Abstract
Vascular endothelial growth factor (VEGF) plays an important role during endochondral bone formation in hypertrophic cartilage remodelling. We examined VEGF and VEGF receptor expression in tibiae from fetuses, newborns and children immunohistochemically. Expression of mRNA for the different VEGF splice forms and for VEGF receptors KDR and FLT-1 was analysed by reverse transcription-polymerase chain reaction (RT-PCR). VEGF could be immunolocalized intracellularly in the hypertrophic chondrocytes of the growth plate and in the chondrocytes around cartilage canals of the epiphysis, respectively. The resting zone and the proliferative zone of the growth plate were VEGF-negative. In cartilage samples of all growth plates analysed, VEGF121 and VEGF165 were identified as the only VEGF splice forms expressed. RT-PCR for VEGF mRNA of normal hyaline cartilage was negative. At vessels growing into the hypertrophic cartilage FLT-1 (VEGFR-1) and KDR (VGEFR-2) could be visualized. Reverse transcriptionpolymerase chain reaction (RT-PCR) substantiated the results regarding FLT-1 and KDR expression. The results of our study suggest that the splice forms VEGF121 and VEGF165 and the receptors KDR and FLT-1 of the known angiogenetic peptide VEGF play a role in process of endochondral ossification.
Keywords: growth plate/hypertrophic chondrocyte, VEGF/splice form
Introduction
Vascular endothelial growth factor (VEGF, also termed vascular permeability factor VPF) is an important mediator of angiogenesis during embryogenesis and during tissue remodelling, wound healing, in malignant or certain inflammatory diseases of the adult (Ferrara, 1999; Pufe et al. 2001a, b, c). Apart from inducing endothelial cell proliferation and migration, VEGF acts as a chemotactic factor for monocytes and is a procoagulant (Ferrara, 1999). In cartilage, VEGF has recently been identified as the essential factor for endochondral ossification, the process whereby cartilage is replaced by bone and long bones are formed (Gerber et al. 1999; Horner et al. 1999; Carvalero et al. 2000; Garcia-Ramirez et al. 2000). The invasion of blood vessels into cartilage, which is normally avascular, is a crucial step in this process, and the vasculature provides a conduit for the recruitment of the cell types involved in cartilage resorption and bone deposition (Gerber et al. 1999). During normal bone morphogenesis, chondrocytes in the epiphyseal plate become hypertrophic (Von der Mark et al. 1992), produce angiogenic factors, and finally undergo apoptosis (Gerber et al. 1999).
VEGF is a homodimeric, heavily glycosylated protein of 46–48 kDa (Ferrara, 1999). The two subunits of about 24 kDa are linked by disulphide bridges. The human factor occurs in several isoforms of 121, 165, 189, or 206 amino acids, arising by alternative splicing of the single mRNA (Eckart et al. 1999). These isoforms differ in their molecular masses and in their biological properties such as their ability to bind to heparin or heparan-sulphate proteoglycans and to different VEGF receptors. The splice forms VEGF121, VEGF145 and VEGF165 are secreted, whereas VEGF189 is tightly bound to cell surface heparan-sulphate and VEGF206 is an integral membrane protein (Eckart et al. 1999). In contrast to the other forms, VEGF121 does not bind to heparin or extracellular matrix proteoglycans. The signalling tyrosine kinase receptor FLT-1 (fms-like tyrosine kinase-1) binds VEGF121 and VEGF165. The KDR (kinase domain region/flk-1, fetal liver kinase-1) binds additionally VEGF145 (apart from certain VEGF-related peptides). The co-receptors neuropilin-1 and -2 bind selectively VEGF165 which is the most common isoform in the majority of tissues (Ferrara, 1999).
Since the VEGF splice variants differ in their receptor specificity and their distance of action, we determined their expression in growth plate and epihyseal cartilage.
Materials and methods
Tissues
Growth plates were obtained at routine autopsies from tibiae of eight human cadavers (six fetuses [14, 23, 26, 29, 30, 34 weeks of gestation] and two children of 1 and 13 years).
The study was perfored with permission of the ethical commission of the Medical Faculty of the Christian-Albrechts-University of Kiel. One half of the specimen was fixed in 4% formaldehyde in phosphate-buffered saline, pH 7.4 (PBS), for histological and immunhistochemical analysis. Then, the cartilage was removed from the other half using a sharp spoon and prepared for reverse transcription polymerase chain reaction (RT-PCR).
Histology and immunohistochemistry
Specimen were decalcified in 10% EDTA (dissolved in aqua dest.) at 37 °C for several hours or days until X-ray examination revealed complete decalcification. The temperature of 37 °C was chosen to speed up decalcification. No antibiotics were added. Then, the tissue was dehydrated and embedded in paraffin. Deparaffinized 8-μm sections were stained with toluidine blue and by Goldners staining and examined by light microscopy.
For immunohistochemistry, sections were dewaxed, incubated with testicular hyaluronidase (2 mg mL−1 in PBS, pH 5.0, for 30 min at room temperature) and pronase (1 mg mL−1 in PBS, pH 7.4, 30 min at room temperature) and with H2O2 (3% for 10 min) for quenching of the endogenous peroxidase, immunostained with anti-VEGF (5 μg mL−1 in tris-buffered saline, 60 min, mouse monoclonal IgG, Santa Cruz Biotechnology, CA, USA), anti-KDR (5 µg mL−1 in tris-buffered saline, 60 min; mouse monoclonal IgG, Santa Cruz Biotechnology), or anti-FLT-1 (5 μg mL−1 in tris-buffered saline, 60 min; rabbit affinity-purified polyclonal antibody, Santa Cruz Biotechnology) followed by biotinylated secondary antibodies and a peroxidase-labelled streptavidin-biotin staining technique; nuclei were counterstained with haemalaun.
Tissues with defined antigen sites (gliomas, synovial membrane of patients with rheumatoid arthritis) were used to control the specifity of the immunoreaction. Knee joint cartilage of a 23-year-old male served as negative control. To control unspecific binding of the secondary antibody one section of every specimen was incubated with the secondary antibody alone.
Reverse transcription-polymerase chain reaction (RT-PCR) for VEGF splice variants
For RT-PCR, frozen samples (1 g) were crushed in an achate mortar under liquid nitrogen, then homogenized in 5 mL peqgold RNA Puer solution (peqLab Biotechnologie, Erlangen, Germany) with a Polytron homogenizer, insoluble material removed by centrifugation (12 000 g, 5 min, 4 °C), and RNA was isolated as described by the manufacturer (phenol-guanidinium thiocyanate method). Crude RNA was purified by isopropanol and repeated ethanol precipitation, and contaminating DNA was destroyed by digestion with RNase-free DNase I (27.27 Kunitz units; 20 min at 25 °C; Boehringer, Mannheim, Germany). RNA quantity was measured photometrically, so that equal amounts were loaded (500 ng). After inactivation of the DNase (15 min at 65 °C), cDNA was generated with 50 ng μL−1 (20 pmol) oligo (dT)15 primer (Amersham Pharmacia Biotech, Uppsala, Sweden) and 0.8 μL superscript RNase H− reverse transcriptase (100 U; Gibco, Paisley, UK) for 60 min at 37 °C as described (Von Der Mark et al. 1992). For PCR, 4 μL cDNA (40 ng) was incubated with 30.5 μL water, 4 μL 25 mm MgCl2, 1 μL dNTP (10 mm), 5 μL 10× PCR buffer, and 0.5 μL (2.5 U) Platinum Taq DNA polymerase (Gibco) and different primers following primers (2.5 µL each containing 10 pmol, see Table 1): (i) non-selective for all VEGF splice variants 5′-ATG-GCA-GAA-GGA-GGG-CAG-CAT-3′ (sense) and 5′-TTG-GTG-AGG-TTT-GAT-CCG-CAT-CAT-3′ (antisense) yielding a 255-bp fragment (40 cycles, annealing temperature 55 °C); (ii) selective for VEGF splice variants 5′-CCA-TGA-ACT-TTC-TGC-TGT-CTT-3′ (sense) and 5′-TCG-ATC-GTT-CTG-TAT-CAG-TCT-3′ (antisense) yielding different bp fragments [40 cycles, annealing temperature 55 °C (Eckart et al. 1999). A separate RT-PCR for glycerinaldehyde-3-phosphate dehydrogenase (GAPDH) with an intron spanning primer pair served as control for intactness of the mRNA and a control without cDNA guaranteed the absence of contaminating DNA.
Table 1.
| Primer | Sequence |
|---|---|
| VEGF sense | 5′-ATG GCA GAA GGA GGG CAG CAT-3′ |
| VEGF antisense | 5′-TTG GTG AGG TTT GAT CCG CAT CAT-3′ |
| VEGF splice sense | 5′-CCA TGA ACT TTC TGC TGT CTT-3′ |
| VEGF splice antisense | 5′-TCG ATC GTT CTG TAT CAG TCT-3′ |
| FLT-1 sense | 5′-GTA GCT GGC AAG CGG TCT TAC CGG CTC-3′ |
| FLT-1 antisense | 5′-GGA TTT GTC TGC TGC CCA GTG GGT AGA GA-3′ |
| KDR sense | 5′-TAT AGA TGG TGT AAC CCG GA-3′ |
| KDR antisense | 5′-TTT GTC ACT GAG ACA GCT TGG-3′ |
| GAP DH sense | 5′-TGA AGG TCG GAG TCA ACG GAT TTG GT-3′ |
| GAP DH antisense | 5′-CAT GTG GGC CAT GAG GTC CAC CAC-3′ |
Results
VEGF can be immunostained in hypertrophic chondrocytes
Within all sections, VEGF could be immunolocalized within the intracellulary in the hypertrophic chondrocytes of the growth plate and in chondrocytes around cartilage canals, respectively (Fig. 1). VEGF immunostaining was restricted to the hypertrophic chondrocytes. The proliferative zone and sections of normal articular cartilage were VEGF negative.
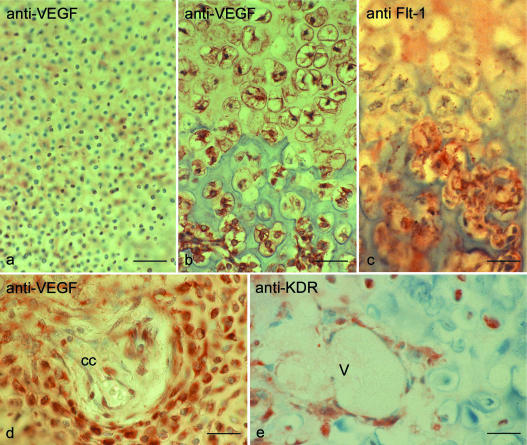
Fig. 1.
(a) VEGF immunostaining in the proliferative zone of the growth plate (a–c: tibia, fetus, 23 weeks after gestation). In the proliferative zone immunostaining for VEGF was negative. (b) Within the zone of hypertrophic cartilage immunolabelling for VEGF was positive. (c) Immunostaining for the VEGF receptor FLT-1 in the growth plate. FLT-1 immunostaining was restricted to vascular endothelial cells, smooth muscle cells and perivascular fibroblasts. FLT-1 was absent in the proliferative and hypertrophic zone. (d) Immunstaining for VEGF in epiphyseal cartilage (d,e: tibial epiphysis in fetus, week 23 of gestation). Chondrocytes around cartilage channels (cc) were VEGF positive. (e) Immunostaining for the VEGF receptor KDR in epiphyseal cartilage. KDR could be immunostained on invading blood vessels (V) but also on single chondrocytes. Scale bars = 65 μm.
The splice forms VEGF121 and VEGF165 can be detected in growth plate cartilage
Since the VEGF antibody detects all splice forms, we analysed the expression of the isoform mRNA with different biological activities by RT-PCR with primers that yield different-sized products (Fig. 2). In all specimens analysed, mRNA of VEGF121 could be amplified whereas cartilage from normal tibial plateaus was VEGF negative. VEGF165 was also detectable in the majority of samples. Only in the growth plate of a 14-week-old fetus was no mRNA of VEGF165 detectable (Fig. 2).
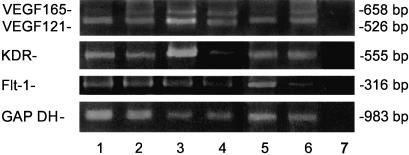
Fig. 2.
RT-PCR amplification with primers allowing the discrimination of VEGF splice variants VEGF121 and VEGF165 in growth plate (lanes 1–3, 1: 14 weeks, 2: 23 weeks, 3: 30 weeks) and epiphyseal cartilage (lanes 4–6, 4: 34 weeks, 5: 29 weeks, 6: 23 weeks). Lane 7 is the contamination control (without RNA added). Four products are detectable in growth plate and epiphyseal cartilage: a 526-bp band derives from VEGF121 with exons 6 and 7 spliced out, and a 658-bp band is from VEGF165 with exon 6 spliced out. mRNA of the VEGF receptors KDR and FLT-1 was also detectable: a 316-bp band derives from FLT-1, and a 555-bp band is from KDR. A separate RT-PCR for glycerinaldehyde-3-phosphate dehydrogenase (GAPDH) yielding a 983-bp product severed as control for intactness of the mRNA applied.
The VEGF receptors FLT-1 and KDR are expressed in human growth plate and in epiphyseal cartilage
The possible target for VEGF, the receptors FLT-1 (VEGFR-1) and KDR (VEGFR-2) could be visualized on blood vessels of the subchondral bone (Fig. 1). FLT-1 was restricted to vascular endothelial cells, smooth muscle cells and perivascular fibroblasts. KDR could be immunostained on vascular endothelial cells, smooth muscle cells and perivascular fibroblasts and on single chondrocytes.
From all samples investigated, two PCR products were obtained (Fig. 2): one with 316 bp, corresponding to FLT-1, and one with 555 bp, corresponding to KDR. In adult articular cartilage no PCR signal for any of both VEGF receptors was detectable.
Discussion
Hypertrophic chondrocytes promote vascularization into the zone of hypertrophic cartilage in the growth plate by production of VEGF; however, with fusion of the growth plates at skeletal maturity, VEGF synthesis and vascularization ceases (Gerber et al. 1999). In the present paper we demonstrate the regular occurrence of VEGF protein and mRNA for the VEGF isoforms VEGF121 and VEGF165 in the human growth plate and in epiphyseal cartilage.
VEGF exists in several isoforms which are generated by alternative splicing from single VEGF gene. These isoforms differ in their biological properties, such as their ability to bind cell-surface heparan-sulphate proteoglycans (Neufeld et al. 1999). Their expression is tissue dependent (Pufe et al. 2001a, b, c). In some cell types multiple VEGF splice forms are detected, e.g. human neonatal fibroblasts express VEGF121, VEGF165, VEGF189 and VEGF206 (Eckart et al. 1999), other cell types express few specific splice forms, e.g. cells from rheumatoid synovium (Pufe et al. 2001c) or cells from osteoarthritic cartilage (Pufe et al. 2001b). In the current study we have demonstrated that hypertrophic chondrocytes express mRNA for both VEGF121 and VEGF165. Interestingly, synovial tissues of patients with rheumatoid arthritis expressed the same VEGF splice forms (VEGF121 and VEGF165, Pufe et al. 2001a, b, c) while samples from osteoarthritic cartilage expressed the splice forms VEGF121 and VEGF189 (Pufe et al. 2001a, b, c). This finding illustrates also that the method has the necessary resolution to detect the different VEGF splice variants.
VEGF121 is freely diffusible in tissues and therefore more angiogenic in vivo than other forms (Zhang et al. 2000) whereas VEGF165 can be bound partially by heperan sulphate at the cell surface or in the extracellular matrix and acts more locally (Neufeld et al. 1999). Since diffusion is an important mechanism in the exchange of molecules between hypertrophic chondrocytes and endothelial cells it seems likely that hypertrophic chondrocytes are capable of stimulating vascular proliferation via VEGF121
However, apart from endothelial cells that express the VEGF receptors FLT-1 and KDR also cartilage cells could be stimulated by locally produced VEGF (Carvalero et al. 2000). KDR has been detected in hypertrophic chondrocytes suggesting a possible autocrine activation which could be mediated by the less-diffusible VEGF165. The experimental inhibition of VEGF in vivo by a soluble receptor prevented hypertrophic chondrocyte programmed cell death leading to an increase in the size of the growth plate (Gerber et al. 1999). Additionally chondroclasts are activated by VEGF (Gerber et al. 1999), and they presumably express the FLT-1 as do other cells of the macrophage lineage (Claus et al. 1996).
In conclusion, we have demonstrated the expression of VEGF121 and VEGF165 isoforms and the VEGF receptors KDR and FLT-1 in growth plate chondrocytes and blood vessels at the protein and mRNA levels. Whilst the exact role of these molecules in growth plate maturation and vascularization remains unknown, we suggest that VEGF may mediate blood vessel invasion into the zone of hypertrophic cartilage. But VEGF may also act in a paracrine or autocrine fashion on other cell types in growth plate cartilage such as in hypertrophic chondrocytes.
References
- Carvalero MF, Cermelli S, Cancedda R, Descalzi-Cancedda F. Vascular endothelial growth factor (VEGF) in cartilage neovascularization and chondrocyte differentiation: auto-paracrine role during endochondral bone formation. J. Cell. Sci. 2000;113:59–69. doi: 10.1242/jcs.113.1.59. [DOI] [PubMed] [Google Scholar]
- Clauss M, Weich H, Breier G, Knies U, Rockel W, Walteneberger J, et al. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J. Biol. Chem. 1996;271:17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- Eckart L, Ban J, Ballaun C, Weniger W, Tschachler E. Reverse transcription-polymerase chain reaction products of alternatively spliced mRNAs form heteroduplexes and heteroduplex complexes. J. Biol. Chem. 1999;274:2613–2615. doi: 10.1074/jbc.274.5.2613. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J. Mol. Med. 1999;77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- Garcia-Ramirez M, Toran N, Andaluz P, Carrasosska A, Audi L. Vascular endothelial growth factor is expressed in human fetal growth cartilage. J. Bone Miner. Res. 2000;15:534–540. doi: 10.1359/jbmr.2000.15.3.534. [DOI] [PubMed] [Google Scholar]
- Gerber HP, Vu TH, Ryan AM, Kolwalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- Horner A, Bishop NJ, Bord S, Beeton C, Kelsall AW, Coleman N, et al. Immunolocalisation of vascular endothelial growth factor (VEGF) in human neonatal growth plate cartilage. J. Anat. 1999;194:519–524. doi: 10.1046/j.1469-7580.1999.19440519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, Poltak Z. Vascular endothelial growth factor and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- Pufe T, Petersen W, Tillmann B, Mentlein R. Expression of the vascular endothelial growth factor in degenerative tendons. Virch. Arch. 2001a;439:355–359. doi: 10.1007/s004280100422. [DOI] [PubMed] [Google Scholar]
- Pufe T, Petersen W, Tillmann B, Mentlein R. Expression of the vascular endothelial growth factor in osteoarthritic cartilage. Arthr. Rheum. 2001b;44:1082–1088. doi: 10.1002/1529-0131(200105)44:5<1082::AID-ANR188>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Pufe T, Petersen W, Tillmann B, Mentlein R. The splice variants VEGF 121 and VEGF 165 are expressed in synovium of rheumatoid arthritis. J. Rheumatol. 2001c;28:1482–1486. [PubMed] [Google Scholar]
- Von der Mark Kirsch T, Nerlich A, Kuss A, Weseloh G, Gluckert K, Stoss H. Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis Rheum. 1992;35:806–811. doi: 10.1002/art.1780350715. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Scott PA, Morbidelli L, Peak S, Moore J, Turley H, et al. The 121 amino acid isoform of vascular endothelial growth factor is more strongly tumorigenic than other splice variants in vivo. Br. J. Cancer. 2000;83:63–68. doi: 10.1054/bjoc.2000.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]