Abstract
Using specific anti-β keratin and general anti-α keratin antibodies, keratins were located in the epidermis of the alligator during the final developmental stages by ultrastructural and immunocytochemical methods. The maturation of the bilayered periderm (= embryonic epidermis) coincides with the disappearance of cell organelles, including the 25–35-nm-thick coarse filaments, and the coalescing of α-keratin filaments into a compact mass. The plasmalemma of peridermal cells forms a 15–25-nm-thick electron-dense corneous envelope. These changes start at stage 25, about 3 weeks before hatching, and continue until hatching when the embryonic epidermis is shed. Immature β-keratogenic cells beneath the embryonic epidermis accumulate immunolabelled β-filaments which are packed into thin, electron-pale β-keratogenic cells in the corneous layer. Together, electron-pale and electron-dense materials form a compact 3–4-nm filament pattern of β-keratin. Melanosomes from epidermal melanocytes, incorporated into β-cells, give rise to the banded skin pattern of hatchlings. β-keratin production is much reduced in the hinge regions, where many α-filaments remain packed together with lipid droplets or mucous granules into thinner, more electron-dense, α-cells. The keratinaceous material of the α-cells is mostly concentrated along the cell membrane, while the lipid/mucous material remains centrally located, as in sebokeratinocytes of the apteric areas of avian skin. Some lipid and mucus is also incorporated into typical β-cells of the outer scale surface, so that lipids are part of the fully keratinized hard keratin layer of the alligator. Lipids within β-cells of outer scale surfaces and α-cells of the hinge region are probably responsible for limiting water loss and ion movements across the skin. Neither typical mammalian keratohyalin granules nor lepidosaurian keratohyalin-like granules were detected anywhere in alligator epidermis. The combination of anti-β and anti-α keratin antibodies revealed different distributions of β- and α-keratins. In late embryonic stages (25–26 to hatching), β-keratin occurs only in the upper suprabasal cells, in prekeratinized and keratinized layers, whereas α-keratin bundles (tonofilaments) remain only in the lowest layers. The cross-reactivity of the β-antibody, produced against a chick scale keratin, further shows that avian and crocodilian hard (β) keratins share common antigenic sites, reflecting a phylogenetic affinity between these taxa.
Keywords: alligator, alpha-keratin, beta-keratin, development, epidermis, immunocytochemistry, ultrastructure
Introduction
Crocodilians are the only surviving archosaurian reptiles (Pough et al. 1998). Most species of crocodilian occur in fresh-water, but some invade brackish or saltwater environments, requiring stringent structural and permeability qualities in the skin (Davis et al. 1980; Dunson & Mazzotti, 1988). Crocodilian skin is generally tough, although the toughness varies in different body regions; it is harder and thicker in dorso-caudal regions where it often contains osteoderms, and less so in ventral and lateral regions (Brazaitis, 1987). The toughness results from both the strongly collagenous dermis (Alibardi & Thompson, 2000) and the presence in the epidermal layers of a hard form of keratin, called β-keratin (Alexander, 1970; Baden & Maderson, 1970; Sawyer et al. 2000; Alibardi & Thompson, 2001). In comparison to α-keratins, β-keratins are less elastic, harder, have a beta-pleated molecular conformation, are rich in glycine and proline, and are produced from specific betakeratin genes (Baden et al. 1974; Wyld & Brush, 1979, 1983; Gregg & Rogers, 1985; Sawyer et al. 2000). β-keratin molecules have tail–head regions that act as a matrix to link the central core with the resulting 3–4-nm-thick fibrillar pattern, and a specific X-ray diffraction pattern Baden & Maderson, 1970; Frazer et al. 1972; Baden et al. 1974). The packing of β-keratin during terminal differentiation of β-keratinocytes forms very hard cellular (turtle, crocodilian, birds, and Sphenodon), or syncitial (lepidosaurian reptiles), very hard corneous layers that provide very effective mechanical protection in both reptilian and avian skin.
In addition to the strong mechanical protection provided by β-keratin, crocodilian epidermis must also be able to control water-loss and ion flux in aquatic environments. Crocodilian epidermis is not very efficient at retarding water loss in comparison to more terrestrial reptiles (Bentley & Schmidt-Nielsen, 1966; Davis et al. 1980; Lillywhite & Maderson, 1982; Dunson & Mazzotti, 1988), although the rate of trans-epidermal water loss is a third to a quarter of that found in amphibians, including terrestrial species. Thus, skin of crocodilians appears to be physiologically intermediate between that of amphibians and terrestrial amniotes. The main molecules involved in retarding water flux across the skin of vertebrates are extracellular and intracellular, neutral and polar lipids present in the stratum corneum and associated with alpha-keratin (Landmann, 1980; Lillywhite & Maderson, 1982; Menon et al. 1996; Menon & Menon, 2000). Since only β-keratin or, in the hinge regions, a mixture of α- and β-keratin are known in crocodilians (Alexander, 1970), it is important to understand how cell differentiation occurs in these regions before emergence from the egg.
The first cells to develop in the epidermis of embryonic alligators contain β-keratin, but they also store a large amount of lipid, probably associated with keratin bundles (Alibardi & Thompson, 2000, 2001) as in birds Sawyer & Borg, 1979; Menon & Menon, 2000; Alibardi, 2002). In contrast, the adult epidermis has little lipid in the β-cells, although some phospholipids and free fatty acids have been detected histochemically in the corneous layer of the outer scale surface and in the hinge regions (Spearman & Riley, 1969). The hinge regions probably contain α-keratin mixed with lipids (Alexander, 1970), but there are no descriptions of the ontogeny of keratinization in these areas. These studies suggest that crocodilian keratinocytes possibly show a primitive stage in keratogenesis (mix of α- and β-keratin) that precedes the confining of α-keratin into β-cells and α-keratin into α-cells, as in modern lepidosaurian reptiles (Maderson et al. 1998). Coexistence of α- and β-keratins in corneous cells also occurs in the shell of turtles and in the epidermis of Sphenodon, both extant representatives of ancient reptilian groups. In Sphenodon, however, only the transition region between α- and β-cells shows a mixture of characteristics (Alibardi, 1999; Alibardi & Maderson, personal observations), probably connected to the evolution of a shedding layer. In more modern lepidosaurians (snakes and lizards), β-cells are much more clearly distinguishable from α-cells than in Sphenodon (Maderson, 1985; Landmann, 1986).
The structure and ontogeny of crocodilian epidermis is not known in detail (Spearman & Riley, 1969; Alexander, 1970). Apart from the important comparative value of describing and understanding the structure and ontogeny of crocodilian skin, it may be of value commercially. Crocodilian skin is still widely collected from wild populations, but commercial farming is becoming more important (Pough et al. 1998). Alligator skin is among the most exploited skin of crocodilians for the leather industry, and the species is extensively farmed in the USA. Despite its importance, very little is known about the skin structure, the origin of the pigmentation pattern, and the role of lipids in affecting texture and skin softness. Consequently, to better understand the structure that determines epidermal physiology in alligators, we studied epidermal ultrastructure and the specific distribution of β-keratin (for the first time using a specific antibeta antibody, anti-β-KAB) and the general distribution of α-keratins (using the AE3 anti-α-KAB) of embryonic and hatchling alligators.
Materials and methods
Eggs of alligators (Alligator missippiensis) from the Australian Reptilian Park at Gosford, NSW. The eggs were incubated at two different temperatures for inducing the development of both males and females (Lang & Andrews, 1994). At 33 °C (male-determining temperature), incubation to hatching takes 67–70 days, compared to 72–80 days at 29 °C (female-determining temperature).
Eggs were opened and embryos of different ages were collected from stage 25 to stage 28, which is just before hatching (Ferguson, 1985). Embryos from eggs incubated at 29 °C were 1–2 stages behind those maintained at 33 °C. The embryos were decapitated and tissues were immediately collected for fixation. The following stages were used (n = sum of embryos collected at both temperatures): stage 24 (n = 3), stage 25 (n = 5), stage 25+ (n = 4; 25 stage sampled 5–7 days later than stage 25; there is no proper stage 26 in alligators, Ferguson, 1985), stage 27 (n = 4), stage 28 (n = 5).
Pieces of skin (2–5 mm square) were sampled from the belly (from the umbilical cord to lateral areas) to the lateral trunk, and from the trunk to the proximal areas of the tail. Some samples (to be used for normal ultrastructural study) were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer at pH 7.4, post-fixed in 2% osmium for 60–90 min, then in 1% uranyl acetate for 60–90 min, dehydrated and embedded in Spurr's resin. Other samples (to be used for immunocytochemistry) were fixed in Carnoy's fluid or in 4% paraformaldehyde in buffer (as above) for 4–6 h, dehydrated in up to 90% ethanol and embedded in Lowicryl K4M at 0–4 °C under UV polymerization.
Tissues were sectioned using an ultramicrotome and thick sections (1–4 µm) were collected for toluidine blue staining or light microscope immunocytochemistry, as previously described (Alibardi, 2000). A rabbit polyclonal anti-β-KAB (β-1) from chicken scales Shames et al. 1988, 1989; Sawyer et al. 2000). and anti-α-KAB (AE1, AE2, AE3) from Progen, Heidelberg, Germany, were used to localize keratins in alligator skin. These antibodies recognize most acidic keratins (AE1, molecular weight range 40–58), basic keratins (AE3, molecular weigh range 50–67), and keratins typical of cornification (AE2, molecular weight of 56.5 and 66–67) (Sun et al. 1983). Briefly, sections were pre-incubated for 20 min at room temperature in 2% BSA in buffer containing 5% normal goat serum, and incubated overnight at 0–4 °C with the anti-β-KAB (1 : 100 to 1 : 200) or anti-α-KAB (1 : 40 to 1 : 100) in 2% BSA in buffer (in controls the primary antibody was omitted). After three rinses in buffer, sections were incubated at room temperature with a goat-anti- mouse- or anti-rabbit-FITC conjugated secondary antibody (1 : 100, Sigma, USA) for 1 h, rinsed, mounted, and viewed under a Zeiss epifluorescence microscope.
Thin sections (40–90 nm thick) of glutaraldehyde-fixed tissues were collected on copper grids, stained with uranyl acetate-lead citrate, and viewed with a Philips CM 100 electron microscope. Thin sections of paraformaldehyde-fixed tissues were collected on nickel-grids and immunostained with the Beta-1 or the AE3 antibody as above. The secondary antibody in this case was a goat anti-rabbit IgG or anti-mouse IgG conjugated to 10-nm-diameter gold particles (1 : 50, Sigma, USA). The primary antibody was omitted in control sections.
Results
Gross aspects of skin in late embryos
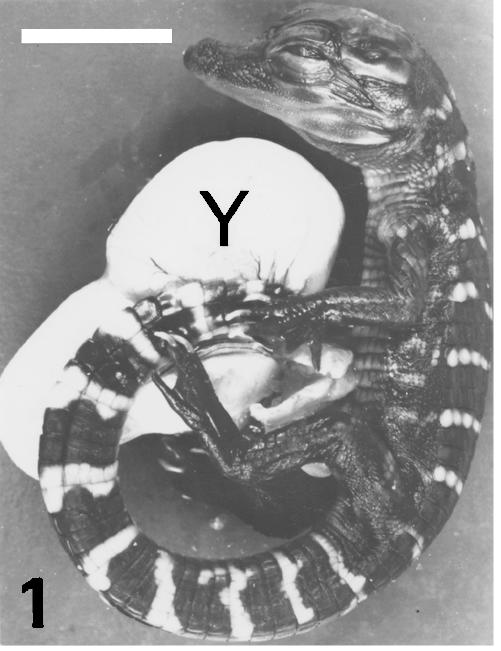
The banded pattern, initiated at stage 23 (Ferguson, 1985; Alibardi & Thompson, 2000, 2001), is more marked at stage 25+, when the body is entirely scaled (Fig. 1). At stage 25+, the embryo has still a large yolk sac, but the scalation, skin toughness and pigment pattern (pale bands on the dorsal body and tail, and less marked ventrally) are similar to those of the last two stages. At stage 27, most of the yolk is internalized and the embryo is bigger, but other features are similar to the previous stage. Finally, at stage 28 the yolk sac is completely internalized and the hatchling takes 2–3 days to emerge from the egg after pipping through the eggshell (Fig. 2). All the skin is tough, particularly that in dorsal and tail regions where large scutes are present.
Fig. 1.
Alligator embryo at stage 25+ showing the almost complete skin banding, and a still large yolk sac (Y). Scale bar = 20 mm.
Fig. 2.
Alligator embryo at stage 28, near hatching, with no yolk sac and mature skin pattern. Scale bar = 20 mm.
Light microscopy
At stage 24 the embryonic epidermis (30–50 µm thick) is bilayered, comprising a thinner (1–3 µm) outer periderm and a thicker inner periderm (3–8 µm). Between the inner periderm and a cuboidal basal layer lies a beta-layer composed of fusiform toluidinophilic beta-cells, and 2–3 paler layers of suprabasal cells (forming beta-cells) above.
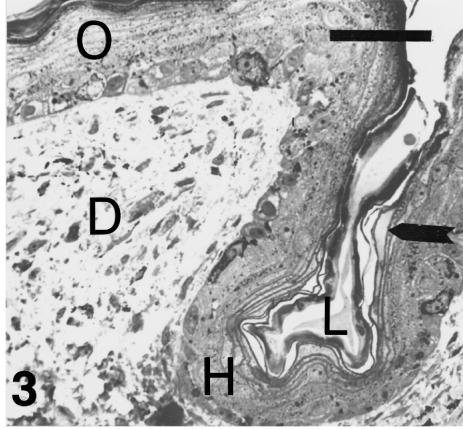
Embryos at stage 25 and 25+ have an epidermis whose thickness varies from about 15–25 µm in the hinge regions to 30–40 µm in the central part of the outer scale surface (Fig. 3). The cells of the basal layer are cuboidal or flat in the hinge region and the inner surface of scales, and cuboidal to polygonal in the outer scale surface. Over the outer scale surface, suprabasal cells become fusiform, and flat and elongate in the upper 6–8 layers before the compact corneum. Pigments are incorporated into these cells, especially within the dark-bands. Three to five layers of very flat, elongate cells overlie a thin germinal layer in the hinge region, and a thin toluidinophilic corneous layer is formed. Pale material, probably lipids, lies between the dense plasma lemma (as later confirmed with the electron microscope) and the more superficial keratinocytes, and in the extracellular hinge space (Fig. 3). The embryonic epidermis (composed of two peridermal layers, see Alibardi & Thompson, 2001) appears as a 4–8-μm-thick pale layer above the darker corneous layer (= β-layer).
Fig. 3.
Section through a mid-body lateral scale at stage 25–26. The thicker corneous layer on the outer surface of the scale (O) becomes thinner in the hinge region (H). The epithelium in the hinge region is flat and less stratified than on the outer surface where pale beta-cells lie under the corneal layer. The arrow indicates pale lipidic material in a flat keratinocyte of the corneous layer. D, dermis. Toluidine blue stain. Scale bar = 40 μm.
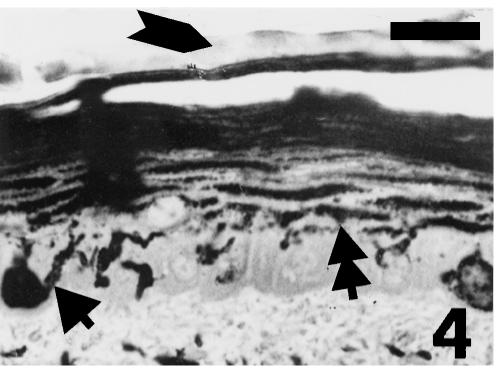
The corneous layer is thicker (15–20 µm) at stage 27 than earlier stages, while the living layers are unchanged (total thickness > 40 µm). Epidermal melanocytes transfer a large number of melanosomes (as later confirmed by the ultrastructural observation) into all flat suprabasal cells especially in the dark bands of dorsal scales (Fig. 4)
Fig. 4.
Dorsal mid-body scale at stage 27 showing intense pigmentation (double arrowhead) in prekeratinizing beta-cells derived from epidermal melanocytes (arrowhead). The arrow indicates the pale embryonic epidermis. Toluidine blue stain. Scale bar = 20 μm.
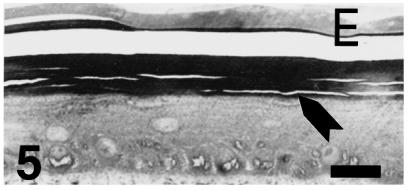
The corneous layer is even thicker (over 20 µm) at stage 28-hatching, and the pale epidermis is partially or completely present. The corneous layer shows artefactual cracks that indicate that it is composed of stratified flat corneocytes (as confirmed with the electron microscope) (Fig. 5). The stratification of cells in living layers resembles that of younger embryos, including the hinge regions (Fig. 6). No formation of osteoderms was observed in the dermis, where large extracellular collagen fibrils formed a dense fibrous network.
Fig. 5.
Thicker epidermis in a lateral scale at stage 28 showing a stratified epithelium, and a toluidinophilic corneal layer (arrow) beneath the embryonic epidermis (E, artifactual split). Scale bar = 20 μm.
Fig. 6.
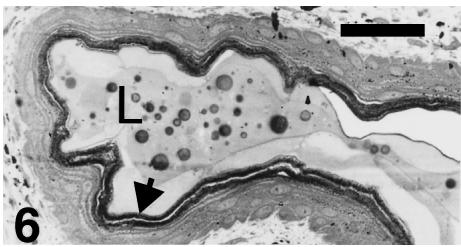
Detail of a hinge region of a ventral scale at stage 28 showing the thin keratinocytes (arrowhead) derived from flattened prekeratinizing cells. L indicates extracellular lipid-like, particulate (yolk) material within the hinge region. Scale bar = 40 μm.
Ultrastructure
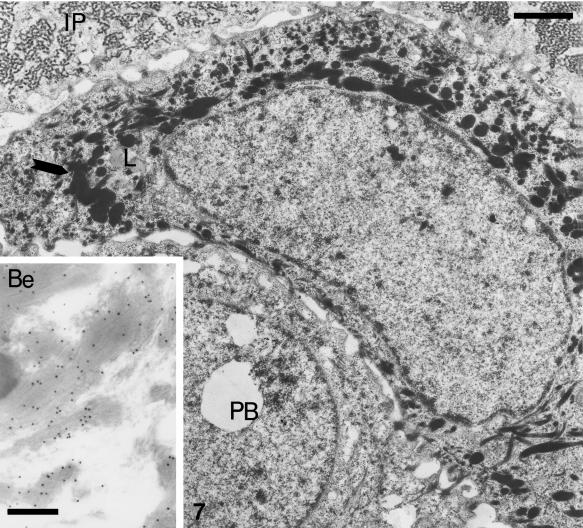
At stage 24, the differentiation of the first layer of fusiform beta-cells, located beneath the bilayered embryonic epidermis, is advanced and their β-keratin packets are intensely immunolabelled with the Beta-1 antibody (Fig. 7 and inset). Little or no labelling is present in keratin fascicles of adjacent, pre-β cells. Pale vacuoles or lipid droplets are more numerous in immature β-keratogenic cells than in the uppermost β-cells.
Fig. 7.
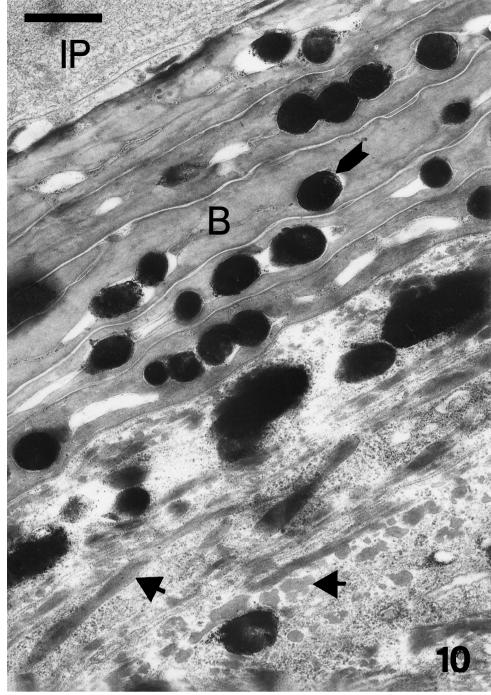
Embryonic stage 25. Ultrastructure of differentiating beta-cell beneath the inner peridermis (IP) filled by a network of coarse filaments. The merging beta-keratin packets (arrows) form large beta-filaments near the nucleus. The inset shows betapackets immunolabelled using the Beta-1 antibody (scale bar = 200 nm). Few lipid droplets (L) are visible in beta-cells, but pale lipidic vaquoles are more abundant in prebeta cell (PB). Scale bar = 1 μm.
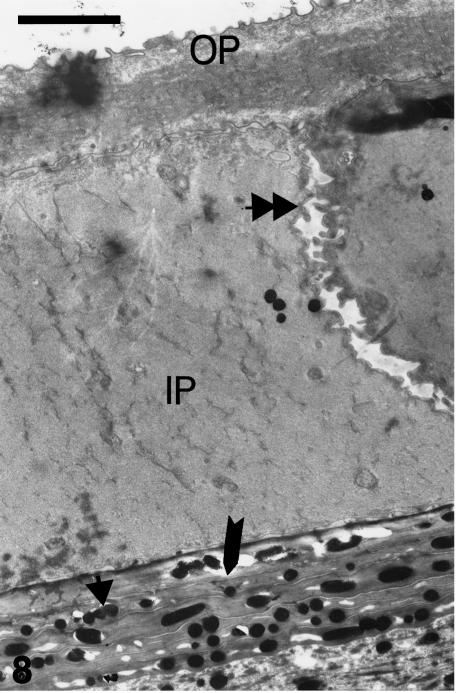
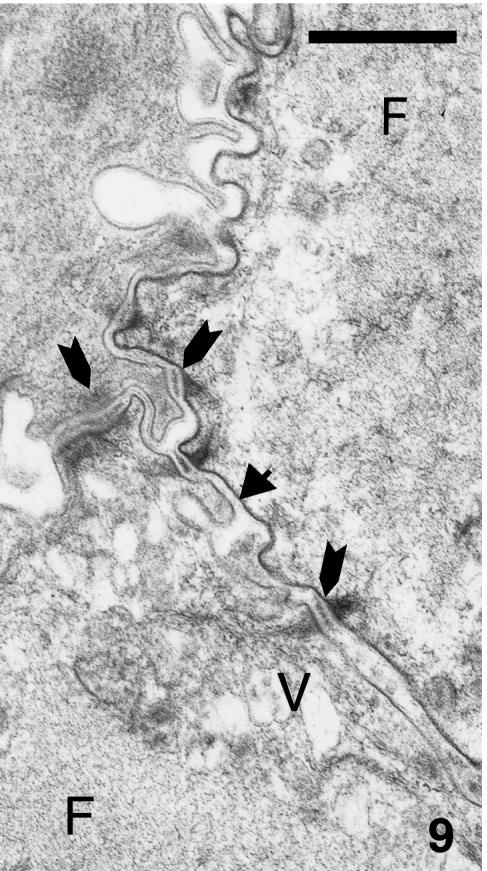
At stages 25 and 25+, a flat outer periderm contains forming keratin filaments and a thickened plasmalemma (8–15 nm thick). The peridermal surface has numerous microvilli, and sparse micropinocytotic vesicles, but most organelles have disappeared and mitochondrial remnants are visible. The thicker inner periderm is mostly filled with a dense network of keratin filaments (7–8 nm), few cell organelles, some mucus-like dense granules, and few coarse filaments, especially in the marginal cytoplasm adjacent to the microvillous surface (Fig. 8). The embryonic epidermis lacks melanosomes, and nuclei are clumped or degenerating. Characteristic corneous cell envelopes occur along the plasma membranes of outer and inner periderm cells, which include the microvillous surface where desmosomes are still visible (Fig. 9).
Fig. 8.
Superficial layers of outer surface of a dorsal scale at stage 26. OP, outer periderm with superficial microvilli and dense cytoplasmic fibrous material. IP, inner periderm, most containing homogenous dense fibrous material instead of the coarse filaments seen in the previous stage. Numerous microvilli (double arrowhead) are present on opposing faces of peridermal cells. The first 5–6 compact layers of thin beta-cells (arrow) contain numerous melanosomes (arrowhead). Scale bar = 2 μm.
Fig. 9.
Detail of fibrous network (F) in inner peridermal cells at stage 26, still joined by numerous desmosomes (arrows). The electron-dense corneous cell envelope is clearly visible (arrowhead). Scale bar = 0.5 μm.
Four to six flat β-keratin cells (0.4–1.0 µm thick), containing a variable number of melanosomes, occur beneath the embryonic epidermis (Figs 8 and 10). Melanosomes are concentrated along the central core of the keratinocyte, and sometimes a central small vacuole is present, probably storing lipids, residual ribosomes or rare glycogen particles. The β-matrix (3–4 nm pattern) is mostly electron-pale with sparse electron-dense areas. β-packets or larger β-filaments (0.05–0.15 μm thick) are irregularly orientated among free ribosomes in prekeratinized cells. These beta-fibrils are homogeneous and no filaments are visible. No keratohyalin granules (with surrounding ribosomes) occur in prekeratinizing β-cells of the outer scale surface, where the β-layer is thicker.
Fig. 10.
Detail of the first layers of beta-cells (B) in a mid-body lateral scale at stage 26, showing their relatively linear surface and melanosomes (arrow). Pale spaces within the core of some keratinocytes represent lipidic material. Irregular beta-keratin filaments (arrowheads, cross or obliquely sectioned) are aggregating in prekeratinized cells. IP, fibrous network within inner periderm. Scale bar = 0.5 μm.
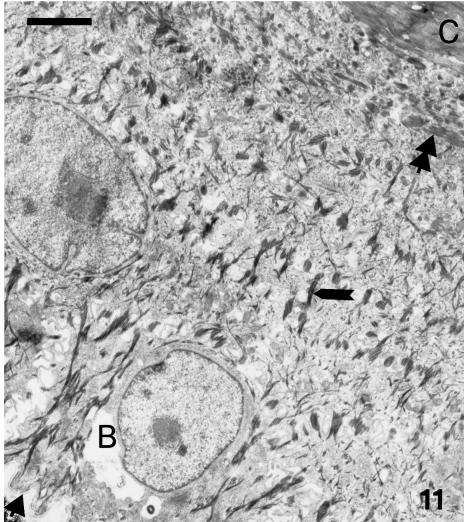
At stage 27, bundles of beta-keratin parallel to the epidermal surface disappear toward the basal layer and are replaced by darker tonofilaments bundles of α-keratin filaments, with irregular and perpendicular orientation (Figs 11 13). When sectioned at random the tonofilaments may resemble keratohyalin granules (Fig. 13). Pale vacuoles of 0.2–0.5 μm, presumed to contain lipid, are sparse in all these living cells. The basement membrane is often undulating.
Fig. 11.
Survey of whole epidermis on the outer surface of a ventral scale at stage 27, from the basal (B) layer (arrowhead on the tortuous basement lamella) to the corneous (C) layer. Tonofilaments are numerous (arrow) in lower cells whereas bundles of (beta-) keratin filaments are present (double arrowhead) in uppermost and prekeratinizing cells. Scale bar = 2 μm.
Fig. 13.
Detail of mixture of bundles of tonofilaments (arrowhead) within the beta-keratin matrix. Scale bar = 200 nm.
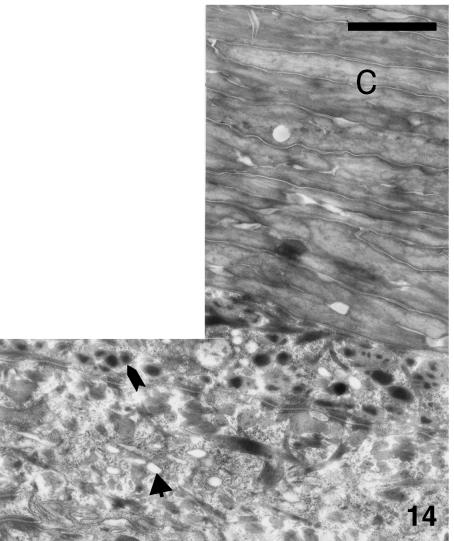
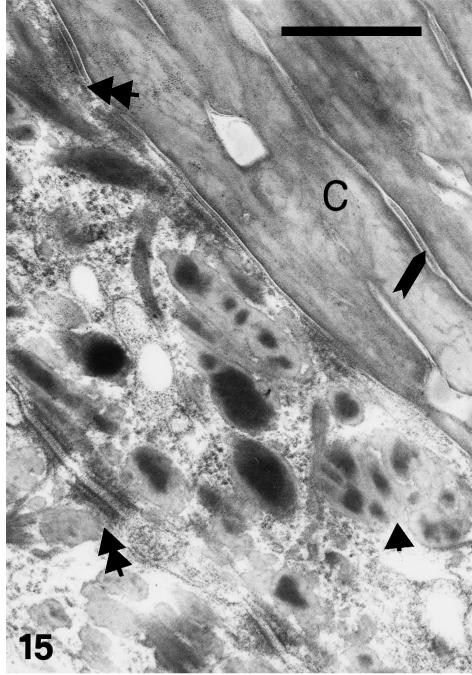
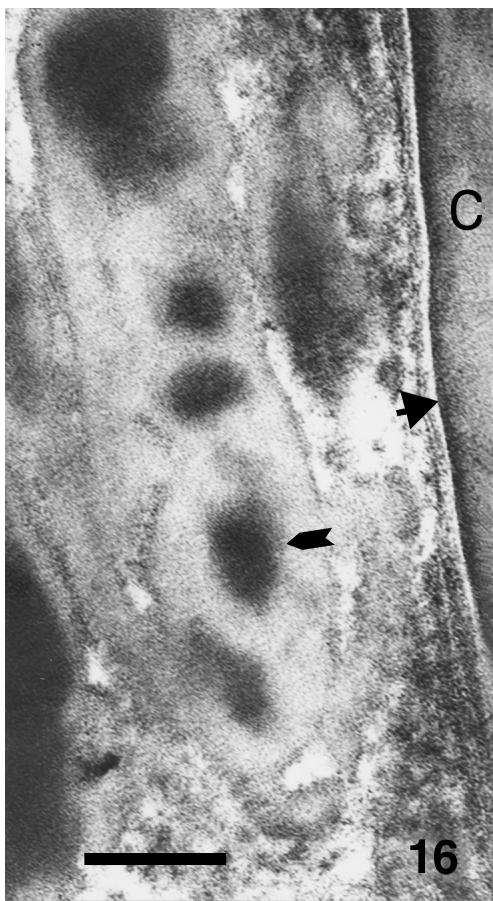
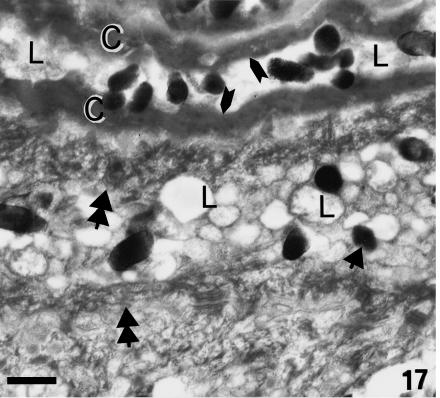
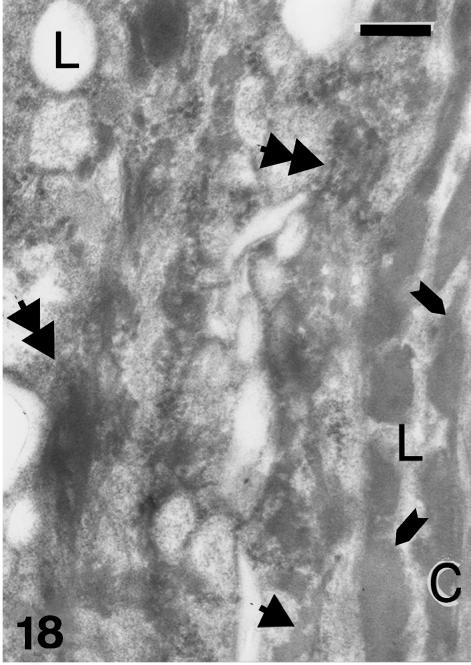
Although β-cells are still very thin (0.5–1.0 µm) at stage 28, a more irregular, even spinulated, surface of β-cells is visible in the thicker and harder areas of the central regions of the outer scale surface (Fig. 14). Desmosomal remnants still occur among the β-cells, while electron-dense material is sparse in the betakeratin matrix, but is deposited along the plasma membrane to form a peripheral dense band of 8–16 nm (Figs 15 and 16). The intracellular attachment plaques of desmosomes are largely infiltrated with homogenous, β-keratin. Although visible in prekeratinizing cells, few vacuoles are present in mature β-cells of the outer scale surface. A different ultrastructure occurs in the hinge region where β-packets and larger β-filaments are reduced or absent (Fig. 17). The cytoplasm of prekeratinizing prekeratinizing cells contained many pale vacuoles of lipid-like material (0.1–0.7 μm), and vacuoles with some amorphous (possibly lipid or mucus) material. A dense α- keratin network forms in the peripheral areas along the plasmalemma. In terminal keratinocytes (0.05–0.3 μm thick), the α-keratin network condenses into dense external bands along the plasma membrane, while the core is occupied by lipids, amorphous material, and melanosomes (Figs 17 and 18). Typical granules, as keratohyalin in mammalian epidermis or keratohyalin-like granules in lizard epidermis, are absent from cells of the hinge region in alligator.
Fig. 14.
Thick electron-pale corneous layer (C) of outer scale surface at stage 28 showing a tortuous surface of some beta cells. Pre-keratinous cells contain some dense spots within merging beta-keratin filaments (arrow) and occasional pale lipid-like vacuoles (arrow). Scale bar = 1 μm.
Fig. 15.
Detail of prekeratinized beta-cells featuring large beta-packets (arrowhead) merging with the intracellular portion of desmosomes (double arrowheads). Desmosomal remnants (arrow) occur in the corneous layer (C). Scale bar = 0.5 μm.
Fig. 16.
Higher magnification of large beta-keratin packet with dense areas (arrow) close to corneous cells (C) where denser material is concentrated along the cell surface (arrowhead). Scale bar = 100 nm.
Fig. 17.
Detail of keratinization in the hinge region of ventral scale at stage 28. Tonofilament or fibrous bundles (double arrowheads) occur in precorneous cells, whereas there are few or no beta-packets. In the first corneous cell, lipid-like material or vacuoles (L) and some melanosomes (arrow) are present in the central core, while fibrous material and keratin form the external thickenings (arrows). Scale bar = 0.5 μm.
Fig. 18.
Higher magnification of the fibrous network (double arrowheads) in prekeratinizing cells of the hinge region. Sparse, non-fibrous keratinaceus material (arrowhead), which is similar to that along the external borders (arrows) of corneous cells (C), is visible at high magnification. L, lipid core. Scale bar = 250 nm.
Immunocytochemistry
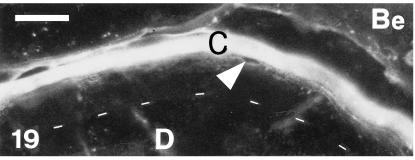
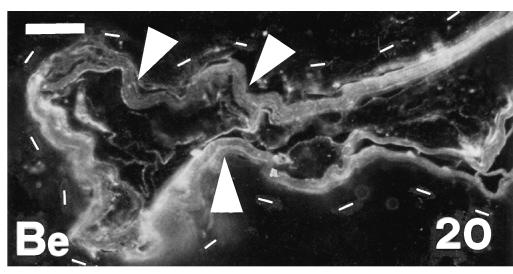
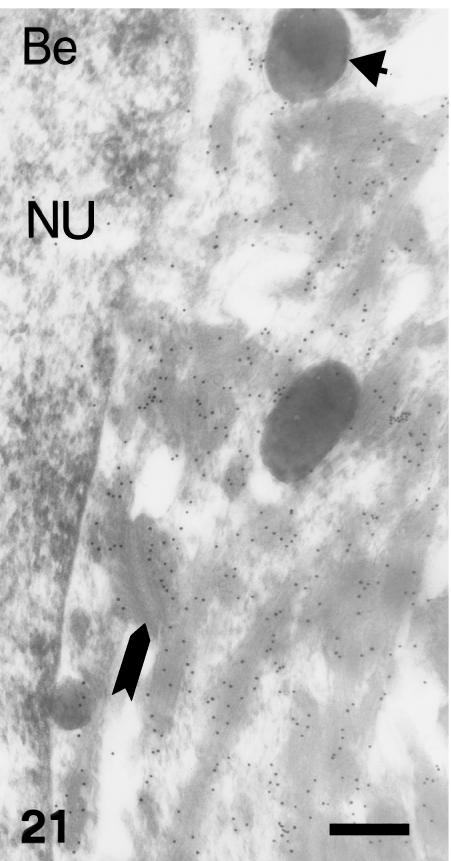
Only the corneous layers of the outer and inner scale surfaces, and some of the prekeratinized layers, labelled with the anti-β-KAB (Fig. 19). The embryonic epidermis and all living layers of the epidermis were immunonegative. Also the hinge regions showed progressive or total disappearance of immunolabelling in the thin corneous layer, while the basal and suprabasal layers were completely negative (Fig. 20). There was intense and homogeneous gold immunolabelling over large beta-keratin filaments in cells of the upper, intermediate and prekeratinized layers at the ultrastructural level (Figs 21 and 22). The labelling was homogeneously diffuse in pale and denser areas of compact keratin in the corneous layer. Only the extracellular desmosomal component of residual desmosomes remained unlabelled with this antibody.
Fig. 19.
Beta-1 (Be)-positive immunofluorescence of the corneous and precorneous layer of the outer surface of a lateral scale at stage 28. The lower suprabasal layers and the basal layer (underlain by dots) are immunonegative, like the dermis (D). Scale bar = 20 μm.
Fig. 20.
Detail of the complete or partial disappearance of Beta-1 (Be) immunoreactivity (arrowheads) in the hinge region at stage 28. Dots underlie the basal layer, which (like suprabasal layers) is completely immunonegative. Scale bar = 25 μm.
Fig. 21.
Beta-1 (Be) immunolabelled beta-packets in a prekeratinized cell (NU, nucleus) at stage 28. The arrow points to desmosomal remnants fused to beta-keratin filaments. The arrowhead indicates incorporated melanosomes. Scale bar = 250 nm.
Fig. 22.
Detail of immunogold-positive beta-keratin filaments in a prekeratinized cell at stage 28. The arrow indicates labelling of beta-keratin filaments merged with the cytoplasmic portion of a desmosome. C, diffuse immunolabelling of the corneous layer. Scale bar = 0.5 μm.
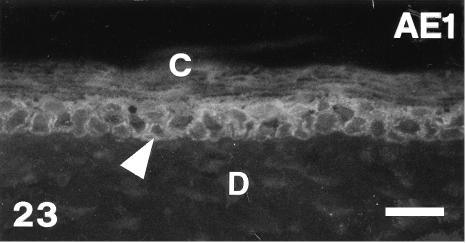
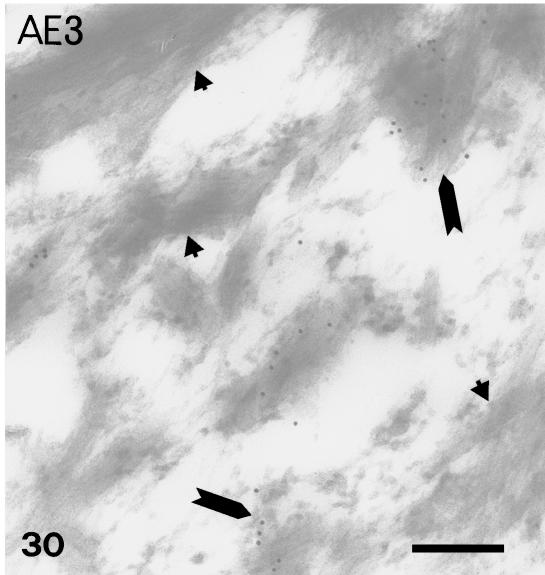
In contrast, the anti-α-KAB, AE1, AE2, AE3 showed a similar pattern of labelling from stage 25 to stage 28 which was the reverse of that for β-keratin. The AE1 antibody labelled mainly the basal-most layers or even only the germ layer in the dorsal outer surface and in the hinge region, but not in the prekeratinized and corneous layer (Figs 23 and 24). The AE2 antibody did not stain any living layers, but labelled the inner periderm at stage 25+ (Fig. 25). The β-layer was not stained specifically by this antibody. The AE3 antibody showed a staining pattern similar to that of AE1, but also labelled intermediate and prekeratinized layers of the outer surface, inner surface and hinge region (Figs 26 and 28). Only the corneous layer was not labelled with this antibody. Ultrastructural analysis confirmed that, while tonofilaments of the basal and suprabasal cells were more or less labelled (Fig. 29), those of upper keratinocyets, and particularly the prekeratinized and keratinized layer (where β-keratin labelling was instead intense), were poorly or not labelled with AE3 (Fig. 30).
Fig. 23.
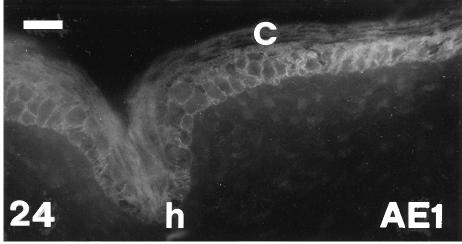
AE1 immunofluorescence of outer surface of lateral mid-body scale at stage 26. The basal-most layer (arrowhead) is fluorescent while the corneous layer (C = beta layer) is mainly negative. D, immunonegative dermis. Scale bar = 20 μm.
Fig. 24.
AE1 immunofluorescence of hinge region (h) of ventral scale at stage 26; the corneous layer (C) is negative. Scale bar = 20 μm.
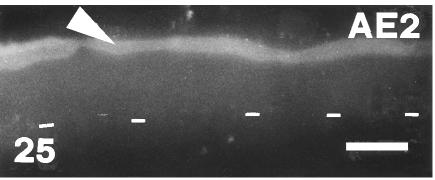
Fig. 25.
AE2 immunofluorescence concentrated on the embryonic epidermis and corneous layers (arrow) of ventral outer scale surface at stage 26. Dashes indicate base of the basal layer. Scale bar = 20 μm.
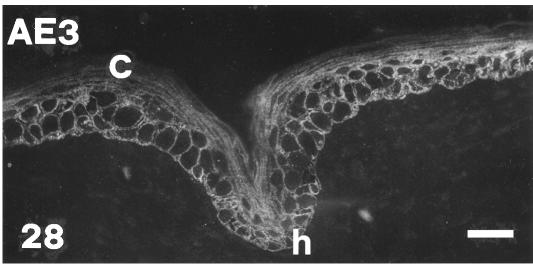
Fig. 26.
AE3 immunofluorescence of part of the outer surface of limb scale at stage 26 showing immunopositivity in the basal layer, less in the corneous layer (C), and none in the embryonic epidermis (E). Scale bar = 20 μm.
Fig. 28.
AE3 immunoreactivity in the hinge region of a ventral scale at stage 26 showing that most basal and suprabasal flat layers are labelled, while the external corneous layer (C) is mostly negative. Scale bar = 20 μm.
Fig. 29.
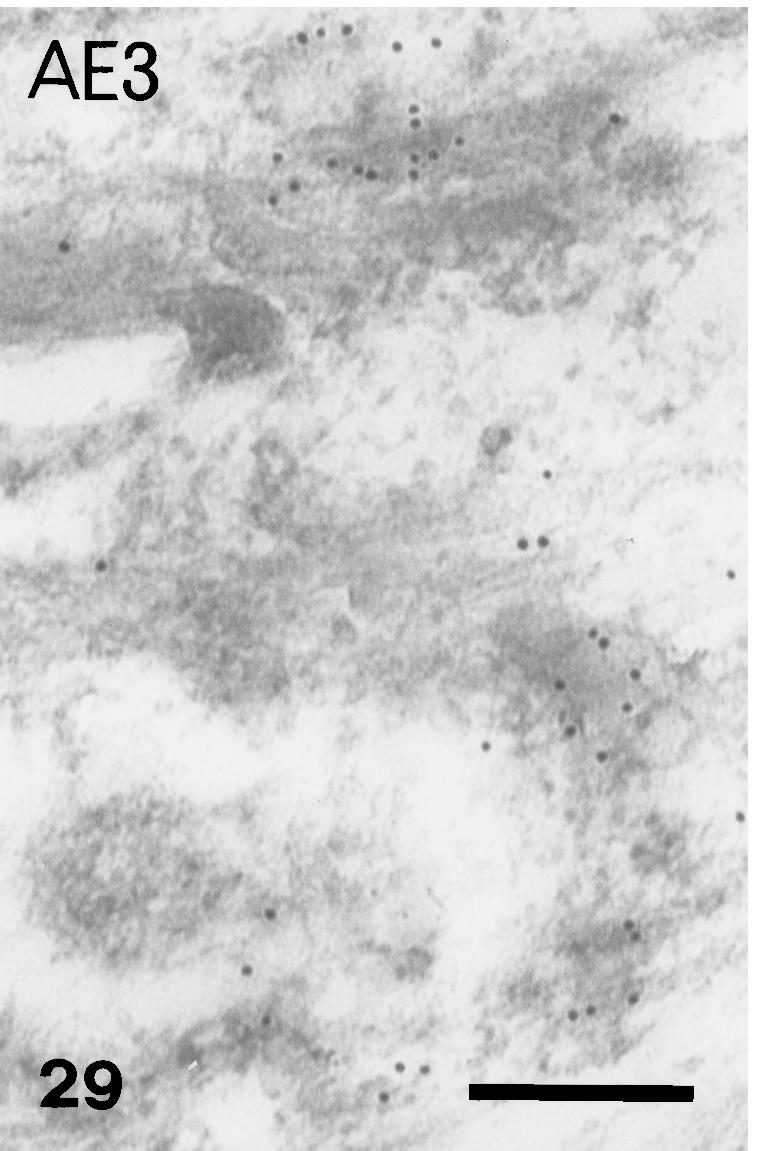
AE3 immunolabelled keratin bundles of suprabasal cell. Scale bar = 250 nm.
Fig. 30.
AE3 immunolabelling in some bundles (arrows), but not others (arrowheads), composed of homogenous beta-keratin (see Fig. 22) of prekeratinizing cells. Scale bar = 200 nm.
Discussion
Final differentiation of the embryonic epidermis
The outer and inner peridermis (= embryonic epidermis) accumulate keratin filaments and show alpha-keratinization similar to that of avian (Mottet & Jensen, 1968; Parakkal & Matoltsy, 1968; Matulionis, 1970; Sawyer & Borg, 1979) and mammalian peridermis (Holbrook, 1991; Akiyama et al. 1999). Additionally, both inner and outer periderm form thickened corneous cell envelopes during the final embryonic stages, suggesting the presence of specific molecules in these areas. The disappearance of the 25–40-nm-thick filaments into the periderm matrix suggests a role as an interfilamentous matrix material. Similarly, in avian epidermis, structures similar to the coarse filaments, and resembling those in alligators, initially coalesce to form periderm granules, and later dissolve within the filamentous component of apteric periderm or in sheath cells around embryonic feathers (Mottet & Jensen, 1968; Parakkal & Matoltsy, 1968; Matulionis, 1970; Alibardi, 2002).
Thus, embryonic epidermis keratinizes with α-keratin pattern (confirmed with positive AE2 staining), as in other amniotes (Weiss & Zelickson, 1975a, 1975b, 1975c; Holbrook, 1991; Alibardi, 1998; Akiyama et al. 1999; Alibardi & Thompson, 1999), but expression of such an α-keratin layer is completely lost after hatching. Instead, the barrier role is immediately taken over by β-cells produced beneath the periderm. The final detachment of the periderm from the β-keratin layer is due both to degradation of desmosomes between the two layers and the tension between the soft external periderm and the tough β-keratin layer.
Formation of the stratum corneum
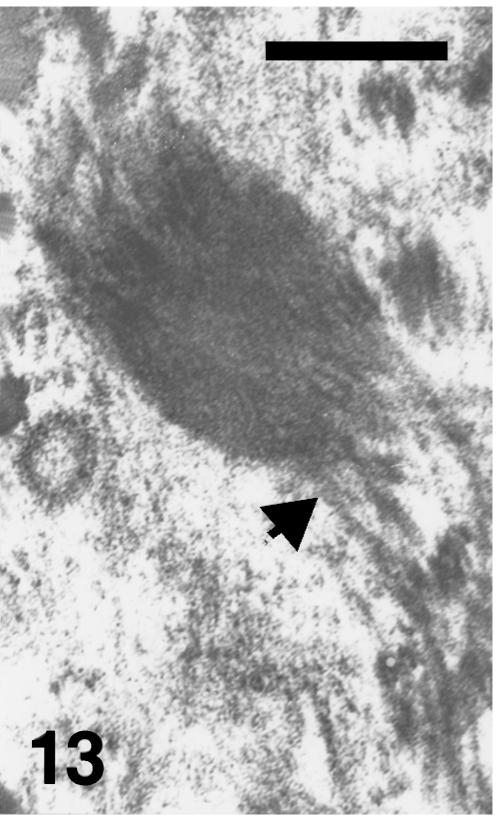
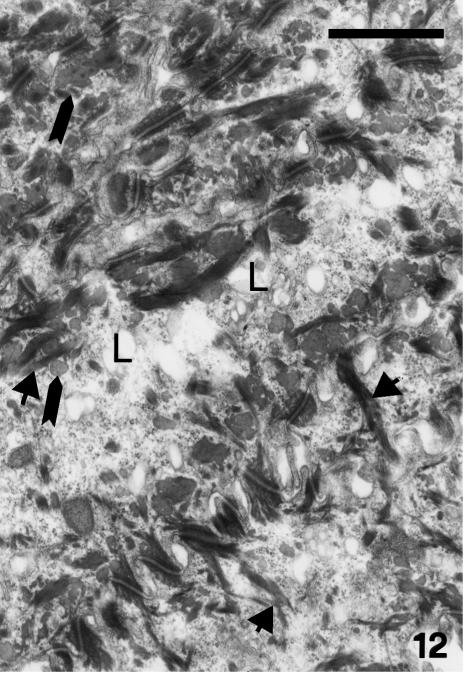
Maturation of the epidermis is almost complete by stage 25 when 5–7 β-cell layers form beneath the embryonic epidermis, and enable the animal to live on land. No keratohyalin is ever present in prekeratinized layers of alligator epidermis, which therefore keratinizes according to a typical β-keratin modality (Alexander, 1970; Alibardi & Thompson, 1999, 2001). The apparent keratohyalin-like material observed in the hinge and outer scale surface represents oblique/irregular sectioning of tonofilaments (see Figs 12 and 13).
Fig. 12.
Details of uppermost, immature beta-keratogenic cells of outer scale surface at stage 27 where electron-dense tonofilament/beta-keratin bundles (arrowheads) are mixed with paler and homogenous beta-keratin packets (arrows). L, lipid droplets or vacuoles. Scale bar = 1 μm.
Cross-reactivity with the antibody against beta-keratin of chicken scales further reflects the shared phylogenetically conserved epitopes of reptilian and avian β-keratins (Sawyer et al. 2000). The ability of peridermal cells to synthesize α-keratin is quickly replaced by the synthesis of β-keratin, which is initially deposited over the α-keratin as in avian scutate scales (Shames et al. 1988, 1989). Together, the ultrastructure and anti-β and anti-α antibodies labelling confirm that β-keratin increases in the initial β-cells and eventually completely obscures the presence of α-keratin (AE1–AE3 reactive). The masking effect of β- over α-keratin, and the degradation of α-keratin or its dilution by β-keratin are possible processes involved in the reduction of alpha-keratin immunoreactivity. The initial beta-cells are thin, with a smooth surface, and few spinulated protrusions. Spinulated protrusions develop in later beta-cells, and in those of the adult (Alibardi, personal observations) The beta-cells are particularly spinulated where the beta-layer is thick and hard, such as in dorsal and tail scales. In contrast to other regions, keratinocytes of hinges (Alexander, 1970) express less β-keratin, allowing α-keratin and lipogenesis to become prevalent.
Lipids and beta-keratinization
Keratinization in the epidermis of alligators resembles that of birds where lipids are stored intracellularly, sandwiched by keratin along the cell periphery (Menon et al. 1986, 1996; Menon & Menon, 2000). A similar process occurs in β-keratinizing cells of avian epidermis (Alibardi, 2002), but is much enhanced in interfollicular and apteric areas where lipids and probably waxes are increased several fold while keratin is considerably reduced (Menon et al. 1986, 1996; Menon & Menon, 2000).
As an extant archosaur, the alligator possesses lipid-rich β-cells, and they were probably also present in the archosaurian progenitors of birds (Maderson, 1972). Keratinocytes containing lipids mixed with α-keratin were probably already more prevalent than β-keratin in the expanding hinge regions of ancient archosaurs. Thus, our data support the hypothesis that the apteric epidermis of birds originated from the expansion of the hinge areas in pre-avian archosaurs. As no keratohyalin was present in the epidermis of the archosaurs, including the hinge region among scales, no keratohyalin is present in apteric avian epidermis. The keratinization of apteric epidermis is different from that of feathered regions (Maderson & Alibardi, 2000; Menon & Menon, 2000).
The alligator has a low water efflux rate compared to other aquatic reptiles (turtles and snakes), indicating that its skin is not specifically adapted to an environment with broad water accessibility. However, compared to other reptiles, the skin of the alligator shows evaporative water-loss values intermediate between aquatic and terrestrial reptiles, a fact that indicates some terrestrial (dry) skin adaptation (Davis et al. 1980; Dunson & Mazzotti, 1988). The mix of â-keratin and lipids probably determines both the skin toughness and the waterproof properties of the skin of alligators. Knowledge of the lipid composition of crocodilian epidermis is therefore essential to understanding its permeability and softness properties vs. its toughness. Apart from water, sodium also cannot diffuse through the skin of crocodilians (Davis et al. 1980; Lillywhite & Maderson, 1982; Dunson & Mazzotti, 1988). No typical lamellar bodies have been described in crocodilian epidermis, so the barrier limiting movements of water and ions through the beta-layer of the epidermis must be lipids that are not incorporated into lamellar membranes. The use of ruthenium tetroxide may reveal some lamellation inside organelles so far identified as dense bodies, and presumed to contain mainly mucus, in crocodilian epidermis (Alibardi & Thompson, 2001). The absence of extracellular lipid lamellae from the stratum corneum of alligators may be responsible for the relatively poor water barrier of crocodilian epidermis compared to species that have lamellae (Landmann, 1980; Menon et al. 1996; Menon & Menon, 2000). The lack of typical lamellate bodies in crocodilians may express the early tendency of the group to adapt to an amphibious lifestyle so that a selection for a very effective barrier to cutaneous water loss was never operating.
The limitation of water and ion movement through the skin of alligators may be due to the richness of hydrophobic lipids among the keratin network of the corneous layer of alligator epidermis. In fact, saturated lipids, which appear as low electron-dense or pale lipid droplets or extracted vacuoles, are visible under the electron microscope after routine staining.
Pigmentation
The large dark brown pigment bands in hatchling alligator skin result from the receipt of melanosomes from epidermal melanocytes by β-cells of specific areas of the epidermis (the dark bands). Similar cyclical injection of pigment from epidermal melanocytes occurs in snake epidermis (β- and α-layer) (Szabo et al. 1973). In contrast to lepidosaurian epidermis where the pigmentation is lost during the following moult (Szabo et al. 1973; Maderson, 1985) in crocodilians (and turtles) the pattern remains, and pigmentation continues to be provided from the underlying pigment deposition. The process that determines the pigmentation pattern of crocodilian skin is, nevertheless, poorly understood and warrants further investigation.
Fig. 27.
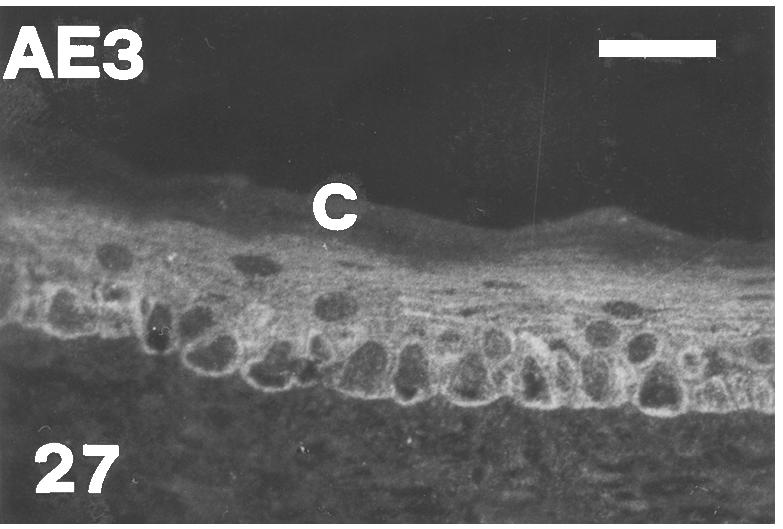
AE3 immunopositive basal and intermediate layers in the central part of outer scale surface of a mid-body lateral scale at stage 26. There is no immunofluorescence in the corneous layer (C). Scale bar = 20 μm.
Acknowledgments
This study was partially financed by a grant by the University of Bologna (LA, ‘segnali molecolari dello sviluppo’) and an ARC Large Grant (MBT). We are grateful to Prof R. H. Sawyer (Biology Department, University of South Carolina at Columbia, USA) for the gift of the antibody against chick beta-keratin, M. Louden for assisting with alligator incubation, Mrs L. Dipietrangelo for photographic and computer-imaging assistance, and the Australian Reptile Park for the alligator eggs.
References
- Akiyama M, Smith LT, Yoneda K, Holbrook KA, Hohl D, Shimizu H. Peridermal cells form cornified cell envelope in their regression process during human epidermal development. J. Invest. Dermatol. 1999;112:903–909. doi: 10.1046/j.1523-1747.1999.00592.x. [DOI] [PubMed] [Google Scholar]
- Alexander NJ. Comparison of α and β keratin in reptiles. Z. Z. Zellforschung. 1970;110:153–165. doi: 10.1007/BF00335521. [DOI] [PubMed] [Google Scholar]
- Alibardi L. Differentiation of the epidermis during scale formation in embryos of lizard. J. Anat. 1998;192:173–186. doi: 10.1046/j.1469-7580.1998.19220173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alibardi L. Keratohyalin-like granules in embryonic and regenerating epidermis of lizards and Sphenodon punctatus. Amphibia-Reptilia. 1999;20:11–23. [Google Scholar]
- Alibardi L, Thompson MB. Epidermal differentiation during carapace and plastron formation in the embryonic turtle Emydura macquarii. J. Anat. 1999;194:531–545. doi: 10.1046/j.1469-7580.1999.19440531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alibardi L. Ultrastructural localization of alpha-keratins in the regenerating epidermis of the lizard Podarcis muralis. Tissue Cell. 2000;32:153–162. doi: 10.1054/tice.2000.0099. [DOI] [PubMed] [Google Scholar]
- Alibardi L, Thompson MB. Scale morphogenesis and ultrastructure of dermis during embryo development in the alligator (Alligator mississippiensis. Acta Zool.(Stockholm) 2000;81:325–338. [Google Scholar]
- Alibardi L, Thompson MB. Fine structure of the developing epidermis in the embryo of the American alligator (Alligator mississippiensis. J. Anat. 2001;198:265–282. doi: 10.1046/j.1469-7580.2001.19830265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alibardi L. Keratinization and lipogenesis in epidermal derivatives of the zebra finch, Taeniopigia guttata castanotis. J. Morphol. 2002;251:294–308. doi: 10.1002/jmor.1090. [DOI] [PubMed] [Google Scholar]
- Baden HP, Maderson PFA. Morphological and biophysical identification of fibrous proteins in the amniote epidermis. J. Exp. Zool. 1970;174:225–232. doi: 10.1002/jez.1401740211. [DOI] [PubMed] [Google Scholar]
- Baden HP, Sviokla S, Roth I. The structural protein of reptilian scales. J. Exp. Zool. 1974;187:287–294. doi: 10.1002/jez.1401870212. [DOI] [PubMed] [Google Scholar]
- Bentley PJ, Schmidt-Nielsen K. Cutaneous water loss in reptiles. Science. 1966;151:1547–1549. doi: 10.1126/science.151.3717.1547. [DOI] [PubMed] [Google Scholar]
- Brazaitis P. The identification of crocodilian skins and products. In: Webb G, Manolis S, Whitehead PJ, editors. Wildlife Managements: Crocodiles and Alligators. Surrey Beatty & Sons Pty Limited & Conservation commission of the Northern Territory; 1987. pp. 373–386. In. [Google Scholar]
- Davis JE, Spotila JR, Schefler WC. Evaporative water loss from the American alligator, Alligator mississippiensis. Comparative Biochem. Physiol. 1980;67A:439–446. [Google Scholar]
- Dunson WA, Mazzotti FJ. Some aspects of water and sodium exchange of freshwater crocodilians in fresh water and sea water: role of the integument. Comparative Biochem. 1988;90A:391–396. [Google Scholar]
- Ferguson MWJ. The reproductive biology and embryology of crocodilians. In: Gans C, Billett F, Maderson PFA, editors. Biology of the Reptilia. Vol. 14. New York: John Wiley & Sons.; 1985. pp. 329–491. In: [Google Scholar]
- Frazer RDB, McRae TP, Rogers GE. Their composition, structure and biosynthesis. Springfield, IL, USA: Charles Thomas.; 1972. Keratins. [Google Scholar]
- Gregg K, Rogers G. Feather keratin: composition, structure and biogenesis. In: Bereither-Hahn J, Matoltsy AG, Sylvia-Richard K, editors. Biology of the Integument. Vol. 2. Berlin: Springer-Verlag; 1985. pp. 666–694. In. [Google Scholar]
- Holbrook KA. Structure and function of the developing human skin. In: Goldsmith LA, editor. Physiology, Biochemistry and Molecular Biology of the Skin. Vol. 1. NY & Oxford: Oxford University Press; 1991. pp. 63–110. [Google Scholar]
- Landmann L. Lamellar granules in mammalian, avian, and reptilian epidermis. J. Ultrastruct. Res. 1980;72:245–263. doi: 10.1016/s0022-5320(80)90062-3. [DOI] [PubMed] [Google Scholar]
- Landmann L. The skin of Reptiles: epidermis and dermis. In: Bereither-Hahn J, Matoltsy AG, Sylvia-Richard K, editors. Biology of the Integument. Vol. 2. Berlin: Springer-Verlag; 1986. pp. 150–187. In. [Google Scholar]
- Lang JW, Andrews HA. Temperature-dependent sex determination in crocodilians. J. Exp. Zool. 1994;270:28–44. [Google Scholar]
- Lillywhite HB, Maderson PFA. Skin structure and permeability. In: Gans C, Pough FH, editors. Biology of the Reptilia. Physiological Ecology. Vol. 12. New York and London: Academic Press; 1982. pp. 397–442. In. [Google Scholar]
- Maderson PFA. On how an archosaurian scale might have given rise to an avian feather. Am. Natur. 1972;176:424–428. [Google Scholar]
- Maderson PFA. Gans C, Billett F, Maderson PFA. Biology of the Reptilia. Vol. 14. New York: John Wiley & Sons; 1985. Some developmental problems of the reptilian integument; pp. 525–598. [Google Scholar]
- Maderson PFA, Rabinovitz T, Tandler B, Alibardi L. Ultrastructural contributions to an understanding of the cellular mechanisms in lizard skin shedding with comments on the function and evolution of a unique lepidosaurian phenomenon. J. Morphol. 1998;136:1–24. doi: 10.1002/(SICI)1097-4687(199804)236:1<1::AID-JMOR1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Maderson PFA, Alibardi L. The development of the sauropsid integument: a contribution to the problem of the origin and evolution of feathers. Am. Zool. 2000;40:513–529. [Google Scholar]
- Matulionis DH. Morphology of the developing down feathers of chick embryos. Z. Anat. Entwicklungs-Geschichte. 1970;132:107–157. doi: 10.1007/BF00523275. [DOI] [PubMed] [Google Scholar]
- Menon GK, Brown BE, Elias PM. Avian epidermal differentiation: role of lipids in permeability barrier function. Tissues Cell. 1986;18:71–82. doi: 10.1016/0040-8166(86)90008-x. [DOI] [PubMed] [Google Scholar]
- Menon GK, Maderson PFA, Drewes RC, Baptista LF, Price LF, Elias PM. Ultrastructural organization of avian stratum corneum lipids as the basis for facultative cutaneous waterproofing. J. Morphol. 1996;227:1–13. doi: 10.1002/(SICI)1097-4687(199601)227:1<1::AID-JMOR1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Menon GK, Menon J. Avian epidermal lipids: functional considerations and relationship to feathering. Am. Zool. 2000;40:540–552. [Google Scholar]
- Mottet NK, Jensen HM. The differentiation of chick embryonic skin. Exp. Cell Res. 1968;52:261–283. doi: 10.1016/0014-4827(68)90564-8. [DOI] [PubMed] [Google Scholar]
- Parakkal PF, Matoltsy GA. An electron microscopic study of developing chick skin. J. Ultrastrastruct. Res. 1968;23:403–416. doi: 10.1016/s0022-5320(68)80106-6. [DOI] [PubMed] [Google Scholar]
- Pough HF, Andrews RM, Cadle JE, Savitsky AH, Wells KD. Herpetology. Upper Saddle River, New Jersey, USA: Prentice Hall Inc.; 1998. [Google Scholar]
- Sawyer RH, Borg TK. Avian scale development. VI. Ultrastructure of the keratinizing cells of reticulate scales. J. Morphol. 1979;161:111–122. doi: 10.1002/jmor.1051610107. [DOI] [PubMed] [Google Scholar]
- Sawyer RH, Glenn T, French JO, Mays B, Shames RB, Barnes GL, Rhodes W, Ishikawa Y. The expression of beta-keratins in the epidermal appendages of reptiles and birds. Am. Zool. 2000;40:530–539. [Google Scholar]
- Shames RB, Knapp LW, Carver WE, Sawyer RH. Identification, expression, and localization of keratin gene products during development of avian scuatate scales. Differentiation. 1988;38:115–123. doi: 10.1111/j.1432-0436.1988.tb00205.x. [DOI] [PubMed] [Google Scholar]
- Shames RB, Knapp LW, Carver WE, Washington LD, Sawyer RH. Keratinization of the outer surface of the avian scuatate scale: interrelationship of alpha and beta keratin filaments in a cornifying tissue. Cell Tissue Res. 1989;257:85–92. doi: 10.1007/BF00221637. [DOI] [PubMed] [Google Scholar]
- Spearman RIC, Riley PA. A comparison of the epidermis and pigment cells of the crocodile with those in two lizard species. Zool. J. Linnean Soc. 1969;48:453–466. [Google Scholar]
- Sun TT, Eichner R, Nelson WG, Tseng SCG, Weiss RA, Jarvine M, Woodcock-Mitchell J. Keratin classes: molecular markers for different types of epithelial differentiation. J. Invest. Dermatol. 1983;81:109–115. doi: 10.1111/1523-1747.ep12540831. [DOI] [PubMed] [Google Scholar]
- Szabo G, Maderson PFA, Roth SI, Kostick RM. Melanocyte activity in the epidermis of the boa constrictor (Constrictor constrictor. Anat. Rec. 1973;176:377–388. doi: 10.1002/ar.1091760402. [DOI] [PubMed] [Google Scholar]
- Weiss LW, Zelickson AS. Embryology of the epidermis: Ultrastructural aspects. (I) Formation and early differentiation of the mouse with mammalian comparison. Acta Dermatovener (Stockolm) 1975a;55:161–168. [PubMed] [Google Scholar]
- Weiss LW, Zelickson AS. Embryology of the epidermis: Ultrastructural aspects. (II) Period of differentiation in the mouse with mammalian comparison. Acta Dermatovener (Stockolm) 1975b;55:321–329. [PubMed] [Google Scholar]
- Weiss LW, Zelickson AS. Embryology of the epidermis: Ultrastructural aspects. (III) Maturation and primary appearance of dendritic cells in the mouse with mammalian comparison. Acta Dermatovener (Stockolm) 1975c;55:431–442. [PubMed] [Google Scholar]
- Wyld JA, Brush AH. The molecular heterogeneity and diversity of reptilian Keratins. J. Mol. Evol. 1979;12:331–347. doi: 10.1007/BF01732028. [DOI] [PubMed] [Google Scholar]
- Wyld JA, Brush AH. Keratin diversity in the reptilian epidermis. J. Exp. Zool. 1983;225:387–396. [Google Scholar]