Abstract
Chronic lung disease in humans is frequently complicated by the development of secondary pulmonary hypertension, which is associated with increased morbidity and mortality. Hypoxia, inflammation and increased shear stress are the primary stimuli although the exact pathways through which these initiating events lead to pulmonary hypertension remain to be completely elucidated. The increase in pulmonary vascular resistance is attributed, in part, to remodelling of the walls of resistance vessels. This consists of intimal, medial and adventitial hypertrophy, which can lead to encroachment into and reduction of the vascular lumen. In addition, it has been reported that there is a reduction in the number of blood vessels in the hypertensive lung, which could also contribute to increased vascular resistance. The pulmonary endothelium plays a key role in mediating and modulating these changes. These structural alterations in the pulmonary vasculature contrast sharply with the responses of the systemic vasculature to the same stimuli. In systemic organs, both hypoxia and inflammation cause angiogenesis. Furthermore, remodelling of the walls of resistance vessels is not observed in these conditions. Thus it has been generally stated that, in the adult pulmonary circulation, angiogenesis does not occur. Prompted by previous observations that chronic airway inflammation can lead to pulmonary vascular remodelling without hypertension, we have recently shown, using quantitative stereological techniques, that angiogenesis can occur in the adult pulmonary circulation. Pulmonary angiogenesis has also been reported in some other conditions including post-pneumonectomy lung growth, metastatic disease of the lung and in biliary cirrhosis. Such angiogenesis may serve to prevent or attenuate increased vascular resistance in lung disease. In view of these more recent data, the role of structural alterations in the pulmonary vasculature in the development of pulmonary hypertension should be carefully reconsidered.
Keywords: angiogenesis, chronic inflammation, endothelium, hypoxia, nitric oxide, pulmonary hypetension, remodelling
Introduction
Sustained pulmonary hypertension is a common complication of chronic lung diseases including chronic obstructive pulmonary disease, cystic fibrosis and bronchiectasis. Particularly in the presence of hypoxaemia, such secondary pulmonary hypertension is strongly associated with increased morbidity and reduced survival (Semmens & Reid, 1974; Ryland & Reid, 1975; MacNee, 1994a, b). Furthermore, the presence of cor pulmonale in these conditions is an independent predictor of increased mortality (Incalzi et al. 1999; Skwarski et al. 1991), suggesting that pulmonary hypertension contributes directly to increased mortality.
The poor prognosis observed in untreated primary pulmonary hypertension supports the view that secondary pulmonary hypertension increases mortality. Primary pulmonary hypertension is a syndrome characterized by chronically increased pulmonary vascular resistance in the absence of a known cause, which, if untreated, usually leads to death within four years (Rubin, 1997; Haworth, 1998; Archer & Rich, 2000). Furthermore, successful treatment, which reduces pulmonary arterial pressure and vascular resistance, leads to long-term survival (Archer & Rich, 2000; Barst et al. 1996; Rubin, 1997). These findings demonstrate that pulmonary hypertension per se can shorten life.
Taken together, this evidence suggests that secondary pulmonary hypertension contributes directly to premature mortality in human lung and cardiac diseases and causes substantial added morbidity. Because of this, intensive research efforts are focused on investigating the underlying mechanisms of this condition in order to identify potential novel therapeutic interventions.
Altered vascular structure in pulmonary hypertension
Secondary pulmonary hypertension results from sustained vasoconstriction and structural alterations to the pulmonary vascular bed. The major stimuli that are responsible for these changes are chronic alveolar hypoxia, chronic inflammation and excessive shear stress (Voelkel & Tuder, 1995). Most commonly, these stimuli act together to produce increased pulmonary vascular resistance (Voelkel & Tuder, 1995). Regardless of the stimuli that cause pulmonary hypertension, the structural changes that are thought to underlie the increased vascular resistance can be broadly classified into two processes: first, remodelling of the pulmonary resistance vessels and, second, a reduction in the total number of blood vessels in the lung, sometimes termed rarefaction or pruning.
Remodelling is a process that causes thickening of the arterial walls and is thought to increase resistance by causing the vessel walls to encroach into the lumen and reduce its diameter. A prominent feature of vascular remodelling is medial thickening, a change that is of particular interest because of the ability of medial smooth muscle to alter lumen size by contraction and relaxation. In previously muscularized, more proximal pulmonary vessels, medial enlargement is caused by hypertrophy and hyperplasia of the pre-existing vascular smooth muscle cells (Meyrick & Reid, 1978, 1979a; Meyrick et al. 1980; Meyrick & Brigham, 1986). In addition, the smooth muscle cells elaborate extracellular matrix proteins, which also contribute to medial enlargement (Poiani et al. 1990). In previously non-muscular precapillary arterioles, the de novo development of a muscular media is observed. The smooth muscle cells, which form this new media, are derived by differentiation of intermediate cells that are present in the normal walls of these vessels (Meyrick & Reid, 1978; Jones, 1992). Smooth muscle cells are also formed by migration and differentiation of interstitial fibroblasts (Jones, 1992). A new internal elastic lamina is formed in these remodelled vessels so that their structure comes to resemble that of the resistance vessels of the systemic circulation (Fig. 1). Adventitial hypertrophy is also a prominent feature of pulmonary hypertension. Resident fibroblasts proliferate and produce matrix proteins in response to hypoxia and chronic inflammation (Meyrick & Reid, 1979b).
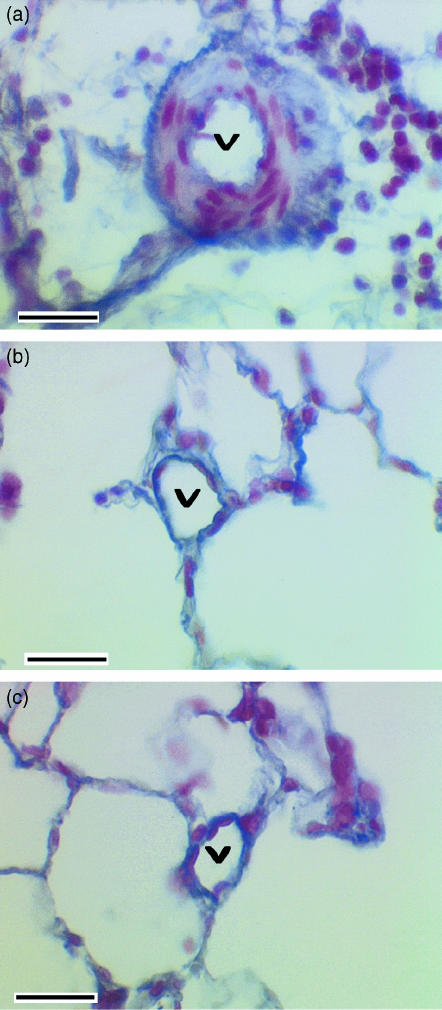
Fig. 1.
Photomicrographs showing intra-acinar pulmonary blood vessels (v) from (a) a rat lung in which chronic airway infection had been induced by inoculation of Pseudomonas aeruginosa in agar beads, (b) lung inoculated with sterile agar beads alone, and (c) lung that had not been inoculated. Sections were stained with Miller's stain, which shows elastin as blue, and counter stained with haematoxylin to demonstrate nuclei. Note well-developed tunica media in chronically infected lungs with an internal and external elastic lamina, whereas no tunica media is seen and there is a single elastic lamina in non-inoculated lung and in lung inoculated with sterile agar beads. Scale bar = 40 μm in all panels. (Figure reproduced with permission from Hopkins et al. Journal of Applied Physiology, 91: 919–928, 2001.)
Reduction in the total number of blood vessels in a vascular bed will increase vascular resistance by reducing the number of paralleled pathways through that circulation. Rarefaction of vessels in the pulmonary circulation has been reported in human subjects with pulmonary hypertension (Ryland & Reid, 1975; Rabinovitch et al. 1979) and in animal models (Hislop & Reid, 1976, 1977; Meyrick & Reid, 1979a; Meyrick et al. 1980; Meyrick & Brigham, 1986; Jones & Reid, 1995; Partovian et al. 2000). This loss of blood vessels has been detected as a reduction in the ratio of the number of blood vessels to the number of alveoli in the intra-acinar (gas exchange) regions of the lung. A similar process of blood vessel loss is well recognized in systemic hypertension and contributes significantly to increased peripheral vascular resistance (Bohlen, 1989; Prewitt et al. 1982; Greene et al. 1989; Hansen-Smith et al. 1990; Schiffrin, 1992; Hernandez et al. 1999).
Hypoxia and vascular remodelling
The evidence that chronic hypoxia leads to pulmonary hypertension is extensive and has been reviewed previously (Fishman, 1985; Heath et al. 1973; Rabinovitch et al. 1979; Grover et al. 1983; Meyrick & Reid, 1983; MacNee, 1994a; Jones & Reid, 1995). In brief, exposure of humans and a wide variety of animal species to hypoxic environments causes pulmonary hypertension associated with right ventricular hypertrophy and pulmonary vascular remodelling. There is a great variation in the magnitude of this response in different species and in individuals within species (Fishman, 1985; Grover et al. 1983). In some susceptible individuals, exposure to high altitude leads to progressively worsening pulmonary hypertension, right ventricular failure and ultimately death, e.g. chronic mountain sickness in human children and Brisket disease in cattle (Hecht et al. 1962; Sui et al. 1988). Return to an environment with a normal partial pressure of oxygen reverses these changes in normal and susceptible individuals (Heath et al. 1973; Hislop & Reid, 1977; Grover et al. 1983; Fishman, 1985).
During the early period of hypoxic exposure, vascular resistance is elevated largely due to hypoxic vasoconstriction. However, following sustained hypoxia return to a normal pO2 causes an immediate small fall in pulmonary arterial pressure to a value that is substantially above normal (Fried et al. 1983; Sime et al. 1971; Lockhart et al. 1976), suggesting that structural changes in the pulmonary vascular bed become a major determinant of vascular resistance. Most attention has focused on the well-described remodelling of the precapillary resistance vessels as the cause of pulmonary hypertension. These structural changes include muscularization of previously non-muscular arterioles, increased medial thickness of previously partially and completely muscular arterioles and deposition of additional matrix components, including collagen and elastin, in the vascular walls, as described above (Fishman, 1985; Rabinovitch et al. 1979; Grover et al. 1983; Stenmark & Mecham, 1997; Rabinovitch, 1999, 2001).
It is interesting to contrast this response of the pulmonary circulation with that of the systemic circulation. In rats, exposure to chronic hypoxia leads to reduced vascular response to vasoconstrictors both in vivo and in isolated systemic vessels (Doyle & Walker, 1991). Chronic hypoxia has also been shown to reduce systemic arterial blood pressure and increase maximal vascular conductance in normal rats (Smith & Marshall, 1999). Furthermore, when spontaneously hypertensive rats are maintained in chronic hypoxia, systemic hypertension is abrogated (Henley & Tucker, 1987). Studies of humans show that high altitude dwellers in the Andes have a lower incidence of systemic hypertension than age, socio-economic and racially matched sea-level residents (Ruiz & Penaloza, 1977). Marticorena et al. (1969) reported that long-term residence of native low-landers at high altitude leads to a reduction in systemic blood pressure Thus, the evidence from both animal and human studies demonstrates that chronic hypoxia leads to reduction in systemic blood pressure in direct contrast to the sustained hypertension observed in the pulmonary circulation.
Chronic inflammation and vascular remodelling
The second major stimulus to the development of pulmonary hypertension is the presence of chronic inflammation in the lungs. One of the most common forms of chronic lung inflammation is chronic airway inflammation, such as that produced by cigarette smoking or chronic airways infection. Experimentally induced chronic airway inflammation in rats leads to remodelling of pulmonary resistance vessels (Cash et al. 1978; Herget et al. 1981; Graham et al. 1990; McCormack & Paterson, 1993; Cadogan et al. 1999) and the development of pulmonary hypertension (Herget et al. 1981; McCormack & Paterson, 1993). The vascular remodelling induced by chronic airway inflammation is structurally similar to that observed in chronic hypoxic pulmonary hypertension (Fig. 1). Most notably it causes marked medial hypertrophy suggesting that encroachment of the vessel wall on the vascular lumen contributes to increased resistance, in a manner similar to that described in chronic hypoxia. Airway inflammation caused by long-term inhalation of cigarette smoke also causes pulmonary vascular remodelling in rats (Sekhon et al. 1994). In human subjects who are cigarette smokers, the pulmonary vascular remodelling that is observed is disproportionate to the degree of arterial hypoxaemia, suggesting that it results in large part from chronic airway inflammation. Thus structural abnormalities of pulmonary vessels are observed in asymptomatic smokers (Hale et al. 1984; Peinado et al. 1999) and medial thickness in patients with COPD is unrelated to arterial pO2 (Barbera et al. 1994). Furthermore, vascular remodelling is observed in COPD even when arterial pO2 is greater than 8 kPa (Wright et al. 1983; Magee et al. 1988; Peinado et al. 1999), whereas in normal individuals chronic hypoxia in isolation does not cause pulmonary hypertension unless arterial pO2 is less than 8 kPa (Grover et al. 1983). Collectively, these data suggest that chronic airway inflammation leads to pulmonary vascular remodelling and contributes to the development of pulmonary hypertension by mechanisms that are independent of hypoxia.
Inflammation of the pulmonary vasculature and diffuse interstitial inflammatory disease can also lead to remodelling and pulmonary hypertension. In animals, interventions that lead to vascular inflammation, including monocrotaline injection, Crotalaria ingestion and repeated injection of endotoxin, cause remodelling of resistance vessels and pulmonary hypertension (Kay et al. 1967; Heath, 1969; Hislop & Reid, 1974; Meyrick et al. 1980; Meyrick & Reid, 1982; Meyrick & Brigham, 1986; Meyrick & Perkett, 1989; Reindel et al. 1990). Diffuse interstitial inflammatory disease of the lung can also cause pulmonary vascular remodelling and hypertension in animal models (Bishop et al. 1990; Lippmann, 1977; Michel et al. 1988; Champion et al. 1999) and in human disease conditions, including adult respiratory distress syndrome (Katz et al. 1984; Zapol & Snider, 1977; Snow et al. 1982; Tomashefski et al. 1983; Leeman, 1999; Wyncoll & Evans, 1999), cryptogenic fibrosing alveolitis (Giaid et al. 1993) and systemic autoimmune diseases (Gurubhagavatula & Palevsky, 1997).
Shear stress and vascular remodelling
It is well recognized that abnormal haemodynamic shear stress is a potent stimulus to vascular remodelling and the development of pulmonary hypertension. Congenital heart diseases that cause left to right shunt, including patent ductus arteriosus, atrial and ventricular septal defects, chronically augment vascular shear stress in the lung. If left uncorrected, these conditions frequently lead to pulmonary vascular remodelling, increased pulmonary vascular resistance, pulmonary hypertension, shunt reversal, right heart failure and death (Heath & Edwards, 1958; Rabinovitch et al. 1981; Meyrick & Reid, 1983). In animal models, it has been shown that increasing pulmonary blood flow, by the creation of left to right shunts, leads to vascular remodelling, increased resistance and pulmonary hypertension (Bousamra et al. 2000; Friedli et al. 1975; Rendas et al. 1979; Everett et al. 1998).
Increased shear stress may be a significant contributory stimulus to the development of pulmonary hypertension in circumstances in which hypoxia and inflammation are the primary initiating stimulus. In a uniform rigid cylinder, shear stress (τ) at the interface between fluid and vessel wall is given by
where Q is flow rate, η is the viscosity of the fluid and r is the lumen radius. Since the pulmonary circulation must normally accommodate the whole cardiac output, total pulmonary blood flow must remain constant following luminal narrowing whether due to vasoconstriction or remodelling of the vessel wall. This means that shear stress at the vessel wall will increase markedly as it is inversely related to the third power of the radius. The importance of this contribution is clearly illustrated by the work of Rabinovitch et al. (1983). They reduced the vascular shear stress in hypoxic lungs by constricting the left main branch of the pulmonary artery with a ‘band’ and demonstrated marked reduction of medial hypertrophy and the degree of extension of vascular smooth muscle into normally non-muscular vessels (Rabinovitch et al. 1983). Conversely, increased shear stress produced by pneumonectomy worsens monocrotaline-induced pulmonary hypertension in rats (Okada et al. 1997). In contrast to the pulmonary vascular response, long-term increases in blood flow in systemic vessels causes increased luminal diameter and reduced resistance (Langille & O'Donnell, 1986; Gibbons & Dzau, 1994).
Altered endothelial function and vascular remodelling
The pulmonary endothelial cell produces a number of important mediators that modulate pulmonary vascular smooth muscle tone and have important influences on vascular smooth muscle cell proliferation and wall remodelling. Amongst the more important vasodilators produced by the endothelium are nitric oxide (NO) and prostacyclin (PGI2), which are also potent inhibitors of wall remodelling. The endothelium-derived vasoconstrictor endothelin is a key mediator of resistance vessel wall remodelling (MacLean, 1999). Other mediators that also stimulate smooth muscle proliferation include prostaglandin F2α (PGF2α) and platelet-derived growth factor-B. Furthermore, the endothelium has a key role in the uptake and catabolism of mediators that can promote remodelling, such as serotonin (MacLean, 1999). Alteration in endothelial production or catabolism of vasoactive mediators can play a central role in vascular remodelling.
Considerable attention has been focused on the role of altered NO production in the development of hypoxia-induced pulmonary hypertension. In chronically hypoxic rat lungs, impaired endothelium-dependent relaxation has been demonstrated (Adnot et al. 1991; Maruyama & Maruyama, 1994) and a similar impairment has been demonstrated in patients with chronic hypoxic lung disease (Dinh-Xuan et al. 1991), suggesting that impaired endothelium-dependent relaxation may contribute to the increased pulmonary vascular resistance of chronic hypoxia. Expression of endothelial nitric oxide synthase is increased in experimental hypoxic pulmonary hypertension (Le Cras et al. 1996, 1998; Tyler et al. 1999) but, despite this, NO production is reduced (Le Cras & McMurtry, 2001). Hypoxic vascular remodelling and pulmonary arterial pressures are increased in endothelial nitric oxide synthase (eNOS) knockout mice (Steudel et al. 1998; Fagan et al. 1999b) while chronic administration of inhaled NO attenuates hypoxic vascular remodelling (Kouyoumdjian et al. 1994; Roberts et al. 1995). Stimulation of endogenous NO production by dietary supplementation with L-arginine also protects against hypoxia-induced vascular remodelling (Mitani et al. 1997; Fagan et al. 1999a). Taken together, these data suggest that impaired endothelial production of NO may contribute to the development of chronic hypoxic pulmonary hypertension.
The potential role of altered endothelial NO synthase expression and activity in chronic inflammatory lung disease has received much less attention. We have found that chronic airway infection in rats leads to reduced eNOS expression in the pulmonary vasculature (Fig. 2) and that these chronically infected lungs are hypersensitive to the vasodilator effects of NO (Cadogan et al. 1999). Furthermore, isolated pulmonary arteries from these lungs demonstrate impaired endothelium-dependent relaxation (Fig. 3). In human subjects with chronic inflammatory airway disease secondary to cigarette smoking, who are not hypoxic, pulmonary vascular endothelium-dependent relaxation is also impaired and eNOS expression is reduced (Peinado et al. 1998; Barbera et al. 2001). Taken together, these data demonstrate that chronic airway inflammation leads to reduced eNOS expression and activity and suggest that reduced NO production may contribute to the vascular remodelling observed in these conditions.
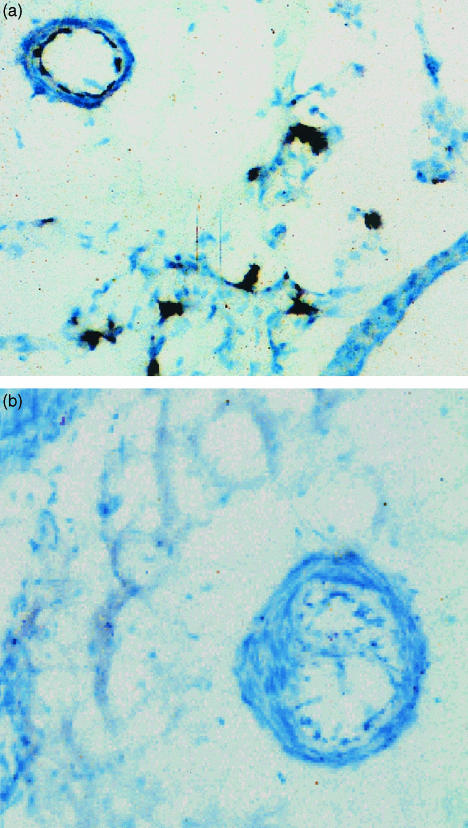
Fig. 2.
Immunoperoxidase staining of endothelial NOS in control lungs, and in lung with chronic airway infection following inoculation with Pseudomonas aeruginosa in agar beads. (a) Photomicrograph showing staining of eNOS in endothelium of blood vessel of control lung and patchy staining in alveolar walls (blue-black colour). (b) Photomicrograph showing absence of staining of eNOS in endothelium of blood vessel of Pseudomonasinoculated lung. All original magnifications ×380. (Figure reproduced with permission from Cadogan et al. American Journal of Physiology, 277: L616–627, 1999.)
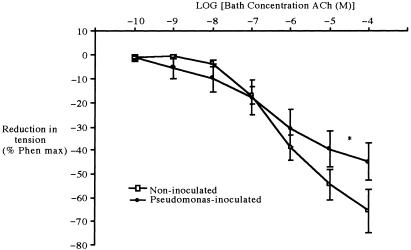
Fig. 3.
Mean (± SEM) reduction in wall tension in response to increasing concentrations of acetylcholine (ACh) in preconstricted pulmonary arterial rings isolated from control (n = 9) and chronically infected (n = 6) rats. Asterisk indicates significant difference between the two groups (P < 0.05, ANOVA).
In other forms of chronic lung inflammation, the behaviour of endothelial NO synthase is controversial. Tyler et al. (1999) reported that in monocrotaline-induced pulmonary hypertension in rats, eNOS expression is reduced. In support of these findings, lipopolysacharide and pro-inflammatory cytokines reduced endothelial NO expression and endothelium-dependent relaxation in pulmonary arteries in organ culture (Ziesche et al. 1996). However, others have reported that monocrotaline causes increased eNOS expression and increased endothelium-dependent relaxation in rat lungs (Resta et al. 1997). It has also been reported that hyperoxia-induced lung inflammation reduced endothelial NO synthase expression (Steudel et al. 1999). Thus, the role of endothelial NO synthase in these conditions is not as clear as its role in chronic airway inflammation and requires further investigation.
The position of the vascular endothelium, immediately adjacent to the blood vessel lumen, means that it must be the site at which alterations in shear stress are detected and transduced. Acute increases in shear stress can immediately activate shear stress-dependent channels, elevate intracellular inositol 1,4,5-triphosphate and diacylglycerol, activate the mitogen-activated and stress-activated protein kinase pathways and cause cytoskeletal reorganization in endothelial cells (Ballermann et al. 1998; Fisher et al. 2001). Change in shear stress also alters production of vasoactive mediators, including NO, prostacyclin and endothelin in cultured endothelial cells (Frangos et al. 1985; Grabowski et al. 1985; Buga et al. 1991; Malek & Izumo, 1992; Malek et al. 1993; Korenaga et al. 1994). Shear stress response elements have been identified in the sequence of genes encoding important vascular growth factors (Ballermann et al. 1998). Furthermore, there is evidence that increased shear stress alters endothelial cell membrane potential and increases NO production in the isolated intact lung and in the whole organism (Nakache & Gaub, 1988; Hakim, 1994; Storme et al. 1999; Tworetzky et al. 2000; Ogasa et al. 2001).
While these data provide good evidence that shear stress can regulate endothelial cell function, they largely demonstrate changes, such as increased NO and PGI2 synthesis, that might be expected to minimize pulmonary vascular resistance, inhibit wall remodelling and prevent the development of pulmonary hypertension. However, prolonged excessive shear stress can lead to endothelial cell damage, reduce the production of vasodilator agents that inhibit wall remodelling, promote the production of mediators that lead to vascular wall remodelling, and increase the expression of adhesion molecules and cytokines that promote an inflammatory response in the vascular wall (Esterly et al. 1968; Reidy & Bowyer, 1977; Zhu et al. 1997; Nomura et al. 2001). Chronic increase in shear stress caused by left to right shunting leads to impaired endothelium-dependent relaxation in pulmonary arteries (Fullerton et al. 1996). In support of these findings, endothelium-dependent arterial relaxation is attenuated and circulating endothelin and thromboxane concentrations are increased in patients with Eisenmengers syndrome (Dinh Xuan et al. 1990; Yoshibayashi et al. 1991; Cacoub et al. 1993; Celermajer et al. 1993; Tweddell et al. 1994). Thus, chronic excessive shear stress may promote the development of pulmonary hypertension, at least in part, by altering endothelial secretion of mediators that regulate vascular wall structure.
Vascular rarefaction in pulmonary hypertension
Intriguingly, in the pulmonary circulation it is reported that chronic hypoxia leads to a reduction in the pulmonary vascular bed in adult animals and man, rather than stimulating angiogenesis. In animal models of chronic hypoxic lung disease, the ratio of pulmonary arterioles to pulmonary alveoli is reduced, suggesting loss of these blood vessels (Hislop & Reid, 1976; Rabinovitch et al. 1979; Jones & Reid, 1995; Partovian et al. 2000). No remnants of these lost vessels could be found (Hislop & Reid, 1976; Rabinovitch et al. 1979) and on re-introduction of animals to normoxic environments, some but not all of these ‘lost’ vessels were restored, suggesting a permanent reduction in the pulmonary vascular bed (Hislop & Reid, 1977). Additionally, in human subjects suffering from congenital heart disease and pulmonary hypertension, a similar alteration in alveolar to arterial ratio has been observed in the lungs (Rabinovitch et al. 1979). Thus, it has been postulated that rarefaction is an important component of the structural basis for hypoxic pulmonary hypertension.
This hypoxia-induced loss of blood vessels in the pulmonary circulations is in stark contrast to the marked angiogenesis observed in the systemic circulation in hypoxia (LaManna et al. 1992; Smith, 1997; Deveci et al. 1998; Marti & Risau, 1999; Smith & Marshall, 1999; Griffioen & Molema, 2000) and has led to the suggestion that the pulmonary circulation in the adult is incapable of angiogenesis. Sobin et al. (1983), using transmission electronic microscopy specifically sought, but could not find, evidence of new capillary formation in chronically hypoxic rat lungs. Furthermore, adenovirally mediated over-expression of the specific endothelial cell mitogen vascular endothelial growth factor (VEGF) in the lung did not alter the ratio of vessels to alveoli (Partovian et al. 2000), in contrast to its potent angiogenic effects in systemic vascular beds.
Rarefaction is also thought to contribute significantly to the development of hypertension in chronic inflammatory conditions of the lung. Pulmonary hypertension produced by crotalaria ingestion led to a reduction in the ratio of arterioles to alveoli in the rat (Meyrick & Reid, 1979a; Meyrick et al. 1980), while repeated endotoxin infusion in sheep led to a similar reduction (Meyrick & Brigham, 1986). In human subjects suffering from cystic fibrosis-induced chronic airway infection, it has also been reported that the arterial to alveoli ratio is reduced (Ryland & Reid, 1975). These findings have been interpreted to indicate a loss of blood vessels, a response that is again markedly different from the response of the systemic vasculature in chronic inflammation, where angiogenesis is prominent and plays a key role in the disease process (Griffioen & Molema, 2000; Sullivan et al. 2000). Schraufnagel and colleagues used scanning electron microscopy to seek evidence of angiogenesis in moncrotaline-induced inflammation and pulmonary hypertension in rats. While they reported extensive new vessel formation in the peribronchial region and on the pleural surface arising from systemic vessels, they could find no evidence of new vessels in the alveolar walls, again suggesting that angiogenesis cannot occur in the adult pulmonary circulation (Schraufnagel et al. 1986; Schraufnagel, 1990).
However, not all investigators agree that rarefaction occurs in pulmonary hypertension. Several studies of pulmonary hypertension in the rat report that the number of blood vessels in the lung was unaltered following chronic hypoxia (Meyrick & Reid, 1979b; Emery et al. 1981; Kay et al. 1982; Finlay et al. 1986) and following chronic inflammatory lung disease (Kay et al. 1982).
Pulmonary vascular remodelling without pulmonary hypertension
A close association between remodelling of pulmonary resistance vessels and the development of hypertension is not invariably observed. We and others have reported that, in chronically infected lungs in rats, pulmonary hypertension did not occur and right ventricular hypertrophy was not observed, despite the development of thickened walls in pulmonary vessels (Graham, 1990; Cadogan et al. 1999). In a similar observation in human subjects with chronic obstructive pulmonary disease, Wright et al. (1983) have reported marked thickening of the walls of pulmonary arterial vessels in the absence of pulmonary hypertension.
One possible explanation of these findings is that wall thickening occurred in an outward direction so that the size of the vascular lumen was not compromised. This phenomenon, termed compensatory enlargement, is well recognized in the systemic circulation (Glagov et al. 1993) but not in the pulmonary vasculature. We tested this hypothesis in chronically infected rat lungs produced using a well-described method of intratracheal inoculation of Pseudomonas aeruginosa organisms that have been incorporated into agar beads. To obtain estimates of the absolute dimensions of maximally dilated, intra-acinar resistance vessels, we used quantitative stereological techniques (Hopkins et al. 2001). These demonstrated that chronic infection did not cause a reduction in mean lumen radius although wall thickness was increased. Thus compensatory hypertrophy had occurred, which may account, at least in part, for the absence of increased pulmonary vascular resistance in such chronically infected lungs (Cadogan et al. 1999).
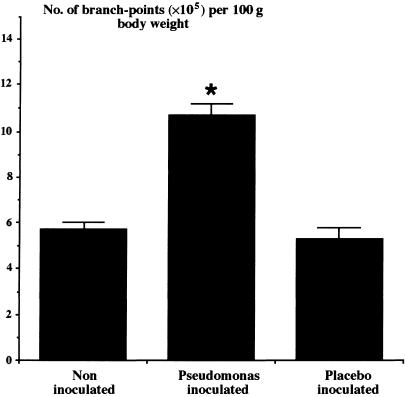
A second possible mechanism that might have prevented the development of pulmonary hypertension is that angiogenesis had occurred in the pulmonary circulation in response to chronic airway infection. To test this hypothesis, we determined total vessel length in the gas exchange (intra-acinar) region of the lung, excluding capillaries, and the number of vascular branch points in those vessels in chronically infected rat lungs. These vessels included the arterioles accompanying respiratory bronchioles, those running in the alveolar walls and all the intra-acinar venules; the diameter of such vessels is approximately 35–45 µm. We observed a substantial increase in total vessel length (Fig. 4) and in the total number of branch-points (Fig. 5) in the pulmonary circulation, suggesting that angiogenesis had occurred (Hopkins et al. 2001). In particular, the evidence of increased branching suggests that angiogenesis led to formation of new arteriolar and venular vessels in addition to the pre-existing pathways. In the systemic circulation, capillary angiogenesis is accompanied by formation of new arterial vessels, so-called arteriogenesis (Carmeliet, 2000). Our evidence suggests that a similar behaviour can occur in the pulmonary circulation and may contribute to the maintenance of a normal low pulmonary vascular resistance.
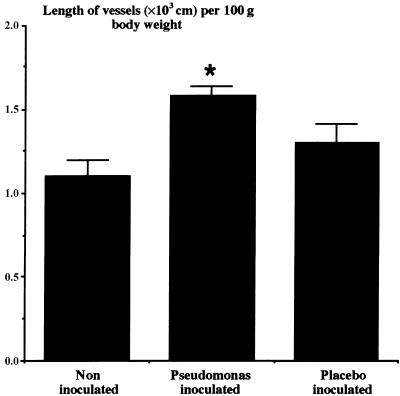
Fig. 4.
The mean (± SEM) total length of intra-acinar pulmonary blood vessels in the left lungs of normal control lungs (n = 8), lungs with chronic airway infection following inoculation with Pseudomonas aeruginosa in agar beads (n = 10), and lungs inoculated with sterile agar beads alone (n = 6). Asterisk indicates significant difference from noninoculated and placebo-inoculated groups (P < 0.05, ANOVA). (Figure reproduced with permission from Hopkins et al. Journal of Applied Physiology, 91: 919–928, 2001.)
Fig. 5.
The mean (± SEM) number of branch-points of intraacinar pulmonary blood vessels in the left lungs of control rats (n = 8), lungs with chronic airway infection following inoculation with Pseudomonas aeruginosa in agar beads (n = 10), and lungs inoculated with sterile agar beads alone (n = 6). Asterisk indicates significant difference from noninoculated and placebo-inoculated groups (P < 0.05, ANOVA). (Figure reproduced with permission from Hopkins et al. Journal of Applied Physiology, 91: 919–928, 2001.)
More recent evidence supports our findings of new vessel formation in the pulmonary circulation. Hsia et al. (1994) have shown that in adult dogs following right pneumonectomy there is proliferation of new alveolar septa and alveolar capillaries in the residual left lung. Other reports demonstrate that endothelial cells of the pulmonary circulation can replicate in a variety of circumstances. Following pneumonectomy, the total number of endothelial cells in the remaining lung increases significantly in rats (Thet & Law, 1984). Sekhon et al. (1994) have shown that airway inflammation caused by cigarette smoke stimulated endothelial cell proliferation in the lung as demonstrated by labelling of endothelial cell nuclei with bromodeoxyuridine. Endothelial cell proliferation in the pulmonary circulation has also been observed during the development of monocrotaline-induced pulmonary hypertension in rats (Meyrick & Reid, 1982). Schraufnagel et al. (1997) have reported evidence of pulmonary angiogensis in rat lung following the induction of biliary cirrhosis. Studies in humans with lung disease have also provided evidence of angiogenesis in the pulmonary circulation. Schraufnagel et al. (1996), using scanning electron microscopic techniques, found evidence of new vessel formation in the lungs of a patient with pulmonary veno-occlusive disease. In addition, it has been demonstrated radiologically that the majority of metastatic tumours in the lung receive their blood supply either exclusively (46% of metastases) or predominantly (36% of metastases) from the pulmonary circulation (Milne & Zerhouni, 1987). Taken together, these reports suggest that pulmonary endothelial cells can proliferate and form new vessels in the adult lung.
The role played by vascular remodelling in the development of pulmonary hypertension in chronic lung infection is complex. Our data suggest that, under certain conditions, wall remodelling may be not result in reduction of the mean lumen diameter (Hopkins et al. 2001). Furthermore, the addition of parallel vascular pathways through the lungs may serve to oppose any increase in total pulmonary vascular resistance that might result from reductions in vascular lumen diameter in some vessels. If these two processes occur together, vascular resistance may not increase despite thickening of the vessel wall. However, in other circumstances, such as more persistent or more severe lung disease, remodelling of the vascular wall, including the wall of newly formed vessels, might lead to significant reductions in mean lumen diameter. Under such conditions, the increase in resistance caused by narrowed vessels might outweigh the extent to which new vessel formation could reduce resistance and the net effect might then become an increase in total pulmonary vascular resistance.
Quantification of vascular structure in the lung
It is clear that there are directly conflicting reports regarding rarefaction and angiogenesis in the pulmonary circulation in a variety of circumstances. The possibility that these apparent discrepancies might arise because of methodological problems is worthy of consideration. The normal lung is a highly vascular structure with a vast vascular network, which is required to provide adequate surface area for gas exchange. Given the extent of the normal vasculature when compared with other organs, it is extremely difficult to detect newly formed blood vessels in the lung. This problem has been reviewed and commented upon previously (Mooi & Wagenvoort, 1983; Schraufnagel et al. 1996). In view of this, it is interesting to note that those studies that have clearly demonstrated angiogenesis in the pulmonary circulation have used the techniques of quantitative stereology (Thet & Law, 1984; Hsia et al. 1994; Hopkins et al. 2001). Stereology allows statistical inferences about the three-dimensional structural parameters of objects based on two-dimensional information such as that provided by histological images. In addition, by using strict random sampling protocols, stereological approaches ensure that the data obtained are unbiased and representative of the whole lung. A further feature of stereology is that it carefully defines and quantifies a reference volume within which a particular parameter is to be measured. This step allows absolute quantities to be measured, e.g. the total length of intra-acinar resistance vessels in the lung.
The task of identifying angiogenesis provides an example of the importance of these issues. Angiogenesis in the lung would increase the total length of vessels in the lung. If the lung increased in volume during the course of our experimental intervention (e.g. hypoxia), but the length of vessel per unit volume of lung were to remain constant, then the number of vessel transections per unit area of cross-section would remain constant, i.e. the number of vessels observed per microscopic field of view would be unaltered. A conventional approach such as this would conclude that no angiogenesis had taken place. However, a stereological approach, by recognizing the altered reference volume (total lung volume), would identify the increase in total vessel length (Bolender et al. 1993; Gundersen et al. 1988a, b). Use of inappropriate or undefined reference spaces can lead to significant errors. For example, an increased ratio of wall thickness to lumen diameter in a vessel might arise either because lumen diameter had reduced, or because wall thickness had increased without change in the lumen size. This implies that changes in this ratio do not allow any direct inference to be made about changes in vascular resistance.
Conclusions
Standard teaching suggests that secondary pulmonary hypertension results, in large part, from two major structural alterations in the pulmonary circulation. The first is thickening of the walls of resistance vessels that causes a reduction of the vascular lumen. The second structural alteration is a reduction in the total number of pulmonary vessels or rarefaction. Recent evidence demonstrates that vascular wall thickening can occur without structural alteration in lumen diameter. Furthermore, angiogenesis can occur in the pulmonary circulation and may serve to prevent or attenuate increased vascular resistance in lung disease. Quantitative stereological approaches are required to allow determination of the three-dimensional parameters that characterize this vascular bed. In view of these more recent data, the potential role of structural alterations in the pulmonary vasculature in the development of pulmonary hypertension should be carefully reconsidered.
References
- Adnot S, Raffestin B, Eddahibi S, Braquet P, Chabrier PE. Loss of endothelium-dependent relaxant activity in the pulmonary circulation of rats exposed to chronic hypoxia. J. Clin. Invest. 1991;87:155–162. doi: 10.1172/JCI114965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer S, Rich S. Primary pulmonary hypertension: a vascular biology and translational research ‘Work in progress’. Circulation. 2000;102:2781–2791. doi: 10.1161/01.cir.102.22.2781. [DOI] [PubMed] [Google Scholar]
- Ballermann BJ, Dardik A, Eng E, Liu A. Shear stress and the endothelium. Kidney Int. Suppl. 1998;67:S100–S108. doi: 10.1046/j.1523-1755.1998.06720.x. [DOI] [PubMed] [Google Scholar]
- Barbera JA, Riverola A, Roca J, Ramirez J, Wagner PD, Ros D, et al. Pulmonary vascular abnormalities and ventilation-perfusion relationships in mild chronic obstructive pulmonary disease. Am. J. Respir. Crit Care Med. 1994;149:423–429. doi: 10.1164/ajrccm.149.2.8306040. [DOI] [PubMed] [Google Scholar]
- Barbera JA, Peinado VI, Santos S, Ramirez J, Roca J, Rodriguez-Roisin R. Reduced expression of endothelial nitric oxide synthase in pulmonary arteries of smokers. Am. J. Respir. CritCare Med. 2001;164:709–713. doi: 10.1164/ajrccm.164.4.2101023. [DOI] [PubMed] [Google Scholar]
- Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N. Engl. J. Med. 1996;334:296–302. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- Bishop JE, Guerreiro D, Laurent GJ. Changes in the composition and metabolism of arterial collagens during the development of pulmonary hypertension in rabbits. Am. Rev. Respir. Dis. 1990;141:450–455. doi: 10.1164/ajrccm/141.2.450. [DOI] [PubMed] [Google Scholar]
- Bohlen HG. The microcirculation in hypertension. J. Hypertens. Suppl. 1989;7:S117–S124. [PubMed] [Google Scholar]
- Bolender RP, Hyde DM, Dehoff RT. Lung morphometry: a new generation of tools and experiments for organ, tissue, cell, and molecular biology. Am. J. Physiol. 1993;265:L521–L548. doi: 10.1152/ajplung.1993.265.6.L521. [DOI] [PubMed] [Google Scholar]
- Bousamra M, Rossi R, Jacobs E, Parviz M, Busch C, Nelin LD, Haworth S, Dawson CA. Systemic lobar shunting induces advanced pulmonary vasculopathy. J. Thorac. Cardiovasc. Surg. 2000;120:88–98. doi: 10.1067/mtc.2000.106654. [DOI] [PubMed] [Google Scholar]
- Buga GM, Gold ME, Fukuto JM, Ignarro LJ. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension. 1991;17:187–193. doi: 10.1161/01.hyp.17.2.187. [DOI] [PubMed] [Google Scholar]
- Cacoub P, Dorent R, Maistre G, Nataf P, Carayon A, Piette C, et al. Endothelin-1 in primary pulmonary hypertension and the Eisenmenger syndrome. Am. J. Cardiol. 1993;71:448–450. doi: 10.1016/0002-9149(93)90452-i. [DOI] [PubMed] [Google Scholar]
- Cadogan E, Hopkins N, Giles S, Bannigan J, Moynihan J, McLoughlin P. Enhanced expression of inducible nitric oxide synthase without vasodilator effect in chronically infected lung. Am. J. Physiol. 1999;277:L616–L627. doi: 10.1152/ajplung.1999.277.3.L616. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Cash HA, Woods DE, McCullough B, Johanson WG, Bass JA. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am. Rev. Respir. Dis. 1978;119:453–459. doi: 10.1164/arrd.1979.119.3.453. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Cullen S, Deanfield JE. Impairment of endothelium-dependent pulmonary artery relaxation in children with congenital heart disease and abnormal pulmonary hemodynamics. Circulation. 1993;87:440–446. doi: 10.1161/01.cir.87.2.440. [DOI] [PubMed] [Google Scholar]
- Champion HC, Bivalacqua TJ, D'Souza FM, Ortiz LA, Jeter JR, Toyoda K, et al. Gene transfer of endothelial nitric oxide synthase to the lung of the mouse in vivo. Effect on agonist-induced and flow-mediated vascular responses. Circ. Res. 1999;84:1422–1432. doi: 10.1161/01.res.84.12.1422. [DOI] [PubMed] [Google Scholar]
- Deveci D, Bryan P, Egginton S, Marshall JM. Chronic hypoxia induces angiogenesis in striated muscle of the rat. J. Physiol. 1998;513P:86P. [Google Scholar]
- Dinh Xuan AT, Higenbottam TW, Clelland C, Pepke-Zaba J, Cremona G, Wallwork J. Impairment of pulmonary endothelium-dependent relaxation in patients with Eisenmenger's syndrome. Br. J. Pharmacol. 1990;99:9–10. doi: 10.1111/j.1476-5381.1990.tb14643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh-Xuan AT, Higgenbottam TW, Clelland CA, Pepke-Zaba J, Cremona G, Butt AY, et al. Impairment of endothelium-dependent pulmonary-artery relaxation in chronic obstructive lung disease. N. Engl. J. Med. 1991;324:1539–1547. doi: 10.1056/NEJM199105303242203. [DOI] [PubMed] [Google Scholar]
- Doyle MP, Walker BR. Attentuation of systemic vasoreactivity in chronically hypoxic rats. Am. J. Physiol. 1991;260:R1114–R1122. doi: 10.1152/ajpregu.1991.260.6.R1114. [DOI] [PubMed] [Google Scholar]
- Emery CJ, Bee D, Barer GR. Mechanical properties and reactivity of vessels in isolated perfused lungs of chronically hypoxic rats. Clin. Sci. (Colch) 1981;61:569–580. doi: 10.1042/cs0610569. [DOI] [PubMed] [Google Scholar]
- Esterly JA, Glagov S, Ferguson DJ. Morphogenesis of intimal obliterative hyperplasia of small arteries in experimental pulmonary hypertension. An ultrastructural study of the role of smooth-muscle cells. Am. J. Pathol. 1968;52:325–347. [PMC free article] [PubMed] [Google Scholar]
- Everett AD, Le Cras TD, Xue C, Johns RA. eNOS expression is not altered in pulmonary vascular remodeling due to increased pulmonary blood flow. Am. J. Physiol. 1998;274:L1058–L1065. doi: 10.1152/ajplung.1998.274.6.L1058. [DOI] [PubMed] [Google Scholar]
- Fagan JM, Rex SE, Hayes-Licitra SA, Waxman L. 1-arginine reduces right heart hypertrophy in hypoxia-induced pulmonary hypertension. Biochem. Biophys. Res. Commun. 1999a;254:100–103. doi: 10.1006/bbrc.1998.9887. [DOI] [PubMed] [Google Scholar]
- Fagan KA, Fouty BW, Tyler RC, Morris KG, Jr, Hepler LK, Sato K, et al. The pulmonary circulation of homozygous or heterozygous eNOS-null mice is hyperresponsive to mild hypoxia. J. Clin. Invest. 1999b;103:291–299. doi: 10.1172/JCI3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay M, Barer GR, Suggett AJ. Quantitative changes in the rat pulmonary vasculature in chronic hypoxia – relation to haemodynamic changes. Q. J. Exp. Physiol. 1986;71:151–163. doi: 10.1113/expphysiol.1986.sp002975. [DOI] [PubMed] [Google Scholar]
- Fisher AB, Chien S, Barakat AI, Nerem RM. Endothelial cellular response to altered shear stress. Am. J. Physiol. Lung Cell Mol Physiol. 2001;281:L529–L533. doi: 10.1152/ajplung.2001.281.3.L529. [DOI] [PubMed] [Google Scholar]
- Fishman AP. The respiratory system. In: Fishman AP, Fisher AB, editors. American Handbook of Physiology. Bethesda, MD: American Physiological Society; 1985. pp. 93–165. [Google Scholar]
- Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- Fried R, Meyrick B, Rabinovitch M, Reid L. Polycythemia and the acute hypoxic response in awake rats following chronic hypoxia. J. Appl. Physiol. 1983;55:1167–1172. doi: 10.1152/jappl.1983.55.4.1167. [DOI] [PubMed] [Google Scholar]
- Friedli B, Kent G, Kidd BS. The effect of increased pulmonary blood flow on the pulmonary vascular bed in pigs. Pediatr. Res. 1975;9:547–553. doi: 10.1203/00006450-197506000-00007. [DOI] [PubMed] [Google Scholar]
- Fullerton DA, Mitchell MB, Jones DN, Maki A, McIntyre RC., Jr Pulmonary vasomotor dysfunction is produced with chronically high pulmonary blood flow. J. Thorac. Cardiovasc. Surg. 1996;111:190–197. doi: 10.1016/S0022-5223(96)70416-6. [DOI] [PubMed] [Google Scholar]
- Giaid A, Michel RP, Stewart DJ, Sheppard M, Corrin B, Hamid Q. Expression of endothelin-1 in lungs of patients with cryptogenic fibrosing alveolitis. Lancet. 1993;341:1550–1554. doi: 10.1016/0140-6736(93)90694-c. [DOI] [PubMed] [Google Scholar]
- Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N. Engl. J. Med. 1994;330:1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N. Engl. J. Med. 1993;22:1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- Grabowski EF, Jaffe EA, Weksler BB. Prostacyclin production by cultured endothelial cell monolayers exposed to step increases in shear stress. J. Lab. Clin. Med. 1985;105:36–43. [PubMed] [Google Scholar]
- Graham LM, Vasil A, Vasil ML, Voelkel NF, Stenmark KR. Decreased pulmonary vasoreactivity in an animal model of chronic Pseudomonas pneumonia. Am. Rev. Respir. Dis. 1990;142:221–229. doi: 10.1164/ajrccm/142.1.221. [DOI] [PubMed] [Google Scholar]
- Greene AS, Tonellato PJ, Lui J, Lombard JH, Cowley AW., Jr Microvascular rarefaction and tissue vascular resistance in hypertension. Am. J. Physiol. 1989;256:H126–H131. doi: 10.1152/ajpheart.1989.256.1.H126. [DOI] [PubMed] [Google Scholar]
- Griffioen AW, Molema G. Angiogenesis. Potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol. Rev. 2000;52:237–268. [PubMed] [Google Scholar]
- Grover RF, Wagner WW, McMurtry IF, Reeves JT. In: The Cardiovascular System. Shepard JT, Aboud FM, editors. Bethesda, MD: American Physiological Society; 1983. pp. 103–136. [Google Scholar]
- Gundersen HJG, Bagger P, Bendtsen TF, Evans TF, Korbo L, Marcussen N, et al. The new stereological tools: Disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988a;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, et al. Some new simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988b;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Gurubhagavatula I, Palevsky HI. Pulmonary hypertension in systemic autoimmune disease. Rheum. Dis. Clin. North Am. 1997;23:365–394. doi: 10.1016/s0889-857x(05)70335-5. [DOI] [PubMed] [Google Scholar]
- Hakim TS. Flow-induced release of EDRF in the pulmonary vasculature: site of release and action. Am. J. Physiol. 1994;267:H363–H369. doi: 10.1152/ajpheart.1994.267.1.H363. [DOI] [PubMed] [Google Scholar]
- Hale KA, Ewing SL, Gosnell BA, Niewoehner DE. Lung disease in long-term cigarette smokers with and without chronic air-flow obstruction. Am. Rev. Respir. Dis. 1984;130:716–721. doi: 10.1164/arrd.1984.130.5.716. [DOI] [PubMed] [Google Scholar]
- Hansen-Smith F, Greene AS, Cowley AW, Jr, Lombard JH. Structural changes during microvascular rarefaction in chronic hypertension. Hypertension. 1990;15:922–928. doi: 10.1161/01.hyp.15.6.922. [DOI] [PubMed] [Google Scholar]
- Haworth SG. Primary pulmonary hypertension in childhood. Arch. Dis. Child. 1998;79:452–455. doi: 10.1136/adc.79.5.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath D, Edwards JE. The pathology of hypertensive pulmonary vascular disease. Circulation. 1958;18:533–547. doi: 10.1161/01.cir.18.4.533. [DOI] [PubMed] [Google Scholar]
- Heath D. In: Pulmonary Circulaiton and the Interstitial Space. Fishman AP, Hecht HH, editors. Chicago: University of Chicago Press; 1969. pp. 305–319. [Google Scholar]
- Heath D, Edwards C, Winson M, Smith P. Effects on the right ventricle, pulmonary vasculature, and carotid bodies of the rat of exposure to, and recovery from, simulated high altitude. Thorax. 1973;28:24–28. doi: 10.1136/thx.28.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht HH, Kuida H, Lange RL, Thorne JL, Brown AM. Clinical features and hemodynamic observations in altitude-dependent right heart failure of cattle. Am. J. Med. 1962;32:171–183. doi: 10.1016/0002-9343(62)90288-7. [DOI] [PubMed] [Google Scholar]
- Henley WN, Tucker A. Hypoxic moderation of systemic hypertension in the spontaneously hypertensive rat. Am. J. Physiol. 1987;252:R554–R561. doi: 10.1152/ajpregu.1987.252.3.R554. [DOI] [PubMed] [Google Scholar]
- Herget J, Palecek F, Preclik P, Cermakova M, Vizek M, Petrovicka M. Pulmonary hypertension induced by repeated pulmonary inflammation in the rat. J. Appl. Physiol. 1981;51:755–761. doi: 10.1152/jappl.1981.51.3.755. [DOI] [PubMed] [Google Scholar]
- Hernandez N, Torres SH, Finol HJ, Vera O. Capillary changes in skeletal muscle of patients with essential hypertension. Anat. Rec. 1999;256:425–432. doi: 10.1002/(SICI)1097-0185(19991201)256:4<425::AID-AR9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Hislop A, Reid L. Arterial changes in Crotalaria spectabilis-induced pulmonary hypertension in rats. Br. J. Exp. Pathol. 1974;55:153–163. [PMC free article] [PubMed] [Google Scholar]
- Hislop A, Reid L. New findings in pulmonary arteries of rats with hypoxia-induced pulmonary hypertension. Br. J. Exp Pathol. 1976;57:542–554. [PMC free article] [PubMed] [Google Scholar]
- Hislop A, Reid L. Changes in the pulmonary arteries of the rat during recovery from hypoxia-induced pulmonary hypertension. Br. J. Exp. Pathol. 1977;58:653–662. [PMC free article] [PubMed] [Google Scholar]
- Hopkins N, Cadogan E, Giles S, McLoughlin P. Chronic airway infection leads to angiogenesis in the pulmonary circulation. J. Appl. Physiol. 2001;91:919–928. doi: 10.1152/jappl.2001.91.2.919. [DOI] [PubMed] [Google Scholar]
- Hsia CC, Herazo LF, Fryder-Doffey F, Weibel ER. Compensatory lung growth occurs in adult dogs after right pneumonectomy. J. Clin. Invest. 1994;94:405–412. doi: 10.1172/JCI117337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incalzi RA, Fuso L, De Rosa M, Di Napoli A, Basso S, Pagliari G, Pistelli R. Electrocardiographic signs of chronic cor pulmonale: a negative prognostic finding in chronic obstructive pulmonary disease. Circulation. 1999;99:1600–1605. doi: 10.1161/01.cir.99.12.1600. [DOI] [PubMed] [Google Scholar]
- Jones R. Ultrastructural analysis of contractile cell development in lung microvessels in hyperoxic pulmonary hypertension. Fibroblasts and intermediate cells selectively reorganize nonmuscular segments. Am. J. Pathol. 1992;141:1491–1505. [PMC free article] [PubMed] [Google Scholar]
- Jones R, Reid L. In: Pulmonary Vascular Remodelling. Bishop JE, Reeves JT, Laurent GJ, editors. London: Portland Press Ltd; 1995. pp. 47–116. [Google Scholar]
- Katz R, Pollack M, Spady D. Cardiopulmonary abnormalities in severe acute respiratory failure. J. Pediatr. 1984;104:357–364. doi: 10.1016/s0022-3476(84)81095-1. [DOI] [PubMed] [Google Scholar]
- Kay JM, Harris P, Heath D. Pulmonary hypertension produced in rats by ingestion of Crotalaria spectabilis seeds. Thorax. 1967;22:176–179. doi: 10.1136/thx.22.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay JM, Suyama KL, Keane PM. Failure to show decrease in small pulmonary blood vessels in rats with experimental pulmonary hypertension. Thorax. 1982;37:927–930. doi: 10.1136/thx.37.12.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenaga R, Ando J, Tsuboi H, Yang W, Sakuma I, Toyo-oka T, et al. Laminar flow stimulates ATP- and shear stress-dependent nitric oxide production in cultured bovine endothelial cells. Biochem. Biophys. Res. Commun. 1994;198:213–219. doi: 10.1006/bbrc.1994.1030. [DOI] [PubMed] [Google Scholar]
- Kouyoumdjian C, Adnot S, Levame M, Eddahibi S, Bousbaa H, Raffestin B. Continuous inhalation of nitric oxide protects against development of pulmonary hypertension in chronically hypoxic rats. J. Clin. Invest. 1994;94:578–584. doi: 10.1172/JCI117372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaManna JC, Vendel LM, Farrell RM. Brain adaptation to chronic hypobaric hypoxia in rats. J. Appl. Physiol. 1992;72:2238–2243. doi: 10.1152/jappl.1992.72.6.2238. [DOI] [PubMed] [Google Scholar]
- Langille BL, O'Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science. 1986;231:405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- Le Cras TD, McMurtry IF. Nitric oxide production in the hypoxic lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280:L575–L582. doi: 10.1152/ajplung.2001.280.4.L575. [DOI] [PubMed] [Google Scholar]
- Le Cras TD, Tyler RC, Horan MP, Morris KG, Tuder RM, McMurtry IF, et al. Effects of chronic hypoxia and altered hemodynamics on endothelial nitric oxide synthase expression in the adult rat lung. J. Clin. Invest. 1998;101:795–801. doi: 10.1172/JCI786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Cras TD, Xue C, Rengasamy A, Johns RA. Chronic hypoxia upregulates endothelial and inducible NO synthase gene and protein expression in rat lung. Am. J. Physiol. 1996;270:L164–L170. doi: 10.1152/ajplung.1996.270.1.L164. [DOI] [PubMed] [Google Scholar]
- Leeman M. Pulmonary hypertension in acute respiratory distress syndrome. Monaldi Arch. Chest Dis. 1999;54:146–149. [PubMed] [Google Scholar]
- Lippmann M. Pulmonary reactions to drugs. Med. Clin. North Am. 1977;61:1353–1367. doi: 10.1016/s0025-7125(16)31266-4. [DOI] [PubMed] [Google Scholar]
- Lockhart A, Zelter M, Mensch-Dechene J, Antezana G, Paz-Zamora M, Vargas E, et al. Pressure-flow-Volume relationships in pulmonary circulation of normal highlanders. J. Appl. Physiol. 1976;41:449–456. doi: 10.1152/jappl.1976.41.4.449. [DOI] [PubMed] [Google Scholar]
- MacLean MR. Endothelin-1 and serotonin: mediators of primary and secondary pulmonary hypertension? J. Lab. Clin. Med. 1999;134:105–114. doi: 10.1016/s0022-2143(99)90114-2. [DOI] [PubMed] [Google Scholar]
- MacNee W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part one. Am. J. Respir. Crit. Care Med. 1994a;150:833–852. doi: 10.1164/ajrccm.150.3.8087359. [DOI] [PubMed] [Google Scholar]
- MacNee W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part two. Am. J. Respir. Crit Care Med. 1994b;150:1158–1168. doi: 10.1164/ajrccm.150.4.7921453. [DOI] [PubMed] [Google Scholar]
- Magee F, Wright JL, Wiggs BR, Pare PD, Hogg JC. Pulmonary vascular structure and function in chronic obstructive pulmonary disease. Thorax. 1988;43:183–189. doi: 10.1136/thx.43.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek A, Izumo S. Physiological fluid shear stress causes downregulation of endothelin-1 mRNA in bovine aortic endothelium. Am. J. Physiol. 1992;263:C389–C396. doi: 10.1152/ajpcell.1992.263.2.C389. [DOI] [PubMed] [Google Scholar]
- Malek AM, Greene AL, Izumo S. Regulation of endothelin 1 gene by fluid shear stress is transcriptionally mediated and independent of protein kinase C and cAMP. Proc. Natl Acad. Sci. USA. 1993;90:5999–6003. doi: 10.1073/pnas.90.13.5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti HH, Risau W. Angiogenesis in ischemic disease. Thromb. Haemost. 1999;82(Suppl. 1):44–52. [PubMed] [Google Scholar]
- Marticorena E, Ruiz L, Severino J, Galvez J, Penaloza D. Systemic blood pressure in white men born at sea level: changes after long residence at high altitudes. Am. J. Cardiol. 1969;23:364–368. doi: 10.1016/0002-9149(69)90516-5. [DOI] [PubMed] [Google Scholar]
- Maruyama J, Maruyama K. Impaired nitric oxide-dependent responses and their recovery in hypertensive pulmonary arteries of rats. Am. J. Physiol. 1994;266:H2476–H2488. doi: 10.1152/ajpheart.1994.266.6.H2476. [DOI] [PubMed] [Google Scholar]
- McCormack DG, Paterson NA. Loss of hypoxic pulmonary vasoconstriction in chronic pneumonia is not mediated by nitric oxide. Am. J. Physiol. 1993;265:H1523–H1528. doi: 10.1152/ajpheart.1993.265.5.H1523. [DOI] [PubMed] [Google Scholar]
- Meyrick B, Brigham KL. Repeated Escherichia coli endotoxin-induced pulmonary inflammation causes chronic pulmonary hypertension in sheep. Structural and functional changes. Lab. Invest. 1986;55:164–176. [PubMed] [Google Scholar]
- Meyrick B, Reid L. The effect of continued hypoxia on rat pulmonary arterial circulation. An ultrastructural study. Lab. Invest. 1978;38:188–200. [PubMed] [Google Scholar]
- Meyrick B, Reid L. Development of pulmonary arterial changes in rats fed Crotalaria spectabilis. Am. J. Pathol. 1979a;94:37–51. [PMC free article] [PubMed] [Google Scholar]
- Meyrick B, Reid L. Hypoxia and incorporation of 3H-thymidine by cells of the rat pulmonary arteries and alveolar wall. Am. J. Pathol. 1979b;96:51–70. [PMC free article] [PubMed] [Google Scholar]
- Meyrick B, Gamble W, Reid L. Development of Crotalaria pulmonary hypertension: hemodynamic and structural study. Am. J. Physiol. 1980;239:H692–H702. doi: 10.1152/ajpheart.1980.239.5.H692. [DOI] [PubMed] [Google Scholar]
- Meyrick BO, Reid LM. Crotalaria-induced pulmonary hypertension. Uptake of 3H-thymidine by the cells of the pulmonary circulation and alveolar walls. Am. J. Pathol. 1982;106:84–94. [PMC free article] [PubMed] [Google Scholar]
- Meyrick B, Reid L. Pulmonary hypertension. Anatomic and physiologic correlates. Clin. Chest Med. 1983;4:199–217. [PubMed] [Google Scholar]
- Meyrick BO, Perkett EA. The sequence of cellular and hemodynamic changes of chronic pulmonary hypertension induced by hypoxia and other stimuli. Am. Rev. Respir Dis. 1989;140:1486–1489. doi: 10.1164/ajrccm/140.5.1486. [DOI] [PubMed] [Google Scholar]
- Michel RP, Hakim TS, Freeman CR. Distribution of pulmonary vascular resistance in experimental fibrosis. J. Appl. Physiol. 1988;65:1180–1190. doi: 10.1152/jappl.1988.65.3.1180. [DOI] [PubMed] [Google Scholar]
- Milne EN, Zerhouni EA. Blood supply of pulmonary metastases. J. Thorac. Imaging. 1987;2:15–23. doi: 10.1097/00005382-198710000-00005. [DOI] [PubMed] [Google Scholar]
- Mitani Y, Maruyama K, Sakurai M. Prolonged administration of 1-arginine ameliorates chronic pulmonary hypertension and pulmonary vascular remodeling in rats [see comments] Circulation. 1997;96:689–697. [PubMed] [Google Scholar]
- Mooi W, Wagenvoort CA. Decreased numbers of pulmonary blood vessels: reality or artifact? J. Pathol. 1983;141:441–447. doi: 10.1002/path.1711410403. [DOI] [PubMed] [Google Scholar]
- Nakache M, Gaub HE. Hydrodynamic hyperpolarization of endothelial cells. Proc. Natl Acad. Sci. USA. 1988;85:1841–1843. doi: 10.1073/pnas.85.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura S, Tandon NN, Nakamura T, Cone J, Fukuhara S, Kambayashi J. High-shear-stress-induced activation of platelets and microparticles enhances expression of cell adhesion molecules in THP-1 and endothelial cells. Atherosclerosis. 2001;158:277–287. doi: 10.1016/s0021-9150(01)00433-6. [DOI] [PubMed] [Google Scholar]
- Ogasa T, Nakano H, Ide H, Yamamoto Y, Sasaki N, Osanai S, Akiba Y, Kikuchi K, Iwamoto J. Flow-mediated release of nitric oxide in isolated, perfused rabbit lungs. J. Appl. Physiol. 2001;91:363–370. doi: 10.1152/jappl.2001.91.1.363. [DOI] [PubMed] [Google Scholar]
- Okada K, Tanaka Y, Bernstein M, Zhang W, Patterson GA, Botney MD. Pulmonary hemodynamics modify the rat pulmonary artery response to injury. A neointimal model of pulmonary hypertension. Am. J. Pathol. 1997;151:1019–1025. [PMC free article] [PubMed] [Google Scholar]
- Partovian C, Adnot S, Raffestin B, Louzier V, Levame M, Mavier IM, et al. Adenovirus-mediated lung vascular endothelial growth factor overexpression protects against hypoxic pulmonary hypertension in rats. Am. J. Respir. Cell Mol. Biol. 2000;23:762–771. doi: 10.1165/ajrcmb.23.6.4106. [DOI] [PubMed] [Google Scholar]
- Peinado VI, Barbera JA, Ramirez J, Gomez FP, Roca J, Jover L, et al. Endothelial dysfunction in pulmonary arteries of patients with mild COPD. Am. J. Physiol. 1998;274:L908–L913. doi: 10.1152/ajplung.1998.274.6.L908. [DOI] [PubMed] [Google Scholar]
- Peinado VI, Barbera JA, Abate P, Ramirez J, Roca J, Santos S, et al. Inflammatory reaction in pulmonary muscular arteries of patients with mild chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1999;159:1605–1611. doi: 10.1164/ajrccm.159.5.9807059. [DOI] [PubMed] [Google Scholar]
- Poiani GJ, Tozzi CA, Yohn SE, Pierce RA, Belsky SA, Berg RA, et al. Collagen and elastin metabolism in hypertensive pulmonary arteries of rats. Circ. Res. 1990;66:968–978. doi: 10.1161/01.res.66.4.968. [DOI] [PubMed] [Google Scholar]
- Prewitt RL, Chen II, Dowell R. Development of microvascular rarefaction in the spontaneously hypertensive rat. Am. J. Physiol. 1982;243:H243–H251. doi: 10.1152/ajpheart.1982.243.2.H243. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M, Gamble W, Nadas AS, Miettinen OS, Reid L. Rat pulmonary circulation after chronic hypoxia: hemodynamic and structural features. Am. J. Physiol. 1979;236:H818–H827. doi: 10.1152/ajpheart.1979.236.6.H818. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M, Keane JF, Fellows KE, Castaneda AR, Reid L. Quantitative analysis of the pulmonary wedge angiogram in congenital heart defects. Correlation with hemodynamic data and morphometric findings in lung biopsy tissue. Circulation. 1981;63:152–164. doi: 10.1161/01.cir.63.1.152. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M, Konstam MA, Gamble WJ, Papanicolaou N, Aronovitz MJ, Treves S, et al. Changes in pulmonary blood flow affect vascular response to chronic hypoxia in rats. Circ. Res. 1983;52:432–441. doi: 10.1161/01.res.52.4.432. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M. EVE and beyond, retro and prospective insights. Am. J. Physiol. 1999;277:L5–L12. doi: 10.1152/ajplung.1999.277.1.L5. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M. Pathobiology of pulmonary hypertension. Extracellular matrix. Clin. Chest Med. 2001;22:433–449. doi: 10.1016/s0272-5231(05)70282-3. viii. [DOI] [PubMed] [Google Scholar]
- Reidy MA, Bowyer DE. Scanning electron microscopy of arteries. The morphology of aortic endothelium in haemodynamically stressed areas associated with branches. Atherosclerosis. 1977;26:181–194. doi: 10.1016/0021-9150(77)90101-0. [DOI] [PubMed] [Google Scholar]
- Reindel JF, Ganey PE, Wagner JG, Slocombe RF, Roth RA. Development of morphologic, hemodynamic, and biochemical changes in lungs of rats given monocrotaline pyrrole. Toxicol. Appl. Pharmacol. 1990;106:179–200. doi: 10.1016/0041-008x(90)90239-q. [DOI] [PubMed] [Google Scholar]
- Rendas A, Lennox S, Reid L. Aorta-pulmonary shunts in growing pigs. Functional and structural assessment of the changes in the pulmonary circulation. J. Thorac. Cardiovasc. Surg. 1979;77:109–118. [PubMed] [Google Scholar]
- Resta TC, Gonzales RJ, Dail WG, Sanders TC, Walker BR. Selective upregulation of arterial endothelial nitric oxide synthase in pulmonary hypertension. Am. J. Physiol. 1997;272:H806–H813. doi: 10.1152/ajpheart.1997.272.2.H806. [DOI] [PubMed] [Google Scholar]
- Roberts JD, Jr, Roberts CT, Jones RC, Zapol WM, Bloch KD. Continuous nitric oxide inhalation reduces pulmonary arterial structural changes, right ventricular hypertrophy, and growth retardation in the hypoxic newborn rat. Circ. Res. 1995;76:215–222. doi: 10.1161/01.res.76.2.215. [DOI] [PubMed] [Google Scholar]
- Rubin LJ. Primary pulmonary hypertension. N. Engl. J. Med. 1997;336:111–117. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- Ruiz L, Penaloza D. Altitude and hypertension. Mayo Clin. Proc. 1977;52:442–445. [PubMed] [Google Scholar]
- Ryland D, Reid L. The pulmonary circulation in cystic fibrosis. Thorax. 1975;30:285–292. doi: 10.1136/thx.30.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffrin EL. Reactivity of small blood vessels in hypertension: relation with structural changes. State of the art lecture. Hypertension. 1992;19:II1–9. doi: 10.1161/01.hyp.19.2_suppl.ii1-a. [DOI] [PubMed] [Google Scholar]
- Schraufnagel DE, Mehta D, Harshbarger R, Treviranus K, Wang NS. Capillary remodeling in bleomycin-induced pulmonary fibrosis. Am. J. Pathol. 1986;125:97–106. [PMC free article] [PubMed] [Google Scholar]
- Schraufnagel DE. Monocrotaline-induced angiogenesis. Differences in the bronchial and pulmonary vasculature. Am. J. Pathol. 1990;137:1083–1090. [PMC free article] [PubMed] [Google Scholar]
- Schraufnagel DE, Sekosan M, McGee T, Thakkar MB. Human alveolar capillaries undergo angiogenesis in pulmonary veno- occlusive disease. Eur. Respir. J. 1996;9:346–350. doi: 10.1183/09031936.96.09020346. [DOI] [PubMed] [Google Scholar]
- Schraufnagel DE, Malik R, Goel V, Ohara N, Chang SW. Lung capillary changes in hepatic cirrhosis in rats. Am. J. Physiol. 1997;272:L139–L147. doi: 10.1152/ajplung.1997.272.1.L139. [DOI] [PubMed] [Google Scholar]
- Sekhon HS, Wright JL, Churg A. Cigarette smoke causes rapid cell proliferation in small airways and associated pulmonary arteries. Am. J. Physiol. 1994;267:L557–L563. doi: 10.1152/ajplung.1994.267.5.L557. [DOI] [PubMed] [Google Scholar]
- Semmens M, Reid L. Pulmonary arterial muscularity and right ventricular hypertrophy in chronic bronchitis and emphysema. Br. J. Dis. Chest. 1974;68:253–263. doi: 10.1016/0007-0971(74)90049-7. [DOI] [PubMed] [Google Scholar]
- Sime F, Penaloza D, Ruiz L. Bradycardia, increased cardiac output, and reversal of pulmonary hypertension in altitude natives living at sea level. Br. Heart J. 1971;33:647–657. doi: 10.1136/hrt.33.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skwarski K, MacNee W, Wraith PK, Sliwinski P, Zielinski J. Predictors of survival in patients with chronic obstructive pulmonary disease treated with long-term oxygen therapy. Chest. 1991;100:1522–1527. doi: 10.1378/chest.100.6.1522. [DOI] [PubMed] [Google Scholar]
- Smith KAM. Does chronic hypoxia induce angiogenesis in skeletal muscle of the rat? J. Physiol. 1997;499P:118–119P. [Google Scholar]
- Smith K, Marshall JM. Physiological adjustments and arteriolar remodelling within skeletal muscle during acclimation to chronic hypoxia in the rat. J. Physiol. 1999;521 Part 1:261–272. doi: 10.1111/j.1469-7793.1999.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow RL, Davies P, Pontoppidan H, Zapol WM, Reid L. Pulmonary vascular remodeling in adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1982;126:887–892. doi: 10.1164/arrd.1982.126.5.887. [DOI] [PubMed] [Google Scholar]
- Sobin SS, Tremer HM, Hardy JD, Chiodi HP. Changes in arteriole in acute and chronic hypoxic pulmonary hypertension and recovery in rat. J. Appl. Physiol. 1983;55:1445–1455. doi: 10.1152/jappl.1983.55.5.1445. [DOI] [PubMed] [Google Scholar]
- Stenmark KR, Mecham RP. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu. Rev. Physiol. 1997;59:89–144. doi: 10.1146/annurev.physiol.59.1.89. [DOI] [PubMed] [Google Scholar]
- Steudel W, Scherrer-Crosbie M, Bloch KD, Weimann J, Huang PL, Jones RC, et al. Sustained pulmonary hypertension and right ventricular hypertrophy after chronic hypoxia in mice with congenital deficiency of nitric oxide synthase 3. J. Clin. Invest. 1998;101:2468–2477. doi: 10.1172/JCI2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudel W, Watanabe M, Dikranian K, Jacobson M, Jones RC. Expression of nitric oxide synthase isoforms (NOS II and NOS III) in adult rat lung in hyperoxic pulmonary hypertension. Cell Tissue Res. 1999;295:317–329. doi: 10.1007/s004410051238. [DOI] [PubMed] [Google Scholar]
- Storme L, Rairigh RL, Parker TA, Cornfield DN, Kinsella JP, Abman SH. K+-channel blockade inhibits shear stress-induced pulmonary vasodilation in the ovine fetus. Am. J. Physiol. 1999;276:L220–L228. doi: 10.1152/ajplung.1999.276.2.L220. [DOI] [PubMed] [Google Scholar]
- Sui GJ, Liu YH, Cheng XS, Anand IS, Harris E, Harris P, Heath D. Subacute infantile mountain sickness. J. Pathol. 1988;155:161–170. doi: 10.1002/path.1711550213. [DOI] [PubMed] [Google Scholar]
- Sullivan GW, Sarembock IJ, Linden J. The role of inflammation in vascular diseases. J. Leukoc. Biol. 2000;67:591–602. doi: 10.1002/jlb.67.5.591. [DOI] [PubMed] [Google Scholar]
- Thet LA, Law DJ. Changes in cell number and lung morphology during early postpneumonectomy lung growth. J. Appl. Physiol. 1984;56:975–978. doi: 10.1152/jappl.1984.56.4.975. [DOI] [PubMed] [Google Scholar]
- Tomashefski JF, Jr, Davies P, Boggis C, Greene R, Zapol WM, Reid LM. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am. J. Pathol. 1983;112:112–126. [PMC free article] [PubMed] [Google Scholar]
- Tweddell JS, Rokkas CK, Harada A, Pirolo JS, Branham BH, Schuessler RB, et al. Anterior septal coronary artery infarction in the canine: a model of ventricular tachycardia with a subendocardial origin. Ablation and activation sequence mapping. Circulation. 1994;90:2982–2992. doi: 10.1161/01.cir.90.6.2982. [DOI] [PubMed] [Google Scholar]
- Tworetzky W, Moore P, Bekker JM, Bristow J, Black SM, Fineman JR. Pulmonary blood flow alters nitric oxide production in patients undergoing device closure of atrial septal defects. J. Am. Coll. Cardiol. 2000;35:463–467. doi: 10.1016/s0735-1097(99)00576-8. [DOI] [PubMed] [Google Scholar]
- Tyler RC, Muramatsu M, Abman SH, Stelzner TJ, Rodman DM, Bloch KD, et al. Variable expression of endothelial NO synthase in three forms of rat pulmonary hypertension. Am. J. Physiol. 1999;276:L297–L303. doi: 10.1152/ajplung.1999.276.2.L297. [DOI] [PubMed] [Google Scholar]
- Voelkel NF, Tuder RM. Cellular and molecular mechanisms in the pathogenesis of severe pulmonary hypertension. Eur. Respir J. 1995;8:2129–2138. doi: 10.1183/09031936.95.08122129. [DOI] [PubMed] [Google Scholar]
- Wright JL, Lawson L, Pare PD, Hooper RO, Peretz DI, Nelems JM, et al. The structure and function of the pulmonary vasculature in mild chronic obstructive pulmonary disease. The effect of oxygen and exercise. Am. Rev. Respir Dis. 1983;128:702–707. doi: 10.1164/arrd.1983.128.4.702. [DOI] [PubMed] [Google Scholar]
- Wyncoll DL, Evans TW. Acute respiratory distress syndrome. Lancet. 1999;354:497–501. doi: 10.1016/S0140-6736(98)08129-X. [DOI] [PubMed] [Google Scholar]
- Yoshibayashi M, Nishioka K, Nakao K, Saito Y, Matsumura M, Ueda T, et al. Plasma endothelin concentrations in patients with pulmonary hypertension associated with congenital heart defects. Evidence for increased production of endothelin in pulmonary circulation. Circulation. 1991;84:2280–2285. doi: 10.1161/01.cir.84.6.2280. [DOI] [PubMed] [Google Scholar]
- Zapol WM, Snider MT. Pulmonary hypertension in severe acute respiratory failure. N. Engl. J. Med. 1977;296:476–480. doi: 10.1056/NEJM197703032960903. [DOI] [PubMed] [Google Scholar]
- Zhu ZG, Li HH, Zhang BR. Expression of endothelin-1 and constitutional nitric oxide synthase messenger RNA in saphenous vein endothelial cells exposed to arterial flow shear stress. Ann. Thorac. Surg. 1997;64:1333–1338. doi: 10.1016/S0003-4975(97)00859-X. [DOI] [PubMed] [Google Scholar]
- Ziesche R, Petkov V, Williams J, Zakeri SM, Mosgoller W, Knofler M, et al. Lipopolysaccharide and interleukin 1 augment the effects of hypoxia and inflammation in human pulmonary arterial tissue. Proc. Natl Acad. Sci. USA. 1996;93:12478–12483. doi: 10.1073/pnas.93.22.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]