Abstract
The purpose of the present study was to obtain insight into the natural development of adaptive intimal thickening and atherosclerosis in the arterial tree of human species. The morphometry and composition of the intimal layer were studied in the arterial system of elderly individuals. Post mortem, a total of 703 arterial segments were dissected from 24 subjects (age 81.9 ± 9.9 years). From each subject, segments were dissected from 31 different arteries. Area stenosis [(plaque area/vessel area) × 100%] was determined in each segment. By (immuno)histochemistry, lipid content and the presence of inflammatory cells (macrophages) were assessed in the coronary, common carotid, brachial, radial and internal iliac arteries. Adaptive intimal thickening or advanced atherosclerosis was observed in all studied artery types. Area stenosis was highest in the coronary arteries (median, 30%) and lowest in the arteries supplying the brain (median, ≤ 7%). Plaques hiding a lipid-rich core and plaques with macrophage infiltration were observed in all five selected artery types. In summary, the present observation demonstrates that intimal thickening is a systemic process involving most artery types. Within elderly humans, features of advanced atherosclerotic disease, a lipid-rich core and macrophages, can be observed in the intimal layer of artery types that are recognised for their relation with clinical syndroms as well as artery types that remain clinically silent.
Keywords: adaptive intimal hyperplasia, aging, artery, atherosclerosis, autopsy, macrophages, peripheral vascular disease, plaque instability
Introduction
With advancing age, modifications of the cardiovascular system occur. Adaptive intimal thickening is an adaptation of the arterial wall to mechanical stresses secondary to variations in flow and wall tension (Stary et al. 1992). Thickening of the intima exists in arteries obtained from healthy human subjects and from many other species and could therefore be considered as a physiological adaptation. In contrast to adaptive intimal thickening, atherosclerotic plaque formation may lead to clinically manifest arterial occlusive disease. Atherosclerotic lesions frequently develop in regions with intimal thickening (Stary et al. 1992). Early lesions preceding advanced atherosclerotic plaques consist of small lipid deposits and the accumulation of macrophages and foam cells in the intima (Stary et al. 1994). Rupture of the atherosclerotic plaque surface is probably the most important mechanism underlying rapid plaque progression and acute thrombotic occlusion (Fuster et al. 1992). The occurrence of plaque rupture is associated with the composition of the atherosclerotic plaque. Determinants that have been associated with rupture-prone plaques are a large extracellular lipid-pool and presence of inflammatory cells in the plaque (Davies et al. 1993; van der Wal et al. 1994).
Certain artery types are more prone to develop clinically manifest atherosclerosis, while other artery types are considered to remain free of atherosclerotic disease. The purpose of the present study was to obtain insight into the natural development of adaptive intimal thickening and atherosclerosis in the arterial tree of human species. In the first part of the study, multiple artery types were obtained and analysed morphometrically. In the second part of the study the composition of the intimal layer was determined in a selection of artery types to identify advanced atherosclerotic lesions. Two artery types known for their development of clinically manifest atherosclerotic disease and two artery types that are considered not to develop overt atherosclerosis were selected. Arterial modification develops slowly during life. Therefore, we used elderly subjects. This may be of particular interest because the fastest growing segment of Western society is that aged over 60 years.
Methods
Post-mortem material
Arteries of 24 donated cadavers (11 men and 13 women, age 81.9 ± 9.9 years, history of cardiovascular disease and risk factors unknown) were pressure fixed with 4% formalin in situ (pressure: age + 100 mmHg). From each cadaver, arterial segments were dissected at 31 locations: left and right (L & R) anterior, middle and posterior (0.5–1 cm distal to origin) cerebral arteries, L & R common (just proximal to carotid sinus) and internal (1–2 cm distal to carotid bifurcation) carotid arteries, L & R brachial arteries (5 cm proximal to elbow), L & R radial arteries (3–5 cm proximal to wrist), ascending (3–5 cm distal to aortic valves) and abdominal (just distal to renal arteries) aorta, left anterior descending coronary artery (between origin and first diagonal side branch), left circumflex coronary artery (between origin and first major side branch), right coronary artery (2 cm distal to origin), superior mesenteric artery (1–2 cm distal to origin), L & R renal arteries (just distal to origin), L & R common (2 cm distal to aortic bifurcation), internal (3–5 cm distal to iliac bifurcation) and external (middle part) iliac arteries and the L & R femoral (10 distal to inguinal ligament) arteries.
Morphometric analysis
Of each artery segment, one paraffin section was stained with elastin-van Gieson. To assess the local cross-sectional area of the intimal layer, microscopic images of the stained sections were recorded on VHS videotape with a 3CCD video camera. In each cross-section, the lumen area and the area encompassed by the internal elastic lamina (IEL area) were measured by computerised planimetry. The intimal area was calculated by subtracting the lumen area from the IEL area. This morphological definition includes normal intima, adaptive intimal thickening and atherosclerotic plaque. Area stenosis, a measure of the size of the intima in a cross-section corrected for arterial size, was calculated as (intimal area/IEL area) × 100%.
(Immuno)histochemistry
Determinants that make an atherosclerotic plaque vulnerable for rupture are a large extracellular lipid-pool and inflammation in cap and shoulders (Davies et al. 1993; van der Wal et al. 1994). These determinants of plaque instability were determined in two artery types that are prone to develop clinically relevant atherosclerosis (coronary and common carotid arteries) and two artery types that are believed not to develop overt atherosclerosis (brachial and radial arteries). Because we observed a high area stenosis in the internal iliac artery, plaque composition of this artery type was also assessed. Only sections that contained an area stenosis of at least 25% were selected to study plaque composition (arbitrary limit). Sections adjacent to the ones that were studied by morphometric analysis were used to study plaque composition. To detect collagen, sections were stained with Picrosirius Red (Junqueira et al. 1979). A mouse anti-human CD68 monoclonal antibody (Dakopatts, Denmark) followed by an indirect horseradish peroxidase technique was used to stain macrophages. As control for the primary antibody, consecutive sections to the ones incubated with anti-CD68 were incubated with an irrelevant antibody of the same isotype (mouse IgG1κ, Dakopatts). To make the CD68 epitope accessible for the anti-CD68 monoclonal antibody, paraffin sections were boiled in sodium citrate buffer (10 mm, pH 6.0) for 15 min.
Analysis of (immuno)histochemical staining
The percentage atheroma of the total intimal area was analysed in the sections stained with Picrosirius Red using polarised light. If collagen was found to be absent, then that part of the plaque was considered to be atheromatous (Mann & Davies, 1996). Thus, the presence of lipid in the plaque was not assessed histochemically. Two groups were considered based on the percentage of atheroma in the intima: lipid-rich core occupying < or ≥ 40% of total plaque area (Davies et al. 1993).
Analysis of macrophages focused on the cap and shoulder of the plaque, where plaque rupture and subsequent thrombus formation is most likely to occur (van der Wal et al. 1994; Falk et al. 1995). Sections were arranged in two groups: −, absent or minor staining of CD68 with no or few scattered cells; +, clusters of cells with > 10 cells present.
Statistics
The Fisher's exact test was used to compare proportions of categorical variables. Student's t-test was used to compare continuous variables.
Results
Morphometry
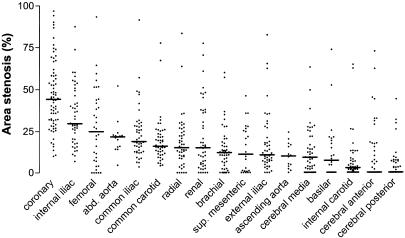
From 24 elderly individuals, 703 arterial segments were harvested (29.3 ± 1.3 per individual). Figure 1 shows the percentage area stenosis for each segment per artery type. The average lumen, intimal and IEL areas of the different artery types are shown in Table 1. Area stenosis was highest in the coronary arteries (median 44.3%; interquartile range: 31.7–58.4%). The internal iliac artery appeared to be the site with a relatively high percentage area stenosis (median: 29.7%; interquartile range: 19.5–47.9%) as compared to artery types that are well recognised for their atherosclerotic clinical syndroms. Area stenosis in the radial artery was similar to area stenosis in the common carotid artery (median: 15.1% vs. 16.0%, respectively). The lowest area stenosis was observed in the arteries supplying the brain (median area stenosis: basilar artery 7.2%, internal carotid artery 2.7%, anterior and posterior cerebral arteries 0%). The average area stenosis of all measured arteries of an individual was 19.8 ± 8.4%. The average area stenosis of all arteries was 22.4 ± 10.1 and 17.6 ± 6.3% for males and females, respectively (P = 0.19).
Fig. 1. Percentage area stenosis for each segment per artery type. Median values are indicated by a horizontal line.
Table 1.
Area stenosis and morphometric parameters of the different artery types
| Artery type | Area stenosis (%) | Lumen area (mm2) | Intimal area (mm2) | IEL area (mm2) |
|---|---|---|---|---|
| Coronary | 44.3 (31.7–58.4) | 4.2 ± 2.8 | 3.9 ± 2.7 | 8.1 ± 4.2 |
| Internal iliac | 29.7 (19.5–47.9) | 24.5 ± 11.0 | 13.2 ± 8.1 | 37.7 ± 14.9 |
| Femoral | 24.9 (5.5–44.1) | 23.9 ± 10.9 | 9.0 ± 8.1 | 32.9 ± 11.2 |
| Abdominal aorta | 21.7 (15.8–23.6) | 158.7 ± 42.7 | 49.6 ± 41.3 | 208.3 ± 70.2 |
| Common iliac | 18.8 (13.2–29.0) | 70.1 ± 32.0 | 22.3 ± 16.3 | 92.4 ± 38.7 |
| Common carotid | 16.0 (12.2–23.4) | 27.3 ± 11.5 | 7.3 ± 6.5 | 34.6 ± 14.1 |
| Radial | 15.1 (9.3–24.6) | 2.7 ± 1.4 | 0.5 ± 0.5 | 3.3 ± 1.5 |
| Renal | 15.0 (4.7–36.9) | 10.3 ± 4.3 | 3.7 ± 4.4 | 14.0 ± 6.4 |
| Brachial | 12.1 (5.2–18.1) | 9.4 ± 4.5 | 2.2 ± 3.6 | 11.6 ± 6.9 |
| Superior mesenteric | 11.1 (0.8–26.0) | 18.8 ± 8.2 | 4.2 ± 5.2 | 23.0 ± 11.4 |
| External iliac | 10.6 (6.6–19.9) | 36.3 ± 13.5 | 7.4 ± 7.4 | 43.7 ± 14.1 |
| Ascending aorta | 9.9 (3.0–17.1) | 323.6 ± 65.4 | 39.3 ± 34.7 | 362.8 ± 78.8 |
| Middle cerebral | 9.1 (2.1–19.2) | 4.5 ± 1.7 | 0.8 ± 1.1 | 5.3 ± 2.3 |
| Basilar | 7.2 (0.0–19.9) | 6.3 ± 3.7 | 1.2 ± 2.2 | 7.5 ± 4.7 |
| Internal carotid | 2.7 (1.1–8.3) | 11.8 ± 5.0 | 1.3 ± 2.6 | 13.0 ± 6.0 |
| Anterior cerebral | 0.0 (0.0–17.4) | 2.9 ± 0.8 | 0.4 ± 0.9 | 3.3 ± 1.0 |
| Posterior cerebral | 0.0 (0.0–6.8) | 3.1 ± 1.6 | 0.3 ± 0.7 | 3.4 ± 2.0 |
Values for area stenosis are median (25-75th percentile); other values are mean ± SD; IEL, internal elastic lamina.
Composition
Intimal composition was studied in five selected artery types. Of these artery types, the composition of the intima was examined in cross-sections with an area stenosis of at least 25% (n = 128 cross-sections).
The presence of lipid in the core of the plaque was inferred from the Picrosirius Red staining. The percentages of sections with a lipid-rich core occupying ≥ 40% of the total plaque area were as follows: coronary artery 16/65 (25%), common carotid artery 5/11 (45%), brachial artery 2/8 (25%), radial artery 2/12 (17%), internal iliac artery 10/32 (31%) (P = 0.58 among groups, Table 2).
Table 2.
Prevalence of lipid-rich core and macrophages in stenotic cross-sections of a selection of artery types
| Cross sections with AS > 25% | ||||
|---|---|---|---|---|
| Artery type | AS > 25%, n (%) | Lipid core > 40%, n (%) | Macrophages, n (%) | Both, n (%) |
| Coronary | 65/72 (90) | 16 (25) | 30 (46) | 13 (20) |
| Com. carotid | 11/48 (23) | 5 (45) | 8 (73) | 5 (45) |
| Brachial | 8/48 (17) | 2 (25) | 6 (75) | 1 (13) |
| Radial | 12/48 (25) | 2 (17) | 1 (8) | 1 (8) |
| Internal iliac | 32/46 (70) | 10 (31) | 26 (81) | 8 (25) |
As, area stenosis.
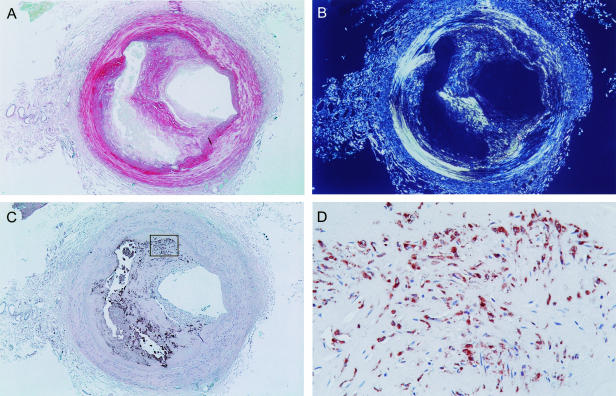
The percentages of sections with staining of CD68 (macrophages) were as follows: coronary artery 30/65 (46%), common carotid artery 8/11 (73%), brachial artery 6/8 (75%), radial artery 1/12 (8%), internal iliac artery 26/32 (81%) (P < 0.001 among groups, Table 2). An example of a plaque with a lipid-rich core and macrophages in the shoulder is shown in Fig. 2.
Fig. 2. Atherosclerotic plaque in radial artery of a 77-year-old-male. (A) Picrosirius Red staining (magnification 20×). (B) Picrosirius Red staining under polarised light. The large area without staining indicates a large lipid-rich core. (C) Staining for macrophages (CD68, magnification 20×). (D) Magnification of the shoulder of the plaque stained for macrophages (magnification 400×).
Discussion
With advancing age, an adaptive thickening of the intima can be observed within the human arterial system. Adaptive intimal thickening could be considered as a physiological adaptation of the intima to variations in flow, wall tension or both (Stary et al. 1992). When atherosclerosis develops, atherosclerotic lesions develop in regions with adaptive intimal thickening. Rupture of a vulnerable atherosclerotic lesion may lead to acute thrombus formation and subsequent occlusion of the artery, causing clinical syndromes like myocardial infarction. Although the development of atherosclerosis is influenced by systemic risk factors like hypercholesterolaemia and hypertension, certain artery types are more prone to develop clinically manifest atherosclerosis than other artery types.
Distribution throughout arterial system
A considerable area stenosis was observed throughout the arterial system, indicating that thickening of the intimal layer is a phenomenon that is present in almost all artery types of elderly individuals. As expected, area stenosis was highest in the coronary arteries. However, artery types that are regarded not to develop overt atherosclerosis, such as the brachial (Zeiher et al. 1995) and radial artery, also revealed a significant area stenosis. The percentage area stenosis in these ‘non-atherosclerosis-prone’ artery types was comparable with area stenosis in the ‘atherosclerosis-prone’ common iliac, common carotid and renal arteries. It might be possible that arterial stenosis leads to clinical symptoms in certain artery types, while no symptoms develop in other artery types. Unilateral occlusion of the internal iliac artery, for example, is in most cases well tolerated. A possible explanation could be that stenosis of clinically silent artery types is compensated for by collateral arteries. Another explanation could be expansive remodelling, in which plaque formation is compensated for by enlargement of the vessel wall (Pasterkamp et al. 1997).
Composition of intimal layer
The intimal layer may consist of relatively harmless adaptive intimal thickening or more advanced atherosclerotic lesions that may be the origin of clinically relevant pathology. Atherosclerotic plaque rupture exposes the lipid-rich core of the plaque to the bloodstream. Subsequently, thrombus formation and fibrotic organisation may occur (Fuster et al. 1992). Plaque rupture probably plays an important role in accelerated growth of atherosclerotic lesions and in the development of acute ischaemic events like myocardial infarction (Fuster et al. 1992; Falk et al. 1995). Plaques with a large extracellular lipid pool and inflammation appear to be vulnerable for rupture (van der Wal et al. 1994; Falk et al. 1995). Moreover, superficial inflammation of the plaque without plaque rupture has also frequently directly been observed underlying thrombus (Arbustini et al. 1999). In a selection of artery types we studied the composition of the intimal layer of ‘stenotic’ cross-sections (area stenosis > 25%). This arbitrary limit was used to prevent inclusion of non-atherosclerotic arteries and to include more advanced atherosclerotic lesion types.
The percentage of stenotic sections with a lipid-rich plaque was not significantly different between ‘atherosclerosis-prone’ artery types, like the coronary and common carotid artery, and artery types which are considered not to develop overt atherosclerosis, like the brachial and radial artery. This observation implies that the development of a large extracellular lipid pool in atherosclerotic plaques is homogeneously distributed in the arterial system. The prevalence of macrophage infiltration, however, showed a wide variation among different artery types. This observation suggests that inflammation in the intimal layer is a common phenomenon that is present with local preference. A high prevalence was observed in the brachial artery, suggesting that inflammation may not be limited to artery types that are linked with clinically symptomatic atherosclerosis. The observed prevalence of inflammation in the coronary artery is consistent with the results of a previous post-mortem study of macrophages in coronary and femoral arteries (Pasterkamp et al. 1999).
The percentage of plaques with both features of advanced atherosclerotic disease (large lipid pool and inflammation) was highest in the common carotid artery. However, the interpretation of this observation merits careful consideration due to the low number of atherosclerotic lesions. Overall, we observed a lipid-rich pool in combination with inflammatory cells in 28 of 128 (22%) plaques of elderly individuals, suggesting that atherosclerotic lesions in elderly individuals are still active and might be considered progressive.
Clinical relevance
Endothelial dysfunction is considered an early event in atherogenesis (Fish et al. 1988). Recently, the measurement of endothelial dysfunction in the brachial artery has been suggested as a non-invasive method to detect presymptomatic atherosclerosis (Celermajer et al. 1992). Ultrasound studies of flow-dependent vascular reactivity of the brachial artery are used to study early atherosclerosis. However, little is known about the development of atherosclerotic lesions in the brachial artery. In the present study, we observed advanced atherosclerosis in the brachial arteries of elderly individuals. This result is consistent with the findings of a previous autopsy study (Sorensen et al. 1997).
There is increasing interest in using the radial artery as a coronary artery bypass graft (CABG) (Acar et al. 1995). Our results demonstrate that (with a low prevalence) advanced atherosclerosis is present in the radial artery (Fig. 2). This is consistent with the results of previous studies (Kaufer et al. 1997; Ruengsakulrach et al. 1999). Our observations suggest that care should be taken when considering the radial artery as a conduit in CABG in elderly individuals.
Limitations
The mean age of the cadavers used for this study was very high. It should therefore be emphasised that further studies are needed to study at which age the pan-arterial distribution of stenosis and plaque formation develops. The cause of death and risk factors of cardiovascular disease of the studied individuals were unknown. Therefore, no differentiation could be made between arterial modifications purely related to aging or related to specific risk factors of atherosclerotic disease.
In summary
The present observation suggests that intimal thickening is a systemic process involving most artery types. The comprehensiveness of the process suggests that this process might be considered a natural tendency of human arteries during life. Within elderly humans, features of advanced atherosclerotic disease, a lipid-rich core and macrophages, can be observed in the intimal layer of artery types that are recognised for their relation with clinical syndroms as well as artery types that remain clinically silent. These results provide insight in the end-stage of the modification of the intimal layer of human arteries during life.
Acknowledgments
This study was supported by the Sorbo foundation. We gratefully thank Willem J. A. van Wolveren and Simon Plomp (Department of Functional Anatomy, University Medical Center Utrecht) for their technical assistance.
References
- Acar C, Jebara VA, Portoghese M, Beyssen B, Pagny JY, Grare P, et al. Revival of the radial artery for coronary artery bypass grafting. Ann. Thorac. Surg. 1995;54:652–660. doi: 10.1016/0003-4975(92)91007-v. [DOI] [PubMed] [Google Scholar]
- Arbustini E, Dal Bello B, Morbini P, Burke AP, Bocciarelli M, Specchia B, et al. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart. 1999;82:269–272. doi: 10.1136/hrt.82.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Richardson PD, Woolf N, Katz DR, Mann J. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br. Heart J. 1993;69:377–381. doi: 10.1136/hrt.69.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- Fish RD, Nabel EG, Selwyn AP, Ludmer PL, Mudge GH, Kirshenbaum JM, et al. Responses of coronary arteries of cardiac transplant patients to acetylcholine. J. Clin. Invest. 1988;81:21–31. doi: 10.1172/JCI113297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes. N. Engl. J. Med. 1992;326:242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Kaufer E, Factor SM, Frame R, Brodman RF. Pathology of the radial and internal thoracic arteries used as coronary artery bypass grafts. Ann. Thorac. Surg. 1997;63:1118–1122. doi: 10.1016/s0003-4975(96)01393-8. [DOI] [PubMed] [Google Scholar]
- Mann JM, Davies MJ. Vulnerable plaque; Relation of characteristics to degree of stenosis in human coronary arteries. Circulation. 1996;94:928–931. doi: 10.1161/01.cir.94.5.928. [DOI] [PubMed] [Google Scholar]
- Pasterkamp G, Schoneveld AH, van Wolferen W, Hillen B, Clarijs RJG, Haudenschild CC, et al. The impact of atherosclerotic arterial remodeling on percentage of luminal stenosis varies widely within the arterial system; A post mortem study. Arterioscler. Tromb. Vasc. Biol. 1997;17:3057–3063. doi: 10.1161/01.atv.17.11.3057. [DOI] [PubMed] [Google Scholar]
- Pasterkamp G, Schoneveld AH, van der Wal AC, Hijnen DJ, Wolveren WJA, Plomp S, et al. Inflammation of the atherosclerotic cap and shoulder of the plaque is a common and locally observed feature in unruptured plaques of femoral and coronary arteries. Arterioscler. Thromb. Vasc. Biol. 1999;19:54–58. doi: 10.1161/01.atv.19.1.54. [DOI] [PubMed] [Google Scholar]
- Ruengsakulrach P, Sinclair R, Komeda M, Raman J, Gordon I, Buxton B. Comparative histopathology of radial artery versus internal thoracic artery and risk factors for development of intimal hyperplasia and atherosclerosis. Circulation. 1999;100(suppl. II):139–144. doi: 10.1161/01.cir.100.suppl_2.ii-139. [DOI] [PubMed] [Google Scholar]
- Sorensen KE, Kristensen IB, Celermajer DS. Atherosclerosis in the human brachial artery. J. Am. Coll Cardiol. 1997;29:318–322. doi: 10.1016/s0735-1097(96)00474-3. [DOI] [PubMed] [Google Scholar]
- Stary HC, Blankenhorn DH, Chandler AB, Glagov S, Insull W, Jr, Richardson M, et al. A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation. 1992;85:391–405. doi: 10.1161/01.cir.85.1.391. [DOI] [PubMed] [Google Scholar]
- Stary HC, Chandler AB, Glagov S, Guyton JR, Insull W, Jr, Rosenfeld ME, et al. A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler. Thromb. 1994;14:840–856. doi: 10.1161/01.atv.14.5.840. [DOI] [PubMed] [Google Scholar]
- van der Wal AC, Becker AE, van der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994;89:36–44. doi: 10.1161/01.cir.89.1.36. [DOI] [PubMed] [Google Scholar]
- Zeiher AM, Schächinger V, Minners J. Long-term cigarette smoking impairs endothelium-dependent coronary arterial vasodilator function. Circulation. 1995;92:1094–1100. doi: 10.1161/01.cir.92.5.1094. [DOI] [PubMed] [Google Scholar]