Abstract
The thickness of the perineurial cell basement membrane was examined in diabetic and non-diabetic human sural nerve. A significant increase in thickness was found in the diabetic group. The nature of this thickening was investigated using immunohistochemistry and image analysis in order to semi-quantify three of the major intrinsic components of the perineurial cell basement membrane: collagen IV, laminin and fibronectin. Amounts of all three components were shown to be increased in the diabetic group, but not significantly so. However, significant linear correlations between fascicle size and perineurial collagen IV, laminin and fibronectin were identified in both diabetic and non-diabetic nerve.
Keywords: basement membrane, diabetes, perineurium
Introduction
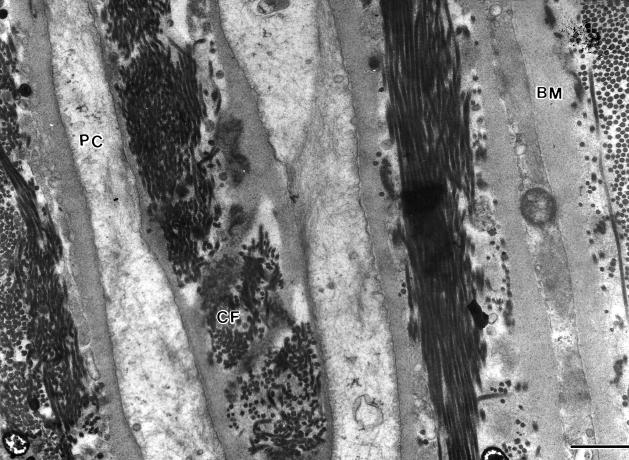
The perineurium of peripheral nerves is a protective structure that ensheathes each individual nerve fascicle. It is constructed of several layers of concentric perineurial cells separated from each other by clefts containing collagen fibrils (Thomas et al. 1993) (Fig. 1). Each perineurial cell is bordered on both surfaces by basement membrane and where two perineurial cells meet they are joined by tight junctions. This combination of tight junctions and basement membrane (BM) allows the perineurium to act as a highly efficient diffusion barrier that regulates and protects the delicate endoneurial environment (Thomas et al. 1993).
Fig. 1.
Part of the perineurium of a human sural nerve. Each perineurial cell (PC) is bordered on both surfaces by basement membrane (BM). Between each perineurial cell layer are collagen fibrils (CF). Scale bar = 1 μm.
In the diabetic state the perineurial cell basement membrane (PCBM) has been reported to increase in thickness (Johnson et al. 1981; King et al. 1989; Bradley et al. 1994; Ghani et al. 1999). It has been suggested that such basement membrane thickening may be involved in the development and progression of diabetic neuropathy. A thickened basement membrane may alter the permeability properties of the perineurium which could slow down the passage of essential macromolecules from the epineurium to the endoneurium and vice versa (Muona & Peltonen, 1994). Increased thickness of the PCBM may produce rigidity of the perineurium which may not only alter its responsiveness to changes in endoneurial osmotic pressure (Olsson, 1990), but might also lead to deformation of the communicating blood vessels that must traverse the perineurium in order to deliver oxygen and nutrients to the endoneurium (Powell et al. 1985). Endoneurial hypoxia has been demonstrated in both humans and animal models of diabetes (Tuck et al. 1984; Newrick et al. 1986. Constriction of the perineurial vessels as a consequence of PCBM thickening may contribute to the development of endoneurial hypoxia. Powell et al. (1985) reported evidence of luminal narrowing and mural thickening that was compounded by thickening of the PCBM, in the perineurial vessels of human diabetic nerve. Thickening of the PCBM is therefore likely to be detrimental to nerve fibre function and so it is essential to determine which components of the PCBM are changing in the diabetic state to account for the increased thickness observed.
Basement membranes other than the PCBM are also known to increase in thickness in the diabetic state. Thickening of the glomerular basement membrane (GBM) is the hallmark of diabetic nephropathy and is accompanied by an increased permeability to negatively charged proteins such as albumin (Adler et al. 1989). A substantial amount of work has been done to elucidate the nature of GBM thickening and most of the evidence to date suggests that changes in the major intrinsic components of the GBM are involved. Work using humans and animal models has suggested that increases in the synthesis of GBM collagen IV, laminin and fibronectin are occurring and contribute to the increased thickness of the GBM (Sato et al. 1975; Brownlee & Spiro, 1979; Spiro & Spiro, 1979; Falk et al. 1983; Killen et al. 1987; Roy et al. 1990). However, in contrast, very little work has examined the changes occurring in the thickened PCBM. Work that has been performed has either not been quantified (Bradley et al. 2000)or has used animal cell culture systems (Muona et al. 1993). Our earlier work examining PCBM collagen IV changes in diabetic nerve was inconclusive (Williams et al. 2000). However, it was performed using tissue from patients with relatively short disease duration, and the PCBM thickness of the samples was not determined.
The aim of this study was therefore first to determine if the PCBM is significantly thicker in the sural nerve taken from patients with advanced diabetes in comparison with those taken from non-diabetics; and second to compare the amounts of three major intrinsic PCBM components whose possible increase may account for any overall increase in basement membrane thickness. The three PCBM components to be examined were collagen IV, laminin and fibronectin.
Collagen IV is the major structural component of all basement membranes. It provides the scaffolding onto which the rest of the basement membrane is built (Timpl et al. 1981). Laminin is the most abundant non-collagenous component of the PCBM (Martin & Timpl, 1987). It has an essential role within the basement membrane, as it is able to interact with itself and with other basement membrane components including collagen IV (Aumailley & Smyth, 1998). Fibronectin is a further major intrinsic component of the PCBM whose properties are thought to be similar to those of laminin (Yamada, 1981).
Materials and methods
Tissue
Ethical permission for obtaining and using human tissue was obtained from Hull and East Yorkshire Ethical and Clinical Trials Committee.
Diabetic nerve samples
Sural nerve samples were taken from the amputated limbs of five diabetic patients (three males and two females) for whom limb removal was necessary due to the combined effects of diabetic neuropathy and lower limb ulceration. In all cases samples were removed at a designated site immediately posterior to the lateral malleolus. All patients were type II diabetics with a disease duration ranging from 6 to 20 years and with a mean age of 71 years.
Non-diabetic control nerve samples
Sural nerve samples were removed from five individuals (four males and one female), who were all free of clinical signs of diabetes and neuropathy. Tissue was taken at post-mortem, within 12 h of death, consent having been obtained from the next of kin. The mean age of the group was 75 years.
There were no significant differences between the two age groups. Non-diabetic control and diabetic nerve samples were processed in an identical manner. A portion of each nerve sample was prepared both for transmission electron microscopy (TEM) and for light microscopy and the remaining tissue was snap-frozen in liquid nitrogen and stored at −70 °C for future use.
Preparation of tissue for PCBM thickness assessment
Briefly, fresh nerve tissue was immediately immersed into glass vials containing 2.5% glutaraldehyde in 0.025 M cacodylate buffer, pH 7.4. Tissue was fixed for a period of 24 h at 10 °C. The temperature chosen for fixation is of significance as fixation performed between 0 °C and 4 °C causes depolymerization of microtubules whilst room temperature enhances the autolysis process. Incubation at 10 °C is therefore chosen in order to minimize these effects (Dyck et al. 1993) and was achieved by incubating the vials in a water bath, set at 10 °C and placed within a ‘cold’ room. Tissue was then washed six times for a 5-min period in fresh cacodylate buffer prior to secondary fixation in 1% osmium tetroxide for 90 min at room temperature. Having been washed again in cacodylate buffer, samples were dehydrated using an ascending ethanol series. Propylene oxide was used as a transitional fluid prior to overnight incubation and eventual embedding in epon resin.
Ultrathin sections were cut from the epon blocks to encompass the full transverse area of each nerve sample. Sections were mounted on formvar-coated copper grids and counter stained with 5% uranyl acetate and lead citrate prior to viewing with a Jeol electron microscope. In order to include the full width of the perineurium it was necessary to make electron micrograph montages. At least two fascicles from each nerve sample were assessed for PCBM thickness. From each fascicle five montages were made in order to sample the complete width of five random areas of the perineurium. Sampling more than one area around the perimeter of the perineurium accounted for any variation in the thickness of the PCBM. Electron micrographs were produced at a final magnification of ×16 500 and thickness measurements were made using a ×7 hand-held magnifier. All measurements were made blind and by the same person. From the montages, measurements were taken at 2-cm intervals along each length of basement membrane bordering both surfaces of each perineurial cell (Fig. 2). Pairs of measurements were therefore obtained at each 2-cm interval.
Fig. 2.
Part of an electron micrograph montage of the perineurium from which basement membrane thickness measurements were made. Measurements were taken at 2-cm intervals along the length of each perineurial cell basement membrane (arrow). Scale bar = 1 μm.
Number of perineurial cell laminae
The mean number of cellular laminae, for both tissue groups, was counted directly from the electron micrograph montages.
Fascicle perimeter measurements
Perimeter measurements were taken from all fascicles used in the assessment of PCBM thickness in order to ensure that meaningful comparisons between the two groups were being made. Semithin sections, encompassing the same area as the ultrathin sections, were cut from all of the epon blocks and stained with toluidine blue. The perimeters of the fascicles used in PCBM thickness assessment were then measured using the tracing function on a Seescan Solitaire image analysis system.
Statistical analysis
PCBM thickness, mean number of cellular laminae and mean fascicle size of the two groups were assessed for statistical difference using the Mann–Whitney U-test
Preparation of tissue for collagen IV, laminin and fibronectin content
Comparisons of the amounts of the three major intrinsic PCBM components were made at the light microscope level. Fresh nerve tissue was fixed in formal saline at 10 °C for 24 h and then washed in fresh phosphate buffer for a further 24 h. An ascending ethanol series was used to dehydrate the tissue prior to eventual embedding in paramat wax. Sections that encompassed the complete transverse area of the nerve sample were cut at a thickness of 8 µm and mounted on polylysine-coated slides.
Fascicle perimeter measurements
A linear relationship is known to exist between fascicle size and the width of the perineurial sheath (Sunderland & Bradley, 1952). Similarly, Lowry et al. (1997) identified a close linear relationship between perineurial collagen IV content per unit of the perineurium and fascicle perimeter in normal sural nerves. It is therefore important to relate the amounts of the different PCBM components to fascicle size. Fascicle perimeter measurements were made from the immunostained paraffin wax sections using a tracing function on the image analyser.
Immunohistochemistry
Collagen IV
Slides were de-waxed and immersed into histoclear for 10 min. Following rehydration through a descending ethanol series, endogenous enzymes were blocked using 1.2% hydrogen peroxide. In order to increase the immunoreactivity of formalin-fixed paraffin wax sections a protease step is necessary. Trypsin has been shown to be superior to pepsin for exposing collagen IV epitopes (Lowry et al. 1997) and slides were therefore incubated in 0.1% trypsin for 90 min at 37 °C. Following a tris buffer wash slides were blocked using normal rabbit serum prior to the application of a 1: 100 concentration of monoclonal mouse antihuman collagen IV primary antibody (MO785, Dako). Two tris buffer rinses preceded secondary antibody application, a biotinylated rabbit antimouse IgG, 1: 300 for 30 min. Collagen IV epitopes were identified using the avidin-biotin (ABC) complex method and visualized with the use of DAB.
Laminin
The protocol for laminin immunostaining was identical to that of collagen IV but with the following changes; sections were blocked using normal horse serum and the primary antibody was a monoclonal mouse antihuman laminin, 1: 1000 (MAB1920, Chemicon) whilst the secondary antibody was biotinylated antimouse IgG.
Fibronectin
The following changes to the basic protocol were made when staining for fibronectin. Only 10 min of trypsinization was necessary in order to achieve optimum fibronectin immunoreactivity. Sections were blocked using goat serum prior to the application of a 1: 1000 concentration of monoclonal rabbit antihuman fibronectin primary antibody (AO245, Dako). A biotinylated goat antirabbit secondary antibody was used prior to ABC and DAB visualization.
For all diabetic and non-diabetic nerve samples a control section was used. Primary antibody was omitted and replaced with the appropriate blocking agent, before application of the secondary antibody.
Semiquantification of the basement membrane components
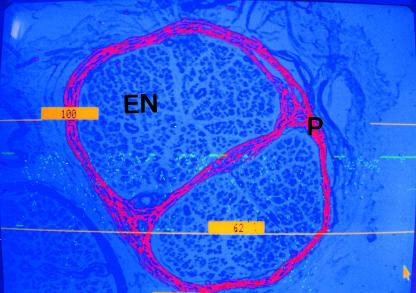
Semiquantitative analysis of all three PCBM components was performed using the method of Lowry et al. (1997). Sections stained for collagen IV, laminin or fibronectin were visualized with a Seescan Solitaire image analysis system. The complete perineurium of each nerve fascicle was viewed using a low-power objective. Each section was shade-corrected to eliminate any variation in background staining prior to thresholding the image. Highlighted pixels that were not part of the PCBM such as blood vessels and Schwann cell basement membranes were excluded (Fig. 3). A user-defined frame was drawn around the outermost layer of the perineurium and the area of highlighted pixels within the frame was then assessed (Lowry et al. 1997). Measurement of the fascicle perimeters allowed the results to be expressed as area of perineurial collagen IV, laminin or fibronectin per unit of the perimeter. Statistical analysis was performed using the SPSS statistical package. For each of the three individual components, between-group comparisons were made using the Mann-Whitney U-test. Correlations between each of the three PCBM components and fascicle perimeter were examined using Spearman’s Rho test.
Fig. 3.
A single human nerve fascicle thresholded for assessment of perineurial collagen IV content, endoneurium (EN), perineurium (P). Highlighted pixels representing collagen IV found elsewhere in the nerve structure have been excluded. (×100).
Results
PCBM thickening
The perineurium was found to be intact and its basement membranes were easily visualized at the electron microscope level. In total, measurements were taken from 26 nerve fascicles (13 from each of the two patient groups). The diabetic group showed a greater overall mean PCBM thickness than the control group (diabetic 524.55 ± 5.9 nm vs. 405.86 ± 4.2 nm control) and this difference was significant (P<0.0001) (Table 1).
Table 1.
Comparisons of mean overall PCBM thickness and fascicle perimeters in the diabetic and control groups
| Group (n = 5) | Basement membrane thickness (nm) | Fascicles used in PCBM thickness measurements (mm) | Fascicles used in collagen IV semiquantification (mm) | Fascicles used in laminin semiquantification (mm) | Fascicles used in fibronectin semiquantification (mm) |
|---|---|---|---|---|---|
| Diabetic | 524.55 ± 5.9* | 1.38 ± 0.07 (NS) | 1.14 ± 0.07 (NS) | 1.04 ± 0.07 (NS) | 1.13 ± 0.06 (NS) |
| (13 fascicles) | (13 fascicles) | (36 fascicles) | (32 fascicles) | (25 fascicles) | |
| Non-diabetic | 405.86 ± 4.2 | 1.18 ± 0.11 | 1.29 ± 0.06 | 0.99 ± 0.05 | 1.09 ± 0.07 |
| (13 fascicles) | (13 fascicles) | (26 fascicles) | (20 fascicles) | (26 fascicles) |
Significantly different from the control group, P < 0.0001. NS, not significantly different from the control group.
Number of perineurial cellular laminae
Comparisons of the mean number of cellular laminae, between the diabetic and non-diabetic groups, gave no significant differences (diabetic 7.4 ± 0.38 vs. control 7.4 ± 0.39, P < 0.05).
Fascicle perimeter measurements
Fascicle perimeter measurements for the two groups were made using the Mann–Whitney U-test. No significant differences were found for fascicles used in either PCBM thickness measurements or in semiquantification of the three basement membrane components (Table 1).
Immunohistochemistry
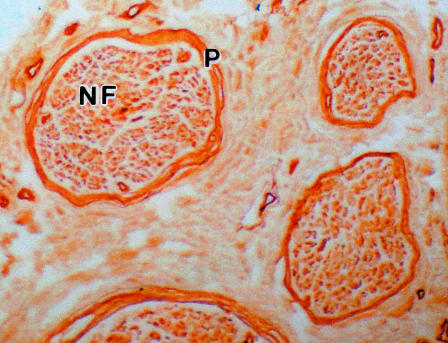
Structural preservation of the nerve tissue was found to be good following immunostaining with collagen IV, laminin and fibronectin (Fig. 4). All three components were found to be located within the perineurium in positions corresponding to the PCBM layers. In all cases background staining was minimal and the control sections where primary antibody was omitted, were completely devoid of staining.
Fig. 4.
A paraffin wax section of a human sural nerve immunostained for collagen IV. The perineurium (P) shows deep staining. Complete nerve fascicles (NF) were easily visualized with the image analyser using a low-power objective (×100).
The diabetic group showed the greater overall mean value for collagen IV per unit of the perineurium than the control group (2.92 ± 0.12 × 10−2 mm vs. 2.60 ± 0.24 × 10−2 mm); however, this difference was not statistically significant. The same was true for both diabetic vs. control laminin (1.38 ± 0.10 × 10−2 mm vs. 1.34 ± 0.10 × 10−2 mm) and fibronectin (2.85 ± 0.1 × 10−2 mm vs. 2.47 ± 0.17 × 10−2 mm) (Table 2). However, significant linear correlations between fascicular size and collagen IV, laminin and fibronectin content per unit of the perineurium were found in both patient groups as determined by Spearman's Rho (Table 3).
Table 2.
Comparisons of mean values for collagen IV, laminin and fibronectin, per unit of the perineurium, between the diabetic and control group
| Group (n = 5) | Collagen IV content per unit of the perineurium (mm × 10−2) | Laminin content per unit of the perineurium (mm × 1010−2) | Fibronectin content per unit of the perineurium (mm × 10−2) |
|---|---|---|---|
| Diabetic | 2.92 ± 0.12 (NS) | 1.38 ± 0.10 (NS) | 2.85 ± 0.1 (NS) |
| (36 fascicles) | (32 fascicles) | (25 fascicles) | |
| Non-diabetic | 2.60 ± 0.24 | 1.34 ± 0.10 | 2.47 ± 0.17 |
| (26 fascicles) | (20 fascicles) | (26 fascicles) |
NS, not significantly different from the control group.
Table 3.
The relationship between perineurial collagen IV, laminin and fibronectin (per unit of the perineurium) and fascicle perimeter in both the diabetic and the control groups
| Group (n = 5) | Correlation between fascicle size (mm) and perineurial collagen IV (mm × 10−2) content | Correlation between fascicle size (mm) and perineurial laminin (mm × 10−2) content | Correlation between fascicle size (mm) and perineurial fibronectin (mm × 10−2) content |
|---|---|---|---|
| Diabetic | rs=0.499, P < 0.01, N = 36 | rs=0.488, P < 0.01, N = 32 | rs=0.455, P < 0.01, N = 25 |
| Control | rs=0.498, P < 0.01, N = 26 | rs=0.565, P < 0.01, N = 20 | rs=0.461, P < 0.01, N = 2 |
Discussion
The results of this study have confirmed that significant thickening of the PCBM does occur in diabetic neuropathic peripheral nerve in comparison with non-diabetic, non-neuropathic nerve (Table 1).
Significant linear relationships between each of the three intrinsic PCBM components, per unit of the perineurium, and fascicle size were demonstrated in both tissue groups (Table 3). However, although the diabetic group showed elevated overall values for collagen IV, laminin and fibronectin in comparison with the non-diabetic group, the differences were not significant (Table 2).
Increased deposition of both collagen IV and laminin were reported in diabetic nerve by Bradley et al. (2000). However this study was based on visual comparisons of light micrographs and was not quantified. Work using cultured rat sciatic nerve cells showed increased production of both collagen IV and fibronectin in response to an elevated glucose concentration (Muona et al. 1993). However, it is not certain how comparable such a system is with the in vivo situation as cellular metabolism is completely separated from other influencing factors present in the in vivo situation (Muona et al. 1993).
Although the results of this current work do not explain the precise nature of the observed increase in PCBM thickness, they do suggest that such changes are not due to increased deposition of the three major components. Several explanations are possible for the observed results. Firstly, it is possible that the diabetic milieu may stimulate increased expression of a normally relatively minor component of the perineurium. For example, collagen I has been identified in the human perineurium (Bradley et al. 2000) and cultured rat sciatic nerve cells have been shown to up-regulate their levels of collagen I mRNA in response to an elevated glucose concentration (Muona & Peltonen, 1994). The presence of collagen VI has also been noted in diabetic nerve. Luse bodies have been found in close proximity to the PCBM of human diabetic nerve (Muona & Peltonen, 1994). Further work in our laboratory is therefore under way to investigate changes in less obvious components of the perineurium.
A further consideration is the effect of advanced glycosylation end products (AGE) on the PCBM. Long-lived proteins such as collagen IV are particularly vulnerable to glycosylation, the effects of which are known to inhibit the lateral association of adjacent molecules and so prevent normal network formation (Brownlee, 1995). The presence of AGE on protein structures can also act as a covalent trap for circulating serum proteins such as albumin, lipoproteins and immunoglobulins (Bucala et al. 1995). It is therefore possible that the presence of AGE products may hinder normal epitope recognition by conventional antibodies. The effect of AGE on protein structures therefore requires further investigation.
References
- Adler S, Anderson P, Xu G, Ihm C, Nast C, Gullermo R, Glassock R. Early renal procollagen ×1 (IV) gene expression in diabetic rats occurs in deep to superficial cortical glomeruli (Abstract) Clin. Res. 1989;37:484A. [Google Scholar]
- Aumailley M, Smyth N. The role of laminin in basement membrane function. J. Anat. 1998;193:1–21. doi: 10.1046/j.1469-7580.1998.19310001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley JL, Thomas PK, King RMH, Watkins PJ. A comparison of perineurial and vascular basal laminal changes in diabetic neuropathy. Acta Neuropathol. 1994;88:426–432. doi: 10.1007/BF00389494. [DOI] [PubMed] [Google Scholar]
- Bradley JL, King RHM, Muddle JR, Thomas PK. The extracellular matrix of peripheral nerve in diabetic polyneuropathy. Acta Neuropath. 2000;99:539–546. doi: 10.1007/s004010051158. [DOI] [PubMed] [Google Scholar]
- Brownlee M, Spiro RG. Glomerular basement membrane metabolism in the diabetic rat. In vivo studies. Diabetes. 1979;28:121–125. doi: 10.2337/diab.28.2.121. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Advanced glycosylation in diabetes and ageing. Annu. Rev. Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- Bucala R, Cerami A, Vlassara H. Advanced glycosylation end products in diabetic complications. Diabetes Rev. 1995;3:258–268. [Google Scholar]
- Dyck PJ, Gianni C, Lais A. Pathologic alterations of nerves. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy. W.B Saunders: 1993. pp. 514–598. [Google Scholar]
- Falk RJ, Scheinman JI, Mauer SM, Michael AF. Poly-antigenic expansion of basement membrane constituents in diabetic nephropathy. Diabetes. 1983;32(Suppl. 2):34–39. doi: 10.2337/diab.32.2.s34. [DOI] [PubMed] [Google Scholar]
- Ghani M, Malik RA, Walker D, Sharma AK, Lowrie CT, Schall WD, et al. Perineurial abnormalities in the spontaneously diabetic dog. Acta Neuropathol. 1999;97:98–102. doi: 10.1007/s004010050961. [DOI] [PubMed] [Google Scholar]
- Johnson PC, Brendel K, Meezan E. Human diabetic perineurial cell basement membrane thickening. Laboratory Invest. 1981;44:265–269. [PubMed] [Google Scholar]
- Killen PD, Ebihara I, Martin GR, Ortola F, Brenner BM. mRNA levels for laminin and collagen IV chains are elevated in diabetic kidneys. (Abstract) Kidney Int. 1987;31:171–174. [Google Scholar]
- King RMH, Llewelyn JG, Thomas PK, Gilbey SG, Watkins PJ. Diabetic neuropathy: Abnormalities of Schwann cell and perineurial basal laminae. Implications for diabetic vasculopathy. Neuropathol. Appl. Neurobiol. 1989;15:339–355. doi: 10.1111/j.1365-2990.1989.tb01234.x. [DOI] [PubMed] [Google Scholar]
- Lowry A, Wilcox D, Masson EA, Williams PE. Immunohistochemical methods for semiquantitative analysis of collagen content in human peripheral nerve. J. Anat. 1997;191:367–374. doi: 10.1046/j.1469-7580.1997.19130367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GR, Timpl R. Laminin and other basement membrane components. Annu. Rev. Cell Biol. 1987;3:57–85. doi: 10.1146/annurev.cb.03.110187.000421. [DOI] [PubMed] [Google Scholar]
- Muona P, Peltonen J. Connective tissue metabolism in diabetic peripheral nerves. Ann. Med. 1994;26:39–43. doi: 10.3109/07853899409147325. [DOI] [PubMed] [Google Scholar]
- Muona P, Peltonen J, Jaakkola S, Zhang R, Pan T, Pelliniemi L, et al. Hyperglycaemic glucose concentrations up-regulate the expression of type IV collagen in vitro. Am. J. Pathol. 1993;142:1586–1597. [PMC free article] [PubMed] [Google Scholar]
- Newrick GM, Wilson AJ, Jakubowski J, Boulton AJM, Ward JD. Sural nerve oxygen tension in diabetes. Br. Med. J. 1986;293:1053–1054. doi: 10.1136/bmj.293.6554.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson Y. Microenvironment of the peripheral nervous system under normal and pathological conditions. Crit. Rev. Neurobiol. 1990;5:265–311. [PubMed] [Google Scholar]
- Powell HC, Rosoff J, Myers RR. Microangiopathy in human diabetic neuropathy. Acta Neuropathol. 1985;68:295–305. doi: 10.1007/BF00690832. [DOI] [PubMed] [Google Scholar]
- Roy S, Sala R, Cagliero E, Lorenzi M. Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc. Natl Acad. Sci. USA. 1990;87:404–408. doi: 10.1073/pnas.87.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Munakata H, Yoshinaga K, Yosizawa Z. Comparison of the chemical composition of glomerular and tubular basement membranes obtained from human kidneys of diabetics and non-diabetics. Clin. Chim. Acta. 1975;61:145–150. doi: 10.1016/0009-8981(75)90308-3. [DOI] [PubMed] [Google Scholar]
- Spiro RG, Spiro MJ. Effect of diabetes on the biosynthesis of the renal glomerular basement membrane. Studies on the glucosyltransferase. Diabetes. 1979;20:641–648. doi: 10.2337/diab.20.10.641. [DOI] [PubMed] [Google Scholar]
- Sunderland S, Bradley KC. The perineurium of peripheral nerves. Anat Rec. 1952;113:125–141. doi: 10.1002/ar.1091130202. [DOI] [PubMed] [Google Scholar]
- Thomas PK, Berthold CH, Ochea J. Microscopic anatomy of the peripheral nervous system. In: Dyck PJ, Thomas PK, editors. Peripheral Neuropathy. Vol. 1. W.B. Saunders Co; 1993. pp. 28–91. [Google Scholar]
- Timpl R, Wiedemann H, Van Delden V, Furthmayr H, Kuhn K. A network model for the organization of type IV collagen molecules in basement membranes. Eur. Neurol. 1981;41(1):35–43. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- Tuck JD, Schmelzer JD, Low PA. Endoneurial blood flow and oxygen tension in the sciatic nerves of rats with experimental diabetic neuropathy. Brain. 1984;107:935–950. doi: 10.1093/brain/107.3.935. [DOI] [PubMed] [Google Scholar]
- Williams PE, Lowry A, Hill RE, Masson EA. Relationship between fascicle size and perineurial collagen IV content in diabetic and control human peripheral nerve. Histopathology. 2000;36:551–555. doi: 10.1046/j.1365-2559.2000.00897.x. [DOI] [PubMed] [Google Scholar]
- Yamada KM. Fibronectin and other structural proteins. In: Hay ED, editor. Cell Biology of the Extracellular Matrix. New York: Plenum Press; 1981. p. 95. [Google Scholar]