Abstract
In the adult lung the pulmonary arteries run alongside the airways and the pulmonary veins show a similar branching pattern to the arteries, though separated from them. During early fetal development the airways act as a template for pulmonary blood vessel development in that the vessels form by vasculogenesis around the branching airways. In later lung development the capillary bed is essential for alveolar formation. This paper reviews evidence for the interaction of the airways and blood vessels in both normal and abnormal lung development.
Keywords: bronchial vessels, pulmonary artery, pulmonary vein
Introduction
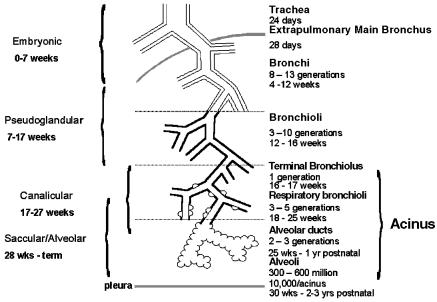
In mammals the lung is made up of conducting airways that carry air to the alveoli, the gas-exchanging units of the lung. The airways branch from the hilum towards the periphery (Fig. 1). From the trachea to the terminal airways the diameter decreases but there is a gradual increase in cross-sectional area because of the increase in number of airways. Within the acinus (the respiratory region of the lung) division does not decrease diameter but rapidly increases surface area particularly at the level of the alveoli. The alveolar region is only an efficient region for gas exchange if there is sufficient blood flow to the area. In the adult human lung the pulmonary arteries run alongside the airways, branching with them and decreasing in diameter. They supply blood to the capillary bed within the alveolar wall with the capillary surface area closely matching that of the alveolar surface area. The pulmonary veins drain the capillary bed and though they do not run alongside the airways they have an equivalent number of branches to the arteries. In addition the lung is supplied with systemic blood for nourishment via the bronchial arteries which arise from the descending aorta. They supply the walls of the airways as far as the respiratory region and the vasa vasorum in the wall of the large vessels.
Fig. 1.
Diagram illustrating the airway structure in the lung and the time period in which each develops.
The close relationship of the blood vessels and the airways is found throughout development of the lung (Fig. 2). The lung is unique in that it grows while not fulfilling its postnatal function but is ready to function efficiently at the moment of birth. In the mammalian lung therefore the majority of structural development and maturation takes place in utero. For the human lung four stages of prenatal lung development have been described based on the appearance of the lung. These are embryonic, pseudoglandular, canalicular and alveolar stages (Loosli & Potter, 1959). The age of change from one stage to another may vary between individuals. The same stages are seen in other species but their duration varies, and the alveolar stage is entirely postnatal in some species (rat and mouse) (Burri, 1974).
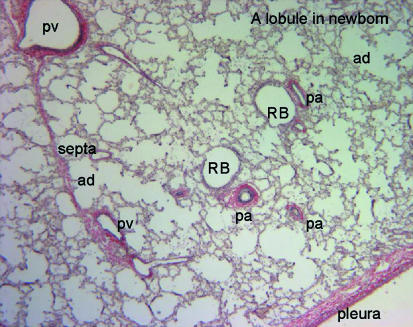
Fig. 2.
Photomicrograph of a section through a newborn lung stained with elastic van Geison stain to show the relationship of airways and blood vessels. pv, pulmonary vein; pa, pulmonary artery; RB, respiratory bronchiolus; ad, alveolar duct.
Lung development
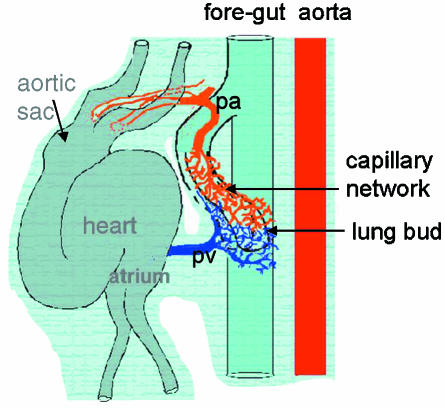
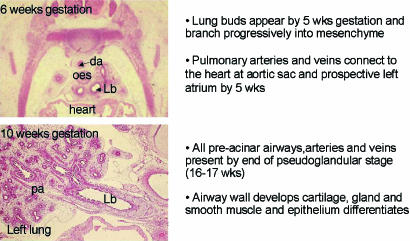
In man within each stage of development the appearance of specific structures is important (Fig. 1). During the embryonic stage (up to 7 weeks of gestation), the lung bud lined by epithelium appears as a ventral diverticulum of the foregut at 4 weeks of gestation. It divides within the surrounding mesenchyme to form the two lung buds that come to lie either side of the prospective oesophagus (Fig. 3). Each of these lung buds further branches within the expanding mesenchyme to form lobar and then segmental airways a total of 10 segments in each lung. By 6 weeks of gestation the two lungs can be distinguished as separate organs in the thorax dorsal to the heart and pericardium (Fig. 4). As early as 34 days of gestation each primitive lung bud is supplied by a pulmonary artery extending from the outflow tract of the heart, which at this time has not divided into separate aortic and pulmonary trunks, and running alongside the trachea into the lung bud. On the ventral side of each lung bud a pulmonary vein connects to the prospective left atrium. Between these arteries and veins is a capillary plexus in the mesenchyme around the epithelial lung bud (Fig. 3) (Hall et al. 2000, 2002).
Fig. 3.
Diagram produced from a serial reconstruction of a 34-day human embryo. The trachea extends from the foregut and the first branches form the lung but on either side of the gut. Around each lung bud is a capillary network which connects cranially to the aortic sac of the heart and caudally to the prospective left atrium. All structures are enveloped in a continuous mesenchymal surround. (Reconstruction by Dr Susan Hall.)
Fig. 4.
Appearance and summary of growth of the lung in the embryonic and pseudoglandular stage. da, dorsal aorta; oes, oesophagus; Lb, left main bronchus; pa, pulmonary artery.
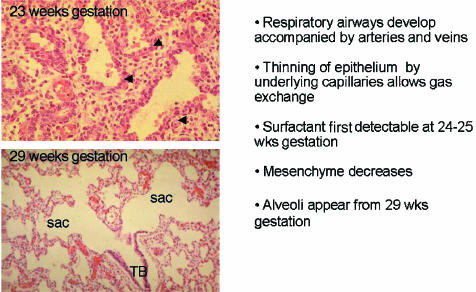
During the pseudoglandular stage (5–17 weeks of gestation in man) further division of the airway buds into the mesenchyme results in all pre-acinar airways being present by the end of the stage (Fig. 3). It is during this phase that the airway wall cells differentiate to form the adult structures of cartilage, submucosal gland, bronchial smooth muscle and epithelial cell types. At the same time all pre-acinar pulmonary arteries and veins are formed. More division occurs during the canalicular stage (16–27 weeks of gestation in man) (Fig. 5) to form the respiratory airways, nearly all of which are present by birth. Thinning of the epithelium by underlying capillaries leads to the formation of a blood gas barrier which is sufficient to sustain life in extremely premature infants. The epithelium has differentiated at this time into Type I and II pneumonocytes. Surfactant is detectable by 24 weeks gestation produced by the Type II pneumonocytes. By the end of this stage the periphery of the lung is made up of thin-walled saccules simple in outline that have been formed by the reduction in the amount of mesenchyme (Fig. 5).
Fig. 5.
Appearance and summary of growth of the lung in the canalicular and saccular stage. TB, terminal bronchiolus; sac, saccule; arrows, capillaries.
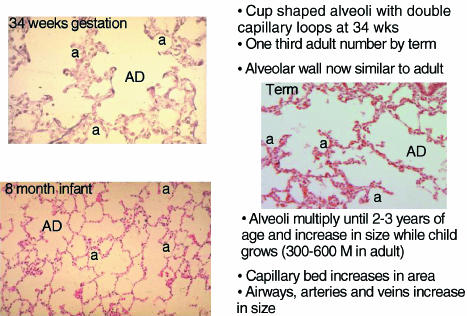
In the alveolar stage (27 weeks of gestation to term) from about 29 weeks of gestation true alveoli appear as indentations in the saccule wall and septae form to produce divisions in the wall. By 34 weeks of gestation cup-shaped alveoli are present. At this time they have a double capillary layer in their walls each under the epithelial cells (Fig. 6). By term about one-third to one-half of the adult number of alveoli are present and the alveolar wall looks similar to that seen in the adult. Postnatally in man there is further multiplication of alveoli and the adult number is reached by 2–3 years of age. Size and surface area of the alveoli increases until after adolescence. At all ages boys have more alveoli than girls for a given height (Thurlbeck, 1982). The evidence for the control of airway development by growth and transcriptional factors has been reviewed recently (Warburton et al. 1999; Minoo, 2000; Cardoso, 2001). Most of these studies are based on the lung development of rats and mice.
Fig. 6.
Appearance and summary of growth of the lung in the alveolar stage. AD, alveolar duct; a, alveolus.
Blood vessel development
It is apparent from the above description that the blood vessels develop at the same time as the airways. In early studies it was assumed that the blood vessels grew by outgrowth from the existing vessels at the same time as the airways, a process of angiogenesis. In those studies it was found that all pre-acinar arteries and veins both conventional and supernumerary were present by 16–17 weeks of gestation and that intra-acinar vessels grew in later fetal life as the respiratory airways developed (Hislop & Reid, 1972, 1973). In a later study on mice it was suggested that the proximal arteries grew in this manner but that peripheral arteries appeared by vasculogenesis in the mesenchyme and joined with the central arteries (deMello et al. 1997).
Vasculogenesis
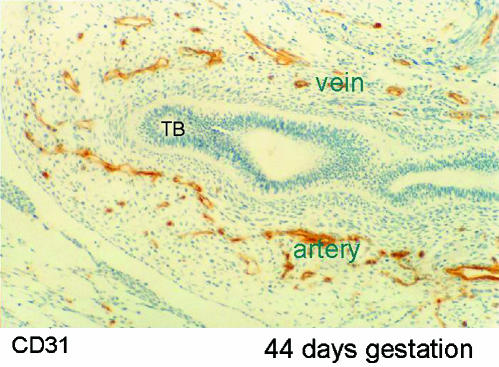
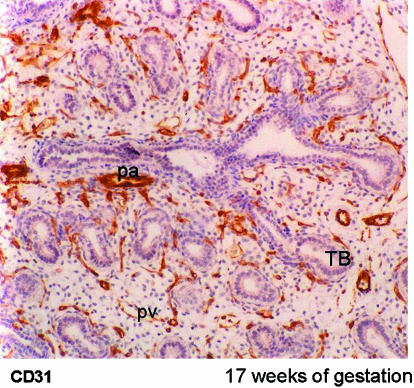
Recently we have studied a series of human embryonic and fetal lungs aged from 28 days of gestation using serial sections immunolabelled specifically for endothelial cells and smooth muscle cells (Hall et al. 2000, 2002). At 28 days of gestation groups of endothelial cells were found in the mesenchyme surrounding the ventral diverticulum. By 34 days of gestation a plexus of capillaries lined by CD31 labelled endothelial cells surrounded the two lung buds and were in connection with both pulmonary artery and pulmonary vein (Fig. 3). As the airways branched into the mesenchyme around each new bud there was a halo of endothelial tubules at a given distance from the epithelium. These tubules coalesced alongside the penultimate airway to form the peripheral pulmonary arteries and veins (Fig. 7). Thus new vessels did not form by angiogenesis but by vasculogenesis of capillaries in the mesenchyme and sustained addition of the newly formed tubes to the existing vessels. This appearance was seen until 17 weeks of gestation when the pre-acinar airway branching was complete (Fig. 8). Thus the airway acts as a template for the pulmonary vessels.
Fig. 7.
Photomicrograph of section through lung bud at 44 days of gestation. The airway, lined by epithelium (ep), ends in a terminal bud (TB). Around the airway are a large number of capillaries staining positively (brown) for CD31 which recognizes endothelium. On one side of the airway these coalesce to form an artery and on the other side a vein.
Fig. 8.
Photomicrograph of lung at 17 weeks of gestation. Arteries accompany airways and veins are found between airways. All vessels stain positively for CD31, the endothelial marker. TB, terminal bud; pa, pulmonary artery; pv, pulmonary vein.
The fact that the endothelial tubes are formed at a constant distance from the terminal bud suggests a factor leading to their formation. There is an increase in cell multiplication within the mesenchymal region around the most distal airway bud suggesting that it is a peripherally driven effect. Vascular endothelial growth factor (VEGF) is known to be involved in angiogenesis and vasculogenesis. It has been demonstrated in the epithelial cells of human fetuses (Shifren et al. 1994; Acarregui et al. 1999) but not in the adjacent capillary bed. In cultured mouse lungs most VEGF is found at the branching points of the peripheral airways (Healy et al. 2000) and grafting of VEGF-coated beads into cultured mouse lung leads to the production of an increased capillary bed (Healy et al. 2000). By contrast mice deficient in the VEGF receptor, Flk 1, have no blood vessel development (Shalaby et al. 1995). VEGF-deficient mice do not survive to term. Another tyrosine kinase receptor expressed on the endothelial cells and involved in vessel assembly is Tie; its ligand is angiopoietin. Null mice for Tie-2 and Ang-1 have abnormal network formation. However, it is likely that these factors are more involved in stabilization of the network rather than its initial appearance (Sato et al. 1995). The mRNA for the growth factors EGF and TGF alpha has been located to the mesenchymal cells surrounding airways and alveoli in human fetal lungs ranging from 12 to 33 weeks of gestation. However, the receptors for these growth factors were demonstrated mainly in the airways and were involved in differentiation (Ruocco et al. 1996).
The capillary bed with its connection to arteries and veins is originally at a similar distance from the epithelial bud. However, the arteries coalesce and run closely associated with the airway wall while the veins after coalescence move away from the airways and eventually run equidistant between them. They seem to be further separated from the influence of the airways as they become surrounded by lymphatic channels within the connective tissue septa (Hall et al. 2002). We do not know what defines the arteries and veins initially. In the systemic vessels of mice the capillaries can be divided early in development by the discriminatory expression of the tyrosine kinase receptor EphB4 and its ligand ephrinB2 which are thought to have a repulsive interaction (Gale & Yancopoulos, 1999). However, this was not the case in the human lungs where though ephrinB2 was only found in arteries, EphB4 was found in both arteries and veins (Hall et al. 2002) and throughout the capillary bed. One might also suggest a gradient of effect from the airways repulsive for the pulmonary veins.
Development of the vessel wall
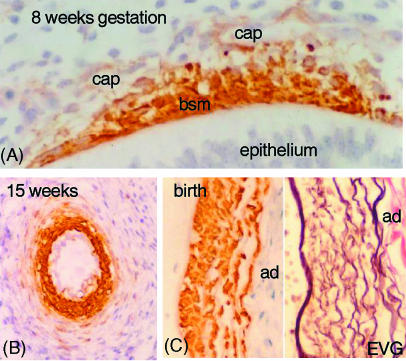
In addition to their different position within the lung, arteries and veins also have a different wall structure. Immediately after their coalescence the pulmonary arteries become invested with smooth muscle cells. Initially these are derived from the bronchial smooth muscle of adjacent airways (Hall et al. 2000). The cells appear to migrate from the bronchial smooth muscle and to line up around the arteries (Fig. 9). This only occurs in one part of the pathway, at the level of the penultimate airway, and these smooth muscle cells make up the innermost layers of the mature vessel. It is the inner layer of smooth muscle cells which in pulmonary hypertension migrate into the intima. Their phenotype may be different. We do not know what stimulates the migration of smooth muscle cells from the bronchial smooth muscle. However, tenascin, versican and fibronectin, which are all involved in cell locomotion, colocate only in this area in the lung between the airway and artery in the penultimate branches.
Fig. 9.
Derivation of smooth muscle cells. (A) Section of airway from a fetus at 8 weeks of gestation, immunostained with α-actin. The bronchial smooth muscle (bsm) stains positively and cells can be seen between this muscle layer and the capillaries (cap) in the mesenchyme. (B) Section of a hilar artery in a fetus of 15 weeks gestation stained for α-actin. The inner layer of cells are strongly labelled and square in shape. Outside are fusiform cells staining weakly for α-actin. (C) A part of a hilar artery stained for α-actin and for elastic van Geison (EVG). The inner smooth muscle cells are a different shape from those in the outer layers though all are within the media. ad, adventitia.
Later in development fibroblasts from the mesenchyme line up around the outer part of the arterial wall and become elongated and develop a smooth muscle cell phenotype expressing α and γ actin and smooth muscle myosin (Fig. 9). A proposed model for this was put forward by Folkman & D'Amore (1996). They suggest that angiopoietin produced by undifferentiated mesenchymal cells binds to and activates the Tie 2 receptor on the endothelial cells resulting in a chemoattractant signal (PDGF or EGF) for the fibroblasts. These are then attracted to the vessel wall and they become committed to becoming smooth muscle cells as a result of further growth factors from the endothelial cells. A third source of arterial smooth muscle cells is seen later in development when the endothelial cells of the capillaries stain positively for α actin and may by division give rise to smooth muscle cells.
The veins do not derive any muscle cells from the bronchial smooth muscle but fibroblasts from the surrounding mesenchyme line up around the endothelium and express smooth-muscle-specific protein.
The smooth muscle cells of the airways, arteries and veins show progressive and gradual maturation of their cytoskeletal structure. During fetal life the bronchial smooth muscle is always the most mature at any age and from 8 weeks gestation desmin is expressed. In the arteries and veins there is no desmin even at birth. Stretch up-regulates myogenic differentiation (Yang et al. 2000) and there is evidence of stretch by fluid in airways from 8 weeks of gestation associated with basal tonic contractions (McCray, 1993; Sparrow et al. 1999). With age there is a gradual increase in the volume of blood flowing through the circulation as the lungs increase in size.
Peripheral blood vessel development
During the canalicular phase the form of development of the blood vessels may change. Airways continue to branch but the mesenchyme around them thins and capillaries come to underlie the epithelium. The capillaries at this stage seem to form by angiogenesis as dividing cells are in the existing capillary tubules rather than in the general mesenchyme (Hall et al. 2002).
We do not know what attracts the capillaries to underlie the epithelium but once there they influence the epithelium to thin and differentiate into Type I and II pneumonocytes. In vitro studies have demonstrated that cell matrix produced by rat pulmonary vascular endothelial cells leads to differentiation of rat Type II to Type I cells (Adamson & Young, 1996). Stretching of fetal rat lungs in culture leads to the epithelial Type II cells differentiating into Type I cells with a reduction in surfactant proteins produced (Gutierrez et al. 1999). Stretch also leads to an increase in VEGF mRNA in vascular smooth muscle cells allowing endothelial cell motility and an increase in capillary number (Smith et al. 2001). Further evidence of the interaction of airway and blood vessel development is shown in transgenic mice with an excess of TGF-β1. They fail to differentiate epithelial cells in the canalicular phase and also the mRNA for both VEGF and Flk-1 are decreased. This was associated with a decrease in the capillaries in the lung periphery (Zeng et al. 2001).
The interaction of the arteries with the airways continues into alveolar development. Burri (1974) first described how in rats a double capillary network was required for normal alveolar development; he also showed in 1986 that a double capillary network was present in the first few months of human life (Zeltner & Burri, 1986). Alveoli form by secondary crests developing from primary septa initially with a capillary loop, which then coalesces to form a single capillary sheet.
Type II pneumonocytes produce mRNA for VEGF during the development of alveoli in mice (Ng et al. 2001) and VEGF is known to promote angiogenesis. In mice absence of VEGF showed retarded alveolarization as well as a reduced capillary number (Ng et al. 2001). VEGF and its receptor Flk 1 have both been shown to increase in perinatal mice during the period of alveolar multiplication (Bhatt et al. 2000). In a study on rat alveolar development the inhibitor of Flk 1 (Su5416) reduced the amount of division of alveoli (Jakkula et al. 2000). The same group also demonstrated that anti-angiogenic factors such as fumagillin and thalidomide reduce the number of small arteries and also the number of alveoli in rats. In another experiment the VEGF receptor inhibitor was found to induce apoptosis in the alveolar walls and the pulmonary vascular bed was pruned (Kasahara et al. 2000). In neonatal rabbit lungs VEGF is located mainly to the alveolar cells suggesting epithelial regulation of capillary formation. Prolonged exposure to hyperoxia decreased the VEGF expression but it increased again on recovery (Maniscalco et al. 1997).
Other factors will also influence both the development of alveoli and the small blood vessels. Inflammation and hyperoxia are involved in abnormal alveolar development in premature infants developing chronic lung disease (bronchopulmonary dysplasia, or BPD) (Jobe, 1999). In many of these cases pulmonary hypertension develops partly because of a reduced pulmonary circulation (Hislop & Haworth, 1990). Hyperoxia in postnatal rats and mice prevents alveolar septation by decreasing cell proliferation and increasing cytokines (Warner et al. 1998; Deng et al. 2000). If the increase in cytokines is prevented by antibodies to neutrophil cytokines alveoli develop relatively normally in hyperoxic exposed newborn rats (Auten et al. 2001). However, cytokines present prenatally appear to enhance lung maturation. Premature babies with chorioamnionitis have a decreased risk of respiratory distress syndrome; however, they also have an increase in their risk of developing BPD (Watterberg et al. 1996). Using the sheep as a model Jobe and his group have shown that giving intra-amniotic endotoxin elevated the cytokines IL-1B, IL-6 and IL-8 and also enhanced maturation of the lung by increasing surfactant production (Kallapur et al. 2001).
For the secondary septa to form, elastin and smooth muscle cells also seem necessary. Elastin null mice do not develop normal alveoli and have a reduced radial alveolar count (Wendel et al. 2000). Retinoic acid receptor deleted mice have reduced elastin and this seems to prevent normal alveolar growth (McGowan et al. 2000). One of the factors which seems to promote alveolar development is stretch. This is likely to be important in the period immediately after birth with the change to air breathing. Increased lung inflation as seen in adult rats post pneumonectomy induces gene expression of c-fos and jun B (Gilbert & Rannels, 1998). Induction of tropoelastin and procollagen I RNA has also been shown in adult rats post pneumonectomy. The deposition was located to sites similar to those seen in alveolar development (Koh et al. 1996). Stretch of mouse and human fetal lung induces serum response factor and myogenesis of airway cells (Yang et al. 2000). Smooth muscle cells are known to produce tropoelastin, which seems essential for alveolar multiplication.
Retinoic acid is a player in development of both arteries and airways. Reduction in vitamin A leads to hypoplastic lungs and increase in retinoic acid will induce alveolar septation in rats even in rats in which septation had previously been reduced due to dexamethasone or in mice with genetic failure of septation (Massaro & Massaro, 2000). It also enhanced post-pneumonectomy lung growth in rats (Kaza et al. 2001); however, it did not prevent the injury done by hyperoxia (Veness-Meehan et al. 2000). Addition of all-trans retinoic acid to human cell lines reduced the production of VEGF (Kini et al. 2001) and reduced endothelial cell migration. Thus it may affect angiogenesis negatively. Vitamin A also inhibits the production of tenascin, which is known to be related to tube formation and endothelial cell sprouting (Schenk et al. 1999).
Most of the studies on control of lung growth have been made on mice or rats. Evidence must accumulate on the importance of these features in the development of alveoli in the normal human infant. It is also important to know if any of them may be used to enhance lung growth in babies with immature or hypoplastic lungs which may be as a result of premature delivery or abnormal growth in utero such as in cases with congenital diaphragmic hernia. A trial on infants of vitamin A supplements showed no improvement in the development of BPD (Wardle et al. 2001). A meta-analysis of several trials does show a reduction in oxygen requirement in surviving premature babies at 36 weeks post menstrual age (Darlow & Graham, 2000).
Development of the bronchial circulation
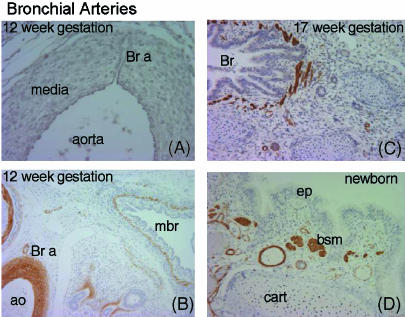
In the human lung there is a second circulatory system, the bronchial circulation. This carries systemic blood to the walls of the airways and the large pulmonary vessels thus giving oxygen and nutrients. The bronchial arteries are found in adult airways as far to the periphery as the alveolar ducts. They do not seem to form at the same time as the pulmonary circulation since they are not found in the lungs until around 8 weeks of gestation. At this time one or two small vessels extend from the dorsal aorta and run into the lung alongside the cartilage plates of the main bronchus (Fig. 10). They extend to the periphery as the airways increase in size and the walls differentiate. They eventually form a network throughout the airway wall but remain small relative to the adjacent pulmonary arteries. They increase in size only in cases where there is a failure of development of the pulmonary circulation as in pulmonary atresia. Many of the small bronchial veins within the airway wall drain into the pulmonary veins. Large bronchial veins are only seen close to the hilum and these drain into the cardinal veins and the right atrium.
Fig. 10.
Development of bronchial arteries. (A) Section through dorsal aorta with a bronchial artery (Br a) passing through the media. (B) Hilar region stained with α-actin. The bronchial artery (Br a) can be seen leading from the aorta (ao) to the wall of the main bronchus (mbr). (C) At 17 weeks of gestation many arteries can be seen in the wall of the bronchus. (D) In the newborn the bronchial vessels have increased in size. bsm, bronchial smooth muscle; cart, cartilage; ep, epithelium.
Abnormal lung growth
Abnormalities in fetal lung development affect both airways and blood vessels. In congenital diaphragmatic hernia there is an equal reduction in arteries and airway number along the main pathway; there is also a reduction in alveolar number (Kitagawa et al. 1971; Ijsselstijn & Tibboel, 1998; Shehata et al. 1999). Such babies also get pulmonary hypertension with an increase in arterial wall thickness. Hypoxic and hyperoxic damage as a result of premature delivery and artificial ventilation lead to an increase in bronchial smooth muscle (Hislop & Haworth, 1989) and airway hyper-responsiveness. There is also a reduction in alveolar formation with abnormal structure (Hislop et al. 1987) and frequently these babies develop pulmonary hypertension partly related to the failure of normal capillary development in the alveolar region (Hislop & Haworth, 1990). Studies on babies developing BPD after premature delivery have emphasized the abnormal capillary formation with decreased VEGF in the walls of the enlarged thick walled alveoli suggesting a direct relationship (Bhatt et al. 2001). Conversely, babies with pulmonary hypertensive congenital heart disease also show an increase in airway smooth muscle and enhanced reactivity (Schindler et al. 1995).
We can only speculate on the mechanisms of interaction between airways and blood vessels. In lung development there are strongly conserved transcription and growth factors in different species. Most have been studied using Drosophila and mouse lungs. The current state of knowledge has been comprehensively reviewed by Warburton et al. (2000). Early formation of the airways is controlled by transcription factors. Later division of airways is influenced by growth factors produced in the mesenchyme acting on the receptors in the epithelium. There is a balance between stimulators usually working via receptor tyrosine kinases including fibroblast growth factors, epidermal growth factor, insulin-like growth factor and platelet-derived growth factor and inhibitors with a serine/threonine kinase receptor, the transforming growth factor –β family. Many of these growth factors are themselves controlled by transcription factors. Pulmonary blood vessels form within the mesenchyme under the influence of growth factors from the endodermal cells (VEGF) as well as some from within the mesenchyme (angiopoietin) (Gale & Yancopoulos, 1999). Later in development the blood vessels control airway growth, particularly alveolar formation.
Acknowledgments
This work was supported by the British Heart Foundation.
References
- Acarregui MJ, Penisten ST, Goss KL, Ramirez K, Snyder JM. Vascular endothelial growth factor gene expresion in human fetal lung in vitro. Am. J. Respir. Cell. Mol. Biol. 1999;20:14–23. doi: 10.1165/ajrcmb.20.1.3251. [DOI] [PubMed] [Google Scholar]
- Adamson IY, Young L. Alveolar type II cell growth on a pulmonary endothelial extracellular matrix. Am. J. Physiol. 1996;270:L1017–L1022. doi: 10.1152/ajplung.1996.270.6.L1017. [DOI] [PubMed] [Google Scholar]
- Auten RL, Jr, Mason SN, Tanaka DT, Welty-Wolf K, Whorton MH. Anti-neutrophil chemokine preserves alveolar development in hyperoxia-exposed newborn rats. Am. J. Physiol. 2001;281:L336–L344. doi: 10.1152/ajplung.2001.281.2.L336. [DOI] [PubMed] [Google Scholar]
- Bhatt AJ, Amin SB, Chess PR, Watkins RH, Maniscalco WM. Expression of vascular endothelial growth factor and Flk-1 in developing and glucocorticoid-treated mouse lung. Pediatr. Res. 2000;47:606–613. doi: 10.1203/00006450-200005000-00009. [DOI] [PubMed] [Google Scholar]
- Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1 and Tie-2 in human infants dying with bronchopulmonary dysplasia. Am. J. Respir. Crit. Care Med. 2001;164:1971–1980. doi: 10.1164/ajrccm.164.10.2101140. [DOI] [PubMed] [Google Scholar]
- Burri PH. The postnatal growth of the rat lung III Morphology. Anat. Rec. 1974;180:77–98. doi: 10.1002/ar.1091800109. [DOI] [PubMed] [Google Scholar]
- Cardoso WV. Molecular regulation of lung development. Annu. Rev. Physiol. 2001;63:471–494. doi: 10.1146/annurev.physiol.63.1.471. [DOI] [PubMed] [Google Scholar]
- Darlow BA, Graham PJ. Vitamin A supplementation for preventing morbidity and mortality in very low birthweight infants. Cochrane Database Syst. Rev. 2000. CD000501. [DOI] [PubMed]
- Deng H, Mason SN, Auten RL., Jr Lung inflammation in hyperoxia can be prevented by antichemokines in newborn rats. Am. J. Respir. Crit. Care Med. 2000;162:2316–2323. doi: 10.1164/ajrccm.162.6.9911020. [DOI] [PubMed] [Google Scholar]
- Folkman J, D'Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996;87:1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- Gale NW, Yancopoulos GD. Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev. 1999;13:1055–1066. doi: 10.1101/gad.13.9.1055. [DOI] [PubMed] [Google Scholar]
- Gilbert KA, Rannels DE. Increased lung inflation induces gene expression after pneumonectomy. Am. J. Physiol. 1998;275:L21–L29. doi: 10.1152/ajplung.1998.275.1.L21. [DOI] [PubMed] [Google Scholar]
- Gutierrez JA, Ertsey R, Scavo LM, Collins E, Dobbs LG. Mechanical distention modulates alveolar epithelial cell phenotopic expression by transcriptional regulation. Am. J. Resp. Cell Mol. Biol. 1999;21:223–229. doi: 10.1165/ajrcmb.21.2.3665. [DOI] [PubMed] [Google Scholar]
- Hall SM, Hislop AA, Pierce C, Haworth SG. Prenatal origins of human intrapulmonary arteries: formation and smooth muscle maturation. Am. J. Resp. Cell Mol. Biol. 2000;23:194–203. doi: 10.1165/ajrcmb.23.2.3975. [DOI] [PubMed] [Google Scholar]
- Hall SM, Hislop AA, Haworth SG. Origin, differentiation and maturation of human pulmonary veins. Am. J. Resp. Cell Mol. Biol. 2002;26:333–340. doi: 10.1165/ajrcmb.26.3.4698. [DOI] [PubMed] [Google Scholar]
- Healy AM, Morgenthau L, Zhu X, Farber HW, Cardoso WV. VEGF is deposited in the subepitelial matrix at the leading edge of branching airways and stimulates neovascularization in the murine embryonic lung. Dev. Dyn. 2000;219:341–352. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1061>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Hislop A, Reid L. Intrapulmonary arterial development during fetal life – branching pattern and structure. J. Anat. 1972;113:35–48. [PMC free article] [PubMed] [Google Scholar]
- Hislop A, Reid L. Fetal and childhood development of the intrapulmonary veins in man – branching pattern and structure. Thorax. 1973;28:313–319. doi: 10.1136/thx.28.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hislop A, Wigglesworth JS, Desai R, Aber V. The effects of preterm delivery and mechanical ventilation on human lung growth. Early Hum. Dev. 1987;15:147–164. doi: 10.1016/0378-3782(87)90003-x. [DOI] [PubMed] [Google Scholar]
- Hislop AA, Haworth SG. Airway size and structure in the normal fetal and infant lung and the effect of premature delivery and artificial ventilation. Am. Rev. Resp. Dis. 1989;140:1717–1726. doi: 10.1164/ajrccm/140.6.1717. [DOI] [PubMed] [Google Scholar]
- Hislop AA, Haworth SG. Pulmonary vascular damage in the development of cor pulmonale following hyaline membrane disease. Paediat. Pulmon. 1990;9:152–161. doi: 10.1002/ppul.1950090306. [DOI] [PubMed] [Google Scholar]
- Ijsselstijn H, Tibboel D. The lungs in congenital diaphragmatic hernia: do we understand? Pediat. Pulmonol. 1998;26:204–218. doi: 10.1002/(sici)1099-0496(199809)26:3<204::aid-ppul8>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, et al. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am. J. Physiol. 2000;279:L600–L607. doi: 10.1152/ajplung.2000.279.3.L600. [DOI] [PubMed] [Google Scholar]
- Jobe AJ. The new BPD: an arrest of lung development. Pediatr. Res. 1999;46:641–643. doi: 10.1203/00006450-199912000-00007. [DOI] [PubMed] [Google Scholar]
- Kallapur SG, Willet KE, Jobe AH, Ikegami M, Bachurski CJ. Intra-amniotic endotoxin: chorioamnionitis precedes lung maturation in preterm lambs. Am. J. Physiol. 2001;280:L527–L536. doi: 10.1152/ajplung.2001.280.3.L527. [DOI] [PubMed] [Google Scholar]
- Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J. Clin. Invest. 2000;106:1311–1319. doi: 10.1172/JCI10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaza AK, Kron IL, Kern JA, Long SM, Fiser SM, Nguyen RP, et al. Retinoic acid enhances lung growth after pneumonectomy. Ann. Thorac. Surg. 2001;71:1645–1650. doi: 10.1016/s0003-4975(01)02478-x. [DOI] [PubMed] [Google Scholar]
- Kini AR, Peterson LA, Tallman MS, Lingen MW. Angiogenesis in acute promyelocytic leukemia: induction by vascular endothelial growth factor and inhibition by all-trans retinoic acid. Blood. 2001;97:3919–3924. doi: 10.1182/blood.v97.12.3919. [DOI] [PubMed] [Google Scholar]
- Kitagawa M, Hislop A, Boyden EA, Reid L. Lung hypoplasia in congenital diaphragmatic hernia. A quantitative study of airway, artery and alveolar development. Br. J. Surg. 1971;58:342–346. doi: 10.1002/bjs.1800580507. [DOI] [PubMed] [Google Scholar]
- Koh DW, Roby JD, Starcher B, Senior RM, Pierce RA. Postpneumonectomy lung growth: a model of reinitiation of tropoelastin and type I collagen production in a normal pattern in adult rat lung. Am. J. Respir. Cell Mol. Biol. 1996;15:611–623. doi: 10.1165/ajrcmb.15.5.8918368. [DOI] [PubMed] [Google Scholar]
- Loosli CG, Potter EL. Pre and postnatal development of the respiratory portion of the human lung. Am. Rev. Resp. Dis. 1959;80:5–20. doi: 10.1164/arrd.1959.80.1P2.5. [DOI] [PubMed] [Google Scholar]
- Maniscalco WM, Watkins RH, D'Angio CT, Ryan RM. Hyperoxic injury decreases alveolar epithelial cell expression of vascular endothelial growth factor (VEGF) in neonatal rabbit lung. Am. J. Respir. Cell Mol. Biol. 1997;16:557–567. doi: 10.1165/ajrcmb.16.5.9160838. [DOI] [PubMed] [Google Scholar]
- Massaro GD, Massaro D. Retinoic acid treatment partially rescues failed septation in rats and in mice. Am. J. Physiol. 2000;278:L955–L960. doi: 10.1152/ajplung.2000.278.5.L955. [DOI] [PubMed] [Google Scholar]
- McCray PB. Spontaneous contractility of human fetal airway smooth muscle. Am. J. Resp. Cell Mol. Biol. 1993;8:573–580. doi: 10.1165/ajrcmb/8.5.573. [DOI] [PubMed] [Google Scholar]
- McGowan S, Jackson SK, Jenkins-Moore M, Dai HH, Chambon P, Snyder JM. Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. Am. J. Respir. Cell Mol. Biol. 2000;23:162–167. doi: 10.1165/ajrcmb.23.2.3904. [DOI] [PubMed] [Google Scholar]
- de Mello DE, Sawyer D, Galvin N, Reid LM. Early fetal development of lung vasculature. Am. J. Respir. Cell Mol. Biol. 1997;16:568–581. doi: 10.1165/ajrcmb.16.5.9160839. [DOI] [PubMed] [Google Scholar]
- Minoo P. Transcriptional regulation of lung development: emergence of specificity (Review) Respir. Res. 2000;1:109–115. doi: 10.1186/rr20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng YS, Rohan R, Sunday ME, deMello DE, D'Amore PA. Differential expression of VEGF isoforms in mouse during development and in the adult. Dev. Dyn. 2001;220:112–121. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1093>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Ruocco S, Lallemand A, Tournier JM, Gaillard D. Expression and localization of epidermal growth factor, transforming growth factor-alpha, and localization of their common receptor in fetal human lung development. Pediatr. Res. 1996;39:448–455. doi: 10.1203/00006450-199603000-00012. [DOI] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolberg-Buchholz K, Fujiwara Y, Gendron-Maguire M, et al. Distinct roles of the receptor tyrosine kinases tie-1 and tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- Schenk S, Chiquet-Ehrismann R, Battegay EJ. The fibrinogen globe of tenascin-C promotes basic fibroblast growth factor-induced endothelial cell elongation. Molec. Biol. Cell. 1999;10:2933–2943. doi: 10.1091/mbc.10.9.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler MB, Bohn DJ, Bryan AC, Cutz E, Rabinovitch M. Increased respiratory system resistance and bronchial smooth muscle hypertrophy in children with acute postoperative pulmonary hypertension. Am. J. Respir. Crit. Care Med. 1995;152:1347–1352. doi: 10.1164/ajrccm.152.4.7551393. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, et al. Failure of blood-island formation and vasculogenesis in Flk-1 deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Shehata SMK, Tibboel D, Sharma HS, Mooi WJ. Impaired structural remodelling of pulmonary arteries n newborns with congenital diaphragmatic hernia: a histological study of 29 cases. J. Pathol. 1999;189:112–118. doi: 10.1002/(SICI)1096-9896(199909)189:1<112::AID-PATH395>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Shifren JL, Doldi N, Ferrara N, Mesiano S, Jaffe RB. In the human fetus, vascular endothelial growth factor is expressed in epithelial cells and myocytes, but not vascular endothelium: implications for mode of action. J. Clin. Endocrin. Metab. 1994;79:316–322. doi: 10.1210/jcem.79.1.8027247. [DOI] [PubMed] [Google Scholar]
- Smith JD, Davies N, Willis AI, Sumpio BE, Zilla P. Cyclic stretch induces the expression of vascular endothelial growth factor in vascular smooth muscle cells. Endothelium. 2001;8:41–48. doi: 10.3109/10623320109063156. [DOI] [PubMed] [Google Scholar]
- Sparrow MP, Weichselbaum Markus McCray PB. Development of the innervation and airway smooth muscle in human fetal lung. Am. J. Resp. Cell Mol. Biol. 1999;20:550–560. doi: 10.1165/ajrcmb.20.4.3385. [DOI] [PubMed] [Google Scholar]
- Thurlbeck WM. Postnatal human lung growth. Thorax. 1982;37:564–571. doi: 10.1136/thx.37.8.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veness-Meehan KA, Bottone FG, Jr, Stiles AD. Effects of retinoic acid on airspace development and lung collagen in hyperoxia-exposed newborn rats. Pediatr. Res. 2000;48:434–444. doi: 10.1203/00006450-200010000-00004. [DOI] [PubMed] [Google Scholar]
- Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech. Devel. 2000;92:55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- Warburton D, Zhao J, Berberich MA, Bernfield M. Molecular embryology of the lung: then, now, and in the future. Am. J. Physiol. 1999;276:L697–L704. doi: 10.1152/ajplung.1999.276.5.L697. [DOI] [PubMed] [Google Scholar]
- Wardle SP, Hughes A, Chen S, Shaw NJ. Randomised controlled trial of oral vitamin A supplementation in infants to prevent chronic lung disease. Arch. Dis. Childh. 2001;84:F9–F13. doi: 10.1136/fn.84.1.F9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner BB, Stuart LA, Papes RA, Wispe JR. Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am. J. Physiol. 1998;275:L110–L117. doi: 10.1152/ajplung.1998.275.1.L110. [DOI] [PubMed] [Google Scholar]
- Watterberg KL, Demers LM, Scott SM, Murphy S. Chorioamnionitis and early lung inflammation in infants in whom bronchopulmonary dysplasia develops. Pediatrics. 1996;97:210–215. [PubMed] [Google Scholar]
- Wendel DP, Taylor DG, Albertine KH, Keating MT, Li DY. Impaired distal airway development in mice lacking elastin. Am. J. Resp. Cell Mol. Biol. 2000;23:320–326. doi: 10.1165/ajrcmb.23.3.3906. [DOI] [PubMed] [Google Scholar]
- Yang Y, Beqaj S, Kemp P, Ariel I, Schuger L. Stretch-induced alternative splicing of serum response factor promotes bronchial myogenesis and is defective in lung hypoplasia. J. Clin. Invest. 2000;106:1321–1330. doi: 10.1172/JCI8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltner TB, Burri PH. The postnatal development and growth of the human lung. II. Morphology. Resp. Physiol. 1986;67:269–282. doi: 10.1016/0034-5687(87)90058-2. [DOI] [PubMed] [Google Scholar]
- Zeng X, Gray M, Stahlman MT, Whitsett JA. TGF-beta1 perturbs vascular development and inhibits epithelial differentiation in fetal lung in vivo. Dev. Dyn. 2001;221:289–301. doi: 10.1002/dvdy.1140. [DOI] [PubMed] [Google Scholar]