Abstract
During the differentiation of secondary lens fibre cells from the lens epithelium, the fibre cells lose all of their cytoplasmic organelles as well as their nuclei. The fibre cells, containing crystallins, which confer optical clarity, then persist in the adult lens. The process of denucleation of these cells has been likened to an apoptotic event which is not followed by the plasma membrane changes that are characteristic of apoptosis. We have examined the expression and subcellular translocation of molecules of the apoptotic cascade in differentiating lens epithelial cells in culture. In this culture system, the epithelial cells differentiate into lentoids composed of lens fibre cells. We find that caspase-9, which is expressed and activated before embryonic day 12 in intact lenses, is localized in the cytosol outside mitochondria in non-differentiating cultured cells. In lentoid cells, caspase-9 migrates into mitochondria after the latter undergo a membrane permeability transition that is characteristic of apoptotic cells. At the same time, caspase-9 co-localizes with cytochrome c in the cytosol. The cytochrome c is apparently released from the mitochondria in lentoid cells after the mitochondrial membrane permeability transition and during the period of nuclear shrinkage. Also during this time, the mitochondria aggregate around the degenerating nuclei. Cytochrome c disappears rapidly, while mitochondrial breakdown occurs approximately coincident with the disappearance of the nuclei, but mitochondrial remnants persist together with cytochrome c oxidase, which is a mitochondrial marker protein. Apaf-1, another cytosolic protein of the apoptotic cascade, also migrates to the permeabilized mitochondria and also co-localizes with caspase-9 and cytochrome c in the cytosol or mitochondria of denucleating cells, thus providing evidence for the formation of an ‘apoptosome’ in these cells, as in apoptotic cells. At no time did we observe the translocation of molecules between cytoplasmic compartments and the nucleus in differentiating lentoid cells. We suggest that the uncoupling of nuclear and membrane apoptotic events in these cells may be due to the early permeability changes in the mitochondria, resulting in the loss of mitochondrial signalling molecules, or to the failure of molecules to migrate to the nucleus in these cells, thus failing to activate nuclear-plasma membrane signalling pathways.
Keywords: apoptosis, caspase-9, cytochrome c, denucleation, embryo, lens, mitochondria
Introduction
Secondary lens fibre cells differentiate from lens epithelial cells by a transformation which involves the loss of cellular organelles, including the nuclei, and the expression of various lens-specific crystallins which endow the lens with its characteristic optical properties (Piatigorsky, 1981; Wride, 1996; Bassnett, 2002). Despite this extreme course of events, the lens fibre cells persist, and their plasma membranes maintain their integrity. Because the degenerating nuclei bear many of the characteristics of the nuclei of apoptotic cells (Sanwal et al. 1986; Bassnett, 1997; Counis et al. 1998; Wride & Sanders, 1998), the nuclear degeneration process has been likened to an apoptotic event which is not accompanied by plasma membrane changes (Lang, 1997; Dahm, 1999; Wride et al. 1999; Wride, 2000). However, this view has been controversial (Bassnett & Mataic, 1997).
During the course of organelle breakdown in lens fibre differentiation, the mitochondria are lost (Bassnett & Beebe, 1992; Bassnett, 1992). If the nuclear degeneration is to be viewed as an apoptotic event, then the timing and the nature of the mitochondrial loss becomes important, because mitochondria are known to play an integral role in the initiation and execution of the apoptotic response (Green & Reed, 1998; Bernardi et al. 1999; Porter, 1999; Goldstein et al. 2000). This connection has led to the suggestion that the signals for the initiation of nuclear degeneration n these cells could be linked to the mitochondria and to the mitochondrial breakdown (Dahm et al. 1998). Further, one can speculate that the loss of the mitochondria in these cells is associated with the failure of the plasma membrane events to follow the nuclear events, as they do in conventional apoptotic cells.
Evidence that lens fibre nuclear degeneration is an apoptotic event is accumulating (Wride, 2000). In transgenic mice over-expressing bcl-2 protein in their lenses, disturbances are seen in lens fibre cell organization and in the degeneration of lens fibre cell nuclei (Fromm & Overbeek, 1997). Bcl-2 is a well-established member of a key family of molecules that regulates the apoptotic cascade (Knudson & Korsmeyer, 1997; Fadeel et al. 1999). Further, there is evidence that caspases are involved in the regulation of nuclear degeneration (Ishizaki et al. 1998), and caspases are crucial to the initiation and execution of the apoptotic response (Stennicke & Salvesen, 1998). We have recently shown that several members of the bcl-2 and caspase families are active during the course of lens fibre cell denucleation (Wride et al. 1999), and that they are expressed in the differentiating lens in developmentally regulated spatio-temporal concentric patterns. Using differentiating lens epithelial cultures, we also showed that peptide inhibitors of caspases-1, -2, -4, -6 and -9 significantly reduced the incidence of nuclear degeneration in the differentiating lentoids in the cultures, while inhibitors of caspases-3 and -8 did not. Further, we provided evidence that one likely substrate of caspases in this system is poly (ADP-ribose) polymerase (PARP).
The central role played by mitochondria in the apoptotic cascade depends on the initiation of the mitochondrial membrane permeability transition (Bernardi et al. 1999; Halestrap et al. 2000), and the release of cytochrome c from the mitochondria into the cytosol (Martinou et al. 1999; Goldstein et al. 2000). The release of cytochrome c is related to the activity and translocation of pro- and anti-apoptotic members of the bcl-2 family of molecules, including bcl-2, bax and bcl-X (Wolter et al. 1997; Eskes et al. 1998; Tsujimoto, 1998; Gross et al. 1999; Putcha et al. 1999). It also appears that the cleavage and migration of the molecule Bid, which is a bcl-2 family member, by caspase-8, is related to the redistribution of mitochondria and the mitochondrial permeability transition during apoptosis (Li et al. 1998), although the timing of Bid cleavage in relation to cytochrome c release is unclear (Granville et al. 1999). Indeed, the relationship between the mitochondrial membrane permeability transition and cytochrome c release is also uncertain (Dinsdale et al. 1999).
The caspases themselves also undergo translocation between cellular compartments during apoptosis (Zhivotovsky et al. 1999; Ritter et al. 2000), and at various times they may be present in, and migrate between, the cytosol, mitochondria, nuclei and the microsomal fraction. One of the caspases that has attracted particular attention in this regard is caspase- 9. Caspase-9 may be present both in the cytoplasm and in the mitochondria of apoptotic cells (Zhivotovsky et al. 1999), and, like cytochrome c, may be released from mitochondria by the activity of bcl-2-family molecules (Krajewski et al. 1999). Pro-caspase-9 may then be activated to caspase-9 in the cytoplasm in association with cytochrome c and apoptotic protease-activating factor-1 (Apaf-1), in the molecular complex known as the ‘apoptosome’ (Li et al. 1997). Once activated, caspase-9 may then activate pro-caspase-3 to caspase-3, which is one of the principal effector caspases which acts on several of the key cellular apoptotic substrates (Stennicke & Salvesen, 1998). The apoptosome complex between cytochrome c, pro-caspase-9 and Apaf-1 is therefore a focal point in caspase-9-mediated apoptosis (Slee et al. 1999; Zou et al. 1999; Cain et al. 2000).
There are therefore two questions of particular interest in relation to the denucleating lens fibre cells: firstly, what is the trigger for denucleation? We have previously implicated tumour necrosis factor-α (TNFα) and its receptors in this process (Wride & Sanders, 1993, 1998; Wride et al. 1994). Secondly, if the denucleation process is an apoptotic event, how does it differ from conventional apoptosis? In this investigation, we attempt to shed light on the second question, by examining the relative timing of the degeneration of the nuclei and mitochondria in lens epithelial cells which are differentiating into small lenses, or ‘lentoids’ (Piatigorsky, 1981), in culture. We have also studied the expression, localization and translocation of cytochrome c, caspase-9 and Apaf-1 during the process of nuclear degeneration, and the possibility that these molecules associate in the cytoplasm of denucleating fibre cells.
Materials and methods
Lens preparation
White Leghorn hens' eggs were incubated at 38 °C in a humid chamber. After various lengths of time, the embryos were removed from their yolk, rinsed and handled in Tyrode's saline and immediately decapitated with a sharp scalpel blade. Lenses were removed from embryos with the vitreous attached at embryonic days (EDs) 8, 12 and 16 of development, as appropriate, using electrolytically sharpened tungsten needles. ED-8 lenses were used because this represents a period of development before nuclear degeneration begins, while ED-12 is the period when nuclear degeneration begins. By ED-16 there is a clear organelle-free zone at the centre of the lens and nuclear degeneration is occurring in a boundary region between the core and outer secondary lens fibres (Bassnett & Mataic, 1997).
When required, ED-12 lenses were dissected into four regions according to the method of Walker & Menko (1999). The four regions dissected consisted of: (1) the central lens epithelium; (2) the equatorial epithelium, including the annular pad where fibre cell differentiation is initiated; (3) the peripheral region of cortical lens fibres, in which cell nuclei are still present; (4) the tightly associated core fibre region, in which the nuclei are in the process of degenerating, or have already degenerated.
Lens epithelial cell culture
Chick embryo lens epithelial cell cultures were prepared according to the method of Menko et al. (1984). We have previously demonstrated (Wride & Sanders, 1998; Wride et al. 1999) that this lens epithelial cell culture system is a reliable one for the study of lens fibre cell nuclear degeneration in culture. Briefly, ED-8 lenses were trypsinized at 38°C in 0.1% trypsin in calcium- and magnesium-free Tyrode's solution for 20 min, or until all the lens capsules were ruptured. The cells were dissociated by repeated pipetting, pelleted to remove the supernatant, resuspended in medium 199 (Gibco) containing 10% fetal calf serum (FCS; Gibco) and gentamycin, and then plated onto coverslips (1.5 µm thickness, for optimum quality confocal microscopy) coated with Matrigel (1.25 mg mL−1Collaborative Biomedical Research). Before plating the cells, the Matrigel was allowed to air dry on the coverslips for approximately 30 min before washing with medium 199 containing 10% FCS. The culture medium was changed each day, and the cultures were maintained at 38 °C for 3–7 days as appropriate.
Antibodies
Antibodies were obtained from the following sources and used at the dilutions indicated. Cytochrome c: Pharmingen Inc., mouse monoclonal; 1 25 in immunocytochemistry (ICC); 1: 50 in immunoblotting (IB). Cytochrome oxidase: Molecular Probes Inc., subunit IV, mouse monoclonal; 1: 50 in ICC; 1: 50 in IB. Caspase-9: Stressgen Inc., rabbit polyclonal; 1: 200 in ICC; 1: 500 in IB; and Santa Cruz Biotechnology Inc., rabbit polyclonal; 1: 50 in ICC; 1: 50 in IB. Caspase-9, 10-kDa fragment: Bio-Source Inc., rabbit polyclonal; 1: 50 in ICC; 1: 500 in IB. Apaf-1: R & D System Inc., rabbit polyclonal; 1: 250 in ICC; and Stressgen Inc., rabbit polyclonal; 1:250 in IB.
Immunocytochemistry and confocal microscopy
Coverslips bearing the cultures were washed with warm Medium 199 and stained with MitoTracker Red® CMXRos (Molecular Probes Inc.) by addition of 5 µL of 10 µm;C MitoTracker® to 1 mL of medium. Cultures were incubated in this reagent for 30 min and 38°C, then washed in warm Tyrode's solution and fixed with 4% buffered paraformaldehyde. Cultures were then permeabilized with acetone at −20°C and non-specific immunoreactivity was blocked with 3% bovine serum albumin in phosphate-buffered saline (PBS). Primary and secondary antibodies were applied sequentially, and finally nuclei were labelled by incubation of cultures with 0.5 µL mL−1 4′-6-diaminido-2-phenylindole (DAPI). In the case of cytochrome oxidase, it was necessary to heat the cultures to 90 °C in 10 mm citrate/citric acid buffer, pH 6.0, before the permeabilization step, according to the method of Marusich et al. (1997). Specimens were mounted in Vectashield (Vector Laboratories Inc.). Negative controls were carried out by replacing the primary antibodies with PBS, and in all cases this resulted in no immunreactivity of the cultures.
In order to monitor the mitochondrial membrane permeability transition, cultures were stained with JC-1 (Molecular Probes Inc.) by incubation with this agent at a dilution of 1: 200 in culture medium for 30 min at 37 °C, and then washing with culture medium. Using this method, mitochondria with high membrane potential are stained red, while depolarized mitochondria with high membrane permeability are stained green.
Specimens were examined using a Zeiss LSM510 confocal microscope equipped with argon, helium/neon and ultraviolet lasers.
Polyacrylamide gel electrophoresis (PAGE) and Western blotting
PAGE and Western blotting were carried out as described previously (Wride & Sanders, 1998; Wride et al. 1999). Briefly, lens tissue was homogenized in protease inhibitor buffer containing 15 µg mL−1 aprotinin, 1 µg mL−1 leupeptin, 5 µg mL−1 pepstatin, and 1.74 mg mL−1 phenylmethyl-sulphonyl fluoride (PMSF). Protein concentrations were determined using the Bio-Rad Bradford-based protein assay method and 20 µg of protein was added to each well of an 8% or 12% polyacrylamide gel. Whole lenses were homogenized at ED-8, -12 and -16, as described previously (Wride et al. 1999). In the case of dissected lenses, the four regions of the lens described above were homogenized and run separately on the gel. Proteins were transferred from the gels onto a supported nitrocellulose membrane at 100 V for 2 h.
For immunoblotting, membrane blocking was carried out using 5% skimmed milk in Tris-buffered saline with 0.1% Tween 20. Primary antibodies were incubated for 18 h at 4 °C followed by four washes for 10 min each, after which biotinylated secondary antibodies were used for 1.5 h at room temperature (RT). Proteins were peroxidase-labelled using the Vectastain ABC Reagent (Vector Laboratories Inc.) for 1.5 h at RT, and visualized by adding Luminol reagent (Santa Cruz Biotechnology Inc.) for 1 min, and then exposing the blot to Hyperfilm ECL (Amersham International Plc).
Where appropriate, negative controls were performed using pre-absorbed primary antibodies, and positive controls were run using lysate from Jurkat cells, which abundantly express many of the proteins examined here. A minimum of three runs were carried out for each antibody used, and representative examples are shown.
Results
We have previously shown that when an inhibitor of caspase-9 is added to cultures of differentiating lens fibre cells, the nuclear degeneration that accompanies this differentiation is suppressed. Further, in these experiments the caspase-9 inhibitor also reduced the cleavage of PARP in the cultured cells (Wride et al. 1999). Both of these observations implicate caspase-9 in the pathways leading to the process of nuclear degeneration that is characteristic of lens fibre cell differentiation.
In the present work, we have extended these observations on caspase-9 by examining the distribution and activity of this caspase in the lens.
Expression of caspase-9
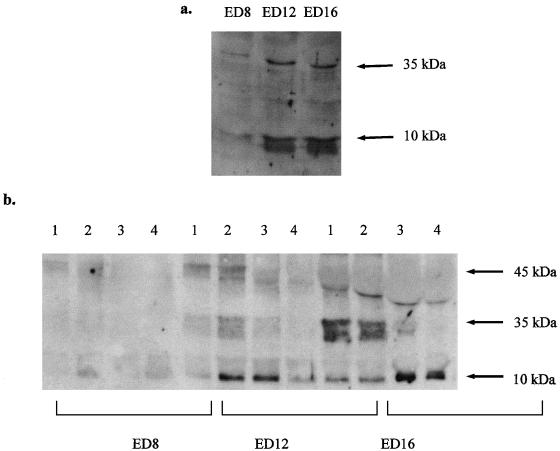
When pro-caspase-9 (approximately 45 kDa) is activated, it is cleaved into fragments of approximately 35 kDa and 10 kDa (Li et al. 1997; Zhivotovsky et al. 1999). Using two different antibodies (from Santa Cruz Biotechnology Inc., and Stressgen Inc.), we were able to detect both cleavage fragments in developing lenses by immunoblotting. Immunoblotting of lysates from ED-8, -12 and -16 whole lenses showed that both cleavage fragments appeared very strongly at a time between days 8 and 12 (Fig. 1a).
Fig. 1.
Western blots for caspase-9 immunoreactivity (antibody from Stressgen Inc.), showing fragments at 10 and 35 kDa indicative of activated caspase-9. Immunoreactivity is shown for lyates of whole lenses from ED-8, -12 and -16. Immunoreactivity for cleaved fragments is strong at ED-12 and ED-16, but weaker at ED-8. (b) Western blots for caspase-9 immunoreactivity (antibody from Stressgen Inc.), showing fragments at 10 and 35 kDa indicative of activated caspase-9. Immunoreactivity is shown for each of the four dissected regions of lenses at ED-8, -12 and -16. Expression of the 10-kDa fragment is seen primarily at ED-12 in regions 2 and 3, and at ED-16 in regions 3 and 4. Expression of pro-caspase-9, at 45 kDa, is seen at ED-12 and -16, but at ED-16 this band is displaced by the abundance of crystallin which is also expressed at this time.
In order to confirm which regions of the lens possessed the highest caspase-9 activity, lysates made from the four dissected regions of ED-8, -12 and -16 embryos were subjected to immunoblotting with the same antibodies. It was clear from examination of these blots (Fig. 1b) that at ED-12, the 10-kDa caspase-9 cleavage fragment was most highly expressed in regions 2 and 3, while at ED-16 expression tended to be highest in regions 3 and 4. As shown in Fig. 1(a), little caspase-9 expression was found at ED-8.
Mitochondrial breakdown and cytochrome c release
It has been shown previously that the loss of the nuclei and mitochondria in differentiating lens fibre cells is coincident (Bassnett & Beebe, 1992; Bassnett, 1992), but that the final DNA degradation and disappearance of the nuclei occurs after the degradation of the mitochondria has been completed (Bassnett & Mataic, 1997). In other words, the degradation of the mitochondria is a more rapid process than the degradation of the nuclei (Dahm et al. 1998; Dahm 1999). However, from the point of view of apoptotic mechanisms, it is the timing of the mitochondrial permeability transition and the consequent release of cytochrome c from the mitochondria that is important, rather than the disappearance of the mitochondria themselves (Bernardi et al. 1999; Halestrap et al. 2000).
We have used differentiating lens epithelial cell cultures to investigate this issue. As markers for mitochondria, we have used MitoTracker Red® (CMXRos) and cytochrome c oxidase, which does not leave the mitochondria after the membrane permeability transition. MitoTracker® is taken up by, and concentrated in, metabolically active mitochondria, and retained after fixation, although the relationship between MitoTracker® staining and the membrane permeability transition is unclear (Bernardi et al. 1999; Mathur et al. 2000).
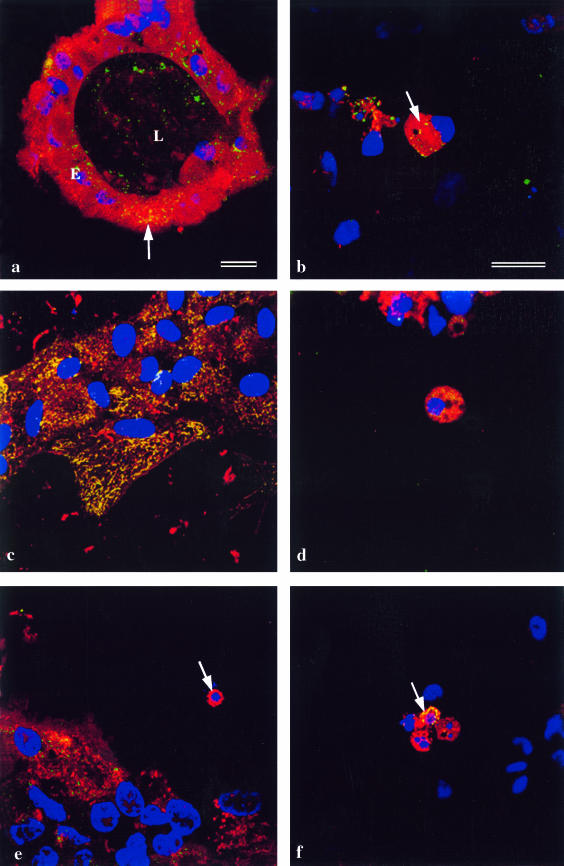
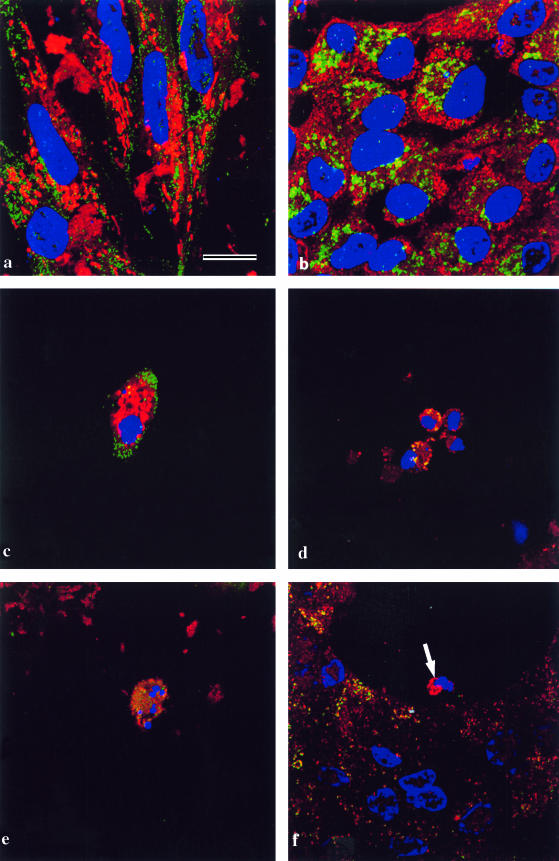
Confocal microscopy on differentiating lens cell cultures showed that, as expected, in normal epithelia, cytochrome oxidase always co-localized with mitochondria (Fig. 2a). In lentoids, in which nuclei had already degenerated (Fig. 2a), mitochondria, as judged by MitoTracker® labelling, had also degenerated, but cytochrome oxidase was still present in the lentoids. This suggests that fragments of mitochondria still remain after nuclear degeneration. When the process of nuclear and mitochondrial degeneration in lentoids was examined more closely, it was found that the first step, nuclear shrinkage and rounding up, is accompanied by the aggregation of mitochondria, still containing cytochrome oxidase (Fig. 2b, arrow, yellow).
Fig. 2.
Lens epithelial cells cultured for 5 days to allow the differentiation of lentoids, and immunostained. Nuclei are labelled with DAPI (blue). Bars = 5 µm (a and b–f). (a) A lentoid (L) surrounded by lens epithelial cells (E). The culture is stained with MitoTracker® (red) and anticytochrome oxidase (green). The cells in the differentiated lentoid contain no nuclei and do not label with MitoTracker®, but show cytochrome oxidase immunoreactivity. In the undifferentiated lens epithelial cells MitoTracker® co-localizes with cytochrome oxidase (yellow; arrow). The epithelial sheet itself is out of the plane of focus. (b) Denucleating cells in a differentiating lentoid immunostained with MitoTracker® (red) and anticytochrome oxidase (green). During the phase of nuclear shrinkage (arrow), cytochrome oxidase co-localizes with MitoTracker® (yellow). (c) Undifferentiated lens epithelial cells stained for cytochrome c (green) and cytochrome oxidase (red). The cytochrome c and cytochrome oxidase co-localize (yellow) in mitochondria. (d) A denucleating cell in a lentoid at the stage of nuclear shrinkage in a lentoid stained with MitoTracker® (red) and cytochrome c (green). The distribution of cytochrome c is diffuse and perinuclear in location. (e) A denucleating cell in a lentoid at the stage of nuclear fragmentation (arrow) stained as in d. The cytochrome c has now disappeared leaving the perinuclear mitochondria labelled red by Mitoracker®. (f) As d and e, stained for cytochrome c (red) and cytochrome oxidase. Mitochondria are located in the perinuclear region, and in one case cytochrome c co-localizes with the cytochrome oxidase labelling (yellow; arrow).
Further fragmentation and degradation of the nuclei was accompanied by the simultaneous and gradual disappearance of MitoTracker® staining. The cytochrome oxidase immunoreactivity then persisted in the lentoids which were now devoid of nuclei, as shown above (Fig. 2a).
Examination of cytochrome c localization during this process showed that, as expected, cytochrome c co-localized with mitochondria in non-differentiating epithelial cells (Fig. 2c). During nuclear degeneration in the lentoids, the distribution of cytochrome c became diffuse (Fig. 2d).Cytochrome c very rapidly disappeared from the cytoplasm of most lentoid cells, and well before the disappearance of the mitochondria (Fig. 2e, arrow), but in cells in which it persisted, it remained co-localized with cytochrome oxidase (Fig. 2f, arrow, yellow), indicating that a small number of mitochondria (possibly those that had not undergone the permeability transition) retained cytochrome c until relatively late in the degradation process.
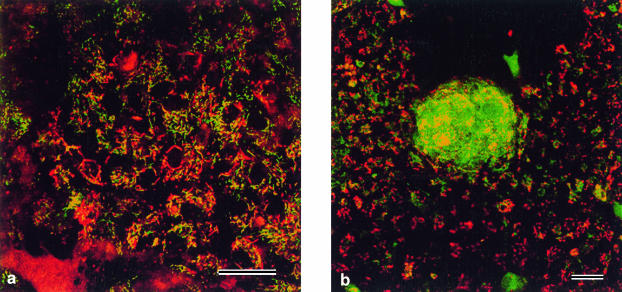
In agreement with the observation that cytochrome c translocated during lentoid differentiation, we observed that JC-1 staining, for the mitochondrial membrane permeability transition (Fig. 3), labelled the mitochondria of early lentoids green (indicating low membrane potential and high permeability) in contrast to the mitochondria of adjacent non-differentiating lens epithelium which stained red (indicating high membrane potential and low permeability). The JC-1 labelling persisted in the lentoids, similar to cytochrome oxidase, supporting the view that fragments of mitochondria linger on after nuclear breakdown.
Fig. 3.
Living cultures labelled with the dual emission dye, JC1. Mitochondria with low membrane permeability label red; mitochondria with high membrane permeability label green. Bars = 5 µm. (a) Undifferentiated lens epithelial cells showing a high proportion of red labelling in normal mitochondria. (b) A differentiating lentoid showing a very high proportion of green-labelled mitochondria indicating that lentoid differentiation is accompanied by increased mitochondrial membrane permeability. Note that the surrounding undifferentiated epithelial cells are similar to those shown in a.
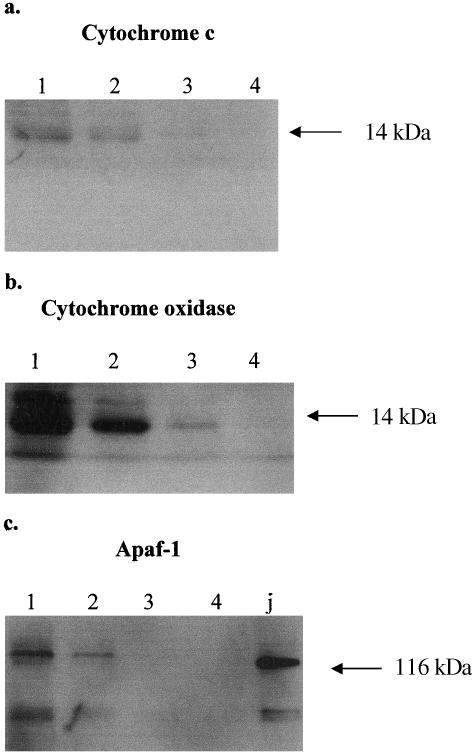
In order to correlate these in vitro results with whole lenses, we carried out Western immunoblots for cytochrome c and cytochrome oxidase on each of the four regions dissected from ED-12 lenses.
As predicted by the confocal microscopy on the cultures, Western blots of the lens regions showed that cytochrome c was detectable only in regions 1 and 2, and not in regions 3 and 4 where organelle breakdown is in progress or complete (Fig. 4a). Cytochrome oxidase was detected in regions 1, 2 and 3 but not 4 (Fig. 4b), which is in agreement with the cultured lentoids in which cytochrome oxidase persisted, while cytochrome c did not (Fig. 2a,d–f).
Fig. 4.
Western blots showing immunoreactivity in homogenates of ED-12 lenses in regions 1, 2, 3 and 4. (a)Cytochrome c is detectable in regions 1 and 2, but not in 3 or 4. (b) Cytochrome oxidase is abundant in regions 1 and 2, still detectable in region 3, but undetectable in region 4. (c) Apaf-1 is detectable in regions 1 and 2, but not in 3 or 4. ‘J’ is a positive control lane using Jurkat cell homogenate.
Caspase-9 localization and translocation
Subcellular localization of caspase-9 was carried out using two different polyclonal antibodies to the entire molecule, as well as with a polyclonal antibody specifically to the 10-kDa fragment of the activated caspase-9. Results were similar in each case, indicating that the activated caspase is being detected, and are illustrated with examples using each reagent.
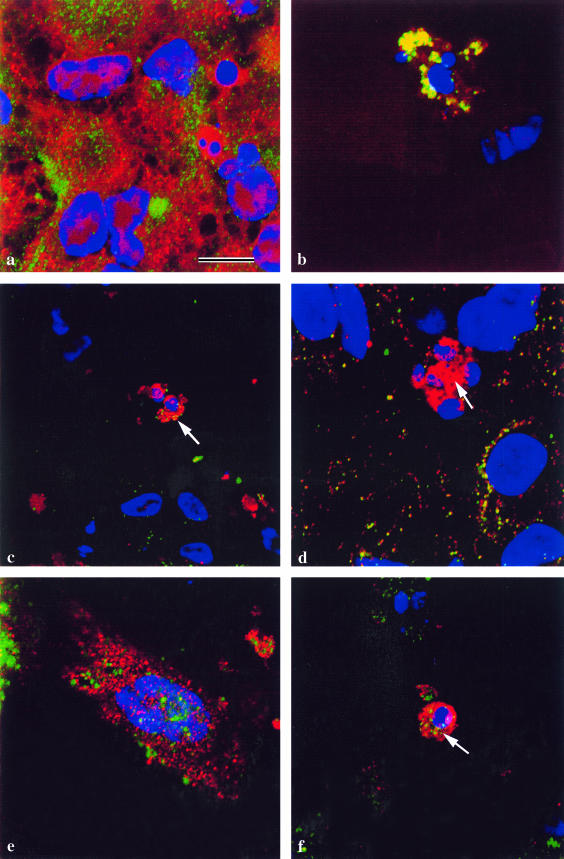
In non-apoptotic epithelial cells in the lens cultures, caspase-9 is not located in the mitochondria as judged by MitoTracker® labelling (Fig. 5a) and double labelling with cytochrome oxidase (not shown). It follows therefore that there is no association between caspase-9 and cytochrome c in non-apoptotic epithelial cells (Fig. 5b).
Fig. 5.
Lens epithelial cells cultured for 5 days to allow the differentiation of lentoids, and immunostained using the caspase-9 antibody from Stressgen Inc. Nuclei are labelled with DAPI (blue). Bar = 5 µm. (a) Undifferentiated cells from a lens cell culture stained with MitoTracker® (red) and anticaspase-9 (green). Caspase-9 is not localized to the mitochondria. (b) As a, but stained for cytochrome c (green) and caspase-9 (red). Cytochrome c and caspase-9 do not co-localize in non-differentiating cells. (c) A denucleating cell in a lentoid stained with MitoTracker® (red) and with an antibody to the 10-kDa fragment of caspase-9 (green). Activated caspase-9 is not present in normal mitochondria, which are in a perinuclear location. (d) As in c, stained for cytochrome oxidase (green) and caspase-9 (red), showing co-localization (yellow) in a perinuclear location. (e) As in c, stained for cytochrome c (green) and caspase-9 (red). The cytochrome c labelling is perinuclear but diffuse. (f) As in c and e, the denucleating cell (arrow) is stained for cytochrome c (green) and caspase-9 (red). Cytochrome c has disappeared, but caspase-9 persists.
In cells in lentoids that are undergoing nuclear degeneration, the exclusion of caspase-9 from metabolically normal mitochondria is maintained (Fig. 5c). However, double labelling with caspase-9 and cytochrome oxidase (Fig. 5d) indicates co-localization of these molecules, suggesting that in apoptotic cells caspase-9 enters mitochondria that no longer label with MitoTracker®. Double labelling for caspase-9 and cytochrome c showed that as long as the latter persisted, it co-localized with caspase-9 (Fig. 5e), but that caspase-9 persisted longer than cytochrome c and became amassed in the perinuclear region (Fig. 5f), apparently now in altered mitochondria.
Apaf-1 expression
The distribution of Apaf-1 in the lens was examined by immunoblotting the four dissected zones of the ED-12 lens (Fig. 4c). Using this method, Apaf-1, at 116 kDa, was found to be expressed in the lens epithelium and in the transitional zone (lanes 1 and 2), but not in the immature or mature lens fibres (lanes 3 and 4).
In non-differentiating epithelial cells and in denucleating cells, Apaf-1 was localized to the cytoplasm, outside normal mitochondria (Fig. 6a). As with caspase-9, evidence was found to suggest that in denucleating cells, Apaf-1 was able to migrate into mitochondria after the latter had undergone the permeability transition, since co-localization between Apaf-1 and cytochrome oxidase was observed (Fig. 6b). As expected therefore Apaf-1 took up a peri-nuclear location with cytochrome oxidase.
Fig. 6.
Lens epithelial cells cultured for 5 days to allow the differentiation of lentoids, and immunostained. Nuclei are labelled with DAPI (blue). Bar = 5 µm. (a) Undifferentiated lens epithelial cells stained with MitoTracker® (red) and anti-Apaf-1 (green). Apaf-1 is not localized in the mitochondria of non-differentiating cells. (b) A denucleating cell in a lentoid stained for cytochrome oxidase (green) and Apaf-1 (red). Apaf-1 co-localizes with cytochrome oxidase (yellow) in a perinuclear location. (c) As b, stained for cytochrome c (green) and Apaf-1 (red). Apaf-1 co-localizes with cytochrome c (yellow) in the cytoplasm (arrow). (d) As b and c, stained for cytochrome c (green) and Apaf-1 (red). Apaf-1 persists in the perinuclear location after cytochrome c disappears (arrow). (e) An undifferentiated cell from a lens epithelial culture stained for caspase-9 (green) and Apaf-1 (red). Apaf-1 is not associated with caspase-9 in the cytosol of non-differentiating cells. (f) A denucleating cell in a lentoid stained for caspase-9 (green) and Apaf-1 (red). Apaf-1 co-localizes with caspase-9 (yellow) in a perinuclear location (arrow).
In denucleating cells in differentiating lentoids, Apaf-1 co-localized with cytochrome c in the cytoplasm (Fig. 6c), and, like caspase-9, persisted after the disappearance of cytochrome c (Fig. 6d). Caspase-9, which did not co-localize with Apaf-1 in non-differentiating epithelial cells (Fig. 6e), did associate with it in the peri-nuclear region of denucleating cells (Fig. 6f).
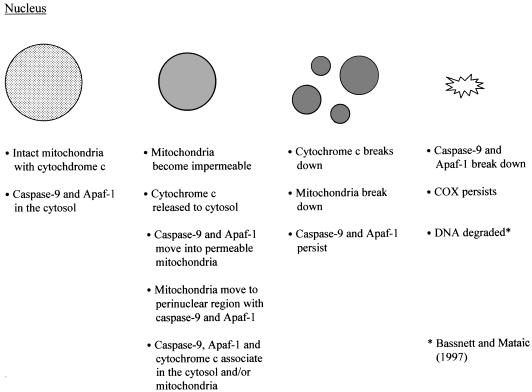
These results, summarized in Fig. 7, suggest therefore that cytochrome c, caspase-9 and Apaf-1 associate transiently in the cytosol in cells undergoing denucleation.
Fig. 7.
The time course of nuclear degeneration in relation to mitochondrial breakdown and molecular translocation in the cytoplasm of cultured lens fibre cells.
Discussion
Caspase-9 expression and translocation
Like apoptosis itself (Hughes et al. 1998), lens fibre cell denucleation appears to be the result of a combination of caspase-dependent and caspase-independent events (Wride et al. 1999). We have previously shown that several caspases are likely involved in the denucleation process, including caspase-9 (Wride et al. 1999), which is a pivotally important caspase in apoptotic pathways (Li et al. 1997; Zhivotovsky et al. 1999).
We show here that caspase-9 activity is maximal by approximately ED-12, at a time when denucleation is beginning, and in the annular pad region of the lens, where the transition into lens fibres is occurring. By immunoblotting, activated caspase-9 was indicated by the detection of a strong cleavage fragment at 10 kDa, while in immunocytochemistry, antibodies to both the 10-kDa fragment and uncleaved caspase-9 fragments gave similar results, showing that we are detecting activated caspase-9 by these methods.
In cultured lens epithelial cells that are not differentiating into lentoids, caspase-9 is clearly present in the cytoplasm, but not in mitochondria. Mitochondria were detected by three different methods: MitoTracker Red®, which labels metabolically active mitochondria; localization of cytochrome c, which is present in metabolically active mitochondria, but which leaves mitochondria in apoptotic cells; and localization of cytochrome c oxidase, which does not leave mitochondria after the apoptotic mitochondrial membrane permeability transition.
In lentoid cells that are undergoing nuclear degeneration, the co-localization of caspase-9 with cytochrome oxidase, but not MitoTracker®, indicates that caspase-9 migrates into the permeabilized mitochondria and, with these mitochondria, caspase-9 aggregates around the degenerating nuclei. At the same time, caspase-9 appears to associate with cytochrome c in the cytoplasm and it maintains this association for as long as the cytochrome c persists. The caspase-9 persists longer than the cytochrome c, but not as long as cytochrome oxidase, which persists in the lentoids, either having been released from degenerating mitochondria, or in mitochondrial fragments.
Caspase-9 has previously been shown to be present in both the mitochondria and the cytosol, and cleaved in both locations after induction of apoptosis (Zhivotovsky et al. 1999). This might be of significance to the denucleation process, because when the mitochondria break down in the lens fibre cells they may take activated caspase-9 with them. This is a situation that would not arise in conventional apoptotic cells, since the mitochondria do not disappear with the same time course, but persist much longer. It is possible to speculate therefore that the removal of mitochondrial activated caspase-9 with the mitochondria might be related to the failure of the plasma membrane events to occur in these cells. On the other hand, it has been demonstrated that very many other proteins are released into the cytosol from mitochondria during apoptosis (Patterson et al. 2000), and the loss of any of these coincident with the early loss of mitochondria in lens fibre cells could disrupt the signalling to the plasma membrane.
We have not observed translocation of molecules into the nucleus during the process of denucleation, likely because of the early nuclear degeneration in these cells. This is in contrast to some other situations that have been described, which include the movement of pro-caspase-9 from mitochondria into the cytosol and subsequently into the nucleus (Krajewski et al. 1999; Ritter et al. 2000), where it is activated. Apaf-1 and apoptosis inducing factor (AIF) have also recently been reported to migrate to the nucleus in apoptotic cells (Chen et al. 2000; Daugas et al. 2000). In the present case, caspase-9 is present neither in the nucleus nor in normal mitochondria. Instead, it appears to migrate to the mitochondria from the cytosol after the permeability change. It is possible therefore that the absence of translocation of caspase-9 to the nucleus, combined with the early permeability changes in the mitochondria, is responsible for the failure of the apoptotic plasma membrane effects to follow nuclear degeneration in these cells, by failure to activate nuclear-cytoplasmic signalling pathways.
Apaf-1 expression and the apoptosome
We find that Apaf-1, like caspase-9, redistributes from the cytosol into permeabilized mitochondria in denucleating cells, and, together with caspase-9, migrates to the peri-nuclear region with these mitochondria. Translocation of ced4, a homologue of Apaf-1, to the nuclear membrane has been shown previously (Chen et al. 2000), but it is unclear how that observation on true apoptosis is related to the current results. Our results also suggest that cytochrome c, caspase-9 and Apaf-1 associate transiently in the cytosol of cells undergoing denucleation, at least until cytochrome c breaks down. It must be assumed that this association is the equivalent of the ‘apoptosome’, described by others (Li et al. 1997; Slee et al. 1999; Zou et al. 1999), and, at least for the present, it must be assumed that this is the mechanism by which caspase-9 is activated, in parallel with the situation in other cell types. No obvious lens defects have been reported in Apaf-1 knock-out mice (Honarpour et al. 1999).
The fact that we observe the migration of caspase-9 and Apaf-1 into the mitochondria of denucleating cells opens the possibility that in these cells an ‘apoptosome’ may form in the mitochondria with residual cytochrome c. The resulting activation of caspase-9 within the mitochondria might then contribute to the demise of the mitochondria, and the corresponding lack of caspase-9 activation in the cytosol might explain the lack of caspase-3 activation (see below) and the consequent lack of membrane events.
Mitochondrial degradation and cytochrome c translocation
In agreement with a report on apoptosis (Li et al. 1998), we found clear evidence that in differentiating lentoids, mitochondria aggregate around the shrunken and fragmenting nuclei before nuclear degeneration. This observation, made here on cultures using MitoTracker®, also agrees with that already reported for intact lenses by Bassnett & Beebe (1992) and Bassnett (1992), who used Rhodamine 123 as a mitochondrial probe. The aggregation occurred at about the time of the membrane permeability transition, but included both mitochondria that label with MitoTracker® and also those that did not.
Examination of the lentoid cells with the dual-emission membrane potential-sensitive dye, JC-1, confirmed that all mitochondria eventually undergo the membrane permeability transition, but that this appears to be a gradual process that starts early in lentoid formation. Similar conclusions were reached by Bassnett & Beebe (1992) and Bassnett (1992) on intact lenses, using Rhodamine 123. Because it is confined to the intermembrane space, cytochrome c release from mitochondria depends on the permeabilization of the mitochondrial outer membrane (OM), whereas the JC-1 fluorescence-change monitors the integrity of the inner membrane (IM). However, release of cytochrome c through OM permeabilization seems to be associated with changes in IM permeability, although the latter change may be transient (Kroemer & Reed, 2000). In some lentoid cells in the current study, cytochrome c remains co-localized with cytochrome oxidase until late in the process, providing further evidence for a heterogeneity of the mitochondrial response to apoptotic stimuli (D'Herde et al. 2000).
Using Rhodamine 123 as a mitochondrial probe in intact lenses, Bassnett & Beebe (1992) showed that the mitochondria and nuclei disappear abruptly and more or less simultaneously from differentiating lens fibre cells, but that DNA degradation occurs after the nuclear breakdown (Bassnett & Mataic, 1997). Using a mitochondrial marker protein, Dahm et al. (1998) suggested that although mitochondrial and nuclear breakdown are simultaneous, the mitochondria actually disappear first. In the present work, we detected cytochrome oxidase and JC-1 labelling in lentoids after complete nuclear breakdown, suggesting that mitochondrial remnants persist longer than was previously thought.
However, although this timing is important, it is likely that the release of cytochrome c from the mitochondria that is crucial to the nuclear degeneration process, not the ultimate breakdown of the mitochondria. In agreement with our results using the permeability-sensitive dye JC-1, we show that generally cytochrome c is released from the mitochondria very early in, or before, the process of nuclear breakdown, and before the mitochondria aggregate around the shrunken nucleus. Thislends support to the view that it is the release of cytochrome c from the mitochondria that initiates denucleation. After its release, in most cells, the cytochrome c became undetectable very quickly and well before the breakdown of the mitochondria. Western blotting for cytochrome c in lens pieces confirms that indeed cytochrome c becomes undetectable in the zones of organelle breakdown, and before cytochrome oxidase disappears.
So the relative timing in these cells appears to be: permeabilization of mitochondrial membrane; cytochrome c release from mitochondria; migration of mitochondria to the peri-nuclear region (all coincident with nuclear shrinkage); cytochrome c breakdown; mitochondrial breakdown; nuclear breakdown; and finally cytochrome oxidase breakdown. This is shown in relation to nuclear degeneration and caspase-9 translocation in Fig. 7.
Lens fibre cell denucleation vs. apoptosis
Comparisons between denucleation and apoptosis have been discussed previously (Chaudun et al. 1994; Wride, 1996; Bassnett & Mataic, 1997; Dahm, 1999). We feel, in agreement with Dahm, (1999), that the evidence favours the view that lens fibre cell denucleation is an apoptotic-like event in which the plasma membrane phenomena associated with apoptosis are absent. From the current results it appears that although caspase-9 forms a cytoplasmic complex (either mitochondrial or cytosolic) with cytochrome c and Apaf-1, the activated caspase-9 does not trigger the signalling pathways that lead to the plasma membrane effects in apoptotic cells. We hypothesize that this could be associated either with the relatively early permeability changes in the mitochondria in these cells and the consequent loss of activated caspase-9 or other mitochondrial proteins, or with the failure of signalling molecules to migrate to the nuclei in these cells, as they appear to do in conventional apoptotic cells.
We are concerned, also, about the role of caspase-3 in the denucleation process. Although caspase-3 is pivotal in the activation and cleavage of downstream substrates during apoptosis (Li et al. 1997; Slee et al. 1999), and it has been claimed to be active in lens fibre cell denucleation (Ishizaki et al. 1998), we have been unable to confirm this activity in lens cells (Wride et al. 1999; unpublished results). If caspase-3 activation is different in these cells from conventional apoptotic cells, then this may be the root of an alternative explanation for the absence of plasma membrane effects in lens cells.
We are concerned, also, about the role of caspase-3 in the denucleation process. Although caspase-3 is pivotal in the activation and cleavage of downstream substrates during apoptosis (Li et al. 1997; Slee et al. 1999), and it has been claimed to be active in lens fibre cell denucleation (Ishizaki et al. 1998), we have been unable to confirm this activity in lens cells (Wride et al. 1999; unpublished results). If caspase-3 activation is different in these cells from conventional apoptotic cells, then this may be the root of an alternative explanation for the absence of plasma membrane effects in lens cells.
Acknowledgments
We thank Dr Mike Wride and Dr Bill Keyes for reading the manuscript and Dr Chris Bleackley for valuable discussion. We thank the Canadian Institutes of Health Research for an operating grant in support of this work.
References
- Bassnett S. Mitochondrial dynamics in differentiating fiber cells of the mammalian lens. Curr. Eye Res. 1992;11:1227–1232. doi: 10.3109/02713689208999548. [DOI] [PubMed] [Google Scholar]
- Bassnett S, Beebe DC. Coincident loss of mitochondria and nuclei during lens fiber cell differentiation. Dev. Dynam. 1992;194:85–93. doi: 10.1002/aja.1001940202. [DOI] [PubMed] [Google Scholar]
- Bassnett S. Fiber cell denucleation in the primate lens. Invest. Ophthal. Vis. Sci. 1997;38:1678–1687. [PubMed] [Google Scholar]
- Bassnett S, Mataic D. Chromatin degradation in differentiating fiber cells of the eye lens. J. Cell Biol. 1997;137:37–49. doi: 10.1083/jcb.137.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassnett S. Lens organelle degradation. Exp. Eye Res. 2002;74:1–6. doi: 10.1006/exer.2001.1111. [DOI] [PubMed] [Google Scholar]
- Bernardi P, Scorrano L, Colonna R, Petronilli V, Delisa F. Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur. J. Biochem. 1999;264:687–701. doi: 10.1046/j.1432-1327.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- Cain K, Bratton SB, Langlais C, Walker G, Brown DG, Sun X-M, et al. Apaf-1 oligomerizes into biologically active ∼700-kDa and inactive ∼1.4-MDa apoptososome complexes. J. Biol. Chem. 2000;275:6067–6070. doi: 10.1074/jbc.275.9.6067. [DOI] [PubMed] [Google Scholar]
- Chaudun E, Arruti C, Courtois Y, Ferrag F, Jeanny JC, Patel BN, et al. DNA strand breakage during physiological apoptosis of the embryonic chick lens: free 3′OH end single strand breaks do not accumulate even in the presence of a cation-independent deoxyribonuclease. J. Cell. Physiol. 1994;158:354–364. doi: 10.1002/jcp.1041580218. [DOI] [PubMed] [Google Scholar]
- Chen F, Hersh BM, Conradt B, Zhou Z, Riemer D, Gruenbaum Y, et al. Translocation of C. elegans CED-4 to nuclear membranes during programmed cell death. Science. 2000;287:1485–1489. doi: 10.1126/science.287.5457.1485. [DOI] [PubMed] [Google Scholar]
- Counis MF, Chaudun EA, Rruti C, Oliver L, Sanwal M, Courtois Y, et al. Analysis of nuclear degradation during lens cell differentiation. Cell Death Differ. 1998;5:251–261. doi: 10.1038/sj.cdd.4400351. [DOI] [PubMed] [Google Scholar]
- D'Herde K, De Prest B, Mussche S, Schotte S, Beyaert R, Van Coster R, et al. Ultrastructural localization of cytochrome c in apoptosis demonstrates mitochondrial heterogeneity. Cell Death Diff. 2000;7:331–337. doi: 10.1038/sj.cdd.4400655. [DOI] [PubMed] [Google Scholar]
- Dahm R, Gribbon C, Quinlan RA, Prescott AR. Changes in the nucleolar and coiled body compartments precede lamina and chromatin reorganization during fibre cell denucleation in the bovine lens. Eur. J. Cell Biol. 1998;75:237–276. doi: 10.1016/S0171-9335(98)80118-0. [DOI] [PubMed] [Google Scholar]
- Dahm R. Lens fibre cell differentiation – a link with apoptosis? Ophthal. Res. 1999;31:163–183. doi: 10.1159/000055530. [DOI] [PubMed] [Google Scholar]
- Daugas E, Susin SA, Zamzami N, Ferri KJ, Iniropoulou T, Larochette N, et al. Miotchondrio-nuclear translocation of AIF in apoptosis and necrosis. FASEB J. 2000;14:729–739. [PubMed] [Google Scholar]
- Dinsdale D, Zhuang J, Cohen GM. Redistribution of cytochrome c precedes the caspase-dependent formation of ultracondensed mitochondria, with a reduced inner membrane potential, in apoptotic monocytes. Am. J. Pathol. 1999;155:607–618. doi: 10.1016/S0002-9440(10)65156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskes R, Antonsson B, Osen-Sand A, Montessuit S, Richter C, Sadoul R, et al. Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ J. Cell Biol. 1998;143:217–224. doi: 10.1083/jcb.143.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadeel B, Zhivotovsky B, Orrenius S. All along the watchtower: on the regulation of apoptosis regulators. FASEB J. 1999;13:1647–1657. doi: 10.1096/fasebj.13.13.1647. [DOI] [PubMed] [Google Scholar]
- Fromm L, Overbeek PA. Inhibition of cell death by lens-specific overexpression of bcl-2 in transgenic mice. Dev. Genet. 1997;20:276–287. doi: 10.1002/(SICI)1520-6408(1997)20:3<276::AID-DVG10>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Goldstein JC, Waterhouse NJ, Juin P, Evan GI, Green DR. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nature Cell Biol. 2000;2:156–162. doi: 10.1038/35004029. [DOI] [PubMed] [Google Scholar]
- Granville DJ, Shaw JR, Leong S, Carthy CM, Margaron P, Hunt DW, et al. Release of cytochrome c, Bax migration, Bid cleavage, and activation of caspases 2, 3, 6, 7, 8, and 9 during endothelial cell apoptosis. Am. J. Pathol. 1999;155:1021–1025. doi: 10.1016/S0002-9440(10)65202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Doran E, Gillespie JP, O'Toole A. Mitochondria and cell death. Biochem. Soc. Trans. 2000;28:170–177. doi: 10.1042/bst0280170. [DOI] [PubMed] [Google Scholar]
- Honarpour N, Du C, Richardson JA, Hammer RE, Wang X, Herz J. Adult Apaf-1-deficient mice exhibit male infertility. Dev. Biol. 1999;218:248–258. doi: 10.1006/dbio.1999.9585. [DOI] [PubMed] [Google Scholar]
- Hughes RM, Evans-Storms RB, Cidlowski JA. Evidence that non-caspase proteases are required for chromatin degradation during apoptosis. Cell Death Diff. 1998;5:1017–1027. doi: 10.1038/sj.cdd.4400418. [DOI] [PubMed] [Google Scholar]
- Ishizaki Y, Jacobson MD, Raff MC. Role for caspases in lens fiber differentiation. J. Cell Biol. 1998;140:153–158. doi: 10.1083/jcb.140.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson CM, Korsmeyer SJ. Bcl-2 and Bax function independently to regulate cell death. Nature Genet. 1997;16:358–363. doi: 10.1038/ng0897-358. [DOI] [PubMed] [Google Scholar]
- Krajewski S, Krajewska M, Ellerby LM, Welsh K, Xie Z, Deveraux QL, et al. Release of caspase-9 from mitochondria during neuronal apoptosis and cerebral ischemia. Proc. Natl. Acad. Sci. USA. 1999;96:5752–5757. doi: 10.1073/pnas.96.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nature Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Lang RA. Apoptosis in mammalian eye development: lens morphogenesis, vascular regression and immune privilege. Cell Death Differ. 1997;4:12–20. doi: 10.1038/sj.cdd.4400211. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Elnemri ES, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Li HL, Zhu H, Xu C-J, Yuan J. Cleavage of BID by caspase-8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- Martinou I, Desagher S, Eekes R, Antonsson B, André E, Fakan S, et al. The release of cytochrome c from mitochondria during apoptosis of NGF-deprived sympathetic neurons is a reversible event. J. Cell Biol. 1999;144:883–889. doi: 10.1083/jcb.144.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich MF, Robinson BH, Taanman J-W, Kim SJ, Schillace R, Smith JL, et al. Expression of mtDNA and nDNA encoded respiratory chain proteins in chemically and genetically-derived RhoO human fibroblasts: a comparison of subunit proteins in normal fibroblasts treated with ethidium bromite and fibroblasts from a patient with mtDNA depletion syndrome. Biochim. Biophys. Acta. 1997;1362:145–159. doi: 10.1016/s0925-4439(97)00061-6. [DOI] [PubMed] [Google Scholar]
- Mathur AH, Ong y Kemp BK, Barrientos AA, Erusalimsky JD. Evaluation of fluorescent dyes for the detection of mitochondrial membrane potential changes in cultured cardiomyocytes. Cardiovasc. Res. 2000;46:126–138. doi: 10.1016/s0008-6363(00)00002-x. [DOI] [PubMed] [Google Scholar]
- Menko AS, Klukas KA, Johnson RS. Chicken embryo lens cultures mimic differentiation in the lens. Dev. Biol. 1984;103:129–141. doi: 10.1016/0012-1606(84)90014-9. [DOI] [PubMed] [Google Scholar]
- Patterson SD, Spahr CS, Daugas E, Susin SA, Irinopoulou T, Koehler C, et al. Mass spectrometric identification of proteins released from mitochondria undergoing permeability transition. Cell Death Differ. 2000;7:137–144. doi: 10.1038/sj.cdd.4400640. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J. Lens differentiation in vertebrates: a review of cellular and molecular features. Differentiation. 1981;19:134–153. doi: 10.1111/j.1432-0436.1981.tb01141.x. [DOI] [PubMed] [Google Scholar]
- Porter AG. Protein translocation in apoptosis. Trends Cell Biol. 1999;9:394–401. doi: 10.1016/s0962-8924(99)01624-4. [DOI] [PubMed] [Google Scholar]
- Putcha GV, Deshmukh M, Johnson EM., Jr BAX translocation is a critical event in neuronal apoptosis: regulation by neuroproteins, BCL-2, and caspases. J. Neurosci. 1999;19:7476–7485. doi: 10.1523/JNEUROSCI.19-17-07476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter PM, Marti A, Blanc C, Baltzer A, Krajewski S, Reed JC, et al. Nuclear localization of procaspase-9 and processing by a caspase-3-like activity in mammary epithelial cells. Eur. J. Cell Biol. 2000;79:358–364. doi: 10.1078/S0171-9335(04)70040-0. [DOI] [PubMed] [Google Scholar]
- Sanwal M, Muel AS, Chaudun E, Courtois Y, Counis MF. Chromatin condensation and terminal differentiation process in embryonic chiken lens in vivo and in vitro. Exp. Cell Res. 1986;167:429–439. doi: 10.1016/0014-4827(86)90183-7. [DOI] [PubMed] [Google Scholar]
- Slee EA, Harte MT, Kluck RM, Wolf BB, Casiano CA, Newmeyer DD, et al. Ordering the cytochrome c-initiated capase cascade: hierarchical activation of caspases-2-3-6, 7–8 and -10 in a caspase-9-dependent manner. J. Cell Biol. 1999;144:281–292. doi: 10.1083/jcb.144.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stennicke HR, Salvesen GS. Properties of the caspases. Biochim. Biophys. Acta. 1998;1387:17–31. doi: 10.1016/s0167-4838(98)00133-2. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y. Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells. 1998;3:697–707. doi: 10.1046/j.1365-2443.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- Walker JL, Menko AS. α6 Integrin is regulated with lens cell differentiation by linkage to the cytoskeleton and isoform switching. Dev. Biol. 1999;210:497–511. doi: 10.1006/dbio.1999.9277. [DOI] [PubMed] [Google Scholar]
- Wolter KG, Hsu Y-T, Smith CL, Nechushtan A, Xi X-G, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wride MA, Sanders EJ. Expression of tumor necrosis factor-α (TNFα)-cross-reactive proteins during early chick embryo development. Dev. Dynam. 1993;198:225–239. doi: 10.1002/aja.1001980308. [DOI] [PubMed] [Google Scholar]
- Wride MA, Lapchak PH, Sanders EJ. Distribution of TNFα-like proteins correlates with some regions of programmed cell death in the chick embryo. Int. J. Dev. Biol. 1994;38:673–682. [PubMed] [Google Scholar]
- Wride MA. Cellular and molecular features of lens differentiation: a review of recent advances. Differentiation. 1996;61:77–93. doi: 10.1046/j.1432-0436.1996.6120077.x. [DOI] [PubMed] [Google Scholar]
- Wride MA, Sanders EJ. Nuclear degeneration in the developing lens and its regulation by TNFα. Exp. Eye Res. 1998;66:371–373. doi: 10.1006/exer.1997.0440. [DOI] [PubMed] [Google Scholar]
- Wride MA, Parker E, Sanders EJ. Members of the Bcl-2 and caspase families regulate nuclear degeneration during chick lens fibre differentiation. Dev. Biol. 1999;213:142–156. doi: 10.1006/dbio.1999.9375. [DOI] [PubMed] [Google Scholar]
- Wride MA. Apoptosis as seen through a lens. Apoptosis. 2000;5:203–209. doi: 10.1023/a:1009653326511. [DOI] [PubMed] [Google Scholar]
- Zhivotovsky B, Samali A, Gahm A, Orrenius S. Caspases: their intracellular localization and translocation during apoptosis. Cell Death Differ. 1999;6:644–651. doi: 10.1038/sj.cdd.4400536. [DOI] [PubMed] [Google Scholar]
- Zou H, Li Y, Liu X, Wang X. An Apaf-1-cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J. Biol. Chem. 1999;274:11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]