyake K, McNeil PL. Contraction-induced cell wounding and release of fibroblast growth factor in heart. Circulation Res. 1995;76:927–934. doi: 10.1161/01.res.76.6.927. [DOI] [PubMed] [Google Scholar]
- Croisier JL, Camus G, Deby-Dupont G, Bertrand F, Lhermerout C, Crielaard JM, et al. Myocellular enzyme leakage, polymorphonuclear neutrophil activation and delayed onset muscle soreness induced by isokinetic eccentric exercise. Arch. Physiol. Biochem. 1996;104:322–329. doi: 10.1076/apab.104.3.322.12904. [DOI] [PubMed] [Google Scholar]
- Driessen-Kletter MF, Amelink GJ, Bar PR, Van Gijn J. Myoglobin is a sensitive marker of increased muscle membrane vulnerability. J. Neurol. 1990;237:234–238. doi: 10.1007/BF00314625. [DOI] [PubMed] [Google Scholar]
- Faulkner JA, Brooks SV. An in situ single skeletal muscle model of contraction-induced injury: mechanistic interpretations. Basic Appl. Myol. 1994;4:17–23. [Google Scholar]
- Fridén J, Sjöström M, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. Int. J. Sports Med. 1983;4:170–176. doi: 10.1055/s-2008-1026030. [DOI] [PubMed] [Google Scholar]
- Fridén J, Lieber RL. Structural basis of muscle cellular damage. Basic Appl. Myol. 1994;4:35–42. [Google Scholar]
- Grounds MD, Robertson TA, Mitchell CA, Papadimitriou JM. Necrosis and regeneration in dystrophic and normal skeletal muscle. In: Kakulas BA, McHowell J, Roses A, editors. Duchenne Muscular Dystrophy: Animal Models and Genetic Manipulation. New York: Raven Press; 1992. pp. 141–153. [Google Scholar]
- Grounds MD. Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. Ann. New York Acad. Sciences. 1998;854:78–91. doi: 10.1111/j.1749-6632.1998.tb09894.x. [DOI] [PubMed] [Google Scholar]
- Hamer PW, Lloyd DG, Wood GA. The design of a small axial torque transducer for a servo-controlled dynamometer. In: Hume PA, Marshall RN, Hunter PJ, Stanley S, Anderson I, McNair P, editors. 2nd Australia and New Zealand Society of Biomechanics Conference Book of Abstracts. Aukland: University of Auckland; 1998. [Google Scholar]
- Matsuda R, Nishikawa A, Tanaka H. Visualization of dystrophic muscle fibres in mdx mouse by vital staining with Evans blue: Evidence of apoptosis in dystrophin-deficient muscle. J. Biochem. 1995;118:959–964. doi: 10.1093/jb/118.5.959. [DOI] [PubMed] [Google Scholar]
- McNeil PL, Khakee R. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am. J. Pathol. 1992;140:1097–1109. [PMC free article] [PubMed] [Google Scholar]
- McNeil PL, Terasaki M. Coping with the inevitable: how cells repair a torn surface membrane. Nature Cell Biol. 2001;3:E124–E129. doi: 10.1038/35074652. [DOI] [PubMed] [Google Scholar]
- Oztas B, Kaya M, Camurcu S. Influence of profound hypothermia on the blood-brain barrier permeability during acute arterial hypertension. Pharmacol. Res. 1992;26:75–84. [PubMed] [Google Scholar]
- Pagel NC, Partridge TA. Covert persistence of mdx mouse myopathy is revealed by acute and chronic effects of irradiation. J. Neurol. Sci. 1999;164:103–116. doi: 10.1016/s0022-510x(99)00061-1. [DOI] [PubMed] [Google Scholar]
- Papadimitriou JM, Robertson TA, Mitchell CA, Grounds MD. The process of new plasmalemma formation in focally injured skeletal muscle fibers. J. Struct. Biol. 1990;103:124–134. doi: 10.1016/1047-8477(90)90016-6. [DOI] [PubMed] [Google Scholar]
- Roberts P, McGeachie JK, Grounds MD, Smith ER. Initiation and duration of myogenic precursor cell replication in transplants of intact skeletal muscles: an autoradiographic study in mice. Anat. Record. 1989;224:1–6. doi: 10.1002/ar.1092240102. [DOI] [PubMed] [Google Scholar]
- Straub V, Rafael JA, Chamberlain JS, Campbell KP. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J. Cell Biol. 1997;139:375–385. doi: 10.1083/jcb.139.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang AT, Hasleton PS, Reid H, Hooper TL. Quantification of early damage in latissiumus dorsi muscle grafts. Muscle Nerve. 1998;21:1451–1456. doi: 10.1002/(sici)1097-4598(199811)21:11<1451::aid-mus13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Berchenko E, Frenette J. Macrophage invasion does not contribute to muscle membrane injury during inflammation. J. Leukocyte Biol. 1999;65:492–498. [PubMed] [Google Scholar]
- Togo T, Alderton JM, Bi GQ, Steinhardt RA. The mechanism of facilitated cell membrane resealing. J. Cell Sci. 1999;112:719–731. doi: 10.1242/jcs.112.5.719. [DOI] [PubMed] [Google Scholar]
- Warren GL, Lowe DA, Armstrong RB. Measurement tools used in the study of eccentric contraction-induced injury. Sports Med. 1999;27:43–49. doi: 10.2165/00007256-199927010-00004. [DOI] [PubMed] [Google Scholar]