Abstract
The significance of the p75 low-affinity neurotrophin receptor, for the maintenance and survival of DRG cells, was studied in p75-deficient mice. Perikarya of the L5 DRG of 12-week-old p75 receptor-deficient mice and healthy Balb C mice were compared using stereological techniques. Following systematic sampling, the optical fractionator and the planar vertical rotator were used to estimate the number and mean volume of the cell bodies of the two neuronal subpopulations. The loss of B-cells was 57% (P < 0.00001), numbers being 7300 (CV = 0.12) in controls and 3100 in p75 receptor-deficient mice (CV = 0.18). Also, A-cells showed a significant loss of 39% (P < 0.0001), numbers being 2600 (CV = 0.12) in control mice and 1500 (CV = 0.16) in p75 receptor-deficient mice. The volume of A-cells was reduced by 30% (P < 0.01), from 24.700 µm3 (CV = 0.17) perikarya in p75 knock-out mice to 15.100 µm3 (CV = 0.17) in controls. B-cell volume did not change significantly. It is concluded that the p75 receptor plays a major role in the survival of DRG cells. The predominant loss of small B-cells indicates that the effect of neurotrophins is dependent upon the presence of the p75 low-affinity receptor.
Keywords: neurotrophic factors, NGF, p75 receptor, stereology
Introduction
Neurotrophins influence sensory neuron survival and development. Nerve growth factor (NGF) (Levi Montalcini, 1987), brain-derived neurotrophic factor (BDNF) (Barde et al. 1982; Leibrock et al. 1989), neurotrophin-3 (NT-3) (Ernfors et al. 1990; Hohn et al. 1990; Jones & Reichardt, 1990; Rosenthal et al. 1990) and neurotrophin 4/5 (NT-4/5) (Berkemeier et al. 1991; Hallbook et al. 1991; Ip et al. 1992) are all present in mammals. Survival of most DRG sensory neurons is dependent on these factors (Goedert et al. 1984; Johnson et al. 1986; Crowley et al. 1994; Farinas et al. 1994). It has been suggested that each of the growth factors affect a distinct set of embryonic neurons at different stages of development (Buchman & Davies, 1993; Snider, 1994; Lewin & Barde, 1996). The effect of neurotrophins is mediated by receptors, subdivided into two groups (Meakin & Shooter, 1992; Dixon & McKinnon, 1994). The high-affinity binding sites are the tyrosine kinase receptors (trkA, -B, and -C) and the low-affinity receptor is a transmembrane protein designated p75 (Chao et al. 1986; Radeke et al. 1987; Chao, 1994; Lee et al. 1994). The p75 receptor is less specific than the trk binding sites and interacts with all the known neurotrophic factors (Squinto et al. 1991).
The retrograde axonal transport of BDNF and NT-4/5 is mediated by the p75 receptor (Curtis et al. 1995). Lack of the p75 receptor results in functional deficits of the peripheral nervous system (Bergmann et al. 1997). Lack of the receptor has no effect on development of neuronal populations of cranial sympathetic and cranial sensory neurons (Lee et al. 1992), but it is well known that DRGs are smaller and that peripheral nerves are thinner. There are no studies on numbers of DRG neurons in the p75-deficient mouse. There are two abstracts indicating loss of peripheral sensory axons (Diamond et al. 1995; Lee et al. 1995). Also, there is one report on retrograde axonal transport of neurotrophins in DRG cells (Curtis et al. 1995). Physiological experiments accomplished by Bergmann et al. showed significant elevation of thresholds to noxious mechanical and to heat stimuli in p75 knock-out mice (personal communication). The neurons mediating these functions are the NGF-dependent, small dark-stained B-cells of the DRGs (Mu et al. 1993). Moreover, some mechanoreceptive neurons whose survival is not regulated by NGF are functionally impaired when the p75 receptor is deficient (Koltzenburg et al. 1995; Lee et al. 1995). This suggests that the p75 receptor plays a role for the A-cells of the DRG, as well.
This is the first study to report neuronal DRG numbers in p75-deficient mice. The aim was to determine the influence of the p75 receptor on survival and maintenance of A- and B-cells in the knock-out model using assumption-free stereological techniques.
Materials and methods
Six 12-week-old p75 knock-out mice (M. Koltzenburg, University of Würzburg, Würzburg, Germany) and six age-matched healthy controls (Balb C mice) were examined. Tissues were preserved by vascular perfusion through the heart under deep anaesthesia with 4% glutaraldehyde dissolved in phosphate buffer (0.08 m).
The lumbar spinal nerves and the fifth DRGs were exposed. To ensure complete DRG sampling the right ganglia were cut with short spinal nerve and dorsal root segments. In one knock-out mouse and in two controls the left DRG was used because the right one was damaged during dissection. The ganglia were embedded in 7% agar, dehydrated in graded concentrations of alcohol (1 h at 70%, 1 h at 96%, 2 h at 99%), infiltrated for 3 days and finally embedded in glycolmethacrylate (Culzer GmbH, Technovit). To obtain transverse sections ganglion alignment was maintained during the entire procedure of embedding, dehydration and infiltration (Baddeley et al. 1986). The entire ganglion was cut into 30-µm-thick transverse serial sections parallel to the longitudinal dorsal root and spinal nerve axis. For the counting procedure neurons were systematically and randomly sampled and counted. Using a random starting point every third section was sampled and stained with cresyl violet acetate.
The number of neurons was estimated using the optical fractionator principle without need for estimation of total DRG volume (West et al. 1991). Neurons were counted within optical dissectors. For the practical procedure a microscope computer set up (Olympus, Denmark) was used. A video camera projected the image from the microscope to a computer screen where counting frames were superimposed (CAST Grid®, Olympus Denmark, Albertslund, Denmark). A microcator was attached to the microscope for measurement of depth of the focal plane and a stepping motor was applied for systematic random sampling of counting fields. For the optical fractionator and the vertical planar rotator measurements a 60× oil immersion lens (NA = 1.4, depth of focus approximately 0.5 µm) was used. The total magnification was 1755×. The same observer (M.D.) evaluated all sections.
The number of neurons was estimated using 15-µm-high optical disectors (Gundersen, 1986; Brændgaard et al. 1990). This implies that cells were sampled in three-dimensional probes of known volume inside the section (Gundersen, 1977). In practice cell counts had a random starting point. Stepping systematically through the section by moving the focal plane, the unique counting points (largest nucleon) appearing within the superimposed counting frame not touching the forbidden sides (Mayhew & Gundersen, 1996) were counted. Approximately 100 A-cells and 100 B-cells were counted per ganglion (Tandrup, 1993). To avoid overestimation correct top and bottom definition of the profiles is necessary (Gundersen, 1986). Cell counts were started approximately 3 µm below the surface of the sections and the nucleus of each cell was used as counting unit. The counting frame for A-cells had an area of 3770 µm2 and for B-cells an area of 1630 µm2. Cell counting and volume estimation took approximately 4 h for each ganglion.
Section thickness varies and staining and cutting procedures can lead to small dimensional changes of the sections. Therefore, actual section thickness was measured at every sixth sampling step using a 100× oil immersion lens.
Neurons were counted according to the optical fractionator principle as a fraction of neurons without need neither for estimation of DRG volume nor number of counting frames (Mayhew, 1992). Numbers (N) were obtained according to the formula:
![]()
where ΣQ− is the total number of neurons counted in the disectors multiplied by 3 as only every third section was sampled. Step x and step y are the constant step lengths in the x and y axis direction of the counting fields. t is the measured mean thickness of the sections, a the area of the counting frame and h the height of the disector.
The volume of every perikarya sampled within the disectors was estimated by a combination of vertical sections (Gunderson & Osterby, 1977; Baddeley et al. 1986) and the vertical planar rotator principle (Vedel Jensen & Gundersen, 1993) to all cells sampled. In principle, using the vertical planar rotator an area of cell profile containing the unique counting point is rotated around the vertical axis and thereby in three dimensions estimates perikarya volume (equation 4.1 in Vedel Jensen & Gundersen, 1993).
![]()
t is one-third of the cell height along the vertical axis through the largest nucleolus and li the length of three perpendicular lines intersected by the vertical axis and the outer cell contours. In case two or more nucleoli of similar size appeared, one was chosen at random. The computer ran a program to measure and estimate magnification and to calculate cell volume.
The neuronal subpopulations were characterised at light microscopy as A-cells and B-cells according to the criteria given by Andres (1961), Lieberman (1976), Duce & Keen (1977) and Rambourg et al. (1983). During the counting procedure it is possible to make multiple optical sections through the thick section and study the cytoplasm and the whole nucleus for classification. The large light A-cells have a big, light nucleus with one large intensively stained nucleolus located centrally (personal observation) and well defined evenly distributed cytoplasmic granules. Usually, the B-cells are smaller, with more irregular shape, dark homogenous cytoplasm and a few nucleoli close to the nuclear membrane. Unclassified cells were counted separately and made up 3% in controls and 4% in knock-out mice.
Unpaired and paired Student's t-tests were used for comparison of the two groups, the level of statistical significance being 5%. The comparison of relative A- and B-cell survival was analysed statistically using logarithmic transformation of cell numbers.
Results
The neuronal subtypes and the nucleolar counting unit were easily identified in both groups. There were no conspicuous pathological differences of DRG neurons between p75 receptor-deficient mice and controls, but neuronal cell bodies of the transgenic mice were less heavily stained and had less distinct outer and inner membranes. Also, their nuclei were stained more intensively and the A-cells appeared smaller with signs of cytolysis in a few cells, whereas B-cells appeared slightly darker compared to controls. The number of non-classified cells was not significant (P < 0.05).
The estimated number of neuronal DRG cells in control and knock-out mice are presented in Table 1. Total numbers per ganglion ranged from 8930 to 11 360 in controls (mean number 10.180; coefficient of variation (CV) 0.10) and from 4000 to 5940 in p75 transgenic mice (4880; (0.16)). Expressed as a percentage the total loss of DRG perikarya in p75 transgenic mice was 52% (P < 0.0001). The A-cell loss was 39% (P < 0.0001) and the loss of B-cells 57% (P < 0.00001).
Table 1.
Numbers of A- and B-cells and non-classified cells of L5 dorsal root ganglia in each of six low-affinity p75 receptor knock-out mice and in each of six Balb C control mice
| A-cells | B-cells | Unclassified | All | |
|---|---|---|---|---|
| control | ||||
| KO-I | 1621 | 3995 | 228 | 5844 |
| KO-II | 1625 | 3051 | 255 | 4931 |
| KO-III | 1277 | 2727 | 139 | 4143 |
| KO-IV | 1298 | 2526 | 175 | 3998 |
| KO-V | 1933 | 3560 | 230 | 5723 |
| KO-VI | 1530 | 2881 | 210 | 4621 |
| Mean | 1547 | 3123 | 206 | 4877 |
| CV | 0.16 | 0.18 | 0.21 | 0.16 |
| p74-mice | ||||
| BALB-I | 2004 | 6724 | 339 | 9068 |
| BALB-II | 2414 | 8602 | 341 | 11357 |
| BALB-III | 2604 | 7446 | 312 | 10362 |
| BALB-IV | 2716 | 7772 | 340 | 10828 |
| BALB-V | 2724 | 5968 | 238 | 8930 |
| BALB-VI | 2899 | 7339 | 316 | 10554 |
| Mean | 2560 | 7309 | 314 | 10183 |
| CV | 0.12 | 0.12 | 0.13 | 0.10 |
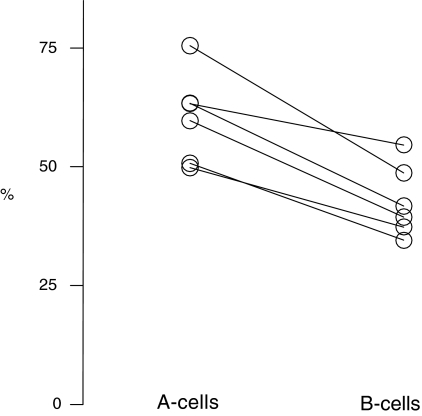
In Fig. 1 individual numbers of A- and B-cells in knock-out mice were compared to the mean number of the same cell type in controls and expressed as a percentage. The figure shows that the B-cell loss is more severe than the A-cell loss in all six transgenic mice (P < 0.002), or that A-cell survival is better.
Fig. 1. Individual and interconnected survival of A- and B-cells in p75 receptor-deficient mice expressed relative to the mean control value, showing a preferential B-cell loss.
Table 2 shows a 30% reduction of A-cell mean perikarya volume in p75 receptor knock-out mice, while the B-cell reduction was insignificant.
Table 2.
Volume (µm3) of A-, B- and non-classified neuronal cells of L5 dorsal root ganglia in each of six low-affinity p75 receptor knock-out mice and in each of six Balb C control mice
| A-cells | B-cells | Unclassified | All | |
|---|---|---|---|---|
| control | ||||
| KO-I | 16415 | 3541 | 8088 | 7739 |
| KO-II | 15232 | 3193 | 8269 | 7404 |
| KO-III | 11456 | 2663 | 8415 | 5913 |
| KO-IV | 19960 | 2773 | 10110 | 8289 |
| KO-V | 13486 | 3897 | 9284 | 8166 |
| KO-VI | 13801 | 3892 | 9061 | 8183 |
| Mean | 15058 | 3327 | 8871 | 7549 |
| CV | 0.19 | 0.16 | 0.09 | 0.12 |
| p75 mice | ||||
| BALB-I | 19272 | 3769 | 9128 | 70484 |
| BALB-II | 17998 | 3001 | 8305 | 6188 |
| BALB-III | 23758 | 5486 | 5973 | 10669 |
| BALB-IV | 23482 | 3681 | 9903 | 8516 |
| BALB-V | 27673 | 4593 | 11870 | 11204 |
| BALB-VI | 19993 | 4401 | 8845 | 9107 |
| Mean | 22020 | 4155 | 10576 | 13458 |
| CV | 0.16 | 0.21 | 0.15 | 0.22 |
Discussion
In mice information about number and volume of lumbar dorsal root ganglia cells is sparse. However, Lawson (1979) estimated that the neuronal population was approximately 6000 neurons in the L3 DRG using nucleoli for profile counts.
Sommer et al. (1985) characterised neuronal subtypes in mice DRG with a combination of ultrastructural and cytochemical techniques allowing identification of A-, B- and C-cells. The relative proportion of A-cells was 36%, of B-cells 63% and C-cells 1%. This is in acceptable accordance with our findings made at the light microscopic level where 25% were A-cells, 71% were B-cells and 3% non-identified cells.
Behavioural and electrophysiological deficits in p75 receptor-deficient mice were reported by Bergmann et al. (1997). Noxious and thermal thresholds, functions known to be NGF dependent, were elevated. Unmyelinated fibres predominantly conduct pain and thermal sensations and originate from small dark staining B-cells (Lawson et al. 1985; Tandrup, 1995). Most of the myelinated fibres are known to originate from large light non-NGF-dependent A-cells. Our findings of a predominant B-cell loss strengthen the presumption that p75 is an important receptor for NGF. However, the loss and shrinkage of the remaining A-cells is remarkable. Whether the A-cells are smaller in general or only the largest A-cells are lost cannot be determined from our study. For clarification further subtyping is necessary including histoimmunochemical staining and electron microscopy. An interesting possibility is that large DRG cells coexpressing trkA and -C receptors are especially dependent on the p75 receptor (Mu et al. 1993; Kashiba et al. 1995). In fact there is evidence that p75 is important for the development of NGF-dependent and NGF-independent sensory neurons (Curtis et al. 1995; Lee et al. 1992), supporting the observations made by Lee et al. (1995) that p75 receptor-deficient mice show behavioural deficits due to loss of mechano receptors.
In p75 knock-out mice a preliminary study by Diamond et al. (1995) reports loss of almost 40% of large and small myelinated sensory nerve fibres and a 60% loss of unmyelinated nerve fibres. These preliminary nerve fibre counts match our results quite well regarding the assumption that unmyelinated fibres arise from the small B-cells and the myelinated fibres from the large A-cells.
Expression of all trk receptors in rat dorsal root ganglia occurs at embryonic day 13, approximately 24–48 h after DRG neurogenesis begins (Mu et al. 1993). From embryonic day 15 to postnatal day 1 trk expressions remain stable. At postnatal day 1 46% of DRG neurons express trkA, 5% express trkB and 10% express trkC receptors. Carroll et al. (1992) and Mu et al. (1993) observed that trkB- and trkC-expressing neurons predominantly are located at the periphery of the DRG while trkA-expressing neuron clusters are distributed at a diffuse pattern. At postnatal day 21 trkA cells mostly are small neurons, trkB-expressing neurons are of intermediate size, whereas large neurons mostly express trkC. Kashiba et al. (1995) presented a study with numerical data very close to those by Mu et al. In both studies it was observed that every neuron expressing trk receptors coexpresses the low-affinity receptor p75. Also, 15% of the neurons express trkA as well as trkC.
Embryos deprived of NGF due to autoimmunity or to passive transfer of antibodies have a loss of 70–80% of DRG neurons (Johnson et al. 1980; Goedert et al. 1984; Carroll et al. 1992; Ruit et al. 1992). NT3 knock-out mice have a loss of 78% of DRG neurons, despite the fact that fewer neurons express the trkC receptor (Kashiba et al. 1995; Mu et al. 1993). Furthermore, neurotrophin knock-outs provide more severe deficits than animals lacking neurotrophin receptors (Klein et al. 1993; Farinas et al. 1994). These observations indicate that a majority of neurons require more than one neurotrophin during embryogenesis for binding to the primary as well as to the additional receptors (Crowley et al. 1994; Klein et al. 1993; Jones et al. 1994).
The p75 receptor has an equal affinity to all of the neurotrophic factors known in mammals (Rodriguez Tebar et al. 1990; Rodriguez Tebar et al. 1992). Furthermore, every DRG neuron expressing a trk receptor coexpresses the low-affinity receptor (Kashiba et al. 1995). Nonetheless, the low-affinity receptor can discriminate ligands and selectively modulate the biological actions of the more specific neurotrophins (Ryden et al. 1995). Several studies strengthen the concept that the p75 receptor interacts with NGF in DRGs where it plays an accessory rather than a direct role in mediating the function of NGF (Meakin et al. 1992; Barbacid, 1993; Valmier et al. 1993).
In-vitro studies have shown that NGF regulates the dimensions of sensory neurons (Yasuda et al. 1990). The modest volume changes of B-cells in p75 receptor knock-out mice observed in the present study might support the hypothesis that NGF regulates the dimensions of DRG cells. However, it is an unexpected finding that number and volume of A-cells are reduced. In conclusion, our findings support the hypothesis that the low-affinity p75 receptor is predominant in small NGF-dependent B-cell neurons of the dorsal root ganglion, and its role for the A-cells attracts attention.
Acknowledgments
Kristen Kandborg is acknowledged for technical assistance.
References
- Andres KH. Untersuchungen über den Feinbau von Spinalganglien. Z. Zellforschung. 1961;55:1–48. [Google Scholar]
- Baddeley AJ, Gundersen HJ, Cruz Orive LM. Estimation of surface area from vertical sections. J. Microsc. 1986;142:259–276. doi: 10.1111/j.1365-2818.1986.tb04282.x. [DOI] [PubMed] [Google Scholar]
- Barbacid M. Nerve growth factor: a tale of two receptors. Oncogene. 1993;8:2033–2042. [PubMed] [Google Scholar]
- Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. Eur. Mol Biol. Organisation. 1982;1:549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann I, Priestley JV, McMahon SB, Brocker EB, Toyka KV, Koltzenburg M. Analysis of cutaneous sensory neurons in transgenic mice lacking the low affinity neurotrophin receptor p75. Eur. J. Neurosci. 1997;9:18–28. doi: 10.1111/j.1460-9568.1997.tb01349.x. [DOI] [PubMed] [Google Scholar]
- Berkemeier LR, Winslow JW, Kaplan DR, Nikolics K, Goeddel DV, Rosenthal A. Neurotrophin-5: a novel neurotrophic factor that activates trk and trkB. Neuron. 1991;7:857–866. doi: 10.1016/0896-6273(91)90287-a. [DOI] [PubMed] [Google Scholar]
- Braendgaard H, Evans SM, Howard CV, Gundersen HJ. The total number of neurons in the human neocortex unbiasedly estimated using the optical disectors. J. Microsc. 1990;157:285–304. doi: 10.1111/j.1365-2818.1990.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Buchman VL, Davies AM. Different neurotrophins are expressed and act in a developmental sequence to promote the survival of embryonic sensory neurons. Development. 1993;118:989–1001. doi: 10.1242/dev.118.3.989. [DOI] [PubMed] [Google Scholar]
- Carroll SL, Silos-Santiago I, Frese SE, Ruit KG, Milbrandt J, Snider WD. Dorsal root ganglion neurons expressing trk are selectively sensitive to NGF deprivation in utero. Neuron. 1992;9:779–788. doi: 10.1016/0896-6273(92)90040-k. [DOI] [PubMed] [Google Scholar]
- Chao MV, Bothwell MA, Ross AH, Koprowski H, Lanahan AA, Buck CR, et al. Gene transfer and molecular cloning of the human NGF receptor. Science. 1986;232:518–521. doi: 10.1126/science.3008331. [DOI] [PubMed] [Google Scholar]
- Chao MV. The p75 neurotrophin receptor. J. Neurobiol. 1994;25:1373–1385. doi: 10.1002/neu.480251106. [DOI] [PubMed] [Google Scholar]
- Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76:1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- Curtis R, Adryan KM, Stark JL, Park JS, Compton DL, Weskamp G, et al. Differential role of the low affinity neurotrophin receptor (p75) in retrograde axonal transport of the neurotrophins. Neuron. 1995;14:1201–1211. doi: 10.1016/0896-6273(95)90267-8. [DOI] [PubMed] [Google Scholar]
- Diamond J, Lourenssen S, Pertens E, Urschel B. Lack of collateral sprouting of noiciceptive nerves in adult p75 knock-out mice. Soc. Neuroscience Abstract. 1995;21:1539. (Abstract) [Google Scholar]
- Dixon JE, McKinnon D. Expression of the trk gene family of neurotrophin receptors in prevertebral sympathetic ganglia. Brain Res. Dev. Brain Research. 1994;77:177–182. doi: 10.1016/0165-3806(94)90194-5. [DOI] [PubMed] [Google Scholar]
- Duce IR, Keen P. An ultrastructural classification of the neuronal cell bodies of the rat dorsal root ganglion using zinc iodideosmium impregnation. Cell Tissue Res. 1977;185:263–277. doi: 10.1007/BF00220670. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Ibanez CF, Ebendal T, Olson L, Persson H. Molecular cloning and neurotrophic activities of a protein with structural similarities to nerve growth factor: developmental and topographical expression in the brain. Proc. Natl. Acadamy Sci. USA. 1990;87:5454–5458. doi: 10.1073/pnas.87.14.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Backus C, Wang XY, Reichardt LF. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:58–661. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- Goedert M, Otten U, Hunt SP, Bond A, Chapman D, Schlumpf M, et al. Biochemical and anatomical effects of antibodies against nerve growth factor on developing rat sensory ganglia. Proc. Natl Acad. Sci. USA. 1984;81:1580–1584. doi: 10.1073/pnas.81.5.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJH. Notes on the estimation of the numerical density of arbitrary porfiles: the edge effect. J. Microsc. 1977;111:219–223. [Google Scholar]
- Gundersen HJ, Osterby R. Glomerular size and structure in diabetes mellitus. II. Late abnormalities. Diabetologia. 1977;13:43–48. doi: 10.1007/BF00996326. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. J. Microsc. 1986;143:3–45. [PubMed] [Google Scholar]
- Hallbook F, Ibanez CF, Persson H. Evolutionary studies of the nerve growth factor family reveal a novel member abundantly expressed in Xenopus ovary. Neuron. 1991;6:845–858. doi: 10.1016/0896-6273(91)90180-8. [DOI] [PubMed] [Google Scholar]
- Hohn A, Leibrock J, Bailey K, Barde YA. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature. 1990;344:339–341. doi: 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- Ip NY, Ibanez CF, Nye SH, McClain J, Jones PF, Gies DR, et al. Mammalian neurotrophin-4: structure, chromosomal localization, tissue distribution, and receptor specificity. Proc. Natl Acad. Sci. USA. 1992;89:3060–3064. doi: 10.1073/pnas.89.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson EM, Gorin PD, Brandeis LD, Pearson J. Dorsal root ganglion neurons are destroyed by exposure in utero to maternal antibody to nerve growth factor. Science. 1980;210:916–918. doi: 10.1126/science.7192014. [DOI] [PubMed] [Google Scholar]
- Johnson JE, Barde YA, Schwab M, Thoenen H. Brain-derived neurotrophic factor supports the survival of cultured rat retinal ganglion cells. J. Neurosci. 1986;6:3031–3038. doi: 10.1523/JNEUROSCI.06-10-03031.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KR, Reichardt LF. Molecular cloning of a human gene that is a member of the nerve growth factor family. Proc. Natl Acad. Sci. USA. 1990;87:8060–8064. doi: 10.1073/pnas.87.20.8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiba H, Noguchi K, Ueda Y, Senba E. Coexpression of trk family members and low-affinity neurotrophin receptors in rat dorsal root ganglion neurons. Brain Res. Mol. Brain Research. 1995;30:158–164. doi: 10.1016/0169-328x(94)00249-e. [DOI] [PubMed] [Google Scholar]
- Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL, et al. Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell. 1993;75:113–122. [PubMed] [Google Scholar]
- Koltzenburg M, Lewin GR, Toyka KV, Thoenen H, Carroll P. Electrophysiological analysis of cutaneous sensory neurons in neonatal wildtype mice and transgenic animals lacking BDNF. Soc. Neurosci. Abstract. 1995;21:1054. (Abstract) [Google Scholar]
- Lawson SN. The postnatal development of large light and small dark neurons in mouse dorsal root ganglia: a statistical analysis of cell numbers and size. J. Neurocytol. 1979;8:275–294. doi: 10.1007/BF01236123. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Harper EI, Harper AA, Garson JA, Coakham HB, Randle BJ. Monoclonal antibody 2C5: a marker for a subpopulation of small neurones in rat dorsal root ganglia. J. Neurosci. 1985;16:365–374. doi: 10.1016/0306-4522(85)90009-0. [DOI] [PubMed] [Google Scholar]
- Lee KF, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, et al. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- Lee KF, Davies AM, Jaenisch R. p75-deficient embryonic dorsal root sensory and neonatal sympathetic neurons display a decreased sensitivity to NGF. Development. 1994;120:1027–1033. doi: 10.1242/dev.120.4.1027. [DOI] [PubMed] [Google Scholar]
- Lee KF, Dickinson-Anson H, Gage FH, Byers M. Low-affinity neurotrophin receptor p75 is required for the development of some mechanoreceptors. Soc. Neuroscience Abstract. 1995;21:1056. (Abstract) [Google Scholar]
- Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P, et al. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989;341:149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- Levi Montalcini R. The nerve growth factor: thirty-five years later. Bioscience Reports. 1987;7:681–699. doi: 10.1007/BF01116861. [DOI] [PubMed] [Google Scholar]
- Lewin GR, Barde YA. Physiology of the neurotrophins. Annual Review of. Neuroscience. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- Lieberman AR. Sensory ganglia. In: London DN, editor. The Peripheral Nerve. London: Chapman and Hall; 1976. pp. 188–278. [Google Scholar]
- Mayhew TM. A review of recent advances in stereology for quantifying neural structure. J. Neurocytol. 1992;21:313–328. doi: 10.1007/BF01191700. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Gundersen HJG. ‘If you assume, you can make an ass out of u and me’: a decade of the disector for stereological counting of particles in 3D space. J. Anat. 1996;188:1–15. [PMC free article] [PubMed] [Google Scholar]
- Meakin SO, Shooter EM. The nerve growth factor family of receptors. Trends Neurosci. 1992;15:323–331. doi: 10.1016/0166-2236(92)90047-c. [DOI] [PubMed] [Google Scholar]
- Mu X, Silos-Santiago I, Carroll SL, Snider WD. Neurotrophin receptor genes are expressed in distinct patterns in developing dorsal root ganglia. J. Neurosci. 1993;13:4029–4041. doi: 10.1523/JNEUROSCI.13-09-04029.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radeke MJ, Misko TP, Hsu C, Herzenberg LA, Shooter EM. Gene transfer and molecular cloning of the rat nerve growth factor receptor. Nature. 1987;325:593–597. doi: 10.1038/325593a0. [DOI] [PubMed] [Google Scholar]
- Rambourg AY, Clermont T, Beaudet A. Ultrastuctural features of six types of neurons in rat dorsal root ganglia. J. Neurocytol. 1983;12:47–66. doi: 10.1007/BF01148087. [DOI] [PubMed] [Google Scholar]
- Rodriguez Tebar A, Dechant G, Barde YA. Binding of brain-derived neurotrophic factor to the nerve growth factor receptor. Neuron. 1990;4:487–492. doi: 10.1016/0896-6273(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Rodriguez Tebar A, Dechant G, Gotz R, Barde YA. Binding of neurotrophin-3 to its neuronal receptors and interactions with nerve growth factor and brain-derived neurotrophic factor. Eur. Mol. Biol. Organization J. 1992;11:917–922. doi: 10.1002/j.1460-2075.1992.tb05130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A, Goeddel DV, Nguyen T, Lewis M, Shih A, Laramee GR, et al. Primary structure and biological activity of a novel human neurotrophic factor. Neuron. 1990;4:767–773. doi: 10.1016/0896-6273(90)90203-r. [DOI] [PubMed] [Google Scholar]
- Ruit KG, et al. Selective dependance of mammalian dorsal root ganglion neurons on nerve growth factor during embryonic development. Neuron. 1992;8:573–587. doi: 10.1016/0896-6273(92)90284-k. [DOI] [PubMed] [Google Scholar]
- Ryden M, Murray-Rust J, Glass D, Ilag LL, Trupp M, Yancopoulos GD, et al. Functional analysis of mutant neurotrophins deficient in low-affinity binding reveals a role for p75LNGFR in NT-4 signalling. Eur. Mol Biol. Organisation. 1995;9:1979–1990. doi: 10.1002/j.1460-2075.1995.tb07190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Sommer EW, Kazimierczak J, Droz B. Neuronal subpopulations in the dorsal root ganglion of the mouse as characterized by combination of ultrastructural and cytochemical features. Brain Res. 1985;346:310–326. doi: 10.1016/0006-8993(85)90865-0. [DOI] [PubMed] [Google Scholar]
- Squinto SP, Stitt TN, Aldrich TH, Davis S, Bianco SM, Radziejewski C, et al. trkB encodes a functional receptor for brain-derived neurotrophic factor and neurotrophin-3 but not nerve growth factor. Cell. 1991;65:885–893. doi: 10.1016/0092-8674(91)90395-F. [DOI] [PubMed] [Google Scholar]
- Tandrup T. A method for unbiased and efficient estimation of number and mean Volume of specified neuron subtypes in rat dorsal root ganglion. J. Comparative Neurolol. 1993;329:269–276. doi: 10.1002/cne.903290208. [DOI] [PubMed] [Google Scholar]
- Tandrup T. Are the neurons in the dorsal root ganglion pseudounipolar? A comparison of the number of neurons and number of myelinated and unmyelinated fibres in the dorsal root. J. Comparative Neurol. 1995;357:341–347. doi: 10.1002/cne.903570302. [DOI] [PubMed] [Google Scholar]
- Valmier J, Mallie S, Baldy Moulinier M. Skeletal muscle extract and nerve growth factor have developmentally regulated survival promoting effects on distinct populations of mammalian sensory neurons. Muscle Nerve. 1993;16:397–403. doi: 10.1002/mus.880160409. [DOI] [PubMed] [Google Scholar]
- Vedel Jensen EB, Gundersen HJG. The rotator. J. Microsc. 1993;170:33–44. [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Sobue G, Ito T, Mitsuma T, Takahashi A. Nerve growth factor enhances neurite arborization of adult sensory neurons; a study in single-cell culture. Brain Res. 1990;524:54–63. doi: 10.1016/0006-8993(90)90491-s. [DOI] [PubMed] [Google Scholar]