Abstract
Studies of the comparative morphology of the tongues of living vertebrates have revealed how variations in the morphology and function of the organ might be related to evolutional events. The tongue, which plays a very important role in food intake by vertebrates, exhibits significant morphological variations that appear to represent adaptation to the current environmental conditions of each respective habitat. This review examines the fundamental importance of morphology in the evolution of the vertebrate tongue, focusing on the origin of the tongue and on the relationship between morphology and environmental conditions. Tongues of various extant vertebrates, including those of amphibians, reptiles, birds and mammals, were analysed in terms of gross anatomy and microanatomy by light microscopy and by scanning and transmission electron microscopy. Comparisons of tongue morphology revealed a relationship between changes in the appearance of the tongue and changes in habitat, from a freshwater environment to a terrestrial environment, as well as a relationship between the extent of keratinization of the lingual epithelium and the transition from a moist or wet environment to a dry environment. The lingual epithelium of amphibians is devoid of keratinization while that of reptilians is keratinized to different extents. Reptiles live in a variety of habitats, from seawater to regions of high temperature and very high or very low humidity. Keratinization of the lingual epithelium is considered to have been acquired concomitantly with the evolution of amniotes. The variations in the extent of keratinization of the lingual epithelium, which is observed between various amniotes, appear to be secondary, reflecting the environmental conditions of different species.
Keywords: keratinization, light and electron microscopy, lingual epithelium, lingual papilla, vertebrate evolution
Introduction
The feeding mechanism is clearly an important factor that determines the success of adaptation of vertebrates to their environment and of their persistence through procreation (Roth & Wake, 1989). In feeding, the tongue plays a principal role, together with other organs within and near the oral cavity, in particular in tetrapods. The tongue has a characteristic form in tetrapods. Fish have a slight elevation of the mucosa on the floor of the mouth but this structure does not contain any voluntary muscles, unlike the tongues of land vertebrates (Kent, 1978), one exception being the African clawed toad Xenopus laevis (Toyoshima & Shimamura, 1982). Most adult amphibians have a tongue (Helff, 1929), as do all known reptiles, birds and mammals. Thus it is likely that the tongue appeared with the establishment of tetrapods and this structure seems to be related, to some extent, to the terrestrial lifestyle (Helff, 1929). We can infer that the main role of the tongue is to facilitate eating on land, in co-operation with other organs within and near the oral cavity. It is proposed that, during adaptation from a wet to a dry habitat in the evolution of vertebrates, stratification and keratinization are the most important changes in the lingual epithelium. In addition, in some vertebrates, the tongue plays additional roles; these are especially significant in reptiles, as discussed below.
The origin of the tongue
A true tongue, which has voluntary muscle and is movable, is found in amphibian animals. As summarized by Youson (1981), a tongue-like piston is found in the oral opening of the adult lamprey but this organ is not homologous to the tongues of gnathostomes. When the lamprey grips its prey with its oral disc, the teeth on the piston abrade and cut the host tissue. The functional role of the piston is very similar to that of the tongue of some tetrapods. Auxiliary structures for food uptake appear to have existed in jawless fishes. They are absent in jawed fishes but are found again in tetrapods. The lamprey tongue and the tongues of tetrapods originated independently during evolution. Ammocoetes, the larvae of lampreys, do not have a tongue-like piston: small particles of food are taken up with fluid via the mouth and are delivered to the alimentary canal after sieving from the ingested fluid (Youson, 1981). Amphibian tadpoles also have little or no tongue-like tissue (Helff, 1929). However, with the exception of a few amphibia (Toyoshima & Shimamura, 1982), all amphibia after metamorphosis have a tongue (Kent, 1978). Teeth, unlike the tongue, are not always present. Extant turtles and also birds lack teeth. Aquatic vertebrates, such as jawed fish and tadpoles, can catch food just by opening their mouths and no tongue is needed to aid in predation, mastication and swallowing. The adult African clawed toad (Xenopus laevis) has no tongue but its life-style is essentially aquatic. The gustatory organs of fishes and tadpoles are located in the epidermis of the face or the epithelium of the oral cavity (Lane & Whitear, 1982; Whitear, 1986; Paulson et al., 1995). On land, effort is required to ingest food and the tongue appears to have evolved in parallel with the movement of vertebrates from water to land.
The musculature of the gnathostome tongue is a derivative of the hypobranchial apparatus, which is present in all vertebrates. In agnathans and gnathostome fishes, the hypobranchial apparatus forms musculature that is related to the gills, which are used for respiration. The same apparatus also functions as the musculature of the gills at embryonic and larval stages in all amphibians. However, gills begin to disappear during metamorphosis and, simultaneously, the hypobranchial apparatus reforms to yield the lingual musculature. As noted by Romer & Parsons (1977), with the reduction in the gills in tetrapods, the gill bars and their muscles became available for other uses, and the mobile tongue, characteristic of most land vertebrates, developed. Thus the tongue musculature is derived from the hypobranchial musculature, which is anchored to the hyoid apparatus.
The precursors to tongue muscle in amniotes have been shown to be derived from the occipital somites. The muscle precursors lose the features of epithelial cells and emigrate as a condensed stream of cells, known as the hypoglossal cord. Their path leads them ventrally and then rostrally through the base of the branchial arches into the mandibular arch. Innervation occurs via the hypoglossal nerve, whose axons follow the same path as the emigrating precursors to tongue muscle (Kuratani et al., 1988; MacKenzie et al., 1998; Huang et al., 1999).
Adaptation of the tongue to the environment
Amphibians usually live in and around freshwater, and the surface of the oral cavity around the tongue is wet. Even on land, amphibians are not generally exposed to extremely dry conditions, and, consistently, no keratinization is found in the amphibian lingual epithelium (Graziadei & DeHan, 1971; Zylberberg, 1977; Iwasaki & Kobayashi, 1989; Iwasaki et al., 1989a, 1989b). In addition, there is no distinct separation of the tongue and the salivary glands. A large part of the lingual epithelium consists of cells with secretory granules. The material secreted by the granules probably plays the same role as saliva. The tongues of amphibians should help both to catch food and to moisten it (Graziadei & DeHan, 1971; Zylberberg, 1977; Iwasaki & Kobayashi, 1989; Iwasaki et al., 1989a, 1989b, 1997b). Moreover, large gustatory papillae with taste buds are widely and densely distributed on the dorsal surface of the tongues of many amphibians (von Düring & Andres, 1976; Iwasaki & Sakata, 1985; Iwasaki & Kobayashi, 1988; Iwasaki et al., 1989a, 1989b). Thus, a gustatory function has been added to the food-eating and salivation functions. Among amphibians, toads have adapted to a comparatively dry habitat and they can live in areas away from ponds and rivers for extended periods. Reflecting this capacity, the lingual epithelium consists partly of cells without secretory granules (Frye, 1991; Fig. 1), especially on the apical side that is exposed to dry conditions. The separation of the tongue from the salivary glands is more conspicuous in terrestrial reptiles, birds and mammals.
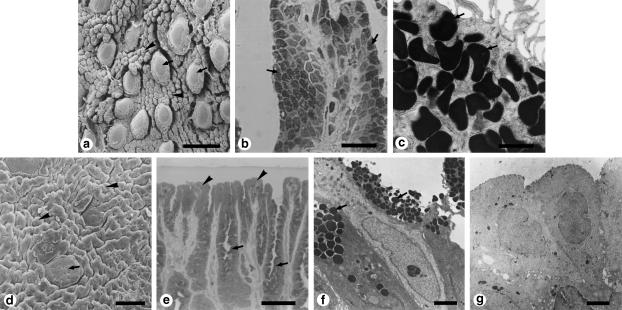
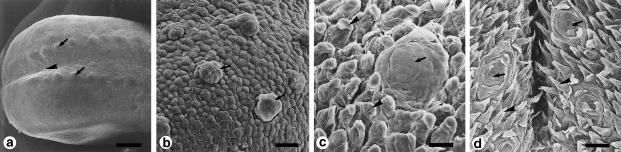
Figure 1.
Surface structure and histology of the dorsal epithelium of the tongues of amphibians. (a-c) Genus Rana. (a) Scanning electron micrograph of the tongue of Rana catesbeiana. (b) Light micrograph of a filiform papilla of Rana rugosa rugosa. (c) High-magnification transmission electron micrograph of the filiform papillae of Rana rugosa rugosa. (d-g) Genus Bufo (Bufo japonicus). (d) Scanning electron micrograph. (e) Light micrograph. (f,g) Transmission electron micrographs. Both Rana and Bufo have sensory discs that contain taste buds (arrows in a,d) and are scattered between filiform papillae (arrowheads in a) or ridge-like papillae (arrowheads in d). In Rana and Bufo, most cells of the lingual epithelium contain secretory granules (arrows in b,c,e,f). However, the cells at the tips of the filiform or ridge-like papillae are significantly different in Rana (b,c) and Bufo (e-g). Arrowheads in (e) show such cells in Bufo; they contain no secretory granules. (g) A high-magnification view of the cells in (e). Scale bars = 300 μm (a); 20μm (b); 1 μm (c); 100 μm (d); 50 μm (e); 2 μm (f); 5μm (g). (a, reproduced from Iwasaki & Sakata, 1985; with permission from Okajimas Folia Anatomica Japonica; b, reproduced from Iwasaki et al., 1997b; with permission from Tissue and Cell; d, reproduced from Iwasaki & Kobayashi, 1988; with permission from Zoological Science; e, reproduced from Iwasaki et al., 1989b; with permission from Zoological Science.)
Reptiles may live in fresh water, sea water and on the land. On land, moreover, habitats vary significantly although temperatures are usually relatively high. Some reptiles live in habitats with extremely high temperatures and high humidity; others live at very low humidity with extreme variations in temperature; still others live at moderate temperatures and humidity (Winokur, 1988). The most interesting features of the histological structures of reptilian tongues reflect adaptations to a dry habitat or to seawater, but stratification and keratinization of the lingual epithelium are common features (Broman, 1920; Iwasaki, 1990; Iwasaki & Kumakura, 1994; Toubeau et al., 1994; Iwasaki et al., 1996a, 1996c, 1996e). Reptilian tongues are characterized by morphological and functional variations among species (Fig. 2). For example, the snake's tongue does not appear to be important for the direct intake of food but might be used exclusively for olfaction in co-operation with Jacobson's vomeronasal organs (Kahmann, 1932; McDowell, 1972; Gillingham & Clark, 1981). The flicking of a snake's tongue is thought to be a way of adsorbing odorants in the air (Halpern et al., 1986). The epithelium of the anterior half of the snake's tongue, which is involved in such flicking, includes numerous lipid-containing granules (Iwasaki et al. unpubl. obs.). The lipids in these granules are suitable for capturing odorants and for their transfer to Jacobson's vomeronasal organs since the affinity of odorants for lipids is generally high (Carmignani & Zaccone, 1975; Nalavade & Varute, 1976; Nomura & Kurihara, 1987a, 1987b; Bouchard et al., 1996; Zhou et al., 1998). The bifurcated apex of the tongue is frequently exposed to dry air and this part of the tongue is shed together with the snake's epidermis (Iwasaki et al. unpubl. obs.). By contrast, the American chameleon's tongue is intimately involved in feeding and a large part of the lingual epithelium consists of cells with secretory granules, many of which are mucous granules and some of which are serous granules (Rabinowitz & Tandler, 1986). Thus the shape and structure of the tongue differ significantly among reptiles, reflecting the various functions of each respective tongue. Turtles provide another interesting case of adaptational differences in the morphology of the lingual epithelium. In freshwater turtles, most of the lingual epithelium is composed of non-keratinized cells, which are filled with secretory granules (Iwasaki et al., 1992a, 1996b, 1996d; Iwasaki, 1992a; Beisser et al., 1995, 1998). By contrast, in terrestrial turtles (Winokur, 1988) and sea turtles (Iwasaki et al., 1996a, 1996c), the lingual epithelium is keratinized and there are no cells with secretory granules (Fig. 3). These differences are thought to reflect differences in habitat and should affect the survival of each reptilian species in different environments. In lizards, similar differences with respect to the morphology and histological structure of the lingual epithelium have been reported among species in different habitats (Schwenk, 1985, 1986, 1989; Smith, 1988; Iwasaki, 1990; Toubeau et al., 1994). The most significant differences are found in the degree and extent of keratinization of the lingual epithelium. Such differences seem to depend on habitat and especially on humidity. However, the alligator is an aquatic reptile and its lingual epithelium is strongly keratinized (Shimada et al., 1990). This phenomenon might be explained by the possibility that the volume of the tongue and the conditions required for maintenance of the lingual tissue might be correlated. Thus, when the alligator is on land, the lingual epithelium would dry out in the absence of keratinization of the surface layer of the epithelium.
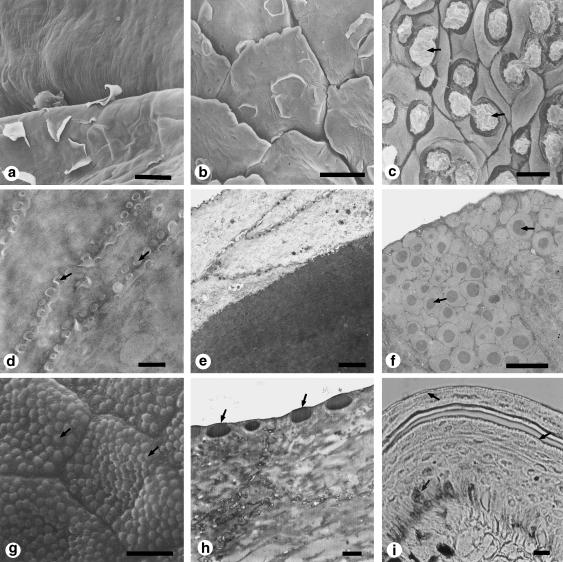
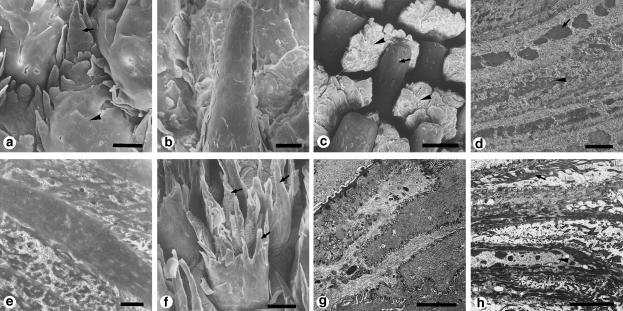
Figure 2.
Surface structure and histology of the epithelium of the tongues of squamate reptiles. (a-c) Scanning electron micrographs of the dorsal surface of the tongue of the Japanese lizard Takydromus tachydromoides. (a) Anterior bifurcated area. (b) Lingual body. (c) Lingual radix. Arrows in c show the secretory fluid. (d-f) Transmission electron micrographs of the dorsal epithelium of the tongues of two lizards. (d) Anterior bifurcated area of the tongue of Takydromus tachydromoides corresponding to a. (e) Lingual body of Takydromus tachydromoides corresponding to b. (f) Lingual radix of Gekko japonicus corresponding to the same region as c in Takydromus tachydromoides. Arrows show bipartite secretory granules with a dense central core. (g-i) The rat snake Elaphe climacophora. (g) Scanning electron micrograph of one of the anterior bifurcated parts of the tongue. Arrows indicate microfacets. (h) Transmission electron micrograph of the outerward face of the a-layer of the epithelium of the anterior bifurcated parts of the tongue. Arrows indicate microfacets. ( j) Light micrograph of the dorsal lingual epithelium showing a frontal section of the lingual apex at the shedding phase. Arrows indicate positive staining with Sudan III. Scale bars = 30 μm (a); 50 μm (b); 5 μm (c,g); 0.5 μm (d); 2 μm (e,f); 1 μm (h); 10 μm (i). (a-c, reproduced from Iwasaki & Miyata, 1985; with permission from Okajimas Folia Anatomica Japonica; g, reproduced from Iwasaki et al., 1996e; with permission from the Anatomical Record.)
Figure 3.
Surface structure and histology of the dorsal epithelium of the tongues of turtles. (a-c) The soft-shell turtle Trionyx cartilagineus, which lives in or near freshwater. (a) Scanning electron micrograph of the surface of a low, disc-like papilla located on the dorsal side of the posterior part of the tongue. (b) Light micrograph of cells in the dorsal epithelium of the tongue. No keratinization is evident in any of the lingual epithelium. (c) Transmission electron micrograph of cells in the dorsal epithelium of the tongue. (d-f) A juvenile sea turtle, the Hawksbill turtle (Eretmochelys imbricata bissa). (d) Scanning electron micrograph. Arrows show the marginal cell border. (e) Light micrograph. Arrow indicates the keratinized, squamous, stratified epithelium.(f) Transmission electron micrograph of the keratinized layer of the epithelium. Scale bars = 100 μm (a); 30 μm (b); 2 μm (c); 3 μm (d); 10 μm (e); 0.5 μm (f). (a,c, reproduced from Iwasaki et al., 1996b; with permission from the Anatomical Record; d-f, reproduced from Iwasaki et al., 1996a; with permission from the Anatomical Record.)
In some groups of lizards, differences among regions of the tongue in terms of the structure of the lingual epithelium have been recognized: epithelial cells at the lingual apex are keratinized; those in the lingual radix are not keratinized; and those in the intermediate region between the lingual apex and the radix exhibit a transition, in terms of the keratinization of the epithelial cells, from one form to the other (Iwasaki & Miyata, 1985; Iwasaki, 1990). Reptiles generally have taste buds in the lingual epithelium (Schwenk, 1985, 1986; Toyoshima & Shimamura, 1987; Delheusy et al., 1994), and taste buds are often also located in the epithelium of the gingiva along the teeth (Iwasaki et al., 1985; Schwenk, 1985). In most snakes, the tongue does not play an important role in feeding. In such cases, there is a direct connection between tasting and biting. In many other squamates, the tongue of which plays an important role in feeding (Schwenk, 1989; Kraklau, 1991), taste buds both on the tongue and on the gingiva seem to function. In birds and mammals, taste buds are located mainly within the lingual epithelium. Furthermore, in terrestrial reptiles, the salivary glands are separate from the tongue (Kent, 1978), and the relative number of cells with secretory granules is relatively low in the lingual epithelium (Iwasaki & Miyata, 1985; Schwenk, 1986, 1988; Iwasaki, 1990; Smith & Mackay, 1990; Iwasaki & Kumakura, 1994; Toubeau et al., 1994). This tendency continues in mammals, where it is even more apparent.
Birds live in the air, on land, and on and around fresh water and sea water. However, keratinization of the lingual epithelium is a common feature (Iwasaki, 1992b; Iwasaki et al., 1997a; Kobayashi et al., 1998), in particular on the ventral side of the tongue, where the so-called ‘lingual nail’ is prominent in all species examined (Susi, 1969; Homberger & Brush, 1986; Carver & Sawyer, 1989). In the ancestors of birds, the lingual epithelium might have become adapted to dry conditions. In birds, the lingual papillae also play an important role in feeding, and birds that eat hard foods have structures similar to teeth in their upper and lower beaks. Moreover, hard processes at the edge of both sides of the tongue are located next to the inside of the beak (Iwasaki et al., 1997a; Fig. 4). These structures act co-operatively during feeding and mastication. The dorsal surface of the lingual epithelium is covered by numerous fine processes, which keep food on the tongue's surface (Iwasaki, 1992b). The same structures are useful for holding food, such as fish, within the mouth (Kobayashi et al., 1998). The taste buds of birds are distributed not only in the lingual epithelium but also in the epithelium of other parts of the oral cavity, as seen also in reptiles (Kutuzov & Sicher, 1951; Gentle, 1971; Ganchrow & Ganchrow, 1985). In some cases, taste buds have been found in the deep area of the lingual epithelium of the dorsal radix, and long ducts connect the buds to the dorsal surface of the tongue. The openings of these ducts at the dorsal surface are called taste pores (Ganchrow & Ganchrow, 1985).
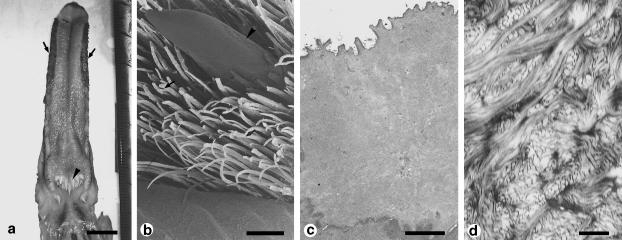
Figure 4.
Surface structure and histology of the dorsal epithelium of the tongue of Middendorff's bean goose, Anser fabalis middendorffii. (a) Macroscopic dorsal view of the tongue. Arrows show lingual hairs on the lateral sides). (b) Scanning electron micrograph of the lateral side of the tongue. Lingual papillae (arrows) are compactly distributed on the tongue, and large cylindrical papillae (arrowhead) are scattered among them. (c) Transmission electron micrograph of cells on the extreme surface side of the keratinized layer of the dorsal epithelium. Keratin filaments are looser than those of cells beneath this area. The surface of the cell membrane has microridges. No other organelles are visible. (d) Higher-magnification transmission electron micrograph of the cytoplasm of a cell in the keratinized layer of the epithelium of a strongly keratinized papilla. Scale bars = 10 μm (a); 500 μm (b); 2 μm (c); 0.2 μm (d). (a-c, reproduced from Iwasaki et al., 1997a; with permission from the Anatomical Record.)
Although stratification is a common feature of all parts of the oral epithelium of mammals, keratinization is normally associated with the masticatory oral mucosa that surrounds the dorsal part of the lingual body and seems to be related to the provision of resistance against damage, through wear, to tissue (Stern, 1980; Avery, 1987). The labial and buccal mucosa, which lie very close to the opening of the mouth, persist in being non-keratinized. Therefore, a dry habitat is no longer a necessary element for keratinization of the oral epithelium, as observed clearly in reptiles. Nevertheless, structural differences in the lingual epithelium between mammalian species can be recognized, related to the habitat of each species. With the exception of primates and some species of Procyonidae (Carnivora), who use their hands during feeding, most mammals use their mouths exclusively for feeding. In all cases, the tongue plays an important role during feeding, together with the teeth. Tongues are also used for grooming. Fundamental and common features of the lingual epithelium of mammals are stratification and keratinization (Kutuzov & Sicher, 1951, 1953; Kubota & Hayama, 1964; Kubota et al., 1966; Farbman, 1970; Baratz & Farbman, 1975; Krause & Cutts, 1982; Steflik et al., 1983; Rentrop et al., 1986; Iwasaki et al., 1987, 1992b; Iwasaki, 1992c, 1992d; MacKenzie & Dabelsteen, 1987; Iwasaki & Miyata, 1989; Agungpriyono et al., 1995; Toyoda et al., 1998) and, as noted for terrestrial reptiles, this phenomenon seems to have been part of the process of adaptation to dry land.
Some evidence for this possibility is provided by the fact that, in most mammals, keratinization of the epithelium begins with the appearance of the non-gustatory lingual papillae, namely, the filiform papillae, just before birth (Baratz & Farbman, 1975; Dougbag, 1987a, 1987b; Iwasaki et al., 1999a, 1999b). By contrast, the gustatory papillae, such as the fungiform, circumvallate and foliate papillae, appear at an earlier embryonic stage without any obvious relationship to the keratinization of the lingual epithelium (Torrey, 1940; Farbman, 1965; Paulson et al., 1985; Dougbag, 1987a, 1987b, 1988; Farbman & Mbien, 1991; Whitehead & Kachele, 1994; Iwasaki et al., 1996f, 1997c; Mistretta & Haus, 1996; Fig. 5). However, there are some exceptions to this scenario. For example, in humans, keratinization of the lingual epithelium starts in the middle of gestation, months before birth (Yamasaki & Takahashi, 1982), perhaps because of the lengthy gestation of humans.
Figure 5.
Surface structure of the dorsal epithelium of the tongues of rats and mice during development, as demonstrated by scanning electron microscopy. (a) Rat fetus on embryonic day 12. Arrows show the rudiments of fungiform papillae. Arrowhead indicates the median sulcus. (b) Mouse fetus on embryonic day 15. Arrows indicate original rudiments of fungiform papillae. (c) Juvenile rat just after birth. Arrow indicates a fungiform papilla. Arrowheads indicate rudiments of filiform papillae. (d) Juvenile mouse 7 days after birth. Arrows indicate fungiform papillae. Arrowheads indicate filiform papillae. Scale bars = 100 μm (a); 20 μm (b, c); 50 μm (d). (a,c, reproduced from Iwasaki et al., 1997b; with permission from the Anatomical Record; b, reproduced from Iwasaki et al., 1996f; with permission from Acta Anatomica.)
The morphological and histological features of mammalian tongues reflect the differences among the life-styles of mammals. In the tongues of rodents, such as rats and mice, there is significant hard keratinization of the epithelium over the entire dorsal area, which includes filiform papillae (Kutuzov & Sicher, 1951, 1953; Kubota & Hayama, 1964; Kubota et al., 1966; Farbman, 1970; Baratz & Farbman, 1975). A plausible explanation for this phenomenon might be that rodents eat hard foods. With some exceptions (Baratz & Farbman, 1975), the histological structure of filiform papillae is similar in almost all mammals. These papillae are commonly inclined towards the lingual radix, and their keratinization is harder than that in the interpapillar area, being similar to that of hair (Boshell et al., 1982). Moreover, the anterior regions of papillae are softer than their posterior regions (Kutuzov & Sicher, 1951, 1953; Farbman, 1970; Baratz & Farbman, 1975; Krause & Cutts, 1982; Rentrop et al., 1986; MacKenzie & Dabelsteen, 1987; Iwasaki & Miyata, 1989; Iwasaki et al., 1992b; Iwasaki, 1992c, 1992d; Agungpriyono et al., 1995). Therefore, the papillae are easily bent in the direction of the radix but not in the opposite direction. This property facilitates retention of food on the dorsal surface of the tongue. The structure of the specialized filiform papillae that are also used for grooming is different from that mentioned above. The keratinization of these papillae is more significant than that of ordinary filiform papillae and the papillae can withstand strong physical force (Kubota, 1968; Iwasaki & Miyata, 1990; Fig. 6). In mammals adapted to sea water, such as the fur seal (Yamasaki et al., 1978), dolphin (Shimoda et al., 1996) and sea otter (Hosley & Oakley, 1987), the extent of development of filiform papillae varies but keratinization of the lingual epithelium is clearly recognizable. The various types of filiform papilla probably reflect the fact that these structures are not of salient importance for feeding in mammals that live in the sea. However, there are no significant differences in terms of the structure and location of the filiform papillae between terrestrial mammals and mammals with an aquatic or semi-aquatic habitat. Primitive mammals may have originated from completely terrestrial reptiles, with keratinization of the lingual epithelium being irreversible during evolution. The same may be true for birds.
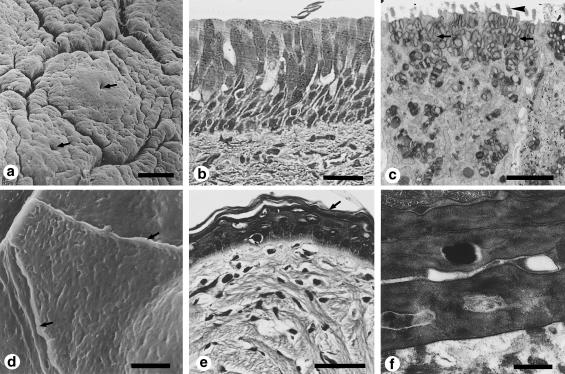
Figure 6.
Surface structure and histology of the epithelium of mammalian tongues. (a-c) Scanning electron micrographs of the dorsal surface of the tongue of the mongoose Herpestes edwardsi. (a) Apex of the dorsal surface. Each filiform papilla has a large bulge (arrowhead) in the baso-frontal area. About 10 small processes surround the bulge in a semicircle on the posterior side. Among these processes, the middle rear one (arrow) is the largest. (b) Anterior part of the dorsal surface. Each papilla consists of a large process without a baso-frontal bulge. (c) Middle dorsal surface. Filiform papillae (arrow) are cylindrical without a baso-frontal bulge. Interpapillar epithelium (arrowheads) appears between these papillae as a series of large protuberances with many folds on their surfaces. (d-e) Transmission electron micrographs of the dorsal epithelium of the tongue of the mongoose Herpestes edwardsi. (d) Superficial intermediate layer of the papillary epithelium. Large, droplet-like keratohyalin granules arrow) and tonofibrils (arrowhead) are recognizable. (e) Keratinized surface layer of the papillary epithelium. Keratinized and prekeratinized cells are arranged in a lamellar pattern. (f) Scanning electron micrograph of the dorsal surface of tongue of the Japanese monkey Macaca fuscata fuscata. Filiform papillae are crown-shaped, with several or more branches (arrows). g-h) Transmission electron micrographs of the dorsal epithelium of the tongue of the Japanese monkey Macaca fuscata fuscata. g) Surface layer of the anterior side of a filiform papilla. Most of the cytoplasm is occupied by filamentous structures that represent tonofilaments. (h) Deep intermediate layer of the posterior side of a filiform papilla. In most of cells, large numbers of tonofibrils are present in the cytoplasm. A large nucleus is recognizable. Scale bars = 30 μm (a); 50 μm (b); 300 μm (c); 2 μm d); 1 μm (e); 5 μm (f, g, h). (a, c, reproduced from Iwasaki et al., 1987; with permission from Acta Anatomica; d, reproduced from Iwasaki & Miyata, 1990; with permission from Journal of Anatomy.)
Another feature of the mammalian tongue is the location of taste buds. Taste buds are distributed over a wide area of the dorsal and lateral surfaces of the tongue (Nickel et al., 1973; Robinson & Winkles, 1990; Takeda et al., 1990; Chamorro et al., 1993; Myers et al., 1995), indicating that the tongue is important for feeding and, at the same time, for taste. The salivary glands are exceedingly well developed in mammals but the major salivary glands, which are the main organs for salivation, are separate from the tongue (Kubota et al., 1963). The minor salivary glands are located in specific areas of the lingual epithelium. Ebner's gland, which is located beneath the circular sulcus around the circumvallate papillae, secretes serous fluid into the space of the sulcus to wash away gustatory materials from the taste buds that face the sulcus (Kubota & Hayama, 1964; Kubota, 1966; Hand, 1970; Graziadei & Graziadei, 1978; Kullaa-Mikkonen et al., 1985; Hosley & Oakley, 1987; Agungpriyono et al., 1995). Then the taste buds of the papillae can receive fresh stimulation by gustatory materials. A similar correlation between taste buds and minor salivary glands exists in the foliate papillae, another type of mammal-specific gustatory papilla (Kubota, 1966; Baratz & Farbman, 1975). The epithelium of the oral mucosa that surrounds the tongue of mammals might generally be softer than that of birds because the wetness of the surface of the oral cavity is significant in mammals due to the high level of development of major salivary glands. The functional importance in feeding of the tongues of Primates and certain other animals, such as some species of Carnivora, is lower than in other mammals because humans and monkeys in Primates and pandas, raccoons (Procyonidae) and sea otters (Enhydra) in Carnivora often use their hands to grasp their food. Furthermore, humans can cook their food and their food is softer than that consumed by other vertebrates. It is noteworthy that the keratinization of the lingual epithelium in humans is not as strong as it is in other mammals (Toyoda et al., 1998).
Morphological evidence for adaptation in extant vertebrates
Comparisons of the morphology and function of the lingual epithelium among extant vertebrates suggest that adaptation has been a factor in the evolution of vertebrates. It seems likely that a movable tongue appeared during adaptation from an aquatic environment to life on land. It is possible that the appearance of the tongue in amphibia proved useful for terrestrial feeding and allowed adaptation to a larger range of habitats. Keratinization of the lingual epithelium might have appeared first in amniotes. The lingual epithelium of some amphibia, such as toads, is of the stratified squamous type, differing from the stratified cuboidal type in frogs (Winokur, 1988). This difference may reflect an adaptational change of the lingual epithelium to drier circumstances since toads can live in drier areas than frogs. Furthermore, the lingual epithelium of freshwater turtles shows no tendency towards keratinization (Iwasaki, 1992a; Iwasaki et al., 1992a, 1996b, 1996d; Beisser et al., 1995, 1998). By contrast, the lingual epithelium of terrestrial turtles exhibits a significant tendency towards keratinization (Winokur, 1988), as does the lingual epithelium of sea turtles (Iwasaki et al., 1996a, 1996c). Thus, the adaptation of the lingual epithelium to a seawater environment resembles that to a terrestrial environment. The maintenance of the homeostasis of the tissues in the oral cavity of animals living in sea water involves the same mechanism as that in terrestrial vertebrates. The possibility that keratinization of the lingual epithelium represents adaptation of terrestrial vertebrates to low humidity is suggested by the rapid keratinization of the lingual epithelium of the fetus just before birth, when mammals move from a wet environment to dry or seawater conditions. Adaptational changes during evolution might have allowed vertebrates to occupy a wider range of habitats than that provided by fresh water.
In conclusion, comparative studies of the morphology of the tongues of extant vertebrates suggest an important role for adaptation of the structure of the tongue during the movement of vertebrates from fresh water to land or sea water, and for keratinization of the lingual epithelium during adaptation from wet conditions to dry or sea water conditions. The evolutional changes in the tongue are thought to be the foundation for progress in the ingestion of food and in the expansion of the range of vertebrate habitats.
Table 1.
Similarities and differences in tongue morphology between various vertebrate species and taxa
| Class | Order | |
|---|---|---|
| Agnatha | No true tongue. Adult lamprey has a tongue-like piston | |
| Osteichthyes | No true tongue. There is the slight elevation of the mucosa from the oral floor without voluntary muscle. | |
| Amphibia | Anura | All species except Xenopus have a tongue, most part of whose epithelium consists of cells with secretory granules. |
| Urodela | Features similar to those in Anura. | |
| Reptilia | Chelonia | Freshwater turtles: most of the lingual epithelium is composed of non-keratinized cells. |
| Terrestrial turtles and sea turtles: the lingual epithelium is keratinized. | ||
| Squamata | Snakes: the lingual epithelium consists of keratinized cells with numerous lipid droplets. | |
| American chameleons: the lingual epithelium consists of non-keratinized cells with secretory granules. | ||
| Lizards: the degree and the extent of keratinization of the lingual epithelium vary among species. | ||
| Crocodilia | Alligators: the lingual epithelium is strongly keratinized. | |
| Aves | Keratinization of the lingual epithelium is a common feature, without regard to habitat. | |
| Mammalia | Stratification and keratinization are common features in all species. However, the morphological and histological features of mammalian tongues reflect differences among the respective life-styles of mammals. |
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (nos 04671110, 06671840, 08672102 and 11671822).
References
- Agungpriyono S, Yamada J, Kitamura N, Nisa C, Sigit K, Yamamoto Y. Morphology of the dorsal lingual papillae in the lesser mouse deer, Tragulus javanicus. J. Anat. 1995;187:635–640. [PMC free article] [PubMed] [Google Scholar]
- Avery JK. Baltimore: Williams & Wilkins; 1987. Oral Development and Histology. [Google Scholar]
- Baratz RS, Farbman AI. Morphogenesis of rat lingual filiform papillae. Am. J. Anat. 1975;143:283–302. doi: 10.1002/aja.1001430303. [DOI] [PubMed] [Google Scholar]
- Beisser CJ, Weisgram J, Hilgers H, Splechtna H. Fine structure of the dorsal lingual epithelium of Trachemys scripta (Chelonia: Emydidae) Anat. rec. 1998;250:127–135. doi: 10.1002/(SICI)1097-0185(199802)250:2<127::AID-AR1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Beisser CJ, Weisgram J, Splechtna H. Dorsal lingual epithelium of Platemys pallidipectoris. J. Morph. 1995;226:267–276. doi: 10.1002/jmor.1052260303. [DOI] [PubMed] [Google Scholar]
- Boshell JL, Wilborn WH, Singh BB. Filiform papillae of cat tongue. Acta Anat. 1982;114:97–105. doi: 10.1159/000145583. [DOI] [PubMed] [Google Scholar]
- Bouchard M, Bouchard N, Auger M. Membrane fluidity response to odorants as seen by 2H-NMR and infrared spectroscopy. Biochim. Biophys. Acta. 1996;1282:233–239. doi: 10.1016/0005-2736(96)00061-2. [DOI] [PubMed] [Google Scholar]
- Broman I. Das Organon vomero-nasale Jacobsoni: Ein Wassergerchsorgan! Anat. Heffe 1. Abteilung. 1920;174:137–192. [Google Scholar]
- Carmignani MPA, Zaccone G. Histochemical distribution of acid mucopolysaccharides in the tongue reptiles I. – Chelonia (Pseudemys scripta Clark. Annales D’histochimie. 1975;20:77–88. [PubMed] [Google Scholar]
- Carver WE, Sawyer RH. Immunocytochemical localization and biochemical analysis of α and β keratins in the avian lingual epithelium. Am. J. Anat. 1989;184:66–75. doi: 10.1002/aja.1001840108. [DOI] [PubMed] [Google Scholar]
- Chamorro CA, de Paz P, Fernández JG, Anel L. Fungiform papillae of the pig and the wild boar analyzed by scanning electron microscopy. Scan. Microsc. 1993;7:313–322. [PubMed] [Google Scholar]
- Delheusy V, Toubeau G, Bels VL. Tongue structure and function in Oplurus cuvieri (Reptilia: Iguanidae) Anat. Rec. 1994;238:263–276. doi: 10.1002/ar.1092380212. [DOI] [PubMed] [Google Scholar]
- Dougbag AEI-S. Scanning electron microscopic studies of the morphogenesis of the lingual gustatory papillae of camel (Camel dromedarius) I. Morphogenesis of the fungiform papillae. Z. Milkrosk. Anat. Forsch. 1987a;101:881–892. [PubMed] [Google Scholar]
- Dougbag AEI-S. Scanning electron microscopic studies of the morphogenesis of the lingual lentiform and corniform papillae in camel (Camel dromedarius) Z. Milkrosk. Anat. Forsch. 1987b;101:893–903. [PubMed] [Google Scholar]
- Dougbag AEI-S. Scanning electron microscopic studies of the morphogenesis of the lingual gustatory papillae of camel (Camel dromedarius) I. Morphogenesis of the circumvallate papillae. Z. Milkrosk. Anat. Forsch. 1988;102:259–271. [PubMed] [Google Scholar]
- von Düring M, Andres KH. The ultrastructure of taste and touch receptors of frog's taste organ. Cell Tissue Res. 1976;169:185–198. doi: 10.1007/BF00226658. [DOI] [PubMed] [Google Scholar]
- Farbman AI. Electron microscope study of the developing taste bud in rat fungiform papilla. Dev. Biol. 1965;11:110–135. doi: 10.1016/0012-1606(65)90040-0. [DOI] [PubMed] [Google Scholar]
- Farbman AI. The dual pattern of keratinization in filiform papillae on rat tongue. J. Anat. 1970;106:233–242. [PMC free article] [PubMed] [Google Scholar]
- Farbman AI, Mbien J-P. Early development and innervation of taste bud-bearing papillae on the rat tongue. J. Comp. Neural. 1991;304:172–186. doi: 10.1002/cne.903040203. [DOI] [PubMed] [Google Scholar]
- Frye FL. Reptile Care – an Atlas of Diseases and Treatment. Neptune: T.F.H. Publications, Inc; 1991. [Google Scholar]
- Ganchrow D, Ganchrow JR. Number and distribution of taste buds in the oral cavity of hatching chicks. Physiol. Behav. 1985;34:889–894. doi: 10.1016/0031-9384(85)90009-5. [DOI] [PubMed] [Google Scholar]
- Gentle MJ. The lingual taste buds of Gallus domesticus. L. Br. Poultry sci. 1971;12:245–248. doi: 10.1080/00071667108415876. [DOI] [PubMed] [Google Scholar]
- Gillingham JC, Clark DL. Snake tongue-flicking: Transfer mechanics to Jacobson's organ. Can. J. Zool. 1981;59:1651–1657. [Google Scholar]
- Graziadei PPC, DeHan RS. The ultrastructure of frog's taste organ. Acta Anat. 1971;80:563–603. doi: 10.1159/000143715. [DOI] [PubMed] [Google Scholar]
- Graziadei PPC, Graziadei AM. Observations on the ultrastructure of ganglion cells in the circumvallate papilla of rat and mouse. Acta Anat. 1978;100:289–305. doi: 10.1159/000144911. [DOI] [PubMed] [Google Scholar]
- Halpern M, Schulman N, Kirschenbaum DM. Characteristics of earthworm washings detected by the vomeronasal system of snakes. In: Duvall D, Müller-Schwartz D, Silverstein RM, editors. In: Chemical Signals in Vertebrates. Vol. 4. New York: Plenum Press; 1986. pp. 63–77. [Google Scholar]
- Hand A. The fine structure of von Ebner's gland of the rat. J. Cell. Biol. 1970;44:340–353. doi: 10.1083/jcb.44.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helff OM. Studies on amphibian metamorphosis. IV. Growth and differentiation of anuran tongue during metamorphosis. Physiol. Zool. 1929;2:334–341. [Google Scholar]
- Homberger DG, Brush A. Functional-morphological and biochemical correlations of the keratinized structures in the African grey parrot, Psittacus erithacus (Aves) Zoomorphology. 1986;106:103–114. [Google Scholar]
- Hosley M, Oakley B. Postnatal development of the vallate papilla and taste buds in rats. Anat. Rec. 1987;218:216–222. doi: 10.1002/ar.1092180217. [DOI] [PubMed] [Google Scholar]
- Huang R, Zhi Q, Izpisua-Belmonte J-C, Christ B, Patel K. Origin and development of the avian tongue muscles. Anat. Embryol. 1999;200:137–152. doi: 10.1007/s004290050268. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Miyata K. Scanning electron microscopy of the lingual dorsal surface of the Japanese lizard, Takydromus tachydromoides. Okajimas Folia Anat. Jpn. 1985;62:15–26. doi: 10.2535/ofaj1936.62.1_15. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Sakata K. Fine structure of the lingual dorsal surface of the bullfrog. Okajimas Folia Anat. Jpn. 1985;61:437–450. doi: 10.2535/ofaj1936.61.6_437. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Miyata K, Kobayashi K. Fine structures of the oral epithelial cell surface in the Japanese lizard, Takydromus tachydromoides. Jpn. J. Oral. Biol. 1985;27:956–964. [Google Scholar]
- Iwasaki S, Miyata K, Kobayashi K. Comparative studies of the dorsal surface of the tongue in three mammalian species by scanning electron microscopy. Acta Anat. 1987;128:140–146. doi: 10.1159/000146330. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Kobayashi K. Fine structure of the dorsal tongue surface in the Japanese toad, Bufo japonicus(Anura, Bufonidae) Zool. Sci. 1988;5:331–336. [Google Scholar]
- Iwasaki S, Kobayashi K. Fine structure of the lingual dorsal epithelium in the bullfrog, Rana catesbeiana. Zool. Sci. 1989;6:259–267. [Google Scholar]
- Iwasaki S, Miyata K. Fine structure of the filiform papilla of beagle dogs. J. Morph. 1989;201:235–242. doi: 10.1002/jmor.1052010303. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Miyata K, Kobayashi K. Fine structures of the filiform papillar epithelium in the tongue of the frog, Rana nigromaculata. Zool. Sci. 1989a;5:61–68. [Google Scholar]
- Iwasaki S, Miyata K, Kobayashi K. Fine structures of the lingual dorsal epithelium of the Japanese toad, Bufo japonicus. Zool. Sci. 1989b;6:681–689. [Google Scholar]
- Iwasaki S. Fine structure of the dorsal lingual epithelium of the lizard, Gekko japonicus (Lacertilla, Gekkonidae) Am. J. Anat. 1990;187:12–20. doi: 10.1002/aja.1001870103. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Miyata K. Fine structure of the dorsal epithelium of the mongoose tongue. J. Anat. 1990;172:201–212. [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S. Fine structure of the dorsal epithelium of the tongue of the freshwater turtle, Geoclemys reevesii (Chelonia, Emydinae) J. Morph. 1992a;211:125–135. [Google Scholar]
- Iwasaki S. Fine structure of the dorsal lingual epithelium of the little tern, Sterna albifrons (Aves, Lari) J. Morph. 1992b;212:13–26. doi: 10.1002/jmor.1052120103. [DOI] [PubMed] [Google Scholar]
- Iwasaki Fine structure of the dorsal lingual epithelium of the domestic, newborn kitten, Felis catus. Ann. Anat. 1992c;174:293–300. doi: 10.1016/s0940-9602(11)80285-2. [DOI] [PubMed] [Google Scholar]
- Iwasaki S. Fine structure of the dorsal lingual epithelium of the crab-eating monkey, Macaca irus. Ann. Anat. 1992d;174:523–529. doi: 10.1016/s0940-9602(11)80315-8. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Asami T, Asami Y, Kobayashi K. Fine structure of the dorsal epithelium of the tongue of the Japanese terrapin, Clemys japonica (Chelonia, Emydinae) Arch. Histol. Cytol. 1992a;55:295–305. doi: 10.1679/aohc.55.295. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Yoshizawa H, Suzuki K. Fine structure of the dorsal lingual epithelium of the Japanese monkey, Macaca fuscata fuscata. Acta Anat. 1992b;144:267–277. doi: 10.1159/000147314. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Kumakura M. An ultrastructural study of the dorsal lingual epithelium of the rat snake, Elaphe quadrivirgata. Ann. Anat. 1994;176:455–462. doi: 10.1016/s0940-9602(11)80478-4. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Asami T, Wanichanon C. Fine structure of the dorsal lingual epithelium of the juvenile Hawksbill turtle, Eretmochelys imbricata bissa. Anat. Rec. 1996a;244:437–443. doi: 10.1002/(SICI)1097-0185(199604)244:4<437::AID-AR2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Asami T, Wanichanon C. Ultrastructural study of the dorsal lingual epithelium of the soft-shell turtle, Trionix cartilagineus(Cehlonia, Trionychidae) Anat. Rec. 1996b;246:305–316. doi: 10.1002/(SICI)1097-0185(199611)246:3<305::AID-AR1>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Wanichanon C, Asami T. Histological and ultrastructural study of the lingual epithelium of the juvenile Pacific ridley turtle, Lepidochelys olivacea (Chelonia, Chelonidae) Ann. Anat. 1996c;178:243–250. doi: 10.1016/s0940-9602(96)80057-4. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Wanichanon C, Asami T. Ultrastructural study of the dorsal lingual epithelium of the Asian snail-eating turtle, Malayemys subtrijuga. Ann. Anat. 1996d;178:145–152. doi: 10.1016/s0940-9602(96)80034-3. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Yoshizawa H, Kawahara I. Three-dimensional ultrastructure of the surface of the tongue of the rat snake, Elaphe climacophora. Anat. Rec. 1996e;245:9–12. doi: 10.1002/(SICI)1097-0185(199605)245:1<9::AID-AR2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Yoshizawa H, Kawahara I. Study by scanning electron microscopy of the morphogenesis of three types of lingual papilla in the mouse. Acta Anat. 1996f;157:41–52. doi: 10.1159/000147865. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Asami T, Chiba A. Ultrastructural study of the keratinization of the dorsal epithelium of the tongue of Middendorff's bean goose, Anser fabalis middendorffii. Anat. Rec. 1997a;247:149–163. doi: 10.1002/(SICI)1097-0185(199702)247:2<149::AID-AR1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Iwabuchi Y, Asami T. Histochemical and ultrastructural study of the effects of cholinergic and adrenergic agonists on salivary secretion from the lingual epithelium and the lingual gland of the Tokyo Daruma pond frog. Tissue Cell. 1997b;29:323–338. doi: 10.1016/s0040-8166(97)80008-0. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Yoshizawa H, Kawahara I. Study by scanning electron microscopy of the morphogenesis of three types of lingual papilla in the rat. Anat. Rec. 1997c;247:528–541. doi: 10.1002/(SICI)1097-0185(199704)247:4<528::AID-AR12>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Okumura Y, Kumakura M. Ultrastructural study of the relationship between the morphogenesis of filiform papillae and the keratinization of the lingual epithelium in the mouse. Acta Anat. 1999a;195:91–103. doi: 10.1159/000016679. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Yoshizawa H, Kawahara I. Ultrastructural study of the relationship between the morphogenesis of filiform papillae and the keratinization of the lingual epithelium in the rat. J. Anat. 1999b;195:27–38. doi: 10.1046/j.1469-7580.1999.19510027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahmann H. Sinnesphysiologische Studien an Reptilien. 1. Experimentelle Untersuchungen über das Jakobsonische Organ der Eidechsen und Schlagen. Zool. Physiologie Tiere. 1932;51:173–238. [Google Scholar]
- Kent GC. Comparative Anatomy of the Vertebrates. Saint Louis: Mosby Co; 1978. [Google Scholar]
- Kobayashi K, Kumakura M, Yoshimura K, Inatomi M, Asami T. Fine structure of the tongue and lingual papillae of the penguin. Arch. Histol. Cytol. 1998;61:37–46. doi: 10.1679/aohc.61.37. [DOI] [PubMed] [Google Scholar]
- Kraklau DM. Kinematics of prey capture and chewing in the lizard Agama agama (Squamata: Agamidae) J. Morph. 1991;210:195–212. doi: 10.1002/jmor.1052100208. [DOI] [PubMed] [Google Scholar]
- Krause WJ, Cutts JH. Morphological observations of the papillae of the opossum tongue. Acta Anat. 1982;113:159–168. doi: 10.1159/000145551. [DOI] [PubMed] [Google Scholar]
- Kubota K, Kubota J, Fukuda N, Asakura S, Nakagawa S. Comparative anatomical and neurohistological observations on the tongue of the marsupials. Anat. Rec. 1963;147:337–353. doi: 10.1002/ar.1091470305. [DOI] [PubMed] [Google Scholar]
- Kubota K, Hayama S. Comparative anatomical and neurohistological observations on the tongues of pigmy and common marmosets. Anat. Rec. 1964;150:473–486. doi: 10.1002/ar.1091500416. [DOI] [PubMed] [Google Scholar]
- Kubota K. Comparative anatomical and neurohistological observations on the tongue of Japanese pika (Ochotona hyperborea yezoensis. Anat. Rec. 1966;154:1–12. doi: 10.1002/ar.1091540102. [DOI] [PubMed] [Google Scholar]
- Kubota K, Fukuda N, Asakura S. Comparative anatomical and neurohistological observations on the tongue of porcupine (Histrix cristata. Anat. Rec. 1966;155:261–268. doi: 10.1002/ar.1091550212. [DOI] [PubMed] [Google Scholar]
- Kubota K. Comparative anatomical and neurohistological observations on the tongue of northern fur seal (Callorhinus ursinus. Anat. Rec. 1968;161:257–266. doi: 10.1002/ar.1091610211. [DOI] [PubMed] [Google Scholar]
- Kullaa-Mikkonen A, Sorvari T, Kotilainen R. Morphological variations on the dorsal surface of the human tongue. Proc. Finnland Dent. Soc. 1985;81:104–110. [PubMed] [Google Scholar]
- Kuratani S, Tanaka S, Ishikawa Y, Zukeran C. Early development of the hypoglossal nerve in the chick embryo as observed by the whole-mount nerve staining method. Am. J. Anat. 1988;182:155–168. doi: 10.1002/aja.1001820206. [DOI] [PubMed] [Google Scholar]
- Kutuzov H, Sicher H. The filiform and the conical papillae of the tongue in the white rat. Anat. Rec. 1951;110:275–288. doi: 10.1002/ar.1091100302. [DOI] [PubMed] [Google Scholar]
- Kutuzov H, Sicher H. Comparative anatomy of the mucosa of the tongue and the palate of the laboratory mouse. Anat. Rec. 1953;116:409–425. doi: 10.1002/ar.1091160403. [DOI] [PubMed] [Google Scholar]
- Lane EB, Whitear M. Sensory structures at the surface of fish skin. I. Putative chemoreceptors. Zool. J. Linnean Soc. 1982;75:141–151. [Google Scholar]
- MacKenzie IC, Dabelsteen E. Connective tissue influences on the expression of epithelial cell-surface antigens. Cell Tissue Res. 1987;248:137–141. doi: 10.1007/BF01239974. [DOI] [PubMed] [Google Scholar]
- MacKenzie S, Walsh FS, Graham A. Migration of hypoglossal myoblast precursors. Dev. Dyn. 1998;213:349–358. doi: 10.1002/(SICI)1097-0177(199812)213:4<349::AID-AJA1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- McDowell SB. The evolution of the tongue of snakes, and its bearing on snake origins. In: Dobzhansky T, Hecht MK, Steere WG, editors. In: Evolutionary Biology. Vol. 6. New York: Meredithe Co; 1972. [Google Scholar]
- Mistretta CM, Haus LF. Temporal and spatial patterns of tenascin and laminin immunoreactivity suggest roles for extracellular matrix in development of gustatory papillae and taste buds. J. Comp. Neural. 1996;364:535–555. doi: 10.1002/(SICI)1096-9861(19960115)364:3<535::AID-CNE11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Myers WE, Hettinger TP, D’Ambrosio JA, Wendt SL, Pearson CB, Barry MA, Frank ME. Visualizing taste papillae in vivo with scanning electron microscopy of a high resolution cast. Chem. Senses. 1995;20:1–8. doi: 10.1093/chemse/20.1.1. [DOI] [PubMed] [Google Scholar]
- Nalavade MN, Varute AT. Histochemical studies on the mucines of the vertebrate tongues. VIII. Histochemical analysis of mucosubstances in the tongues of the turtle. Folia Histochem. Cytochem. 1976;14:123–134. [PubMed] [Google Scholar]
- Nickel R, Schummer A, Seiferle E. The Viscera of the Domestic Mammals. Berlin: Verlag Paul Parey; 1973. pp. 21–74. [Google Scholar]
- Nomura T, Kurihara K. Effects of changed lipid composition on responses of liposomes to various odorants: possible mechanism of odor discrimination. Biochemistry. 1987a;26:6141–6145. doi: 10.1021/bi00393a029. [DOI] [PubMed] [Google Scholar]
- Nomura T, Kurihara K. Liposomes as a model for olfactory cells: changes in membrane potential in the response to various odorants. Biochemistry. 1987b;26:6135–6140. doi: 10.1021/bi00393a028. [DOI] [PubMed] [Google Scholar]
- Paulson RB, Hayes TG, Sucheston ME. Scanning electron microscope study of tongue development in the CD-1 mouse fetus. J. Craniofac. General Dev. 1985;5:59–73. [PubMed] [Google Scholar]
- Paulson RB, Alley KE, Salata LJ, Whitemyer CC. A scanning electron-microscopic study of tongue development in the frog Rana pipiens. Arch. Oral Biol. 1995;40:311–319. doi: 10.1016/0003-9969(94)00172-8. [DOI] [PubMed] [Google Scholar]
- Rabinowitz T, Tandler B. Papillary morphology of the tongue of the American chameleon: Anolis carolinensis. Anat. Rec. 1986;216:483–489. doi: 10.1002/ar.1092160405. [DOI] [PubMed] [Google Scholar]
- Rentrop M, Knapp B, Winter H, Schweizer J. Differential localization of distinct keratin mRNA-species in mouse tongue epithelium by in situ hybridization with specific cDNA probes. J. Cell Biol. 1986;103:2583–2591. doi: 10.1083/jcb.103.6.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PP, Winkles PA. Quantitative study of fungiform papillae and taste buds on the cat's tongue. Anat. Rec. 1990;225:108–111. doi: 10.1002/ar.1092260112. [DOI] [PubMed] [Google Scholar]
- Romer AS, Parsons TS. The Vertebrate Body. 5th edn. Philadelphia: Saunders Co.; 1977. [Google Scholar]
- Roth G, Wake DB. Conservatism and innovation in the evolution of feeding in vertebrates. In: Wake DB, Roth G, editors. In: Complex Organismal Functions: Integration and Evolution in Vertebrates. New York: John Wiley & Sons; 1989. pp. 7–21. [Google Scholar]
- Schwenk K. Occurrence, distribution and functional significance of taste buds in lizards. Copeia. 1985;1985:9–101. [Google Scholar]
- Schwenk K. Morphology of the tongue in the tautara, Sphenodon punctatus (Reptilia: Lepidosauria) J. Morph. 1986;188:129–156. doi: 10.1002/jmor.1051880202. [DOI] [PubMed] [Google Scholar]
- Schwenk K. Comparative morphology of the Lepidosaurian tongue and its relevance to squamata phylogeny. In: Estes R, Pregill G, editors. In: Phylogenetic Relationships of the Lizard Families. Stanford: Stanford University Press; 1988. pp. 569–598. [Google Scholar]
- Schwenk K. Function and evolutionary morphology of lingual feeding in squamata reptiles: phylogenetics and kinematics. J. Zoo. 1989;219:153–175. London. [Google Scholar]
- Shimada K, Sato I, Yokoi A, Kitagawa T, Tezuka M, Ishii T. The fine structure and elemental analysis on the dorsal tongue in the American alligator (Alligator mississippiensis) Okajimas Folia Anat. Jpn. 1990;66:375–392. doi: 10.2535/ofaj1936.66.6_375. [DOI] [PubMed] [Google Scholar]
- Shimoda T, Nakanishi E, Yoshino S, Kobayashi S. Light and scanning electron microscopic study on the lingual papillae in the newborn sea otter Enhydra lutris. Okajimas Folia Anat. Jpn. 1996;73:65–74. doi: 10.2535/ofaj1936.73.1_65. [DOI] [PubMed] [Google Scholar]
- Smith KK. Form and function of the tongue in Agamid lizards with comments on its phylogenetic significance. J. Morph. 1988;196:157–171. doi: 10.1002/jmor.1051960205. [DOI] [PubMed] [Google Scholar]
- Smith KK, Mackay KA. The morphology of the intrinsic tongue musculature in snakes (Reptilia, Ophidia): Functional and phylogenetic implications. J. Morph. 1990;205:307–324. doi: 10.1002/jmor.1052050306. [DOI] [PubMed] [Google Scholar]
- Steflik DE, Singh BB, McKinney RV, Jr, Boshell JL. Correlated TEM, SEM, and histological observations of filiform papillae of the cow tongue. Acta Anat. 1983;117:21–30. doi: 10.1159/000145767. [DOI] [PubMed] [Google Scholar]
- Stern IB. Oral mucous membrane. In: Bhaskar SN, editor. In: Orban's Oral Histology and Embryology. 7th edn. St. Louis: Mosby Co; 1980. pp. 261–335. [Google Scholar]
- Susi FR. Keratinization in the mucosa of the ventral surface of the chicken tongue. J. Anat. 1969;105:477–486. [PMC free article] [PubMed] [Google Scholar]
- Takeda M, Obara N, Suzuki Y. Keratin filaments of epithelial and taste-bud cells in the circumvallate papillae of adult and developing mice. Cell Tissue Res. 1990;260:41–48. doi: 10.1007/BF00297488. [DOI] [PubMed] [Google Scholar]
- Torrey TW. The influence of nerve fibers upon taste buds during embryonic development. Proc. Nat. Acad. Sci. 1940;26:627–634. doi: 10.1073/pnas.26.11.627. USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toubeau G, Cotman C, Bels V. Morphological kinematic study of the tongue and buccal cavity in the lizard Anguis fragilis. Anat. Rec. 1994;240:423–433. doi: 10.1002/ar.1092400315. [DOI] [PubMed] [Google Scholar]
- Toyoda M, Sakita S, Kagoura M, Morohashi M. Electron microscopic characterization of filiform papillae in the normal human tongue. Arch. Histol. Cytol. 1998;61:253–268. doi: 10.1679/aohc.61.253. [DOI] [PubMed] [Google Scholar]
- Toyoshima K, Shimamura A. Comparative study of ultrastructures of the lateral-line organs and the palatal taste organs in the African clawed toad, Xenopus laevis. Anat. Rec. 1982;204:371–381. doi: 10.1002/ar.1092040411. [DOI] [PubMed] [Google Scholar]
- Toyoshima K, Shimamura A. Monoamine-containing basal cells in the taste buds of the newt Triturus pyrrhogaster. Arch. Oral Biol. 1987;32:619–621. doi: 10.1016/0003-9969(87)90034-3. [DOI] [PubMed] [Google Scholar]
- Whitear M. Chapter 2. Epidermis. In: Bereiter-Hahn J, Matoltsy AG, Richards KS, editors. In: Biology of the Integument 2 Vertebrates. Berlin: Springer-Verlag; 1986. pp. 8–38. [Google Scholar]
- Whitehead MC, Kachele DL. Development of fungiform papillae, taste buds, and their innervation in the hamster. J. Comp. Neural. 1994;340:515–530. doi: 10.1002/cne.903400405. [DOI] [PubMed] [Google Scholar]
- Winokur RM. The buccopharyngeal mucosa of the turtles (Testudines) J. Morph. 1988;196:33–52. doi: 10.1002/jmor.1051960105. [DOI] [PubMed] [Google Scholar]
- Yamasaki F, Komatsu S, Kamiya T. Papillary projections at the lingual margin in the striped dolphin, Stenella coeruleoalba. J. Morph. 1978;157:33–48. doi: 10.1002/jmor.1051570104. [DOI] [PubMed] [Google Scholar]
- Yamasaki F, Takahashi K. A description of the times of appearance and regression of marginal lingual papillae in human fetuses and newborns. Anat. Rec. 1982;204:171–173. doi: 10.1002/ar.1092040211. [DOI] [PubMed] [Google Scholar]
- Youson JH. Chapter 25. The alimentary canal. In: Hardisty MW, Potter IC, editors. In: The Biology of Lamprey. New York: Academic Press Inc; 1981. pp. 95–189. [Google Scholar]
- Zhou Q, Yang Y, Chen Z, Sun A. Effects of molecular structures on the olfactory responses of phospholipid membranes to four alcohols. Chem. Phys. 1998;95:1–9. doi: 10.1016/s0009-3084(98)00054-1. [DOI] [PubMed] [Google Scholar]
- Zylberberg L. Histochemistry and ultrastructure of amphibian lingual glands and phylogenetic relations. Histochem. J. 1977;9:505–520. doi: 10.1007/BF01002979. [DOI] [PubMed] [Google Scholar]