Abstract
The properties of cervical–vaginal fluid are under strict hormonal control: and in mammals in which semen is deposited in the anterior vagina, changes produced in these properties can result in a lower or higher resistance to sperm motion. The aim of this study was to determine whether the structural organization of bovine vaginal fluid is related to its rheological properties. Vaginal fluid samples were collected from 41 cows at oestrus: 20 at the middle of oestrus (between 8 and 12 h after starting) and 21 at the end of oestrus (between 18 and 22 h). Flow behaviour was determined using a viscosimeter, and the ultrastructural analysis was performed by scanning electron microscopy. Six samples showed newtonian behaviour: three collected at the middle and three collected at the end of oestrus. Newtonian samples had dense and compact matrices arranged as membranes with rough, irregular surfaces, and sparse, thin filaments (< 150 nm). Non-newtonian samples collected at the end of oestrus (n = 18) had a higher (P = 0.016) consistency index (K = 944 ± 229 mPa.sn) than those collected at the middle of oestrus (n = 17; K = 237 ± 84 mPa.sn). Thick filaments (> 700 nm) that were either randomly arranged with thinner filaments forming a mesh or heavily cross-linked by thin filaments (50–150 nm) were observed in all non-newtonian samples collected at the end of oestrus, while medium-diameter filaments (between 200 and 500 nm) forming loose networks were observed in non-newtonian samples collected at the middle of oestrus. These findings indicate a close relationship between the molecular organization of the structural elements of bovine vaginal fluid and its rheological behaviour. Vaginal fluid dramatically reduces its mechanical barrier effect during the course of oestrus but always appears to maintain its three-dimensional filamentous structure. The images of vaginal fluid showing newtonian behaviour would appear to support previous results, suggesting that this property may be related to bovine infertility.
Keywords: bovine, rheology, ultrastructure, vaginal fluid
Introduction
Interactions between spermatozoa and the luminal micro-environments of the female genital tract play a significant role in mammalian sperm transport. In animals in which semen is deposited into the anterior vagina at the time of mating, such as rabbits, ruminants and primates, vaginal fluid is the first physiological medium that the spermatozoa must cross on its journey to the cranial parts of the tubular genital organs. Vaginal fluid is a biological product of complex composition that is mainly derived from cervical secretions (Odeblad, 1968). The physical and chemical properties of vaginal fluid show cyclical changes that are under hormonal control (El-Banna & Hafez, 1972). Cervical secretions are favourable for sperm penetration only under certain circumstances related to the follicular phase, or under oestrogen administration, and are unfavourable for sperm penetration during the luteal phase, or under administration of gestational agents. The mucus constituents responsible for these biophysical properties are glycoproteins secreted mainly by the cervical epithelium. Glycoproteins consist of a polypeptide backbone (20–25%) that carries numerous heterosaccharide side chains (75–80%) (Doehr & Moghissi, 1973). Associations between these glycoproteins give rise to the so-called mucins Gibbons, 1959; Glover, 1959; Gibbons, 1959which generate intrinsic resistance to sperm motion Yudin et al. 1989; Overstreet et al. 1991
The quality of structural elements of vaginal fluid and molecular interaction between these structures are important determinants of the rheological properties of vaginal fluid Litt et al. 1976; Wolf et al. 1977 (Quemada, 1984). Rheology is the study of the flow and deformation properties of a given system (Davajan et al. 1972). The role played by the rheological properties of vaginal fluid in reproduction is widely recognized, and variations during the ovarian cycle reflect their regulation by the hormonal imbalance El-Banna, 1972; Hafez, 1974; Eliezer, 1974; Tam et al. 1980; Carlstedt, 1989; Sheehan, 1989; López-Gatius et al. 1993; López-Gatius et al, 1997). Several factors have been related to the rheology of vaginal fluid such as flow elasticity (Scott Blair et al. 1941; Scott Blair, 1989; Glover, 1989), plasticity (Chrétien, 1974), tack (Marcus, 1963; Marcus, 1963; Chrétien, 1974), spinnbarkeit (Clift, 1945; Moghissi, 1980) or viscosity and viscoelasticity (Eliezer, 1974; Wolf et al. 1977; Tam et al. 1980; Katz et al. 1982). In earlier experiments performed in dairy cows, we noted wide variation in the rheological properties of vaginal fluid at the time of insemination (López-Gatius et al. 1993; López-Gatius et al, 1994; López-Gatius et al, 1996). The consistency index of vaginal fluid during the course of oestrus indicated that the fluidity of vaginal fluid increased from 8 to 16 h and fell markedly 24 h after the start of manifest oestrus (L´pez-Gatius et al. 1996). Moreover, cows were found to have a higher probability of becoming pregnant when vaginal fluid samples showed non-newtonian behaviour and a low consistency index value, compared to animals in which vaginal fluid samples showed non-newtonian behaviour with a high consistency index value, or compared to animals with newtonian behaviour samples (López-Gatius et al. 1997). It was suggested that vaginal mucus samples showing newtonian behaviour could be abnormal secretions possibly reflecting a reproductive disorder.
We recently showed that the structural component of vaginal fluid at oestrus is entirely comprised of filaments, which are arranged three-dimensionally to form a network (Rutllant et al. 1997; Rutllant et al 1999 The aim of the present study was to characterize the flow behaviour and ultrastructure of bovine vaginal fluid to establish a possible association between its molecular organization and rheological properties.
Materials and methods
Vaginal fluid samples
Translucent vaginal fluid samples were collected from 41 lactating Friesian cows at oestrus using a plastic inseminating sheath and a 50-mL syringe, as described previously (López-Gatius et al. 1993; López-Gatius et al. 1996 previously (Lépez-Gatius et al. 1993; Lépez-Gatius et al. 1996). Twenty samples were collected at the middle (between 8 and 12 h after starting oestrus) and 21 at the end of oestrus (between 18 and 22 h). Samples from each cow were divided into two aliquots: 9 mL for rheological measurements and 1 mL for ultrastructural examination by SEM. After collection, samples were introduced into sterile plastic containers and stored frozen at -20 °C. Prior to use, they were thawed at 38 °C in a water bath.
All animals were between 50 and 80 days in milk and were carefully inspected for signs of oestrus early morning, midday and late afternoon for 30–45 min, plus several times day and night. Standing to be mounted over 3 s was considered as the first sign of oestrus. Cows in which samples were collected at the middle of oestrus showed mounting activity for at least 4 h more. Cows in which samples were collected at the end of oestrus finished oestrous behaviour within either 2 h before or 2 h after fluid collection. Confirmation of oestrus in each cow was performed by palpation of genital organs per rectum (López-Gatius, 1991; Camón-Urgel, 1991 and by evaluation of the crystallization patterns of each sample Abusineina, 1962 A drop of the fluid was placed 1 cm from the end of a glass slide and a uniform, thin smear was obtained by dragging the edge of a second slide across the fluid. The sample was then air dried and its crystallization pattern examined microscopically at a magnification of ×40 for arborization. All samples showed a type A fern-like crystallization pattern Abusineina, 1962 with long, thin stems, clear venation and tiny subvenation, characteristic of oestrus. Crystallization was apparent throughout most of the smear.
Rheological measurements
Flow behaviour was evaluated using a Haake Rotovisco 12 viscosimeter (López-Gatius et al. 1993; López-Gatius et al. 1996 Experimental data were fitted to the power law equation: T = K · (S)n in which T is the shear stress expressed in pascals, S the shear rate in seconds−1, K the index of consistency in pascals per secondn and n the flow behaviour index (dimensionless). Newtonian or ideal fluids show a proportional relationship between shear stress (T) and shear rate (S). By exclusion, all other fluids are designated non-newtonian systems (Dodge, 1959).
Scanning electron microscopy
Samples were prepared as described elsewhere (Rutllant et al. 1997; Rutllant et al. 1999
Following the technique described by Chrétien 1975 samples were fixed in 2.5% glutaraldehyde in 0.1 M PBS at pH 7.2 and maintained for 4 days at 4 °C. The sample tubes were then washed twice in PBS for 15 min each time and rinsed three times in 4 °C double-distilled water for 10 min each time. The water was removed by gentle decanting and the samples were immersed in liquid nitrogen for 1 min. Samples were then gradually thawed in a freeze-dryer (Edwards 12E6, Crawley, West Sussex, UK) at −70 %C for 10 h, −40 %C for 14 h, −20 °C for 10 h, 0 °C for 14 h and at 20 °C for 6–24 h. The tubes were then split along the longitudinal axis, and glued open onto specimen holders, coated with gold at 12 mA for 3 min in a Polaron E-500 Sputter coater (Watford, Herts., UK) and examined using a Hitachi S-570 (Tokyo, Japan) scanning electron microscope at 12 kV.
Filament thicknesses in each sample were measured using an image analyser (UTHSCSA Image Tool 1.25 program, developed at the University of Texas San Antonio, TX, USA, and available from the internet at (http://www.uthscsa.edu), and was expressed as the mean value of the smallest and largest diameters recorded for each filament. The gold coat thickness of the samples (approximately 50 nm) was taken into account for filament measurements. Mean data represented the average of at least 150 measurements of filament thickness in each sample.
Statistical analysis
Data on rheological measurements and filament thickness were recorded for each sample. Numerical data obtained from non-newtonian samples were tested for normal distribution. All values are expressed as the mean ± standard error of mean (SEM).
Rheological values from non-newtonian samples between middle and end oestrus were analysed by the Student's t-test
Results
Rheological measurements
In the flow analysis, 35 samples (85%) presented non-newtonian behaviour while six (15%) behaved as newtonian fluids. The mean consistency index of the newtonian samples was 2±1 mPa.snnewtonian samples was 2 ± 1 mPa.sn (range 0–6) and of the non-newtonian samples was 630 ± 149 mPa.sn (range 41–3881).
Non-newtonian samples collected at the end of oestrus (n = 18) had a higher (P = 0.016) consistency index (K = 944 ± 229 mPa.sn) than those collected at the middle of oestrus (n = 17; K = 237 ± 84 mPa.sn).
Scanning electron microscopy
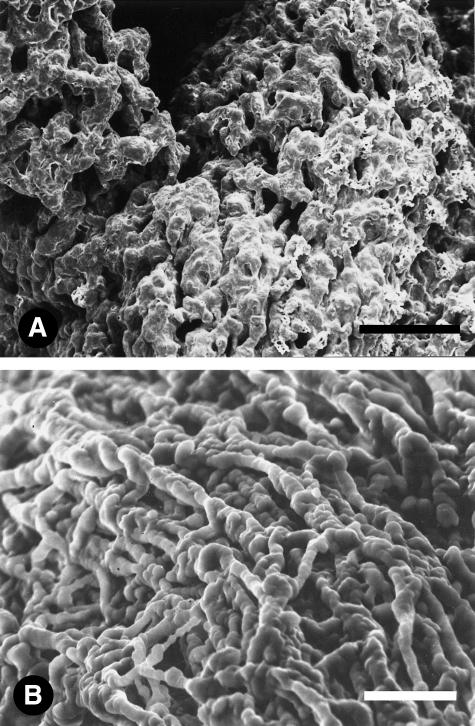
Newtonian and non-newtonian samples showed clear structural differences. The main structural feature of newtonian samples was the presence of a dense and compact matrix arranged as membranes with rough, irregular surfaces (Fig. 1A,B Examination of these membranes at high magnification revealed the presence of thin filaments, 40–70 nm in diameter, penetrating the surface. Fig. 2
Fig. 1.
Scanning electron micrographs of bovine vaginal fluid samples showing newtonian behaviour. Note the dense, compact matrix arranged as membranes with rough, irregular surfaces. Scale bar = 100 (A) and 20 (B) μm.
Fig. 2.
Scanning electron micrograph of bovine vaginal fluid showing newtonian behaviour. Note the presence of thin filaments, 40–70 nm in diameter, penetrating the surface (arrows). Scale bar = 5 μm.
The structural appearance of the non-newtonian samples was similar to that observed in previous studies including the presence of filaments of different thickness forming a network-like structure (Fig. 3 All samples showed the same basic structure, with differences shown in the arrangement and size of filaments related to the consistency index. All non-newtonian samples collected at the end of oestrus (high-consistency samples) had filaments greater than 700 nm (up to 800 nm) in diameter that were either randomly arranged with thinner filaments forming a mesh (Fig. 3), or heavily cross-linked by thin filaments of 50–150 nm (Fig. 4A,B). In some of these samples (n = 5), the thin cross-linking filaments stabilized the thicker filaments in such a way that larger filaments could be seen to lie parallel, leaving narrow spaces between them and, in some areas, the spaces even disappeared (Fig. 5). Samples collected at the middle of oestrus (lowconsistency samples) showed scanty large-diameter filaments (between 200 and 500 nm) and a few or no cross-linking filaments, with entangled and disorganized filaments forming loose networks (Fig. 6A,B).
Fig. 3.
Scanning electron micrograph of bovine vaginal fluid showing non-newtonian behaviour. Filaments of different diameter are arranged in a three-dimensional network-like structure with no dominant organization. Scale bar = 5 μm.
Fig. 4.
Scanning electron micrographs of non-newtonian vaginal fluid samples of high consistency index showing organized filaments with diameters up to 800 nm, heavily cross-linked by thin filaments. Scale bar = 5 (A) and 2.5 (B) μm.
Fig. 5.
Scanning electron micrograph of a non-newtonian vaginal fluid sample of high consistency index showing extensive filament organization. In some areas, spaces between filaments disappear giving the appearance of a filamentous membrane. Scale bar = 20 μm.
Fig. 6.
Photomicrographs of vaginal fluid samples showing non-newtonian behaviour and a low consistency index. Scanty large-diameter filaments and scarce or absent cross-linking filaments may be observed but, despite this, non-aligned filaments forming loose networks can also be seen. Scale bar = 30 (A) and 5 μm (B).
Discussion
The present findings show a very close relationship between the molecular organization of structural elements observed in bovine vaginal fluid and its rheological behaviour. Clear structural differences were observed between samples showing newtonian and non-newtonian behaviour, and between samples presenting a high and low consistency index.
Newtonian samples showed scarce structural elements consisting of thin filaments embedded in a dense matrix of unknown composition. These observations are markedly different to those previously reported for bovine vaginal fluid (Rutllant et al. 1997; Rutllant et al. 1999 yet cannot be considered artefacts since they were observed in all the samples that presented newtonian characteristics. Vaginal fluid consists of two main fractions, a solid phase or insoluble fraction, also known as mucin, and an aqueous phase containing the soluble components Daunter, 1980; Counsilman, 1980The association between the two fractions contributes to the formation of a hydrogelCarlstedt, 1989; Sheehan, 1989; Silberberg, 1989 with a continuous spectrum of physical properties, from a rigid elastic gel to a newtonian fluid Gibbons, 1973; Sellwood, 1973; López-Gatius et al. 1993; López-Gatius et al. 1994; López-Gatius et al. 1996 The present authors previously proposed that samples of vaginal fluid showing Newtonian behaviour had lost their gel nature (López-Gatius et al. 1993) This idea would be consistent with the present findings. Further, Gibbons & Sellwood (1973) reported that vaginal mucus could not exhibit newtonian behaviour since its primary role of becoming anisotropic would not be accomplished and, consequently, spermatozoa would not be guided by orientated filaments towards the site of fertilization Tampion & Gibbons, 1962; Mattner, 1966; 1968). The results reported here would support the hypothesis that vaginal fluid showing newtonian properties might be associated with a reproductive disorder, based on the fact that structural findings are considered abnormal and that cows in which vaginal fluid showed newtonian behaviour have been associated with a higher risk of non-pregnancy (López-Gatius et al. 1997). More studies are needed to confirm whether newtonian properties of the vaginal fluid are related to bovine infertility.
Vaginal fluid samples showing non-newtonian behaviour presented filaments of different diameter and organization, constituting the backbone of vaginal fluid structure. These structural elements are similar to those previously described in bovine vaginal fluid (Sato & Masaki, 1981; Rutllant et al. 1997, 1999 and humanvagial fluid Chrétien et al. 1973; Zaneveld et al. 1975; Takano et al. 1979; Barros et al. 1985; Poon & McCoshen, 1985; Vigil et al. 1991. However, using SEM it was possible to detect notable structural differences between samples with high and low consistency indices. Since Odeblad's, 1968 structural hypothesis for cervical mucus comprised of a three-dimensional molecular network, several attempts have been made to validate this model or to present alternative models using different techniques. Lee et al. (1977), using laser light-scattering spectroscopy, found that the molecular arrangement of cow oestrous cervical mucus might be more accurately described as an ensemble of entangled, randomly coiled macromolecules rather than the arrangement proposed by Odeblad 1968. The structural models from these studies arose from the interpretation of biophysical measurements of vaginal fluid. However, in the present study, ultrastructural images combined with rheological results gave rise to a model which may be described as intermediate to those of Odeblad 1968 and Lee et al. 1977. Nonnewtonian mucus samples with a high consistency index were found to have a large number of thick filaments that might offer support and texture to the fluid. These thick filaments were cross-linked by thin filaments that were usually observed throughout the sample. Both these elements are responsible for a high consistency index at the end of oestrus indicating that high shear stress is needed to break the structure Meyer, 1976. Thus, samples of vaginal fluid of high consistency would seem to approach Odeblad's 1968 model. In contrast, non-newtonian vaginal fluid samples of a low consistency index were seen to contain filaments of medium diameter that were entangled and disorganized to form a loose network. Furthermore, in these samples, cross-linking filaments were virtually undetectable. Combined with the rheological results, these images were compatible with the structural model proposed by Lee et al. 1977. This latter model lends support to a hypothesis in which luteal and folicular mucus are proposed to be structurally equivalent, and differences perceived during the ovarian cycle are attributed to a different level of hydration or ‘swelling“ regulated by fixed charges on the mucins and by nonmucin components trapped in the gel Tam, 1981; Verdugo, 1981; Verdugo et al. 1987. This phenomenon is known as Donnan-mediated swelling Tam & Verdugo, 1981.
In a previous study, the consistency index of bovine vaginal fluid was found to vary throughout the oestrus period so that the fluidity of vaginal fluid had its greatest value at the middle of oestrus (López-Gatius et al. 1996). Thus it would appear feasible that during the course of oestrus modulated by hormonal imbalance, the structural and, consequently, the rheological properties of vaginal fluid continuously vary. Samples collected at the middle of oestrus had an intercross-link spacing of about 56 μm2 (Rutllant et al. 1999). Since sperm head dimensions are about 8–10 μm length and 4–4.5 μm width (Saacke, 1964; Almquist, 1964; Gravance et al. 1996), the filament network should not deter passage of sperm cells. In fact, spermatozoa previously cultured in mid-oestrus samples appeared to be swimming in random directions and did not seem to alter filamentous structure Rutllant et al. 1999). Taken together, all these findings suggest that in mid-oestrus, there only seems to be a short 2–4-h period (8–12 h after first manifestation of oestrus) available for the sperm to reach the uterus. Either side of this time interval, the mucus is mechanically more hostile for the movement of the sperm. Since ovulation in cows occurs 24–35 h following the start of oestrus (Walker et al. 1996), this time interval for sperm passage would allow a period of at least 12 h for sperm transport and capacitation before fertilization (Yanagimachi, 1994).
In conclusion, the present findings indicate a close relationship between the rheological behaviour of bovine vaginal fluid obtained at oestrus and the molecular organization of its structural elements. The present report describes how the ultrastructure of the physiological medium that spermatozoa encounter in the middle of oestrus is a three-dimensional network of thin filaments defining wide spaces. This structure is related to the low consistency index of samples showing non-newtonian behaviour, and can reduce the mechanical effort required for sperm passage to the utmost. It is likely that in mammalian females, in which semen is deposited in the anterior vagina, vaginal fluid is able to achieve a dramatic reduction in its mechanical barrier effect while maintaining its three-dimensional filamentous structure. Moreover, based on previous findings, vaginal fluid showing newtonian behaviour might reflect a reproductive disorder.
Acknowledgments
We would like to thank Dr Ashley Yudin for assistance with the manuscript and Ana Burton for assistance with the English translation.
References
- Abusineina ME. A study of fern-like crystalline patterns in the cervical and vaginal mucus of cattle. Vet. Record. 1962;74:619–621. [Google Scholar]
- Barros C, Argüello B, Jedlicki A, Vigil P, Herrera E. Scanning electron microscopy of human cervical mucus. Gamete Res. 1985;12:85–89. [Google Scholar]
- Carlstedt I, Sheehan JK. Structure and macromolecular properties of cervical mucus glycoproteins. In: Chantler E, Ratcliffe NA, editors. Mucus and Related Topics. Cambridge: Society for Experimental Biology; 1989. pp. 289–316. [PubMed] [Google Scholar]
- Chrétien FC, Gernigon C, David G, Psychoyos A. The ultrastructure of human cervical mucus under scanning electron microscopy. Fertil. Steril. 1973;24:746–757. doi: 10.1016/s0015-0282(16)39969-1. [DOI] [PubMed] [Google Scholar]
- Chrétien FC. La glaire cervicale. Propriétés chimiques et physiques. Ultrastructure. J. Gynaecol. Obstetr. Biol. Reproduction. 1974;3:711–744. [PubMed] [Google Scholar]
- Chrétien FC. Préparation du mucus cervical a l’observation au microscope électronique à balayage. J. Microsc. Biol. cell. 1975;24:23–44. [Google Scholar]
- Clift AF. Observations on certain rheological properties of human cervical secretion. Proc. Royal. Soc. Med. 1945;39:1–9. doi: 10.1177/003591574503900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daunter B, Counsilman C. Cervical mucus: its structure and possible biological functions. Eur. J. 1980;10:141–161. doi: 10.1016/0028-2243(80)90056-8. [DOI] [PubMed] [Google Scholar]
- Davajan V, Nakamura RM, Kharma K. Spermatozoan transport in cervical mucus. Obstetr. 1972;25:1–43. [Google Scholar]
- Dodge DW. Fluid systems. Industrial Engineering Chem. 1959;51:839–840. [Google Scholar]
- Doehr SA, Moghissi KS. Human and bovine cervical mucins. In: Blandau RJ, Moghissi KS, editors. The Biology of the Cervix. Chicago: The University of Chicago Press; 1973. pp. 125–142. [Google Scholar]
- El-Banna AA, Hafez ESE. The uterine cervix in mammals. Am. J. Obstetr. Gynecol. 1972;77:145–164. doi: 10.1016/0002-9378(72)90544-3. [DOI] [PubMed] [Google Scholar]
- Eliezer N. Viscoelastic properties of mucus. Biorheology. 1974;11:61–68. doi: 10.3233/bir-1974-11103. [DOI] [PubMed] [Google Scholar]
- Gibbons RA. Chemical properties of two mucoids from bovine cervical mucin. Biochem. J. 1959;73:209–217. doi: 10.1042/bj0730209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons RA, Glover RA. Physicochemical properties of two mucoids from bovine cervical mucus. Biochem. J. 1959;73:217–225. doi: 10.1042/bj0730217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons RA, Sellwood R. The macromolecular biochemistry of cervical secretions. In: Blandau RJ, Moghissi KS, editors. The Biology of the Cervix. Chicago: The University of Chicago Press; 1973. pp. 251–265. [Google Scholar]
- Gravance CG, Vishwanath R, Pitt C, Casey PJ. computer automated morphometric analysis of bull sperm heads. Theriogenology. 1996;46:1205–1215. doi: 10.1016/s0093-691x(96)00291-9. [DOI] [PubMed] [Google Scholar]
- Katz DF, Tam PY, Berger SA, Sensabaugh GF. Flow permeation analysis of bovine cervical mucus. Biophys. J. 1982;38:153–159. doi: 10.1016/S0006-3495(82)84542-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WI, Verdugo P, Blandau RJ, Gaddum-Rose P. Molecular arrangement of cervical mucus: a reevaluation based on laser light-scattering spectroscopy. Gynecol. 1977;8:254–266. [PubMed] [Google Scholar]
- Litt M, Khan MA, Wolf DP. Mucus rheology: relation to structure and function. Biorheology. 1976;13:37–48. doi: 10.3233/bir-1976-13106. [DOI] [PubMed] [Google Scholar]
- López-Gatius F, Camón-Urgel J. Confirmation of oestrus rates by palpation per rectum of genital organs in normal repeat dairy cows. J. Vet. Med. A. 1991;38:553–558. doi: 10.1111/j.1439-0442.1991.tb01047.x. [DOI] [PubMed] [Google Scholar]
- López-Gatius F, Miró J, Sebastián I, Ibarz A, Labernia J. Rheological properties of the anterior vaginal fluid from the superovulated dairy heifers at estrus. Theriogenology. 1993;40:167–180. doi: 10.1016/0093-691x(93)90350-e. [DOI] [PubMed] [Google Scholar]
- López-Gatius F, Rutllant J, López-Béjar M, Labernia J. Sperm motion and rheological behavior of the vaginal fluid of superovulated dairy heifers. Theriogenology. 1994;41:1523–1531. doi: 10.1016/0093-691x(94)90203-u. [DOI] [PubMed] [Google Scholar]
- López-Gatius F, Rutllant J, Labèrnia J, Ibarz A, Lopez-Bejar M, Santolaria P. Rheological behavior of the vaginal fluid of the dairy cows at estrus. Theriogenology. 1996;46:57–63. [Google Scholar]
- López-Gatius F, Labèrnia J, Santolaria P, Rutllant J, Lopez-Bejar M. The relationship of the rheological behavior of the vaginal fluid at the time of insemination to the pregnancy rate in dairy cows. Theriogenology. 1997;48:865–871. doi: 10.1016/s0093-691x(97)00307-5. [DOI] [PubMed] [Google Scholar]
- Marcus SL, Marcus CC. Cervical mucus and its relation to infertility. Obstetr. 1963;18:749–772. doi: 10.1097/00006254-196310000-00027. [DOI] [PubMed] [Google Scholar]
- Mattner PE. Formation and retention of spermatozoan reservoir in the cervix of the ruminant. Nature. 1966;212:1479–1480. doi: 10.1038/2121479a0. [DOI] [PubMed] [Google Scholar]
- Mattner PE. The distribution of spermatozoa and leucocytes in the female genital tract in goats and cattle. J. Reprod. Fertil. 1968;17:253–261. doi: 10.1530/jrf.0.0170253. [DOI] [PubMed] [Google Scholar]
- Meyer FA. Mucus structure: relation to biological transport function. Biorheology. 1976;13:49–58. doi: 10.3233/bir-1976-13107. [DOI] [PubMed] [Google Scholar]
- Moghissi KS. Prediction and detection of ovulation. Fertil Steril. 1980;34:89–98. doi: 10.1016/s0015-0282(16)44888-0. [DOI] [PubMed] [Google Scholar]
- Odeblad E, editor. The functional structure of human cervical mucus. Acta Obstetr. 1968;47(Suppl. 1):57–79. doi: 10.3109/00016346809156845. [DOI] [PubMed] [Google Scholar]
- Overstreet JW, Katz DF, Yudin AI. Cervical mucus and sperm transport in reproduction. Semin. Perinatol. 1991;15:149–155. [PubMed] [Google Scholar]
- Poon WW, Mccoshen JA. Variances in mucus architecture as a cause of cervical factor infertility. Fertil Steril. 1985;44:361–365. doi: 10.1016/s0015-0282(16)48861-8. [DOI] [PubMed] [Google Scholar]
- Quemada D. Towards a unified model of elasto-tixotropy of biofluids. Biorheology. 1984;21:423–436. doi: 10.3233/bir-1984-21403. [DOI] [PubMed] [Google Scholar]
- Rutllant J, López-Gatius F, Camón J, Lopez-Plana C, Lopez-Bejar M. A scanning electron microscope study of the structural component of the bovine vaginal fluid at oestrus. J. Vet. Med. A. 1997;44:237–241. doi: 10.1111/j.1439-0442.1997.tb01106.x. [DOI] [PubMed] [Google Scholar]
- Rutllant J, López-Gatius F, Camón J, Lopez-Bejar M, Lopez-Plana C. A structural study of the bovine vaginal fluid at estrus. Scanning. 1999;21:204–211. doi: 10.1002/sca.4950210306. [DOI] [PubMed] [Google Scholar]
- Saacke R, Almquist O. Ultrastructure of bovine spermatozoa I. The head of normal ejaculated sperm. Am. J. Anat. 1964;115:143–162. doi: 10.1002/aja.1001150109. [DOI] [PubMed] [Google Scholar]
- Sato M, Masaki J. The ultrastructure of the bovine cervical mucus under scanning electron microscopy. Tohoku J. Agric Res. 1981;32:27–30. [Google Scholar]
- Scott Blair GW, Folley SJ, Coppen FMV, Malpress FH. Rheological properties of bovine cervical secretions during the oestrus cycle. Nature. 1941;147:453–454. [Google Scholar]
- Scott Blair GW, Glover FA. Early pregnancy tests from studies of bovine cervical mucus. Br. Vet. 1959;111:3–11. [Google Scholar]
- Silberberg A. Mucus glycoproteins, its biophysical and gel-forming properties. In: Chantler E, Ratcliffe NA, editors. In Mucus and Related Topics. Cambridge: Society for Experimental Biology; 1989. pp. 43–63. [PubMed] [Google Scholar]
- Takano N, Maekawa I, Takamizawa H. Ultrastructure of human cervical mucus observed by cryo-scanning electron microscopy. Fertil Steril. 1979;32:604–607. doi: 10.1016/s0015-0282(16)44368-2. [DOI] [PubMed] [Google Scholar]
- Tam PY, Katz DF, Berger SA. Non-linear viscoelastic properties of cervical mucus. Biorheology. 1980;17:465–478. [PubMed] [Google Scholar]
- Tam PY, Verdugo P. Control of mucus hydration as a Donnan equilibrium process. Nature. 1981;292:340–342. doi: 10.1038/292340a0. [DOI] [PubMed] [Google Scholar]
- Tampion D, Gibbons RA. The orientation of spermatozoa in mucus of the cervix uteri. Nature. 1962;194:381. doi: 10.1038/194381a0. [DOI] [PubMed] [Google Scholar]
- Verdugo P, Deyrup-Olesen I, Aitken M, Villalon M, Johnson D. Molecular mechanism of mucin secretion: 1. The role of intragranular charge shielding. J. Dentistry Res. 1987;66:506–508. doi: 10.1177/00220345870660022001. [DOI] [PubMed] [Google Scholar]
- Vigil P, Perez A, Neira J, Morales P. Post partum cervical mucus: biological and rheological properties. Human Reprod. 1991;6:475–479. doi: 10.1093/oxfordjournals.humrep.a137364. [DOI] [PubMed] [Google Scholar]
- Walker WL, Nebel RL, Mcgillard ML. Time of ovulation relative to mounting activity in dairy cattle. J. Dairy Sci. 1996;79:1555–1561. doi: 10.3168/jds.S0022-0302(96)76517-7. [DOI] [PubMed] [Google Scholar]
- Wolf DP, Khan MA, Litt M. Human cervical mucus. I. Rheological properties. Fertil. Steril. 1977;28:41–46. [PubMed] [Google Scholar]
- Yanagimachi R. Mammalian fertilisation. In: Knobil E, Neil J, editors. In The Physiology of Reproduction. New York: Raven Press; 1994. pp. 189–317. [Google Scholar]
- Yudin AI, Hanson FW, Katz DF. Human cervical mucus and its interaction with sperm: a fine-structural view. Biol. Reprod. 1989;40:661–671. doi: 10.1095/biolreprod40.3.661. [DOI] [PubMed] [Google Scholar]
- Zaneveld LJD, Tauber PF, Port C, Propping D, Schumacher GFB. Structural aspects of human cervical mucus. Am. J. Obstetr. Gynecol. 1975;122:650–654. doi: 10.1016/0002-9378(75)90065-4. [DOI] [PubMed] [Google Scholar]