Abstract
To the respiratory physiologist or anatomist the diaphragm muscle is of course the prime mover of tidal air. However, gastrointestinal physiologists are becoming increasingly aware of the value of this muscle in helping to stop gastric contents from refluxing into the oesophagus. The diaphragm should be viewed as two distinct muscles, crural and costal, which act in synchrony throughout respiration. However, the activities of these two muscular regions can diverge during certain events such as swallowing and emesis. In addition, transient crural muscle relaxations herald the onset of spontaneous acid reflux episodes. Studying the motor control of this muscular barrier may help elucidate the mechanism of these episodes. In the rat, the phrenic nerve divides into three branches before entering the diaphragm, and it is possible to sample single neuronal activity from the crural and costal branches. This review will discuss our recent findings with regard to the type of motor axons running in the phrenic nerve of the rat. In addition, we will outline our ongoing search for homologous structures in basal vertebrate groups. In particular, the pipid frogs (e.g. the African clawed frog, Xenopus laevis) possess a muscular band around the oesophagus that appears to be homologous to the mammalian crural diaphragm. This structure does not appear to interact directly with the respiratory apparatus, and could suggest a role for this region of the diaphragm, which was not originally respiratory.
Keywords: crural diaphragm, phrenic nerve, Xenopus
The two muscles of the diaphragm and diaphragmatic development
The mammalian diaphragm has traditionally been studied as a respiratory muscle. However, there is mounting evidence that suggests that it should more correctly be characterized as two separate muscles, the crural diaphragm and the costal diaphragm (De Troyer et al. 1981; Mittal, 1993). During human development of the costal diaphragm, myoblasts originating in the body wall, probably derived from the third, fourth and fifth cervical segments, invade two pleuroperitoneal membranes. The primordial target for phrenic nerve outgrowth appears to these pleuroperitoneal folds and not as originally thought the septum transversum (Greer et al. 1999). In contrast, the crura develop in the mesentery of the oesophagus (Langman, 1969). For reasons outlined later in connection with phylogeny, it is of interest to ascertain whether crural myoblasts are of oesophageal or body wall origin.
Motor innervation of the diaphragm
The diaphragm receives its motor innervation via the phrenic nerve, with separate branches innervating the crural and costal regions (Gordon & Richmond, 1990). An older notion of an extra-phrenic motor innervation has almost disappeared from the literature (Sant’Ambrogio et al. 1963). The literature on the topography of phrenic innervation is concerned with three areas: spinal cord organization, nerve root origins and the projection pattern of phrenic nerve branches.
If we consider the diaphragm to be composed of two muscles, the question arises as to whether the phrenic nerve represents the fusion of two different nerves. Also it is relevant to establish whether there is a distinct crural motor nucleus in the spinal cord. Originally, the innervation of the costal and crural diaphragm was indeed thought to be segmental. In the dog, the C5 root has been reported to innervate almost exclusively the costal diaphragm, whilst the C7 root innervates the crural diaphragm (De Troyer et al. 1982). In the cat, Sant’Ambrogio et al. (1963) showed that electrical activity of a hemi-diaphragm requires intact C4−6 rootlets with the vertebral (or crural) region mainly receiving its innervation from C6 and the costal region from C5. However, later more refined topographical mapping studies in the cat showed that there was no such simple segmental organization, but that generally the ventral region of both muscles was innervated by the C5 neurones, and the dorsal portions of both were innervated by the C6 neurones (Fournier & Sieck, 1988). The use of an elegant technique involving electrically evoked glycogen depletion greatly increased the resolution of cervical root and phrenic nerve branch innervation territories (Hammond et al. 1989). Advances have been forthcoming not only in the study of peripheral projection of phrenic nerve roots and branches but also in the analysis of the central origin of crural and costal motoneurones in the spinal cord (Gordon & Richmond, 1990). When three different tracers were applied to cat phrenic nerve branches, retrogradely labelled motoneurones were found to be quite mixed throughout the full extent of the phrenic motor nucleus. Therefore, whilst there is a highly delineated strip-like peripheral projection to muscle fibres, the motoneurones in the spinal cord are relatively intermingled rostrocaudally, dorsoventrally and mediolaterally (Gordon & Richmond, 1990). Although there are no simple, obvious differences between the anatomical arrangement of the crural and costal motor innervation at the spinal cord or root level, recordings of single motor fibres may yet reveal fundamental differences in their motor control.
Sensory innervation of the diaphragm
The afferent feedback from the diaphragm may give some clues as to the function of the crural and costal muscles. Traditionally, diaphragmatic proprioception has been thought to have little or no role in the regulation of breathing (Corda et al. 1965). Histological studies of muscle spindles in the cat by Duron et al. (1978) showed a rather sparse distribution of muscle spindles in the cat diaphragm in comparison with other skeletal muscles, with a mere 10 spindles identified in the entire diaphragm. Perhaps significantly, all 10 spindles were located within the crural diaphragm. On the other hand, Balkowiec et al. (1995) recorded 13 spindle afferents from a single cat phrenic nerve, suggesting a spindle density more typical of skeletal muscle (although, again, their distribution was predominantly, but not exclusively, crural). However, as the projections of the spindle afferents are unknown, the absolute number of spindles may not be relevant; a small number of afferents may still be capable of exerting an influence on the activity of a large number of motoneurones. The dramatic findings by Balkowiec et al. (1995) led Jammes et al. (2000) to revisit the question of diaphragmatic proprioception. In contrast, they found that high-frequency diaphragm mechano-stimulation had no effect on the activity of the crural or costal segments whilst the remote intercostal muscles showed a clear increase of activity. Unfortunately, this study was performed in a different species (the rabbit) compared to the study of Balkowiec et al. (1995). What is clear from the history of diaphragmatic proprioception is that all the studies have been undertaken by respiratory physiologists, who are, by default, obsessed with respiration. If we consider that the crural diaphragm is not a respiratory muscle but a gastrointestinal sphincter then the adequate stimulus for crural mechanoreceptors may be oesophageal distension at the hiatus not vibration of the central tendon; and a more meaningful output to study may be the manometric profile of sphincter pressure.
The uncertainty as to the role of phrenic afferents is not helped by the fact that there is evidence that some of the afferents are not of diaphragmatic origin. Kostreva &Pontus (1993a, b) have shown, in the dog, that the phrenic nerve contains afferents from the pericardium, liver and vena cava. Liver afferents in the phrenic nerve were also suggested by Alexander (1940) in the cat. These must be taken into account in any study of phrenic nerve mechanoreceptor afferents.
Oesophageal distension and crural diaphragm relaxation
In order for a food bolus to pass easily into the stomach, the crural diaphragm briefly ceases to contract with the rest of the diaphragm during inspiration, thus allowing bolus transit across the diaphragm. Dramatic divergence of the activity of the crural and costal diaphragm is seen during swallowing and oesophageal distension. This selective crural inhibition evoked by balloon distension of the oesophagus has been reported by many researchers (Cherniack et al. 1984; Altschuler et al. 1985; Oliven et al. 1989; Oyer et al. 1989a; Holland et al. 1994; Jones et al. 1994; Liu et al. 2000).
The precise mechanism that mediates the reflex inhibition of the crural diaphragm during oesophageal distension is still somewhat unclear. In certain studies, bilateral vagotomy was seen to abolish the reflex (Altschuler et al. 1985; Cherniack et al. 1984; Oyer et al. 1989a; Jones et al. 1994). Although it has been suggested that vagal afferents from the oesophagus exert an inhibitory influence on the brainstem inspiratory neurones controlling the crural diaphragm, Altschuler et al. (1987) could not demonstrate any inhibition of medullary inspiratory neurones in response to oesophageal distension in the cat. This suggests that the selective inhibition of crural fibres could be due to a reflex pathway caudal to the central respiratory pattern generator in the medulla, possibly at the level of the spinal motoneurones themselves. There is very recent evidence, however, that the crural inhibition may be mediated by an even more distal mechanism. Liu et al. (2000) recorded the contraction of the crural diaphragm in cats indirectly from its effect on the oesophageal pressure, while simultaneously stimulating the crural muscle directly. What they found was that, during oesophageal distension, there was a complete relaxation of the oesophagogastric junction, despite a continuous electrical stimulation of the crural diaphragm. This would seem to suggest that a peripheral neural pathway exists between the oesophagus and the crural diaphragm. Another study lends weight to the argument that a peripheral mechanism may be at work here. Oyer et al. (1989a) found that oesophageal distension produced a complete inhibition of the crural diaphragm electromyogram, while at the same time there was only a partial inhibition of the efferent discharge in the crural branch of the phrenic nerve. This observation has been invoked by Liu et al. (2000) to confirm the presence of inhibitory mechanisms distal to the phrenic nerve. There are some hypotheses beginning to form about the possible nature of this peripheral inhibitory mechanism. Neuhuber et al. (1994) showed histologically that myenteric neurones innervate the motor endplates of the oesophageal skeletal muscle. Some of these enteric neurones were positively immunostained for NADPH diaphorase, which in turn indicates the presence of nitric oxide. It is known that nitric oxide can block neurotransmission across the skeletal neuromuscular junction (Wang et al. 1995). Could nitrergic enteric neurones from the oesophagus be innervating and inhibiting the crural diaphragm? This is an intriguing possibility, and suggests that much further work needs to done before the relative importance of central and local mechanisms in oesophageal distension is known.
Another interesting feature of selective inhibition of the crural diaphragm is that it only seems to be selective at low, physiological volumes. In the dog, large volumes inhibit the activity of both the crural and the costal diaphragm (Cherniack et al. 1984). Oliven et al. (1989) found that the peak inspiratory electromyogram of the costal diaphragm decreased with increasing distension volumes, but not as sharply as the crural diaphragm did. With a distension volume of 150 mL, for example, the crural diaphragm activity was completely abolished, whereas the costal diaphragm contractions were attenuated, but still observable. It is known that additional oesophageal afferent fibres, both vagal and spinal, are activated when a distension stimulus increases beyond the normal physiological range to become a noxious stimulus (Pickering et al. 2002). It is logical to assume that the different types of stimuli would produce completely different types of responses, so this change from a selective crural inhibition at low volume to non-selective, total diaphragm inhibition at high volume is rather unsurprising. However, it does highlight the caution needed when selecting the stimulus used to evoke reflex inhibition of the diaphragm.
The role of the diaphragm in emesis
The physiological process of emesis requires the complex integration of muscles of the upper airway, abdomen, gastrointestinal tract and respiratory muscles (Barnes, 1984; Miller, 1990, 1999). As with oesophageal distension and swallowing, the activity profiles of the crural and costal diaphragm diverge during the expulsive phase of vomiting. In the retching phase, the diaphragm contracts strongly as a single muscle, along with the abdominal muscles. This has the effect of increasing the gastric pressure, but the gastric contents cannot readily traverse the diaphragm because of the simultaneous increase in pressure of the oesophagogastric junction due to the crural contraction (Lang, 1990). The mechanics of vomiting are still relatively controversial. The cineradiographic studies of Brizzee (1990) describe a shuttling of vomitus back and forth between stomach and oesophagus during the retching phase.
There is consensus, however, on the next stage of the vomiting cycle. With the switch to the expulsive phase, the crural and costal diaphragm dissociate their activities, with the crural diaphragm relaxing to allow the ejection of the gastric contents and the costal diaphragm contracting to increase the abdominal pressure and thus force the gastric contents outwards (McCarthy & Borison, 1974; Monges et al. 1978; Barnes, 1984; Miller, 1990). Miller et al. (1988) showed that the selective inhibition of the crural diaphragm persisted after bilateral vagotomy, suggesting that the relaxation arises from a central programme and is independent of vagal feedback.
In the case of the rat, a non-emetic species, the situation may be slightly different. Pollard et al. (1985) examined the activity of the crural and costal diaphragm during normal breathing and eating in conscious rats with chronically implanted electrodes in both diaphragm muscles. What they found was that during swallowing, the crural and costal diaphragm relaxed to allow the transit of the bolus. At no time did they observe a dissociation of crural and costal activity. It appears as if, unlike the cat, the rat is unable to exert separate and selective control of either the diaphragmatic muscle. The fact that rats are non-emetic may be intrinsically related to their apparent inability to dissociate the activity of the crural and costal diaphragm.
The diaphragm and the anti-reflux barrier
Boyle et al. (1985) correlated the contractions of the crural diaphragm to the phasic, inspiratory increases in oesophagogastric junction pressure in the cat. Mittal (1993) draws the analogy between the oesophagogastric barrier and the internal and external anal sphincter, suggesting that the smooth muscle lower oesophageal sphincter is an ‘internal’ sphincter, and the skeletal muscle crural diaphragm is an ‘external’ sphincter.
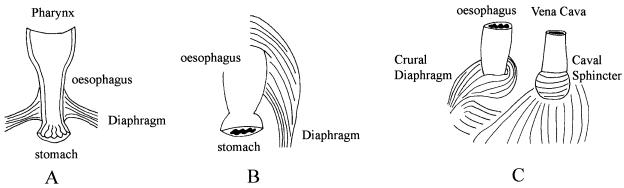
De Troyer et al. (1981, 1982) showed that whilst the costal diaphragm expands the lower rib cage, the crural diaphragm does not change the dimensions of the rib cage appreciably. The crural diaphragm, it seems, has a minor respiratory role, but is greatly involved in gastroesophageal functions, such as swallowing, vomiting, and contributing to the gastroesophageal reflux barrier. The anatomical position and structure of the diaphragm is such that it forms a physical barrier between the abdominal cavity and the thorax. This allows the muscle to generate the negative pressures in the thorax to fill the lungs. This barrier, however, must be traversed by the gastrointestinal system. It is primarily in the facilitation of gastrointestinal transport that the activities of the crural and costal muscles diverge. In contrast, respiratory changes do not generally evoke different response patterns in the two muscles. Oyer et al. (1989b) showed little difference between the activity of costal and crural muscles during normal breathing and in response to hypoxia and hypercapnia. The respiratory rhythm, which the crural muscle exhibits, is appropriate for its function, since descent of the costal diaphragm creates a thoraco-abdominal pressure gradient which favours acid reflux. It is worth noting that the diaphragmatic barrier must not only be traversed by the oesophagus, it must also be traversed by the vena cava. In certain diving mammals such as seals, this region of the diaphragm has become specialized to form a caval sphincter. In a similar fashion to the oesophageal crural sphincter, this caval sphincter is innervated by the phrenic nerve (Harrison & Tomlinson, 1963) (see Fig. 1).
Fig. 1.
Schematic general representation of ‘diaphragms’ from three different vertebrates. A = Frog (Xenopus), B = Frog (Pipa), C = Seal (Phoca). A and B are diagrams of the muscular attachments to the frog oesophagus of the amphibian ‘diaphragm’. C is the diaphragm of a diving mammal (e.g. the seal, Phoca). The crural diaphragm envelops the oesophagus as is typical of mammalian diaphragms, but in addition the diaphragm also forms a funnel-shaped sphincter around the posterior vena cava of the seal.
In cat and human the lower oesophageal sphincter and the crural diaphragm are anatomically superimposed (Biancani et al. 1982) and therefore it is difficult to ascertain the relative contribution of oesophageal and crural muscle to junction pressure. In the rat, however, there is an anatomical separation of the crural diaphragm and the lower oesophageal sphincter. Thus, the contribution of the crural sphincter may be studied in isolation (Soto et al. 1997). In this species a three-dimensional reconstruction of the crural sphincter pressure could be usefully performed at different levels of phrenic nerve electrical stimulation. The increasingly popular use of three-dimensional reconstruction of lower oesophageal pressure is marred by the fact that breathholding is involved in the procedure. The variability amongst studies of the phase of respiration in which breath is held suggests there may be some lack of awareness of the crural sphincter amongst gastroenterologists.
Gastro-oesophageal reflux disease
Gastro-oesophageal reflux occurs when the antireflux barrier is ineffective, allowing acidic gastric contents to pass into the oesophagus (Schoeman et al. 1995). It is of great clinical importance, and has been implicated as a possible contributing factor in sudden infant death syndrome (Thatch, 2000) and the pathogenesis of Barretts oesophagus (DeMeester et al. 1999). This reflux may be due to relaxations of the lower oesophageal sphincter (LES) related to a swallow, or inappropriate transient relaxations. In a study of normal healthy subjects, Schoeman et al. (1995) found that transient lower oesophageal sphincter relaxation was the mechanism of reflux in 82% of episodes, irrespective of activity or body position, whereas swallow-related LES relaxations accounted for 13%. The early history of studies relating to gastro-oesophageal reflux disease tended to concentrate on the lower oesophageal sphincter, i.e. the intrinsic muscle of the oesophagus. More recently, attention is focusing on the extrinsic crural sphincter. It has been pointed out already (vide supra) that crural muscle respiratory rhythm may not be expressed for the purpose of moving air but for rhythmically gripping the oesophagus and opposing the action of the costal diaphragm, which can potentially rhythmically squeeze acid from stomach to oesophagus. If this hypothesis is correct then selective crural myotomy should create a state of frequent gastroesophageal reflux. Mittal et al. (1993) performed the crucial experiment and found that indeed there was a significant increase in spontaneous acid reflux. After crural myotomy, intrinsic oesophageal muscle remains to protect the oesophagus from acid reflux. It is of interest that this barrier cannot fully compensate for the loss of the crural muscle. Martin et al. (1992) also emphasized the unique importance of crural diaphragm activity in episodes of spontaneous acid reflux in dogs. Every episode of acid reflux was heralded by a profound selective diminution of crural diaphragm electrical signal. This transient unexplained reduction of crural muscle electrical activity may lie at the very heart of gastroesophageal reflux disease. From all the considerations above it is pertinent to consider our present state of knowledge about the central control of the crural motoneuronal pool.
Recordings of costal and crural motoneurones
In an excellent electron microscopic analysis of the rat phrenic nerve, Langford & Schmidt (1983) calculated that there are a total of approximately 700 axons, of which there are on average 275 myelinated motor fibres and 123 unmyelinated motor fibres. Most but not all of the unmyelinated motor fibre element is connected with the cervical sympathetic chain. Balkowiec & Szulczyk (1992) have described in detail the firing characteristics of sympathetic post-ganglionic fibres in the phrenic nerve of cat. The discharge is characterized by obvious cardiac and inspiratory rhythmicities and the fibres are putative diaphragmatic vasoconstrictors.
Our laboratory has recently developed a method whereby single motoneuronal electrical activity can be recorded from crural and costal phrenic branches (Pickering et al. 2000). Using a glass suction microelectrode technique and spike-triggered averaging (STA), three types of motor units have been classified in the crural and costal branches of the rat phrenic nerve (Pickering & Jones, unpubl. obs.): alpha-motoneurones, gamma motoneurones and C-fibres. This classification is based only on conduction velocity as determined by STA. In contrast to the results of Balkowiec & Szulczyk (1992), the C-fibre units do not display either respiratory or cardiac rhythm in their discharge. At this stage of the investigation, it is unknown what the slow gamma-units are. The fusimotor control of the diaphragm has been explored in the past (Corda et al. 1965) following the observation that unlike external intercostal motoneurones, phrenic motoneurones cannot augment their discharge to an imposed external load. Corda et al. (1965) showed that there is a paucity of proprioceptive innervation of the cat diaphragm and out of 38 spindle afferents, only seven showed a rhythmical fusimotor activation strong enough to drive the spindle during inspiration.
It is also possible that the gamma units may be myelinated preganglionic (B) fibres (although this is a strange anatomical route for the fibres to adopt), or these slow myelinated fibres may be extrafusal motoneurones with small motor units. Finally, these units may represent a primitive motor system as described in amphibia, that is a β-motoneurone system, which supply both intra- and extrafusal fibres. Clearly much work remains to be done to decide between these various hypotheses.
Evolution of the diaphragm: the natural history of a dual function muscle
If the diaphragm is to be seen as a single structure, it is unusual in that it appears to have two completely different functions. This raises the interesting question as to which came first. How did such a muscle evolve? Did a respiratory muscle become recruited by the digestive system, or did a gastrointestinal muscle become adapted for ventilating the lungs? One might intuitively assume that the respiratory function came first; the contraction of the crural diaphragm during inspiration exists precisely because of the negative pressure caused by the contraction of the costal diaphragm. Although it is tempting to assume this apparently obvious evolutionary history of the diaphragm, the alternative case, that the diaphragm initially had a purely gastrointestinal function, must be considered.
Some hints that the latter case may be true come from a somewhat unlikely source; the pipid frogs. It is not immediately obvious to go looking for a muscle predominantly associated with negative pressure ventilation in an animal that ventilates its lungs under positive pressure buccal pumping. However, Keith (1905) felt that this was where the precursor of the mammalian diaphragm is to be found. Keith suggested that two muscles found in Xenopus laevis (and similar structures in Rana) corresponded to what we now call the crural and costal diaphragm in mammals. These muscles were innervated by nerves emerging from the cervical plexus, much as the phrenic nerve of mammals does. Keith also believed that these muscles were not involved in respiration. Neither did he seem to consider them to have a gastrointestinal function. Instead, he believed that they were involved in the circulatory system, acting in such a manner as to increase venous pressure and aid the return of blood from the abdomen to the heart. The idea may be interesting, but it does not seem to have captured the imagination of other investigators and, to our knowledge, no further work has been carried out to test this hypothesis.
One of these muscles in Xenopus, which seems to correspond to the crural diaphragm in mammals, was examined again many years later (Snapper et al. 1974). In this study, the effect of this muscle on pulmonary pressure was investigated (the anatomical relationship of the muscle under investigation to the lungs in Xenopus must be taken into consideration). As Keith (1905) illustrated, this narrow band of muscle passes dorsal to the top of each lung. This arrangement is such that the only possible effect of contraction, if any, is to ‘squeeze’ the top of the lung. EMG recordings of the muscle during respiration showed that its activity coincided with the first of two buccal pressure spikes. This was interpreted to mean that the muscle acts to squeeze air out of the lung just before the buccal pump then fills the lung. Snapper et al. (1974) concluded that the function of this muscle is to move air out of the lungs during expiration. However, electrical stimulation of the muscle between breaths was found to produce a very slight increase in pulmonary pressure in comparison to the pressure rises seen during buccal pump inflation. In fact the pressure rises are hardly larger than the pressure ripples generated by heartbeats (see figure 7 in Snapper et al. 1974).
And so the question regarding the function of this muscle in Xenopus remains. It is somewhat curious, however, that previous studies have apparently not focused on a gastro-oesophageal function, especially considering the close association the muscle has with the oesophagus (Fig. 1). Our own unpublished observations (Pickering & Jones) show that all the insertions of the muscle are in the oesophagus just above the oesophagogastric junction, and that contraction of the muscle produces an increase in oesophageal pressure. What is less obvious is why such an effect exists during respiration in a positive pressure ventilating animal such as Xenopus. Unlike mammals, positive pressure inspiration would not produce an oesophagogastric pressure gradient which would be conducive to oesophageal reflux. Bearing in mind that members of the genus Xenopus are entirely aquatic, one may theorize that the muscle contracts during inspiration to prevent aerophagy, because an accumulation of air in the stomach may have significant effects on buoyancy, leaving the animals more prone to predation at the surface of the water. It is an appealing hypothesis; the aquatic ancestors of the terrestrial tetrapods developing a mechanism to prevent aerophagy that later became adapted, by changing the muscle architecture but not the phasic contraction properties, to drawing air into the lungs by negative pressure; but it is merely that, an hypothesis. There are other possibilities which are equally worth considering. Xenopus displays both retching and vomiting behaviour which can culminate in oesophageal and stomach prolapse (Naitoh et al. 1989). During the emetic act Xenopus reduces abdominal volume by sliding the sacrum rostrocaudally along the ilium. It is of interest that one attachment of the ‘crural muscle’ in Xenopus is the ilium. Therefore, this muscle could quite conceivably provide the mechanics to re-internalize the prolapsed stomach. Alternatively, in the frog, the crura may also have a role in keeping live prey in the stomach, but the exact function or functions of this muscle in Xenopus remain(s) unknown. More importantly, much more work must be done before we can say with any degree of certainty that this muscle in Xenopus is homologous to the mammalian crural diaphragm. Xenopus belongs to a basal and highly modified family of anurans that are unique in many aspects of their biology, and their use as a representative model system has been called into question (Kellog & Shaffer, 1993). If it is a homologous structure, it would allow us to gain an understanding of the evolutionary origins of the diaphragm, which will in turn lead to insights into how the diaphragm functions in modern mammals.
Concluding remarks
The crural diaphragm is a gastrointestinal sphincter which is implicated strongly in the pathogenesis of gastro-oesophageal reflux disease. Current therapies of this disorder ameliorate the condition (usually by drying up stomach acid) but do not address the fundamental problem of why transient repetitive relaxations of the crural muscle are occurring. Is this a disease of disordered motor control of the crural diaphragm? Is there direct inhibition or disfacilitation of crural motoneurones at the spinal cord level? It may be the case that motor unit recruitment is malfunctioning somehow. A more exotic explanation is inadequate gamma-motor drive. The recent exciting findings concerning a peripheral mechanism of crural muscle inhibition (Liu et al. 2000) should be followed up with enthusiasm.
Acknowledgments
We wish to acknowledge the generous support of the Health Research Board (Ireland).
References
- Alexander WF. The innervation of the biliary system. J. Comparative Neurol. 1940;72:357–370. [Google Scholar]
- Altschuler SM, Boyle JT, Nixon TE, Pack AI, Cohen S. Simultaneous reflex inhibition of lower esophageal sphincter and crural diaphragm in cats. Am. J. Physiol. 1985;249:G586–G591. doi: 10.1152/ajpgi.1985.249.5.G586. [DOI] [PubMed] [Google Scholar]
- Altschuler SM, Davies RO, Pack AI. Role of medullary neurones in the control of the diaphragm during oesophageal stimulation in cats. J. Physiol. 1987;39:289–298. doi: 10.1113/jphysiol.1987.sp016738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Szulczyk P. Properties of postganglionic sympathetic neurons with axons in phrenic nerve. Respiration Physiol. 1992;88:323–331. doi: 10.1016/0034-5687(92)90006-i. [DOI] [PubMed] [Google Scholar]
- Balkowiec A, Kukula K, Szulczyk P. Functional classification of afferent phrenic nerve fibres and diaphragmatic receptors in cats. J. Physiol. 1995;483:759–768. doi: 10.1113/jphysiol.1995.sp020620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes JH. The physiology and pharmacology of emesis. Mol Aspects Med. 1984;7:397–508. doi: 10.1016/0098-2997(84)90006-2. [DOI] [PubMed] [Google Scholar]
- Biancani P, Zabinski M, Kerstein M, Behar J. Lower esophageal sphincter mechanics: anatomic and physiologic relationships of the esophagogastric junction of cat. Gastroenterology. 1982;82:466–475. [PubMed] [Google Scholar]
- Boyle JT, Altschuler SM, Nixon TE, Tuchman DN, Pack AI, Cohen S. Role of the diaphragm in the genesis of lower esophageal sphincter pressure in the cat. Gastroenterology. 1985;88:723–730. doi: 10.1016/0016-5085(85)90143-x. [DOI] [PubMed] [Google Scholar]
- Brizzee KR. Mechanics of vomiting: a minireview. Can. J. Physiol. Pharmacol. 1990;68:221–229. doi: 10.1139/y90-035. [DOI] [PubMed] [Google Scholar]
- Cherniack NS, Haxhiu MA, Mitra J, Strohl K, Lunteren EV. Response of upper airway, intercostal and diaphragm muscle activity to stimulation of oesophageal afferents in dogs. J. Physiol. 1984;349:15–25. doi: 10.1113/jphysiol.1984.sp015139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda M, Von Euler C, Lennerstrand G. Proprioceptive innervation of the diaphragm. J. Physiol. 1965;178:161–177. doi: 10.1113/jphysiol.1965.sp007621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Troyer A, Sampson M, Sigrist S, MacKlem PT. The diaphragm: two muscles. Science. 1981;213:237–238. doi: 10.1126/science.7244632. [DOI] [PubMed] [Google Scholar]
- De Troyer A, Sampson M, Sigrist S, MacKlem PT. Action of the costal and crural parts of the diaphragm on the rib cage in dog. J. Appl. Physiol. 1982;53:30–39. doi: 10.1152/jappl.1982.53.1.30. [DOI] [PubMed] [Google Scholar]
- DeMeester TR, Peters JH, Bremner CG, Chandrasoma P. Biology of gastroesophageal reflux disease: pathophysiology relating to medical and surgical treatment. Annu. Rev. Med. 1999;50:469–506. doi: 10.1146/annurev.med.50.1.469. [DOI] [PubMed] [Google Scholar]
- Duron B, Jung-Caillol MC, Marlot D. Myelinated nerve fibre supply and muscle spindles in the respiratory muscles of the cat: a quantitative study. Anat. Embryol. 1978;152:171–192. doi: 10.1007/BF00315923. [DOI] [PubMed] [Google Scholar]
- Fournier M, Sieck GC. Somatotopy in the segmental innervation of the cat diaphragm. J. Appl. Physiol. 1988;64:291–298. doi: 10.1152/jappl.1988.64.1.291. [DOI] [PubMed] [Google Scholar]
- Gordon DC, Richmond FJ. Topography in the phrenic motoneuron nucleus demonstrated by retrograde multiple-labelling techniques. J. Comparative Neurol. 1990;292:424–434. doi: 10.1002/cne.902920308. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Allan DW, Martin-Caraballo M, Lemke RP. An overview of phrenic nerve and diaphragm muscle development in the perinatal rat. J. Appl. Physiol. 1999;86:779–786. doi: 10.1152/jappl.1999.86.3.779. [DOI] [PubMed] [Google Scholar]
- Hammond CG, Gordon DC, Fisher JT, Richmond FJ. Motor unit territories supplied by primary branches of the phrenic nerve. J. Appl. Physiol. 1989;66:61–71. doi: 10.1152/jappl.1989.66.1.61. [DOI] [PubMed] [Google Scholar]
- Harrison RJ, Tomlinson JDW. Anatomical and physiological adaptations in diving mammals. In: Carthy JD, Duddington CL, editors. Viewpoints in Biology. Vol. 2. London: Butterworths; 1963. pp. 115–162. [Google Scholar]
- Holland CT, Satchell PT, Farrow BRH. Vagal afferent dysfunction in naturally occurring canine esophageal motility disorder. Digestive Dis. Sci. 1994;39:2090–2098. doi: 10.1007/BF02090355. [DOI] [PubMed] [Google Scholar]
- Jammes Y, Arbogast S, De Troyer A. Response of the rabbit diaphragm to tendon vibration. Neuroscience Lett. 2000;290:85–88. doi: 10.1016/s0304-3940(00)01301-x. [DOI] [PubMed] [Google Scholar]
- Jones JFX, Mckeogh D, Nolan P, O'Regan RG. The effects of oesophageal distension on diaphragm and laryngeal muscle activity in the anaesthetized cat. Exp. Physiol. 1994;79:505–513. doi: 10.1113/expphysiol.1994.sp003783. [DOI] [PubMed] [Google Scholar]
- Keith A. The nature of the mammalian diaphragm and pleural cavities. J. Anat. Physiol. 1905;39:243–284. [PMC free article] [PubMed] [Google Scholar]
- Kellog EA, Shaffer HB. Model organisms in evolutionary studies. Syst. Biol. 1993;42:409–414. [Google Scholar]
- Kostreva DR, Pontus SP. Pericardial mechanoreceptors with phrenic afferents. Am. J. Physiol. 1993a;265:H1836–H1846. doi: 10.1152/ajpheart.1993.264.6.H1836. [DOI] [PubMed] [Google Scholar]
- Kostreva DR, Pontus SP. Hepatic vein, hepatic parenchyma, and inferior vena caval mechanoreceptors with phrenic afferents. Am. J. Physiol. 1993b;265:G15–G20. doi: 10.1152/ajpgi.1993.265.1.G15. [DOI] [PubMed] [Google Scholar]
- Lang IM. Digestive tract motor correlates of vomiting and nausea. Can. J. Physiol. Pharmacol. 1990;68:242–253. doi: 10.1139/y90-038. [DOI] [PubMed] [Google Scholar]
- Langford LA, Schmidt RF. An electron microscopic analysis of the left phrenic nerve in the rat. Anat. Record. 1983;205:207–213. doi: 10.1002/ar.1092050211. [DOI] [PubMed] [Google Scholar]
- Langman J. 2. Edinburgh: E: S. Livingstone Ltd; 1969. Medical Embryology; pp. 278–280. [Google Scholar]
- Liu J, Yamamato Y, Schirmer BD, Ross RA, Mittal RK. Evidence for a peripheral mechanism of esophagocrural diaphragm inhibitory reflex in cats. Am. J. Physiol. 2000;278:G281–G288. doi: 10.1152/ajpgi.2000.278.2.G281. [DOI] [PubMed] [Google Scholar]
- Martin C, Dodds W, Liem H, Dantas R, Layman R, Dent J. Diaphragmatic contribution to gastrooesophageal competence and reflux in dogs. Am. J. Physiol. 1992;263:G551–G557. doi: 10.1152/ajpgi.1992.263.4.G551. [DOI] [PubMed] [Google Scholar]
- McCarthy LE Borison. Respiratory mechanics of vomiting in decerebrate cats. Am. J. Physiol. 1974;226:738–743. doi: 10.1152/ajplegacy.1974.226.3.738. [DOI] [PubMed] [Google Scholar]
- Miller AD, Lacos SF, Tan LK. Central motor program for relaxation of perioesophageal diaphragm durin expulsive phase of vomiting. Brain Res. 1988;456:367–370. doi: 10.1016/0006-8993(88)90241-7. [DOI] [PubMed] [Google Scholar]
- Miller AD. Respiratory muscle control during vomiting. Can. J. Physiol. Pharmacol. 1990;68:237–241. doi: 10.1139/y90-037. [DOI] [PubMed] [Google Scholar]
- Miller AD. Central mechanisms of vomiting. Digestive Dis Sci. 1999;44(Suppl):39S–43S. [PubMed] [Google Scholar]
- Mittal RK. The crural diaphragm, an external lower esophageal sphincter: a definitive study. Gastroenterology. 1993;105:1565–1567. doi: 10.1016/0016-5085(93)90167-b. [DOI] [PubMed] [Google Scholar]
- Mittal RK, Sivri B, Schirmer BD, Heine KJ. Effect of crural myotomy on the incidence and mechanism of gastroesophageal reflux in cats. Gastroenterology. 1993;105:740–747. doi: 10.1016/0016-5085(93)90891-f. [DOI] [PubMed] [Google Scholar]
- Monges H, Salducci J, Naudy B. Dissociation between the electrical activity of the diaphragmatic dome and crura muscle fibres during oesophageal distension, vomiting and eructation. J. Physiol. (Paris) 1978;74:541–554. [PubMed] [Google Scholar]
- Naitoh T, Wassersug RJ, Leslie RA. The Physiology, morphology, and ontogeny of emetic behaviour in Anuran Amphibians. Physiol. Zool. 1989;62:819–843. [Google Scholar]
- Neuhuber WL, Worl J, Berthoud HR, Conte B. NADPH diaphorase positive nerve fibres associated with motor endplates in the rat esophagus: new evidence for coinnervation of striated muscle by enteric neurons. Cell Tissue Res. 1994;276:23–30. doi: 10.1007/BF00354780. [DOI] [PubMed] [Google Scholar]
- Oliven A, Haxhiu M, Kelsen SG. Reflex effect of esophageal distension on respiratory muscle activity and pressure. J. Appl. Physiol. 1989;66:536–541. doi: 10.1152/jappl.1989.66.2.536. [DOI] [PubMed] [Google Scholar]
- Oyer LM, Knuth SL, Ward DK, Bartlett D. Reflex inhibition of the crural diaphragmatic activity by esophageal distension in cats. Respiratory Physiol. 1989a;77:195–202. doi: 10.1016/0034-5687(89)90006-6. [DOI] [PubMed] [Google Scholar]
- Oyer LM, Knuth SL, Ward DK, Bartlett D. Patterns of neural and muscular electrical activity in the costal and crural portions of the diaphragm. J. Appl. Physiol. 1989b;66:2092–2100. doi: 10.1152/jappl.1989.66.5.2092. [DOI] [PubMed] [Google Scholar]
- Pickering M, Campion DP, Jones JFX. A technique for recording the electrical activity of single crural phrenic motoneurones in the anaesthetized rat. Irish J. Med. Sci. 2000;169:277. [Google Scholar]
- Pickering M, Campion DP, Jones JFX. Reflex cardiorespiratory effects of nociceptive oesophageal distension in the decerebrate rat. Exp. Physiol. 2002;87:41–48. doi: 10.1113/eph8702241. [DOI] [PubMed] [Google Scholar]
- Pollard MJ, Megirian D, Sherrey JH. Unity of costal and crural diaphragmatic activity in respiration. Exp. Neurol. 1985;90:187–193. doi: 10.1016/0014-4886(85)90051-2. [DOI] [PubMed] [Google Scholar]
- Sant’Ambrogio G, Frazier DT, Wilson MF, Agostini E. Motor innervation and pattern of activity of the cat diaphragm. J. Appl. Physiol. 1963;18:43–46. doi: 10.1152/jappl.1963.18.1.43. [DOI] [PubMed] [Google Scholar]
- Schoeman MN, Tippett MD, Akkermans LM, Dent J, Holloway RH. Mechanisms of gastroesophageal reflux in ambulant healthy human subjects. Gastroenterology. 1995;108:83–91. doi: 10.1016/0016-5085(95)90011-x. [DOI] [PubMed] [Google Scholar]
- Snapper JR, Tenney SM, Mccann FV. Observations on the amphibian ‘diaphragm’. Comparative Biochem. Physiol. A. 1974;49:223–230. doi: 10.1016/0300-9629(74)90111-x. [DOI] [PubMed] [Google Scholar]
- Soto C, Qi B, Diez-Pardo JA, Tovar JA. Identification of diaphragmatic crural component of gastroesophageal barrier in the rat. Digestive Dis. Sci. 1997;42:2420–2425. doi: 10.1023/a:1018831705342. [DOI] [PubMed] [Google Scholar]
- Thatch BT. Sudden infant death syndrome: can gastroesophageal reflux cause sudden infant death? Am. J. Med. 2000;108:144s–148s. doi: 10.1016/s0002-9343(99)00354-x. [DOI] [PubMed] [Google Scholar]
- Wang TI, Xie Z, Bal L. Nitric oxide mediates activity-dependent synaptic suppression at developing neuromuscular synapse. Nature. 1995;374:262–265. doi: 10.1038/374262a0. [DOI] [PubMed] [Google Scholar]