Abstract
This study examines interpopulation variations in the facial skeleton of 10 modern human populations and places these in an ontogenetic perspective. It aims to establish the extent to which the distinctive features of adult representatives of these populations are present in the early post natal period and to what extent population differences in ontogenetic scaling and allometric trajectories contribute to distinct facial forms. The analyses utilize configurations of facial landmarks and are carried out using geometric morphometric methods. The results of this study show that modern human populations can be distinguished based on facial shape alone, irrespective of age or sex, indicating the early presence of differences. Additionally, some populations have statistically distinct facial ontogenetic trajectories that lead to the development of further differences later in ontogeny. We conclude that population-specific facial morphologies develop principally through distinctions in facial shape probably already present at birth and further accentuated and modified to variable degrees during growth. These findings raise interesting questions regarding the plasticity of facial growth patterns in modern humans. Further, they have important implications in relation to the study of growth in the face of fossil hominins and in relation to the possibility of developing effective discriminant functions for the identification of population affinities of immature facial skeletal material. Such tools would be of value in archaeological, forensic and anthropological applications. The findings of this study underline the need to examine more deeply, and in more detail, the ontogenetic basis of other causes of craniometric variation, such as sexual dimorphism and hominin species differentiation.
Keywords: allometric vectors, craniofacial variation, facial ontogeny
Introduction
Anatomically modern humans show considerable geographical variation in the form of the facial skeleton. Although the magnitude and nature of these differences in adults has been extensively documented in metrical analyses (e.g. Howells, 1973, 1989; Froment, 1992; Hanihara, 1993a, 1993b, 1996; Relethford, 1994), little is known about the ontogenetic processes that produce these divergent forms (Lieberman, 2000). Thus, this study explores the ontogenetic basis of population-specific craniofacial variation in 10 distinct groups of modern humans. In particular we are concerned to discover the extent to which population-specific aspects of facial form are present in early infants and the degree to which they develop during later ontogeny.
Relethford (1994) has indicated that the level of intergeographical variation in human craniofacial form is low relative to intrageographical variation. Despite this, measurements of the facial skeleton are recognized as being reliable skeletal indicators of population affinities in modern humans (Giles & Elliot, 1962; Howells, 1973, 1989, 1995; Gill, 1984; Krogman & Iscan, 1986; Ubelaker, 1989). Furthermore, the majority of studies of craniometric variation have found some clear and highly replicable patterns of facial variation between modern human groups (e.g. Howells, 1973, 1989; Guglielmino-Matessi et al. 1977; Froment, 1992; Hanihara, 1996; Lynch et al. 1996). The most notable of these patterns is a strong similarity between Australian and sub-Saharan African groups. Two different explanations have been proposed for this: a correlation between Australian–African morphology and climatic temperature variables (Guglielmino-Matessi et al. 1977); and plesiomorphy (Stringer, 1992). Other trends observed include distinct European and Far Eastern clusters, a less distinct American Indian group, and diversity amongst Polynesian populations (Howells, 1973). Thus, patterns of craniofacial variation between modern human populations neither map directly onto present-day geographical distributions nor onto molecular phylogenies. This said, patterns of molecular variation concord to a much greater degree with geography (Cann et al. 1987; Nei & Roychoudhury, 1993; Batzer et al. 1994; Cavalli-Sforza et al. 1994). Therefore, although human craniofacial form is indicative of the population affinities of individual crania, it is not a reliable indicator of evolutionary history. Similar findings indicate that the same is true of several primates at the species level (Collard & Wood, 2000; Collard & O'Higgins, 2001). This is because the translation of genetic variation into phenetic variation involves multiple, interacting and complex ontogenetic mechanisms. Thus, during ontogeny the genome is translated into the phenome through the processes of development (changes in shape with age); growth (changes in size with age) and allometry (changes in shape with size), all of which are prone to genetically and epigenetically mediated environmental influences (e.g. possible homoplasies between Tierra del Fuegans and North American Eskimo; Hernandez et al. 1997) and are subject to epigenetic interactions between developing tissues. In consequence, the correspondence between genetic and phenetic variation is not direct and phenetic variation reflects genetic and epigenetic influences (e.g. the influence of climate on the cranium of kangaroos, Milne & O'Higgins, 2002).
During growth the facial skeleton changes dramatically in shape as well as in size. It comprises several interdependent bones that grow and develop under the influence of various local and systemic factors. Although different bones and/or different parts of the same bone may grow independently to some degree under the influence of localized factors (Moss, 1964, 1968; Moss & Salentijn, 1969a, 1969b), the facial skeleton remains a functional whole throughout the course of development. This integration is achieved through constant modelling and remodelling regulated at local and more global levels (Frost, 1964; Canalis, 1993). In consequence, several cross-sectional studies of facial ontogeny in humans and other primates show that overall changes in size and shape can be adequately described using linear allometric models reflecting this high degree of integration (O'Higgins & Jones, 1998; O'Higgins & Strand Viðarsdóttir, 1999; Collard & O'Higgins, 2001; O'Higgins et al. 2001; O'Higgins & Collard, 2002). The, perhaps surprising, degree of linearity indicates that shape changes per increment in size remain more or less constant in degree and character throughout ontogeny. Given that even subtle differences in facial muscle composition generate marked differences in form (Hunt, 1998) this constancy implies that the forces (e.g. the influence of soft tissues, mechanical loadings) moulding facial ontogeny also remain relatively constant in location, nature and relative degree.
It is well known (Howells, 1973, 1989; Lynch et al. 1996; O'Higgins & Strand Viðarsdóttir, 1999; Ross et al. 1999; Strand Viðarsdóttir, 1999; Strand Viðarsdóttir & O'Higgins, 2001) that adult modern human populations show significant differences in both facial size and shape. Further, the differences in adult size and shape between populations appear to be uncorrelated, and hence interpopulation differences in shape are not explained simply in terms of static allometry (Strand Viðarsdóttir, 1999). Given the importance of ontogeny for generating variation, it is of interest to consider when and how differences in craniofacial morphology develop. Thus, the present study will examine the extent to which differences in face shape between modern populations are present in infancy and consider the degree to which ontogenetic allometry (i.e. correlated ontogenetic changes in shape and size) further contributes to differences through ontogenetic scaling and differences in allometric trajectories. The results could have wider implications for the study of craniometric variation in the hominids, e.g. sexual dimorphism, and interspecific variation. Further, in considering the ontogeny of fossil hominins (e.g. Ponce de Leon & Zollikofer, 2001) in relation to that of modern humans it may be important to take into account the extent of ontogenetic variation within the latter.
Building on the findings of our previous ontogenetic studies (O'Higgins & Jones, 1998; O'Higgins & Strand Viðarsdóttir, 1999; Collard & O'Higgins, 2001; O'Higgins et al. 2001; O'Higgins & Collard, 2002), we aim to account for adult differences in facial size and shape. These can arise through three interwoven but broadly different ontogenetic mechanisms. First, infants from different populations could develop distinct facial shapes early in ontogeny. Second, further differences could arise through divergent population-specific ontogenetic trajectories or, third, by ontogenetic scaling where populations have different end (adult) points on the same ontogenetic trajectory. Thus, any displacement of ontogenetic trajectories indicates different base morphologies; divergence of trajectories between two populations indicates a difference in allometry between groups; and, where vectors show no difference in trajectory, significant differences in mean adult size imply ontogenetic scaling. The study therefore examines the ontogeny of distinct population face shapes through testing of the following hypotheses.
H1: there is no difference in the shape of the facial skeleton of different populations irrespective of maturation
This hypothesis will be tested by looking for significant population differences in face shape irrespective of age. If we find such differences this indicates early establishment of population differences.
H2: there is no difference between populations in their ontogenetic trajectories
This hypothesis will be tested by examining ontogenetic trajectories in each population and comparing these between populations. Any differences we find in either trajectory (nature of ontogenetic shape change) or, where trajectories do not differ, end point (adult size and shape) on the trajectory (extent of ontogenetic shape change) will falsify this hypothesis and indicate differences in postnatal ontogeny between populations.
Additionally, the relative contribution of these mechanisms to interpopulation adult shape differences is assessed by comparing the nature of any differences between subadults of each population to those between adults (Strand Viðarsdóttir, 1999).
Materials
The study includes 334 individuals, ranging from infancy to adulthood, from 10 geographically distinct populations: Polynesians (POL); Papua New Guineans (PNG); Australians (AUS); Egyptians (EGY); Alaskan Inupiaq Eskimo (ALA); West African Ashanti (ASH); Aleutians (ALE); Arikara Plains Indians (ARIK); African Americans (AFR) and French/British Caucasians (CAUC). The composition and the origins of the population samples are given in Table 1. Great care was taken in specimen selection to avoid sample bias but the availability of material in museum collections did not allow us to gather data from age- and sex-matched samples. Each individual is represented by 26 unilateral homologous landmarks in three dimensions, collected using a Polhemus 3-Space Isotrak II electromagnetic digitizer. Unilateral data were used in preference over bilateral data, in order reduce the number of variables by taking advantage of symmetry (Strand Viðarsdóttir & O'Higgins, 2001) and increase the size of the available sample. The landmarks are listed in Table 2. Error was assessed by repeating the measurement of 10 Caucasian crania, five times, and comparing the variation due to measurement with interspecimen variation. For justification of this approach see O'Higgins & Jones (1998). For each specimen the repeats clustered closely together, and the distance between the repeats of the same specimen were always significantly closer to one another than they were to any other specimen. Furthermore, discriminant analysis of GPA co-ordinates found that the error of measurement was in no case of such magnitude that any one individual repeat was misclassified. In order to aid visual interpretation of results, each face is approximated by a three-dimensional surface, obtained by triangulations of landmarks. The surface thus obtained only approximates the facial skeletal surface and is used solely for visualization purposes; analysis is based only on the three-dimensional coordinates of landmarks.
Table 1.
Description of the data used in the present study. Biological ages are given in years, and are estimated on the basis of the method of Ubelaker (1989). M = males; F = females; U = sex unknown. NHM = The Natural History Museum London; NMNH = The National Museum of Natural History, Smithsonian Institution, Washington DC; AMNH = American Museum of Natural History, New York; CMNH = Cleveland Museum of Natural History, Ohio; UTK = Department of Anthropology, University of Tennessee, Knoxville; RCS = Royal College of Surgeons, London; MH = Musée de l'Homme, Paris
| Polynesian | Papua New Guinean | Australian | Egyptian | Alaskan Inupiaq | Ashanti | Aleutian | African American | Arikara | Caucasian | |
|---|---|---|---|---|---|---|---|---|---|---|
| Number adults | 5 (U) | 6 (2M; 3F) | 8 (3M; 5F) | 9 (5M; 4F) | 9 (4M; 5F) | 10 (5M; 5F) | 11 (6M; 5F) | 12 (7M; 5F) | 10 (5M; 5F) | 10 (5M; 5F) |
| Number subadults | 21 | 18 | 6 | 14 | 34 | 7 | 24 | 22 | 49 | 49 |
| Total | 26 | 24 | 14 | 23 | 43 | 17 | 35 | 34 | 59 | 59 |
| Min. age (year) | ||||||||||
| subadults | 1.5 | 6 | 1 | 4 | 2 | 4 | 0.5 | 0.75 | 2.5 | 0 |
| Max. age (year) | ||||||||||
| subadultsr | 17 | 18 | 14 | 18 | 17.5 | 17.5 | 16 | 18 | 19 | |
| Collection | NHM | NHM, NMNH | NHM, NMNH AMNH, | NHM, NMNH | AMNH | NHM | NMNH | CMNH | UTK | RCS, MH |
Table 2.
Landmarks used in the present study. For further explanation of the anatomical location of individual landmarks, see O'Higgins & Strand Viðarsdóttir (1999) and Strand Viðarsdóttir (1999)
| Nr. | Landmark definition |
|---|---|
| 1 | Bregma |
| 2 | Frontomalare orbitale |
| 3 | Frontomalare temporale |
| 4 | Nasion |
| 5 | Glabella |
| 6 | Stephanion |
| 7 | Frontotemporale |
| 8 | Superior rim of the orbit |
| 9 | Supraorbital torus |
| 10 | Dacryon |
| 11 | Zygotemporale superior |
| 12 | Zygotemporale inferior |
| 13 | Maxillofrontale |
| 14 | Zygoorbitale |
| 15 | Zygomaxillare |
| 16 | Jugale |
| 17 | Orbitale |
| 18 | Alveolare |
| 19 | Nasospinale |
| 20 | Alare |
| 21 | External alveolus at second incisor |
| 22 | External alveolus at canine |
| 23 | External alveolus at most posterior tooth |
| 24 | Palatine-maxillary suture |
| 25 | Infraorbital foramen |
| 26 | Staphylion |
Each subadult individual is assigned a (biological/developmental) age estimate according to the dental standard of Schour & Massler (1941), as adapted for use on non-white populations by Ubelaker (1989). This estimate of maturation is used in this study simply for the purposes of graphing data and not for subsequent statistical analysis. For the purpose of this paper, no attempt has been made at sexing specimens; however, we are currently engaged in research into the possibility of divergent sexual ontogenetic shape trajectories between those populations for which we have good data on sex, and our findings indicate that no such divergence is discernable in any of the populations for which we have sex information. Rather, sexual dimorphism appears to arise in the main through scaling effects. Thus, in those populations where sexual dimorphism can be studied (i.e. those with an adequate number of sexed males and females) there is no statistically significant angle between the ontogenetic shape vectors of males and females, and thus the direction of the combined-sex allometric vector is a reasonable representation of ontogenetic shape changes for the population as a whole. For non-sexed populations we are aware of the limitations of this generalized trajectory, but feel it is unlikely that the findings are significantly compromised.
All adults are assigned the arbitrary age of 21. In this study, individuals are classified as adults if the third permanent molar has fully erupted and the spheno-occipital synchondrosis fused. Care was taken only to include relatively young adult specimens, as determined by degree of dental wear, stage of suture closure (Meindl & Lovejoy, 1985), as well as post-cranial parameters where available (Lovejoy et al. 1985; Brooks & Suchey, 1990).
Methods
The three-dimensional coordinates of landmarks are analysed using techniques from geometric morphometrics. Geometric morphometrics are a group of analytical methods that preserve complete information about the relative spatial configuration of landmarks throughout an analysis and utilize the properties of Kendall's shape space (see below; Slice et al. 1996). The shape spaces and associated statistics of these methods are well understood (Dryden & Mardia, 1998) and yield highly visual and readily interpretable results.
The landmarks are registered using generalized Procrustes analysis (GPA) minimizing the sum of squared distances between homologous landmarks by translating, rotating, reflecting and scaling them to best fit. This registration method does not introduce bias into the distribution of specimens whose landmarks vary independently and according to random error (Rohlf, 2000). Further, it performs best relative to many other approaches in providing a consistent estimate of the mean when variations at each landmark simulate digitizing error (Rohlf, 2002). Additionally, with regard to estimating the variance covariance matrix, if variations are small (in relation to Procrustes distance, not in relation to a vague notion of biological variation) then all registrations will yield approximately similar results (Kent, 1994). Dryden & Mardia (1998, p. 287) suggest that ‘if the data lie within full Procrustes distance of about dF = 0.2 of an average shape then methods give very similar conclusions’.
Scaling is according to centroid size; the square root of the sum of squared Euclidean distances from each landmark to the centroid, which is the mean of landmark coordinates. Centroid size is used in this study as a biologically meaningful expression of the overall scale of the landmark configuration, and thus of the face. We use it here to examine allometry and growth.
As a result, all analyses of shape are carried out on data sets from which centroid size has been partitioned. Information about the centroid size of the individual specimens prior to GPA is retained for the purpose of studying size/shape relationships; allometry.
The registering of landmark coordinates through GPA results in each specimen being represented as a single point in a non-Euclidean shape space of
dimensions, known as Kendall's shape space (Kendall, 1984), where k is equivalent to the number of landmarks, and m denotes the dimensionality of those landmarks. To aid statistical analysis the points are projected into a linear tangent space (Dryden & Mardia, 1992), and statistical analyses carried out within that space using standard multivariate methods. This approach is satisfactory when variations are small, as in these data (see O'Higgins, 2000).
To explore relative relationships between individual shapes, Principal components analysis (PCA) is used to calculate principal axes of variation in the tangent space. Visualization of shape differences along the Principal components (PCs) is obtained by warping the triangulated surface of the mean shape to represent shapes at any position within the plot, using the loadings of original landmark coordinates on these PCs (O'Higgins & Jones, 1998; O'Higgins & Strand Viðarsdóttir, 1999). Cartesian transformation grids, calculated using the method of thin plate splines (Bookstein, 1989), are used to further visualize and interpret shape differences.
Discriminant functions are generated between adults from different populations on the basis of PC scores from GPA/PCA of the adult sample alone, and tested for significance. Subsequently, discriminant analyses with cross-validations are carried out in SAS to assess the power of the discriminant function. These analyses are repeated for the total data set (adults and subadults combined).
In order to investigate ontogenetic trajectories, each population is subjected to a separate GPA and PCA and the relationships between variations in shape (principal component scores), centroid size and maturation are assessed. Centroid size provides a reasonable direct measure of the scale of the landmark configurations of each individual specimen, and thus their faces. In each case only the first principal component showed a large or significant correlation with centroid size and maturation. Thus, the significance of the angle between ontogenetic trajectories (represented by PC1 in each of the populations studied) of pairs of populations is assessed in relation to the distribution of angles between 1000 random re-samplings (Good, 1993).
Having shown that PC1 well represents ontogenetic shape changes in all populations studied, ‘mean adult’ and ‘mean infant’ facial shapes (O'Higgins, 2000) are created for each population, by warping the mean shape to the extremes of the first PC. These estimates of mean shape are subsequently submitted to PCA to allow ready examination and visualization of differences in ontogenetic trajectory between data sets.
Where pairs of populations show no significant angle between their ontogenetic shape change trajectories (as represented by PC1), the possible presence of ontogenetic scaling is assessed by the computation of the mean adult centroid size for each population and relating this to ontogenetic trajectory divergence and shape differences between adults. The significance of any difference in facial centroid size is evaluated using Student's t-test, after testing for normality and equality of variance, with appropriate adjustments made when necessary.
Results
We present the results in two subsections corresponding to the two hypotheses under test. The first section examines differences in face shape between populations throughout postnatal ontogeny while the second looks at the evidence for divergent ontogenetic trajectories and ontogenetic scaling between populations.
H1: there is no difference in the shape of the facial skeleton of different populations irrespective of maturation
First, the statistical significances of interpopulation differences in adult face shape are examined through the computation of Mahalanobis' distances computed from the scores of specimens on PCs 1–16 from the GPA/PCA of adults only. These PCs account for > 80% of the total variance amongst the specimens and were found to provide the greatest discrimination. The significance of these distances was assessed by Hotelling's T2. The results of this analysis are presented in the upper triangle of Table 3. Discriminant analysis with cross-validation was used to assess the extent to which adults can be correctly assigned to populations based on these discriminant functions. The results (Table 4) indicate that between 66.7% and 100% (mean 82.6%) of individuals can be correctly classified depending on the population from which they come. There is no significant correlation between the numbers of representatives of each population used in this study and correct assignation, nor is there a significant correlation between either the number of males or females in each population and their correct assignation.
Table 3.
Results of the discriminant analyses between populations. The upper right triangle gives the Mahalanobis' distances between the adults of each population (based on PC1-16); and the lower left those between the combined adults and age-series (based on PC1-26). The upper value in each table element represents the distance between the populations, and the lower the corresponding p value from Hotelling's T2
| Polynesian | Papua New Guinean | Australian | Egyptian | Alaskan | Ashanti | Aleutian | African American | Arikara | Caucasian | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Polynesian | 5.571714 | 6.436148 | 6.774806 | 5.064287 | 7.784151 | 6.12601 | 5.849701 | 4.445447 | 5.54626 | ||
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||
| Papua New Guinean | 3.239136 | 4.388736 | 4.254409 | 4.89183 | 4.459372 | 5.30773 | 5.249 | 4.346148 | 5.104998 | ||
| p < 0.0001 | p = 0.0003 | p = 0.0002 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||
| Australian | 4.588573 | 2.648396 | 6.745221 | 5.975617 | 4.681346 | 7.136456 | 5.67609 | 5.855169 | 6.873936 | ||
| p < 0.0001 | p = 0.0009 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||
| Egyptian | 3.042532 | 3.826879 | 5.186617 | 5.764026 | 5.514164 | 4.420407 | 5.968417 | 4.590316 | 5.286114 | ||
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||
| Alaskan | 3.682934 | 4.771792 | 5.953654 | 3.798552 | 3.798552 | 5.901356 | 3.875822 | 6.450349 | 3.628085 | 5.890076 | |
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||
| Ashanti | 3.65527 | 2.754451 | 3.534402 | 4.549835 | 5.10196 | 6.109583 | 4.923312 | 5.810938 | 6.374088 | ||
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||
| Aleutian | 4.38372 | 5.525939 | 6.857988 | 3.54415 | 3.664833 | 5.581666 | 6.283868 | 3.946644 | 6.189507 | ||
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||
| African American | 3.872596 | 3.976431 | 4.750263 | 4.556205 | 4.682307 | 3.561601 | 5.370568 | 5.05193 | 4.524268 | ||
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||
| Arikara | 3.319789 | 4.298372 | 5.823725 | 3.207647 | 3.363183 | 4.954796 | 3.349478 | 3.612617 | 5.398796 | ||
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | |||
| Caucasian | 3.580782 | 4.24429 | 5.466352 | 2.920616 | 3.768023 | 4.498444 | 4.346378 | 4.398181 | 3.597777 | ||
| p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 | p < 0.0001 |
Table 4.
Results of the cross-validation test of the adult populations, based on PCs1-16. The table lists the percentage of individuals from the known groups (listed in the first column), assigned to each group (listed in the first row) in the cross-validation test. It should be read horizontally for correct interpretation
| POL | PNG | AUS | EGY | ALA | ASH | ALE | AFR | ARIK | CAUC | |
|---|---|---|---|---|---|---|---|---|---|---|
| POL | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| PNG | 0 | 66.67 | 16.67 | 0 | 0 | 0 | 16.67 | 0 | 0 | 0 |
| AUS | 0 | 12.5 | 87.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| EGY | 0 | 0 | 0 | 90 | 0 | 10 | 0 | 0 | 0 | 0 |
| ALA | 0 | 0 | 0 | 11.11 | 88.89 | 0 | 0 | 0 | 0 | 0 |
| ASH | 0 | 0 | 0 | 0 | 0 | 90 | 0 | 10 | 0 | 0 |
| ALE | 0 | 0 | 0 | 10 | 20 | 0 | 70 | 0 | 0 | 0 |
| AFR | 0 | 0 | 0 | 0 | 0 | 16.67 | 0 | 75 | 0 | 8.33 |
| ARIK | 0 | 0 | 0 | 0 | 0 | 0 | 10 | 0 | 90 | 0 |
| CAUC | 0 | 0 | 0 | 11.11 | 0 | 0 | 0 | 11.11 | 0 | 77.78 |
The lower left triangle of Table 3 lists the Mahalanobis' interpopulation distances and their significance (highly significant in all cases) computed between the combined adults and subadults based on PCs 1–26 from GPA/PCA (> 90% total variance between specimens; providing maximal discrimination for these combined data). All the populations are significantly separated on the basis of facial shape irrespective of age. This is confirmed by discriminant analysis with cross validation (based on PCs 1–26) in which the proportion of individuals correctly assigned to each group ranges from 53 to 88% (Table 5). On average 71% are correctly assigned. The Mahalanobis' distances from the analysis of adults and subadults are moderately but significantly correlated with the corresponding Mahalanobis' distances based on the adult sample alone (r = 0.542, p = 0.0001). Unlike the adult analysis, there is a significant correlation between the number of individuals correctly assigned to each group by the cross-validated discriminant functions and the number of individuals in that particular group (r = 0.724, p = 0.018). Of the 85 individuals incorrectly assigned, 28 (33%) were adults and 57 (67%) subadults. This distribution is proportional to that expected from the composition of the dataset (adults 27% vs. subadults 63%; Table 1). The subadults incorrectly assigned spanned all biological ages from 1 to 18 years.
Table 5.
Results of the cross-validation test of the 10 populations (adults and subadults), based on PC1-26. The table lists the percentage of individuals from the known groups (listed in the first column) assigned to each group (listed in the first row) in the cross-validation test. It should be read horizontally for correct interpretation
| POL | PNG | AUS | EGY | ALA | ASH | ALE | AFR | ARIK | CAUC | |
|---|---|---|---|---|---|---|---|---|---|---|
| POL | 61.54 | 3.85 | 0 | 7.69 | 7.69 | 3.85 | 0 | 7.69 | 7.69 | 0 |
| PNG | 4.17 | 58.33 | 16.67 | 8.33 | 4.17 | 4.17 | 0 | 4.17 | 0 | 0 |
| AUS | 0 | 28.57 | 64.29 | 0 | 0 | 7.14 | 0 | 0 | 0 | 0 |
| EGY | 4.35 | 0 | 0 | 69.57 | 0 | 0 | 4.35 | 0 | 8.7 | 13.04 |
| ALA | 0 | 0 | 0 | 2.33 | 88.37 | 0 | 2.33 | 0 | 6.98 | 0 |
| ASH | 11.76 | 17.65 | 5.88 | 0 | 0 | 52.94 | 0 | 11.76 | 0 | 0 |
| ALE | 0 | 0 | 0 | 5.71 | 8.57 | 0 | 80 | 0 | 5.71 | 0 |
| AFR | 0 | 2.94 | 0 | 2.94 | 0 | 5.88 | 0 | 76.47 | 8.82 | 2.94 |
| ARIK | 5.08 | 0 | 0 | 5.08 | 5.08 | 0 | 1.69 | 1.69 | 79.66 | 1.69 |
| CAUC | 1.69 | 0 | 0 | 11.86 | 0 | 1.69 | 1.69 | 3.39 | 3.39 | 76.27 |
H2: there is no difference between populations in their ontogenetic trajectories
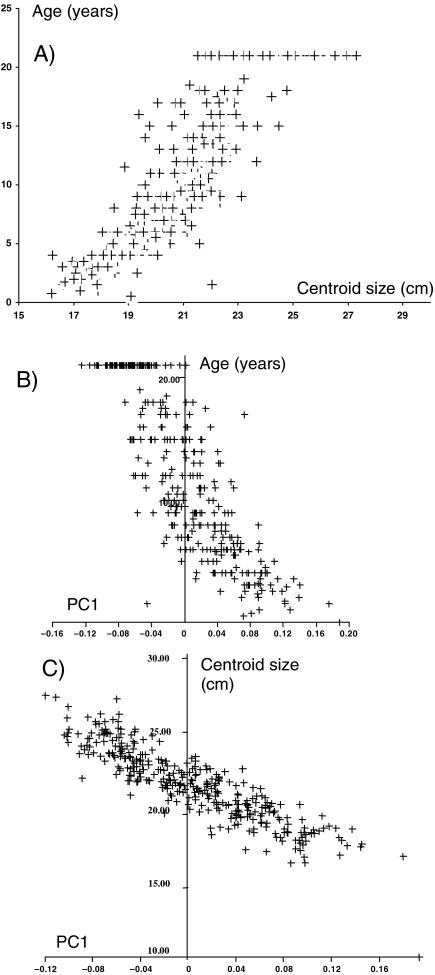
From GPA/PCA of the entire sample PC1 represents 39% of the total shape variance, and is the only PC to show a significant correlation with size or age. Variation described by this PC is therefore a reasonable representation of ontogenetic shape changes and accounts for about 40% of the total variance. Figure 1 illustrates the relationships between size, shape and biological age, these being the components of facial ontogeny. The first graph depicts growth (biological age vs. centroid size), a loose curvilinear relationship, with size remaining fairly constant after early teens; the second shows development (PC1 vs. biological age), again a loose curvilinear relationship; the third depicts allometry (PC1 vs. centroid size), a contrastingly tight and more or less linear relationship between these two variables. The tight and linear correlation between PC1 and centroid size (r = 0.87, p < 0.001), indicates that it well represents aspects of allometry common to all populations in this analysis.
Fig. 1. The full sample: (A) biological age (years) vs. centroid size (cm); (B) biological age vs. PC1; (C) centroid size vs. PC1.
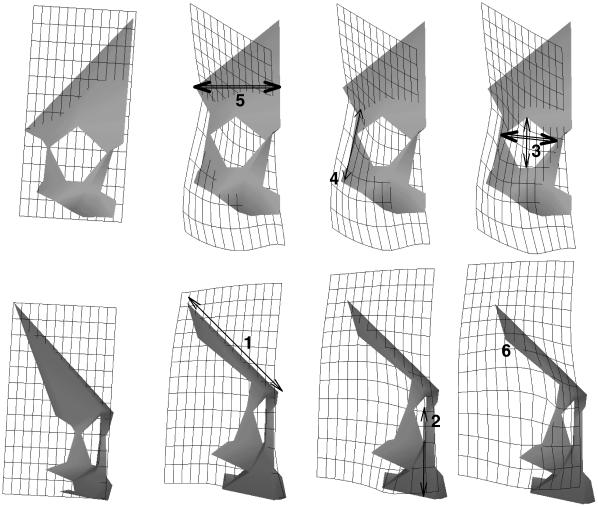
Figure 2 shows the variations in facial shape represented by PC1, from the smallest/youngest specimens (positive PC1 scores) to the largest/oldest (negative PC1 scores): there is a relative reduction in the length of the frontal (as assessed by stretchings andcontractions of the transformation grid; Fig. 2, 1); a relative increase in maxillary height (Fig. 2, 2); a decrease in relative orbit size (Fig. 2, 3); a relative expansion of the zygomatic (Fig. 2, 4); a relative reduction in frontal breadth (Fig. 2, 5) and a relatively more supero-posterior positioning of the point (stephanion; Fig. 2, 6) representing the temporal muscle attachment. These changes in facial shape are shared by all populations studied.
Fig. 2. The warped mean with overlain transformation grids showing the aspects of variation in facial shape of the full sample represented by PC1 (see Fig. 1) from the positive (reference shape; PC1 0.16) to the negative (target shape; PC1 − 0.16) extremes, frontal and lateral views. The first image in each row represents the reference shape; while the other three show the target shape with the transformation grid at three different positions on the shape. Single arrows mark expansions, double arrows contractions. For explanations of labels, see text.
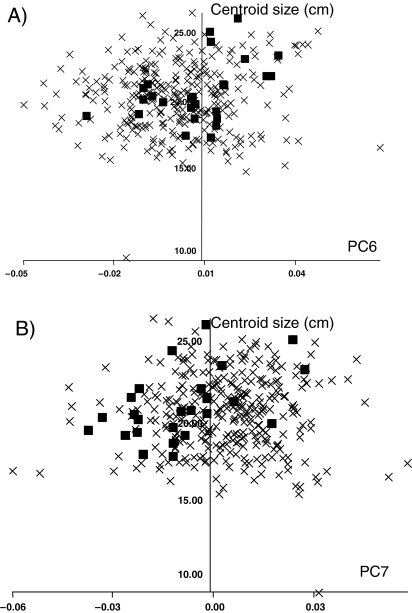
Fig. 3. The full sample: (A) PC6 vs. centroid size and (B) PC7 vs. centroid size. Black squares = Polynesians; X = all other populations.
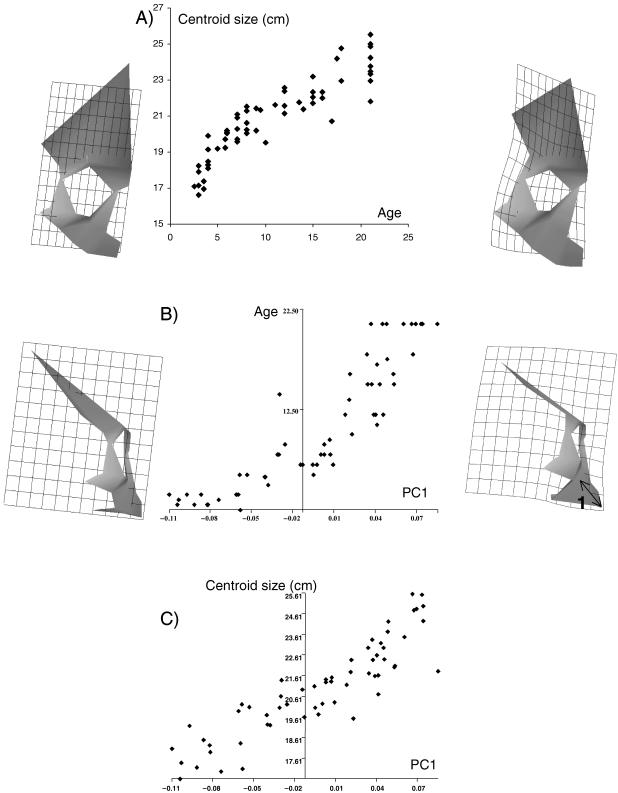
Fig. 4. Arikara Plains Indians: (A) biological age vs. centroid size; (B) biological age vs. PC1; (C) centroid size vs. PC1. Rendered insets and transformation grids show the variation in facial shape represented by PC1, from the negative (left, reference: PC1 −0.10) to the positive extremes (right, target; PC1 0.09), frontal (top) and lateral (bottom) views.
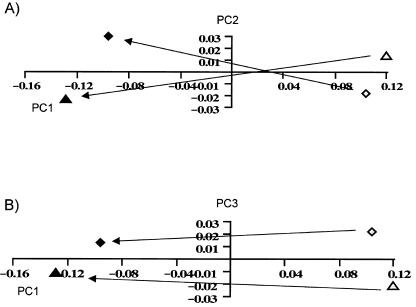
Fig. 5. Ontogenetic trajectories between the mean adult (solid black) and the mean infant (grey fill) of two populations: Arikara (diamonds) and Aleutians (triangles). (A) PC1 vs. PC2; (B) PC1 vs. PC3.
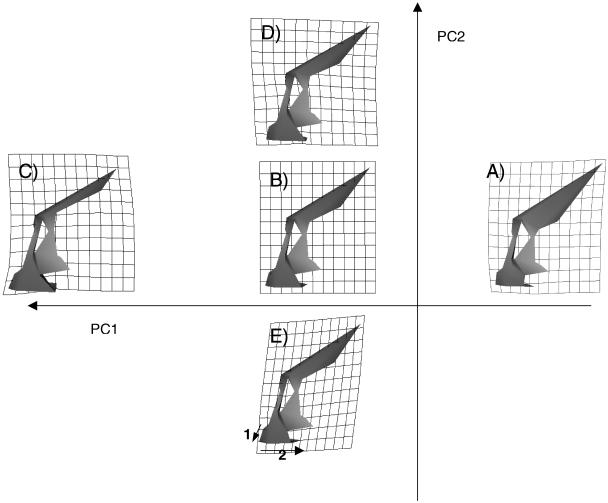
Fig. 6. Warping of the mean shape along PC1 and PC2 (from the analysis of Arikara and Aleutian ontogenetic trajectories corresponding to Fig. 5A), with superimposed Cartesian transformation grids to illustrate the midline shape variability represented by the two PCs. (A) PC1 0.12; (B) PC1 PC2 mean (reference shape); (C) PC1 − 0.12; (D) PC2 0.03; (E) PC2 − 0.03. In (D) and (E) the grid has been calculated to show deformations × 2 to aid visualization.
Despite PC1 being the only PC showing a large or significant correlation with centroid size in the sample as a whole, individual populations within the analysis show correlations between centroid size and some of the higher PCs. As an example, scores on neither PC6 nor PC7 show any evidence of a relationship with centroid size for the sample as a whole; however, both show a modest but significant correlation with centroid size in the Polynesian population (PC6 vs. Centroid size, r = 0.42, p = 0.02; PC7 vs. centroid size, r = 0.50, p < 0.01; Fig. 3). This implies that while they follow the overall human ontogenetic allometric trend they also show some distinct aspects of allometry. Similar findings are evident in several populations when compared to the whole, indicating that there may be distinct ontogenetic trajectories for diverse populations superimposed on the shared one.
In order to formally investigate this notion, separate analyses are carried out on each individual population. In each case, PC1 is the only PC found to show a large (r = 0.9, as opposed to r = 0.2 or less, for higher PCs) and significant correlation with both biological age and centroid size in all the populations analysed (Table 6). Thus the first PC provides a satisfactory representation of the majority of ontogenetic shape changes in each population. An example of these analyses is shown in Fig. 4. It shows the plots of growth, allometry and development in the Arikara, as well as the facial shapes represented at each extreme of PC1. The figure is very similar to that from the sample as a whole (Fig. 1), and PC1 represents very similar shape variability to that found in the whole sample (compare with Fig. 2). However, there is a marked relative increase in alveolar prognathism (Fig. 4, 1) with increasing age/size that was not noted in the whole sample, but is also found in the Alaskan Inupiaq, Aleutian, African American, and Caucasian populations and to a dramatic degree in Australians. It is not found in the remaining four populations. This reinforces the notion that there may be significant population-specific changes in facial shape taking place during ontogeny, indicated by different ontogenetic trajectories. This is further reinforced by the fact that between those populations that share the ontogenetic increase in alveolar prognathism, there is an indication of further subtle differences in allometry. As an example, Fig. 5 visualizes the relationship between the ontogenetic trajectories (represented by the line connecting the ‘mean infant’ and ‘mean adult’ of each population; see Methods) of two populations: the Arikara and the Aleutians. There is a clear divergence between the two trajectories, noted to a great degree on the plot of PC1 and PC2 (Fig. 5a), but also present to some degree on PC1 and PC3 (Fig. 5b). The overall angle of 22° between these trajectories is highly significant (p < 0.001, Table 7). Figure 6 visualizes the similarities and differences in ontogeny between the two populations, as described by PC1 (shared ontogenetic trajectory) and PC2 (divergence of trajectories). It reveals that although both populations share (on PC1) common ontogenetic shape changes very similar to those documented in Fig. 2 as well as the alveolar prognathism documented in Fig. 4, the ontogenetic trajectory of the Aleutians involves an even greater degree of increase in alveolar prognathism (Fig. 6,1) and expansion of the palate postero-inferiorly (Fig. 6, 2), not documented in the Aleut (on PC2). These results indicate a significant divergence in ontogenetic trajectories between these populations.
Table 6.
Correlations between PC1 and facial centroid size; PC1 and biological age; and facial centroid size and biological age, for each of the samples studied
| PC1 vs. size | PC1 vs. age | size vs. age | |
|---|---|---|---|
| Polynesian | r = −0.877 | r = −0.667 | r = 0.745 |
| p =4.19*10−9 | p = 0.0002 | p = 1.29*10−5 | |
| Papua New | r = −0.866 | r = −0.913 | r = 0.750 |
| Guinean | p = 4.7*10−8 | p = 4.86*10−10 | p = 2.42*105 |
| Australian | r = 0.949 | r = 0.941 | r = 0.929 |
| p = 2.37*10−7 | p = 5.48*10−7 | p = 1.52*10−6 | |
| Egyptian | r = 0.940 | r = 0.948 | r = 0.934 |
| p = 2.65*10−11 | p = 6.82*10−12 | p = 7.71*10−11 | |
| Alaskan | r = −0.831 | r = −0.825 | r = 0.888 |
| p = 5.49*10−12 | p = 1.07*10−11 | p = 2.06*10−15 | |
| Ashanti | r = −0.871 | r = −0.820 | r = 0.879 |
| p = 1.13*10−5 | p = 9.88*10−5 | p = 7.53*10−6 | |
| Aleutian | r = −0.955 | r = −0.952 | r = 0.950 |
| p = 6.83*10−19 | p = 1.77*10−18 | p = 3.13*10−18 | |
| African | r = −0.900 | r = −0.896 | r = 0.896 |
| American | p =4.18*10−13 | p = 5.27*10−11 | p = 7.57*10−13 |
| Arikara | r = 0.900 | r = 0.890 | r = 0.90 |
| p = 1.39*10−21 | p = 8.93*10−21 | p = 2.01*10−22 | |
| Caucasian | r = −0.930 | r = −0.901 | r = 0.842 |
| p = 5.11*10−11 | p = 1.25*10−18 | p = 3.56*10−14 |
Table 7.
Pair-wise comparisons of the angle between PC1s. The upper value denotes the angle, in degrees, between the PC1s of the populations being compared, and the lower the corresponding p value assessed by a permutation test. The comparisons in which the angles proved statistically significant are given in bold. The asterisks indicate which of the population pairs shows a significant (p < 0.05) difference in adult size, see text for explanation
| Polynesian | Papua New Guinean | Australian | Egyptian | Alaskan | Ashanti | Aleutian | African American | Arikara | Caucasian | |
|---|---|---|---|---|---|---|---|---|---|---|
| Polynesian | ||||||||||
| Papua New | *39 | |||||||||
| Guinean | p = 0.0649 | |||||||||
| Australian | *48.6 | 33.4 | ||||||||
| p = 0.0069 | p = 0. 2007 | |||||||||
| Egyptian | *42.8 | *38.6 | 29.5 | |||||||
| p = 0.0029 | p = 0.0149 | p = 0.1428 | ||||||||
| Alaskan | 39.3 | *29.8 | 22.8 | 27.8 | ||||||
| p = 0.0349 | p = 0.2847 | p = 0.8781 | p = 0.0889 | |||||||
| Ashanti | *33.1 | 35 | 34 | 26.2 | *27.2 | |||||
| p = 0.4105 | p = 0.1088 | p = 0.3686 | p = 0.4365 | p = 0.6933 | ||||||
| Aleutian | 36.6 | *32.6 | *23.5 | *19.1 | 16.2 | *23.9 | ||||
| p < 0.0009 | p = 0.0069 | p = 0.1448 | p = 0.0639 | p = 0.2507 | p = 0.1018 | |||||
| African American | *37.4 | *37.9 | 31.5 | 25.4 | 24.5 | 23 | *23.3 | |||
| p = 0.0089 | p = 0.0069 | p = 0.3366 | p = 0.1938 | p = 0.3786 | p = 0.924 | p = 0.0089 | ||||
| Arikara | 39.7 | *32.8 | 28.3 | 24.7 | 19.8 | 28.9 | *22 | 21.4 | ||
| p = 0.0029 | p = 0.0129 | p = 0.1718 | p = 0.0299 | p = 0.0669 | p = 0.1598 | p < 0.0009 | p = 0.0669 | |||
| Caucasian | *36 | 37 | 35.1 | *37 | *24.6 | 23.7 | *24.6 | *23.6 | *24.1 | |
| p < 0.0009 | p < 0.0009 | p = 0.0079 | p < 0.0009 | p < 0.0009 | p = 0.2117 | p = 0.0019 | p = 0.0049 | p < 0.0009 |
To assess differences in trajectories between all populations a pair-wise comparison of angles between PC1s is carried out, 45 comparisons in total. The results of this analysis are given in Table 7. There is no significant correlation between these angles and the Mahalanobis' distances calculated from the age series in Table 3.
Twenty-one of the 45 analyses show a statistically significant difference in the direction of PC1 (= ontogenetic trajectory) between the two populations being compared. There is a significant correlation between the level of statistical significance (as measured by the p value) and the number of individuals (r = 0.355, p = 0.0168), and in particular subadult individuals (r = 0.402, p = 0.0062) included in each analysis. However, the success or failure of an analysis to reach statistical significance is not fully explained by these relationships: the comparison between the Polynesian and Australian populations is highly statistically significant despite being based on only 40 individuals, and that between the Arikara and Alaskan Inupiaq is not, despite being based on 102 individuals. In those analyses which reach statistical significance the angle between the two PC1s ranges between 22° and 48.6° (Table 7). The comparisons which include the Oceanic populations (the Polynesians, Papua New Guineans and Australians) tend to have much larger angles than do the others (with the sole exception of the comparison between Egyptians and Caucasians which also shows a large angle: 37°). These Oceanic populations thus appear to show the greatest individuality in postnatal facial ontogeny both when compared with each other and with more geographically distant populations.
The above analyses show that in some populations, differences in adult facial shape arise partially through differences in ontogenetic trajectory as represented by PC1. For other comparisons, however, there was no significant difference in ontogenetic trajectory. This suggests that differences between adults of these populations arise before infancy but could in addition be accentuated by one population achieving larger adult sizes along this common ontogenetic trajectory. The possibility of such ontogenetic scaling is assessed by contrasting population differences in adult mean size (Table 8) with differences in ontogenetic shape trajectories and adult shapes. There are significant (p < 0.05) adult size differences amongst seven of the 24 population pairs where no significant angle is found between ontogenetic allometric trajectories (Table 7). This implies that ontogenetic scaling may contribute to interadult shape differences in these populations. It should be noted however, that these seven significant differences all involve the populations with the smallest or largest adults. This suggests that greater sample sizes might have yielded more significant differences and so it is likely that ontogenetic scaling plays a wider part in generating interpopulation differences in adults than is documented here. Indeed, further examination of the size differences between populations (Table 7) indicates that 15 of the 21 populations that show significant differences in ontogenetic allometry also show significant (p < 0.05) differences in adult size. This finding implies that adult facial shapes may, in part, come to differ between populations due to differences in the extent to which they grow along their divergent ontogenetic allometries. Thus, we have already noted (Fig. 1) that there are common aspects of allometry shared by all populations and that superimposed on this are population-specific ontogenetic shape changes that would be accentuated to varying degree by the extent of ontogenetic changes in size.
Table 8.
Mean facial centroid size (in cm) of each of the 10 populations, sexes pooled
| Population | Mean centroid size (cm) |
|---|---|
| Polynesian | 25.334 |
| Papua New Guinean | 22.542 |
| Australian | 23.494 |
| Egyptian | 24.096 |
| Alaskan | 24.456 |
| Ashanti | 23.211 |
| Aleutian | 25.404 |
| African American | 24.080 |
| Arikara | 24.043 |
| Caucasian | 23.063 |
Discussion
This paper sets out to examine the ontogenetic basis of population-specific facial variation in 10 distinct modern human groups. In so doing it erects two null hypotheses: that there is no difference in the shape of the facial skeleton of the different populations irrespective of maturation; and that there is no difference between populations in their ontogenetic trajectories. It uses geometric morphometric methods because these techniques provide a useful way to explore ontogenetic changes in facial shape in relation to size and age. The discussion of the results is in three sections, the first two corresponding to the two null hypotheses, and the third relating these to their more general implications with regard to evolution, growth, development and allometry.
Differences in the shape of the facial skeleton
The analyses of the adults reveal that all the populations can be distinguished on the basis of aspects of facial shape (PC scores). Thus there are distinct morphological characteristics unique to each of the populations. Similarly, discriminant analysis reveals a statistically significant separation between all the population ontogenetic series. Population-specific morphologies are evident irrespective of their age or sex. This finding is echoed in the cross-validation study. On average, 70%, and in half the populations over 75%, of the individuals are correctly assigned.
The relative relationships between facial morphologies, calculated from the ontogenetic series, mirror those based on the adult shapes alone, assessed by computing correlations between the Mahalanobis' distance matrices. Thus similar morphological features may contribute to distinguishing the adult populations, and the age series, although there may be accentuation or modification during ontogeny (see next section). Population-specific aspects of facial morphology have already developed prenatally or relatively early postnatally and are carried through, accentuated and modified during ontogeny. The limitations (availability and preservation) of infant skeletal material mean that it is impossible to establish when differences develop, although they seem to be evident in the youngest individuals included in this study, i.e. in the first year of life.
The results of these analyses are important in the contexts of archaeology, forensic anthropology and the identification of subadult skeletal remains. There is, at present, no way of reliably assigning subadult remains to ‘racial’ groups on the basis of their skeletal morphology. Given the results of this study it seems feasible to develop an identification system similar to that based on Howells' (1989) dataset (Wright, 1992), for use on subadult individuals. In order to do so, reference samples would have to be collected from a relatively large amount of subadult craniofacial material, representing those groups most likely to have contributed to the skeletal assemblage in the region. It seems that, given that the unidentified cranium came from one of the reference samples, and the samples numbered 50+ individuals, it would be possible to assign it to the correct group with c. 75% confidence (Strand Viðarsdóttir & O'Higgins, 2001).
These results suggest some caution is needed in the study of comparative morphology with subadults of other hominins. Given the significant interpopulation differences in adult and subadult facial shape documented here, studies of comparative development between modern humans and fossils that use ‘conglomerate’ age series based on more than one population (e.g. Ponce de Leon & Zollikofer, 2001) may obscure the subtleties of possible ontogenetic similarities and differences to diverse human groups.
Differences in the ontogenetic trajectory
The analyses of individual groups found that for all populations PC1 was strongly correlated with both facial centroid size and estimated age. No other PC showed a large or significant correlation with either size or developmental age. Therefore, PC1 well represents the ontogenetic allometric vector in all the studied populations. Although most of the larger-scale shape changes noted along the first PCs, from the smallest/youngest extreme to the largest/oldest extreme, were shared between all the populations, some appear to be population specific. Thus, for example, the Australians, Alaskans, African Americans, Arikara and Caucasians share an increase in relative alveolar prognathism not found in the other populations. These differences indicate that there might be variations in the ontogenetic trajectories producing the adult facial morphologies of diverse groups. The pair-wise comparisons of PC1s support this notion. Twenty-one of the 46 comparisons showed a statistically significant angle between the PC1s calculated for each group.
Two populations stand out as being most distinct in this analysis: the Caucasians and Polynesians. The Caucasians show the greatest number of significant differences, their ontogenetic vector being significantly different from that of all other populations apart from the Ashanti. Indeed, the Ashanti vector is not significantly different from that of any other group and this is most likely a function of the small number of subadults within that sample, with resultant failure to define adequately the allometric vector at the ‘younger/smaller’ end of the range. A similar factor is probably operating in the comparisons involving Australians. The sample size of the Australians is small, and they only show a significantly distinct vector when compared with the Polynesians and Caucasians, i.e. the most distinctive groups. Other populations show a varying number of significant and non-significant allometric vectors.
In contrast to Caucasians, the Polynesians show not only a large number of significant differences between vectors, but also a more distinctive ontogenetic trajectory (larger angles). Indeed the ontogenetic trajectory of the Polynesian population is significantly different from that of most other populations, except the Papua New Guineans (which just fails to achieve statistical significance) and Ashanti. Thus, considering only those angles that show statistical significance, there is an indication that the Oceanic group, and in particular the Polynesians, have the most distinctive ontogenetic trajectories.
In light of the discussion above, it should be noted that under a strict Bonferroni approach, all the significant differences between groups would be expected to have a p value of 0.0011(0.05/45). Under this conservative approach, 16 of the 21 apparently significant comparisons are invalidated. A more relaxed Bonferroni approach, in which the significance of the differences between the angle of any one modern group and all others is adjusted to lead us to expect a p value of 0.006(0.05/9), results in 11 of the 21 apparently significant differences being accepted. It should be noted that the suitability of the Bonferroni correction to these sorts of data remains controversial (Sankoh et al. 1997; Perneger, 1998; Bland, 2000) but in any case our results strongly indicate diversity of facial ontogenetic trajectories among modern human populations.
Some of the populations that are similar in shape as assessed from Mahalanobis' distances computed from the combined adult and subadult ontogenetic series (i.e. the Aleutian/Alaskan; Papua New Guinean/Australian) do not have significantly different ontogenetic trajectories. However, others such as the Egyptians/Caucasians and the Arikara/Aleutians grow very differently. Indeed, there is no significant correlation between the PC1 angles between groups and corresponding Mahalanobis' distances, showing that population-specific aspects of facial shape are not purely a function of differences in postnatal ontogenetic allometry and visa versa.
The above analyses have shown that between some populations, differences in adult facial shape arise partly through differences in ontogenetic trajectory, as represented by PC1. However, the differences in the direction of this vector are not sufficient to explain the differences in adult facial shape between all the populations. The analysis of adult centroid size shows that additional differences in facial form can arise through extension of shared ontogenetic vectors in one group relative to another, i.e. ontogenetic scaling. Such a mechanism may produce different adult morphologies in populations with parallel allometries. Similar scaling might further accentuate differences brought on by divergence of ontogenetic trajectories. Thus adult facial shapes come to differ between populations through two allometric mechanisms, working either separately or in conjunction: trajectory divergence and ontogenetic scaling.
General conclusions
The present study has indicated that differences in adult human facial form arise through three interwoven ontogenetic processes or mechanisms. The first is the very early, possibly prenatal, development of major aspects of population-specific morphology. There is no indication from our analyses that modern human infants from diverse groups share a (more or less) common facial form. Second, there are dissimilarities in ontogenetic trajectories between populations. These are not statistically significant between all populations (at least not given the present sample sizes) although some populations, in particular the Caucasians and Polynesians, grow along vectors that are significantly distinct from most other populations in this study. In addition, the members of the Oceanic group as a whole have allometric vectors that are relatively more distinct from the other populations than are those of other groups. There is no significant relationship between the population-specific differences in facial form (as assessed from the Mahalanobis' distance matrix) and the angles between population-specific allometric vectors. Third, greater distinctions arise through variable extension or truncation of ontogenetic allometries into larger size ranges, i.e. scaling. Such scaling may produce different adult morphologies in populations with parallel allometries (ontogenetic scaling), or further accentuate differences brought on by allometric divergence.
The finding that underlying (prenatal and neonatal) population-specific morphology is not directly related to the subsequent ontogenetic trajectory is of great interest here. It implies that the former does not set up the latter in a simple way. The relationship between these aspects of ontogeny needs to be further explored. All in all this study emphasizes the overall plasticity of the human face. It is clear that, when necessary, facial shape can be adapted with relative ease through subtle shifts in neonatal form, ontogenetic trajectory and ontogenetic scaling.
Various factors have been proposed to influence the adult form of the facial skeleton. Although the basic structure is determined in accordance with a genetically regulated blueprint while in utero (Thesleff, 1998; Schilling & Thorogood, 2000), this is modified pre- and postnatally through functional matrices responding to environmental and epigenetic influences, such as climate (Van Vark et al. 1985; Hernandez et al. 1997), activity patterns (Lieberman, 1996) and masticatory function (Ingervall & Bitsanis, 1987). As an example, Van Vark et al. (1985) demonstrated a statistically significant correlation between craniofacial variables (as given by the Mahalanobis'D2) described by Howells (1973) and a variety of climatic variables, in particular daily temperature range and daily humidity range, although they were unable to pinpoint particular morphometric features which might be influenced to a greater degree than others. Climatic variables can also have an indirect effect, by influencing subsistence patterns. Thus facial flatness in the Inuit and related cold-adapted populations is highly correlated with increasing latitude, but latitude is also associated with increasing whaling, and whaling with increased use of the dentition for holding and grabbing (Wanner, 1977). Similarly, correlations between latitude and facial form have recently been demonstrated in macropods by Milne & O'Higgins (2002). It will be of great interest in the future to try to link differences in facial shape and ontogenetic allometry to particular variables, such as climate, altitude or diet.
Overall, the results of this study introduce many interesting avenues for future investigation, while highlighting the caution with which studies of ontogeny have to be approached. As an example, one of the authors has shown that adult sexual dimorphisms can differ to greater or lesser degree, not only between different primate species but also between modern human populations (O'Higgins et al. 1989, 1990a, 1990b; O'Higgins, 1989). Our results shed light on the mechanisms by which such differences might develop and it would be interesting in future to examine the degree to which these are responsible.
Moreover, the results of this study indicate that modern human populations possess generally similar postnatal facial ontogenetic trajectories and that much of the diversity amongst adults is present relatively early. Further distinctions in form arise through ontogenetic divergences and scaling. These divergences (angles) are of comparable magnitude to some interspecific angles previously documented for non-human primates (Cobb, 2001; O'Higgins et al. 2001; O'Higgins & Collard, 2002) but absolute angles have to be interpreted with caution since they give no indication of the particular anatomical features underlying divergence, and thus of the anatomical nature of differences in ontogeny. In this study we have highlighted some of the principal anatomical features underlying the divergences of ontogenetic trajectory between populations. The presence of such differences raises the issue of the extent to which they might be used to infer aspects of evolutionary history. There is evidence that ontogenetic shape divergence (angles between ontogenetic trajectories) is a poor indicator of phylogeny between diverse primates (Collard & O'Higgins, 2001) but it will still be interesting, with reference to the mode of origin of our species, to determine to what extent the ontogeny of archaic forms of Homo (Ponce de Leon & Zollikofer, 2001) resembles that of different modern populations.
Summary
Differences in modern human adult facial form arise through three interwoven ontogenetic mechanisms: an early development of major aspects of population-specific morphologies, a dissimilarity in the direction of the ontogenetic trajectory, and ontogenetic scaling.
Furthermore, there is no direct relationship between underlying (prenatal and neonatal) population-specific morphology and the subsequent ontogenetic trajectory. This implies that the former does not set up the latter in a direct way.
Acknowledgments
We wish to thank the curators of the collections analysed for access to the specimens in their care. We also thank Nicholas Jones for computing support and our colleagues at UCL and Durham for stimulating discussions and comments on this work. We also thank the two anonymous reviewers for their contributions. This study was carried out while U.S.V. held a University of London Scholarship, a NATO Scientific Fellowship, and an Addison Wheeler Fellowship, and was further funded by the UCL Graduate School.
References
- Batzer MA, Stoneking M, Alegriahartman M, Bazan H, Kass DH, Shaik TH, et al. African origin of human-specific polymorphic Alu insertions. Proc. Natl Acad. Sci. USA. 1994;91:12288–12292. doi: 10.1073/pnas.91.25.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland M. An Introduction to Medical Statistics. Oxford: Oxford University Press; 2000. [Google Scholar]
- Bookstein FL. Principal warps: thin-plate splines and the decomposition of deformations. IEEE Trans. Pattern Anal. Machine Intelligence. 1989;11:567–585. [Google Scholar]
- Brooks S, Suchey JM. Skeletal age determination based on the os pubis: a comparison of the Acsadi-Nemiskeri and Suchey-Brooks methods. Human Evol. 1990;5:27–238. [Google Scholar]
- Canalis E. Regulation of bone remodelling. In: Favus M, editor. Primer on Metabolic Bone Diseases and Disorders of Mineral Metabolisms. New York: Rave Press; 1993. pp. 33–37. [Google Scholar]
- Cann RL, Stoneking M, Wilson AC. Mitochondrial-Dna and human-evolution. Nature. 1987;325:31–36. doi: 10.1038/325031a0. [DOI] [PubMed] [Google Scholar]
- Cavalli-Sforza L, Menozzi P, et al. The History and Geography of Human Genes. New Jersey: Princeton University Press; 1994. [Google Scholar]
- Cobb SN. PhD thesis. University College London, Department of Anatomy and Developmental Biology; 2001. Form Variation in the Postnatal Facial Skeleton of the African Apes. [Google Scholar]
- Collard M, Wood B. How reliable are human phylogenetic hypotheses? Proc. Natl Acad. Sci. USA. 2000;97:5003–5006. doi: 10.1073/pnas.97.9.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard M, O'Higgins P. Ontogeny and homoplasy in the papionin monkey face. Dev. Evol. 2001;3:322–331. doi: 10.1046/j.1525-142x.2001.01042.x. [DOI] [PubMed] [Google Scholar]
- Dryden IL, Mardia KV. Size and shape analysis of landmark data. Biometrika. 1992;79:57–68. [Google Scholar]
- Dryden IL, Mardia K. Statistical Shape Analysis. Chichester: John Wiley; 1998. [Google Scholar]
- Froment A. Correspondence between anatomical differentiation and geographic distribution of modern humans studied by craniometry. CR Acad. Des Sci. Serie III-Sci. la Vie-Life Sci. 1992;315:323–329. [PubMed] [Google Scholar]
- Frost H. The Laws of Bone Structure. Springfield, IL: Charles C Thomas; 1964. [Google Scholar]
- Giles E, Elliot O. Race identification from cranial measurements. J. Forensic Sci. 1962;7:147–157. [Google Scholar]
- Gill G. A forensics test case for a new method of geographical race determination. In: Rathbun I, Buikstra J, editors. Human Identification: Case Studies in Forensic Anthropology. Springfield, IL: Charles C Thomas; 1984. pp. 329–339. [Google Scholar]
- Good P. Permutation Tests: a Practical Guide to Resampling Methods for Testing Hypotheses. New York: Springer-Verlag; 1993. [Google Scholar]
- Guglielmino-Matessi C, Gluckman P, Cavalli-Sforza L. Climate and the evolution of skull metrics in man. Am. J. Phys. Anthropol. 1977;50:549–564. doi: 10.1002/ajpa.1330500407. [DOI] [PubMed] [Google Scholar]
- Hanihara T. Population prehistory of East Asia and the Pacific as viewed from craniofacial morphology. Am. J. Phys. Anthropol. 1993a;91:173–187. doi: 10.1002/ajpa.1330910204. [DOI] [PubMed] [Google Scholar]
- Hanihara T. Cranial morphological contrasts between Negritos, Australians, and neighboring populations. Anthropol. Sci. 1993b;101:389–404. [Google Scholar]
- Hanihara T. Comparison of craniofacial features of major human groups. Am. J. Phys. Anthropol. 1996;99:389–412. doi: 10.1002/(SICI)1096-8644(199603)99:3<389::AID-AJPA3>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Hernandez M, Fox CL, GarciaMoro C. Fueguian cranial morphology: The adaptation to a cold, harsh environment. Am. J. Phys. Anthropol. 1997;103:103–117. doi: 10.1002/(SICI)1096-8644(199705)103:1<103::AID-AJPA7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Howells W. Cranial Variation in Man. Cambridge, MA: Harvard University; 1973. [Google Scholar]
- Howells W. Skull Shapes and the Map: Craniometric Analyses in the Dispersion of Modern Homo. Cambridge, MA: Harvard University; 1989. (Papers of the Peabody Museum 79) [Google Scholar]
- Howells W. Who's Who in Skulls: Ethnic Identification of Crania from Measurements. Cambridge, MA: Harvard University; 1995. (Papers of the Peabody Museum 82) [Google Scholar]
- Hunt N. Muscle function and the control of facial form. In: Harris MH, Edgar M, Meghji S, editors. Clinical Oral Science. London: Wright; 1998. pp. 120–133. [Google Scholar]
- Ingervall B, Bitsanis E. A pilot-study of the effect of masticatory muscle training on facial growth in children. Eur. J. Orthodontics. 1987;9:15–23. doi: 10.1093/ejo/9.1.15. [DOI] [PubMed] [Google Scholar]
- Kendall DG. Shape manifolds, Procrustean metrics and complex projective spaces. Bull. London Mathemat. Soc. 1984;16:81–121. [Google Scholar]
- Kent JT. The complex Bingham distribution and shape analysis. J. Royal Statistical Soc. Series B. 1994;56:285–299. [Google Scholar]
- Krogman W, Iscan M. The Human Skeleton in Forensic Medicine. Springfield, IL: Charles C Thomas; 1986. [Google Scholar]
- Lieberman DE. How and why humans grow thin skulls: Experimental evidence for systemic cortical robusticity. Am. J. Phys. Anthropol. 1996;101:217–236. doi: 10.1002/(SICI)1096-8644(199610)101:2<217::AID-AJPA7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Lieberman DE. Ontogeny, homology, and phylogeny in the Hominid craniofacial skeleton: the problem of the browridge. In: O'Higgins P, Cohn M, editors. Development, Growth and Evolution. London: Academic Press; 2000. pp. 85–122. [Google Scholar]
- Lovejoy CO, Meindl RS, Pryzbeck TR, Mensforth RP. Chronological metamorphosis of the auricular surface of the ilium: a new method for the determination of age at death. Am. J. Phys. Anthropol. 1985;68:15–28. doi: 10.1002/ajpa.1330680103. [DOI] [PubMed] [Google Scholar]
- Lynch J, Wood C, Luboga S. Geometric morphometrics in primatology: Craniofacial variation in Homo sapiens and Pan troglodytes. Folia Primatol. 1996;67:15–39. doi: 10.1159/000157203. [DOI] [PubMed] [Google Scholar]
- Meindl RS, Lovejoy CO. Ectocranial suture closure: a revised method for the determination of skeletal age at death and blind tests of its accuracy. Am. J. Phys. Anthropol. 1985;68:57–66. doi: 10.1002/ajpa.1330680106. [DOI] [PubMed] [Google Scholar]
- Milne N, O'Higgins P. Inter-specific variation in Macropus crania: form, function and phylogeny. J. Zool. 2002;256:523–535. [Google Scholar]
- Moss M. Vertical growth of the human face. Am. J. Orthodontics. 1964;50:359–376. [Google Scholar]
- Moss M. The primacy of functional matrices on orofacial growth. Dental Practitioner. 1968;19:65–73. [PubMed] [Google Scholar]
- Moss M, Salentijn L. The primary role of functional matrices in facial growth. Am. J. Orthodontics. 1969a;55:566–577. doi: 10.1016/0002-9416(69)90034-7. [DOI] [PubMed] [Google Scholar]
- Moss M, Salentijn L. The capsular matrix. Am. J. Orthodontics. 1969b;56:474–490. doi: 10.1016/0002-9416(69)90209-7. [DOI] [PubMed] [Google Scholar]
- Nei M, Roychoudhury AK. Evolutionary relationships of human-populations on a global-scale. Mol. Biol. Evol. 1993;10:927–943. doi: 10.1093/oxfordjournals.molbev.a040059. [DOI] [PubMed] [Google Scholar]
- O'Higgins P, Johnson D, Moore W, McAndrew T. Determination of race and sex of the human skull by discriminant function analysis of linear and angular dimensions. Forensic Sci. Int. 1989a;41:41–53. doi: 10.1016/0379-0738(89)90234-x. [DOI] [PubMed] [Google Scholar]
- O'Higgins P, Johnson D, Moore W, McAndrew T. Determination of race and sex of the human skull by discriminant function analysis of linear and angular dimensions – an appendix. Forensic Sci. Int. 1989b;45:1–3. doi: 10.1016/0379-0738(89)90234-x. [DOI] [PubMed] [Google Scholar]
- O'Higgins P, Moore W, Johnson D, McAndrew T, Flinn R. Patterns of cranial sexual dimorphism in certain groups of extant hominoids. J. Zool., London. 1990a;222:399–420. [Google Scholar]
- O'Higgins P, Johnson D, Moore W, Flinn R. The variability of patterns of sexual dimorphism in the primate skull. Experientia. 1990b;46:670–672. doi: 10.1007/BF01939929. [DOI] [PubMed] [Google Scholar]
- O'Higgins P, Jones N. Facial growth in Cercocebus torquatus: an application of three- dimensional geometric morphometric techniques to the study of morphological variation. J. Anat. 1998;193:251–272. doi: 10.1046/j.1469-7580.1998.19320251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Higgins P, Strand Viðarsdóttir U. New approaches to the quantitative analysis of craniofacial growth and variation. In: Hoppa R, Fitzgerald C, editors. Human Growth in the Past: Studies from Bones and Teeth. Cambridge: Cambridge University Press; 1999. pp. 128–160. [Google Scholar]
- O'Higgins P. The study of morphological variation in the hominid fossil record: biology, landmarks & geometry. J. Anat. 2000;197:103–120. doi: 10.1046/j.1469-7580.2000.19710103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Higgins P, Chadfield P, Jones N. Facial growth and the ontogeny of morphological variation within and between Cebus apella and Cercocebus torquatus. J. Zool. 2001;254:337–357. [Google Scholar]
- O'Higgins P, Collard M. Sexual dimorphism and facial growth in Papionin monkeys. J. Zool. 2002;257:255–272. [Google Scholar]
- Perneger T. What is wrong with Bonferroni adjustments. Br. Med. J. 1998;136:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce de Leon M, Zollikofer C. Neanderthal cranial ontogeny and its implications for late hominid diversity. Nature. 2001;412:534–538. doi: 10.1038/35087573. [DOI] [PubMed] [Google Scholar]
- Relethford JH. Craniometric variation among modern human-populations. Am. J. Phys. Anthropol. 1994;95:53–62. doi: 10.1002/ajpa.1330950105. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ. On the use of shape spaces to compare morphometric methods. Hystrix. 2000;11:9–25. [Google Scholar]
- Rohlf FJ. Consistency and bias in morphometric methods. Am. J. Phys. Anthropol. 2002;S34:133. doi: 10.1002/(SICI)1096-8644(200004)111:4<463::AID-AJPA3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Ross AH, McKeown AH, Konigsberg LW. Allocation of crania to groups via the ‘new morphometry’. J. Forensic Sci. 1999;44:584–587. [PubMed] [Google Scholar]
- Sankoh A, Huque M, Dubey S. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Statistics Med. 1997;16:2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Schilling TF, Thorogood PV. Development and evolution of the vertebrate skull. In: O'Higgins P, Cohn M, editors. Development, Growth and Evolution: Implications for the Study of the Hominid Skeleton. Vol. 20. Academic Press; 2000. pp. 57–83. Linnean Society Symposium Series. [Google Scholar]
- Schour I, Massler M. The development of the human dentition. J. Am. Dental Assoc. 1941;28:1153–1160. [Google Scholar]
- Slice DE, Bookstein FL, Marcus LF, Rohlf FJ. A glossary for geometric morphometrics. In: Marcus LF, Corti M, Loy A, Naylor GJP, Slice D, editors. Advances in Morphometrics NATO ASI Series A. New York: Plenum; 1996. pp. 531–551. [Google Scholar]
- Strand Viðarsdóttir U. Changes in the Form of the Facial Skeleton during Growth: a Comparative Morphometric Study of Modern Humans and Neanderthals PhD Thesis. University College London; 1999. [Google Scholar]
- Strand Viðarsdóttir U, O'Higgins P. Geometric morphometrics and the analysis of variations in facial form: Robusticity of biological findings in relation to bilateral versus unilateral and missing landmarks. Statistica. 2001;LXI:315–333. [Google Scholar]
- Stringer C. Reconstructing recent human evolution. Phil. Trans. Royal Soc. London. 1992;B337:217–224. doi: 10.1098/rstb.1992.0099. [DOI] [PubMed] [Google Scholar]
- Thesleff I. The genetic basis of normal and abnormal craniofacial development. Acta Odontol. Scand. 1998;53:144–151. doi: 10.1080/000163598428248. [DOI] [PubMed] [Google Scholar]
- Ubelaker D. Human Skeletal Remains: Excavation, Analysis, Interpretation. Washington DC: Smithsonian Institution; 1989. [Google Scholar]
- Van Vark S, van der Sman P, Hazewindus S. The statistical significance of an association between skull morphology and climatic conditions. Homo. 1985;36:232–241. [Google Scholar]
- Wanner J. Clinical Variation in Eskimo Craniofacial Morphology PhD Thesis. University of Boulder; 1977. [Google Scholar]
- Wright R. Correlation between cranial form and geography in Homo sapiens. Archaeologica Oceania. 1992;27:105–112. [Google Scholar]