Abstract
This article reviews recent studies on the importance of glycine receptors for both the spontaneous and the reflex respiratory modulation of the laryngeal abductors and adductors. Our findings show that strychnine blockade of glycine receptors within the brainstem changes the eupneic three-phase respiratory pattern into two phases. This has major implications for glottal control: (i) the inspiratory glottic abduction and early expiratory adduction were both compromised – a finding mimicked by 5% hypoxia; (ii) closure of the glottis during defensive upper airway reflexes became intermittent and the reflex apnoea reversed to sustained inspiratory discharge. Based on these data, we predict that periods of prolonged hypoxia, such as those that occur during sleep apnoeas, will constrain inspiratory glottic abduction thereby impeding inhalation.
Keywords: apnoea, glycine receptor, respiratory rhythm, strychnine, upper airway
Introduction
Breathing is an automatic activity generated by reciprocal inhibitory synaptic interactions in the pontine-medullary respiratory network (St.-John, 1998; Richter & Spyer, 2001). The output of this network drives numerous types of respiratory muscles to pump air into and out of the lungs. Adequate ventilation depends on efficient pumping of air but also on the patency of the upper airway. In eupnoea the glottis regulates upper airway patency. The recurrent and superior laryngeal nerves both innervate glottal adductor and abductor muscles. These motor nerves are mixed, containing fibres that innervate either the abductor or the adductor muscles, which contract during neural inspiration and post-inspiration, respectively. Accordingly, two classes of laryngeal motoneurones can be found within the ventral respiratory group: inspiratory and post-inspiratory laryngeal motoneurones (Barillot et al. 1990; Bryant et al. 1993).
Post-inspiratory neurones receive profound glycinergic synaptic inhibition during inspiration (Haji et al. 1990; Schmid et al. 1991) which, according to network models, originates from inspiratory neurones (Rybak et al. 1997; Richter & Spyer, 2001). The abrupt firing of post-inspiratory neurones provides an irreversible off-switch mechanism of inspiration (Bianchi et al. 1995; Bonham, 1995; Richter, 1996) but also laryngeal adduction. This post-inspiratory glottic constriction serves multiple functions (see Shiba et al. 1999): it slows expiratory airflow out of the lungs, to increase time for efficient gas exchange and maintains functional residual capacity to prevent lung collapse (Bartlett, 1986).
The former is most significant in neonatal mammals that have a high breathing frequency. Further, interactions occur within the pontomedullary respiratory network that modulate laryngeal motor activity to allow vocalization, suckling and swallowing (Sakamoto et al. 1996; Shiba et al. 1999) as well as defensive reflexes such as sneezing and coughing (Widdicombe, 1986).
A recent study has demonstrated that during glycine receptor blockade post-inspiratory neurones shift their phase of firing to inspiration (Büsselberg et al. 2001). A similar effect was also observed during anoxia (Lieske et al. 2000), which presumably reflects the failure of inhibitory synaptic mechanisms during low oxygen levels (Schmidt et al. 1995; Ramirez et al. 1998). Thus, we hypothesized that an absence of glycinergic inhibition, induced by strychnine or hypoxia, would disrupt both the eupneic and the reflex control of the upper airway.
Comments on methods
We used a cellular and systemic approach to understand the role of glycine receptors within the brainstem for breathing. The working heart–brainstem preparation (Paton, 1996) was employed since it allows kinesiological experiments as well as intracellular recordings of identified respiratory neurones. Further, it generates an eupneic motor pattern of discharge in rats from 1-h-old to mature animals (Dutschmann et al. 2000) and is a good model in which to study the development of central neural control of respiration (and the cardiovascular system) in a single preparation.
In this study we have made intracellular and motor nerve recordings. Importantly, we have directly assessed changes in glottic resistance as measured from subglottic pressure (SGP) recordings during constant air flow perfusion of the upper airway in the expiratory direction (Paton et al. 1999).
Three-phase respiratory rhythm
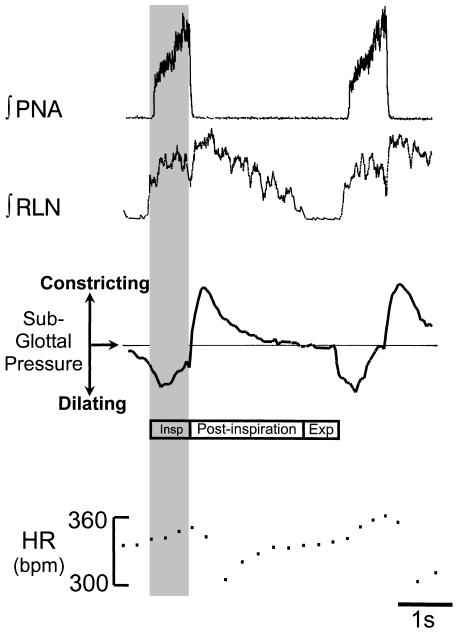
The respiratory rhythm comprises three phases (Richter, 1996): inspiration, post-inspiration (stage I expiration) and expiration (stage II; Fig. 1). The post-inspiratory phase is clearly evident in recordings of cranial motor outflows, such as the recurrent laryngeal nerve, and is essential for the early expiratory glottic constriction (Fig. 1). Functionally, constriction of the vocal fold during early expiration maintains functional residual capacity and prevents lung collapse. We believe that the post-inspiratory phase is an important criterion in the definition of eupnoea.
Fig. 1.
The three phases of eupnea. In the working heart–brainstem preparation (Paton, 1996) eupnea consists of a ramp inspiratory pattern in the phrenic nerve (PNA), inspiratory and post-inspiratory discharges in the recurrent laryngeal nerve (RLNA) with the glottis dilating and constricting with inspiration and early expiration, respectively. There is also a respiratory sinus arrhythmia as revealed from changes in heart rate (HR). Inspiration (Insp), post-inspiration (Post-Insp) and expiration can be clearly seen in both the RLNA and subglottal pressure recording (SGP). SGP was obtained by perfusing the upper airway in the expiratory direction with a constant flow of warmed and moistened air (see Paton et al. 1999).
Physiological role of brainstem glycine receptors for the three-phase rhythm
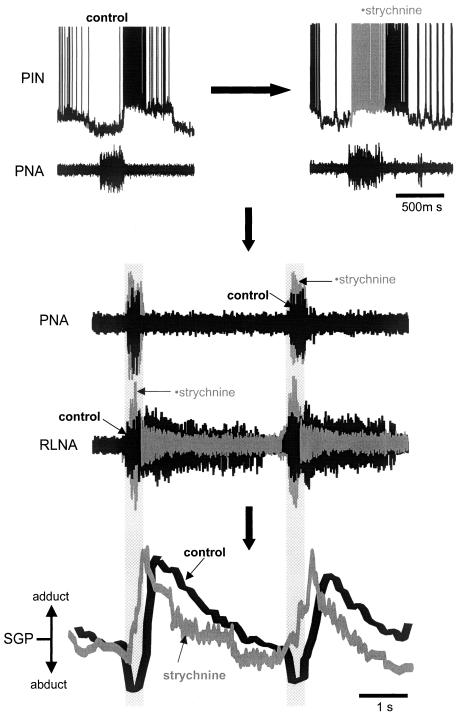
Blockade of glycine receptors with selective doses of strychnine attenuated the post-inspiratory activity in cranial motor outflows (Fig. 2). This was mimicked during isocapnic hypoxia (5% oxygen, 5% carbon dioxide, 90% nitrogen), an effect consistent with that reported previously in anaesthetized cats (see Zhou et al. 1989). In our kinesiological studies of eupneic modulation of glottal function, strychnine reduced the inspiratory laryngeal abduction. Paradoxically, glottal adduction occurred during inspiration (Fig. 2). Again we also observed this during systemic hypoxia (5% oxygen). To understand the cause of this change in glottic function we exposed post-inspiratory neurones to strychnine and found a dramatic change in their phase-related firing pattern. Post-inspiratory neurones were depolarized during inspiration and consequently fired during this phase. Assuming that laryngeal adductor neurones behaved similarly, there would be a concomitant activation of adductors and abductors during neural inspiration. If the contraction of adductor muscles was greater than abductors, this might explain the reduced inspiratory abduction and the paradoxical inspiratory laryngeal adduction after strychnine. Although the eupneic rhythm became less periodic, burst discharges in the phrenic nerve were recorded after glycine receptor blockade. All told, the integrity of glycine receptors within the mammalian respiratory network is essential for the co-ordination of each of the respiratory phases.
Fig. 2.
Respiratory modulation of the upper airway is distorted after blockade of glycine receptors. Glycine receptor blockade causes post-inspiratory neurones (PIN) to fire in inspiration, enhances inspiratory but depresses postinspiratory discharge in the recurrent laryngeal nerve (RLN) and causes a paradoxical inspiratory adduction as indicated by an increase in subglottal pressure (SGP) during PNA. Black traces are control and grey traces represent the changes observed after glycine receptors were blocked with strychnine (0.5 μM) or isocapnic hypoxia (5% oxygen, 5% carbon dioxide and 90% nitrogen). Shaded areas indicate neural inspiration.
Functional role of glycine receptors in reflexes protecting the upper airway
One of the most potent defensive cardiorespiratory reflexes of the upper airways is the diving response. Irrigation of the nasal mucosa with cold water or electrical stimulation of the trigeminal ethmoidal nerve can elicit the diving response consisting of: post-inspiratory apnoea, potent and sustained bradycardia, constriction in the arterial vessels and the glottis (Dutschmann & Herbert, 1996, 1998). The latter ensures that water cannot seep into the lungs. Since this reflex depends on activation of post-inspiratory mechanisms (i.e. inspiratory off-switch and glottic constriction) we predicted that blockade of glycine receptors would abolish the apnoea and reduce the laryngeal constriction.
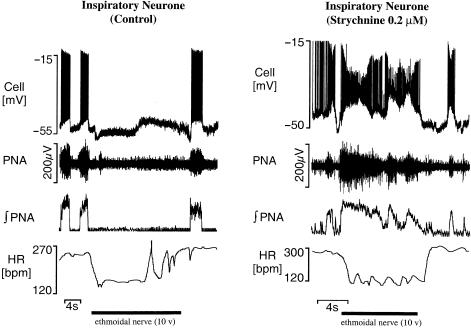
Figure 3 shows the devastating effect that strychnine has on the diving response. The pattern of response of apnoea was converted to a paradoxical activation of phrenic nerve activity (i.e. a loss of the inspiratory off-switch). Indeed, our intracellular recordings of inspiratory neurones show persistent firing after strychnine (Fig. 3). In addition, the diving response evoked sustained glottal adduction was reduced significantly (Dutschmann & Paton, 2002). The latter likely reflects a simultaneous firing of both abductor and adductor motoneurones, so reducing glottal constriction. In terms of the diving response, blockade of glycine receptors would have a devastating consequence. First, an activation of phrenic motoneurones would cause diaphragmatic contraction and generate a negative pressure within the upper airway. Second, the reduced glottal constriction would mean that it is unlikely to be closed properly. The latter combined with the negative pressure in the upper airway is likely to aspirate water into the lungs as is reported in most cases of drowning. Since post-inspiratory neurones/laryngeal adductors are activated during many cardiorespiratory reflexes (e.g. Hering Breuer, cough, aspiration, sneezing, swallowing; Grélot et al. 1992; Shiba et al. 1999; Gestreau et al. 2000), we suggest that these behaviours will also fail if glycine receptor function is affected.
Fig. 3.
Inspiratory component of the diving response is reversed after glycine receptor blockade. The diving response was elicited by electrical stimulation of the ethmoidal nerve (20 Hz, 0.5–10 V, 0.1 ms). The response consisted of a hyperpolarization of inspiratory neurones and an apnoea (i.e. cessation of phrenic nerve activity; PNA). After strychnine the response was converted to a depolarization/persistent firing of inspiratory neurones and activation of PNA. Note there was no obvious change to the heart rate response (HR; see Dutschmann & Paton, 2002).
Potential clinical importance
There are a number of conditions that may seriously alter glycine receptor function. Clearly, strychnine poisoning is rare but a point mutation of the glycine receptor could occur. It is also known that there is a change in the subunit composition of the glycine receptor that occurs with age: in the rat the glycine receptor matures around the 14th postnatal day of life (Becker et al. 1988). Failure of this development may have serious deleterious consequences on post-inspiratory activity affecting eupnea and reflex control of breathing as well as protection of the upper airway. It is predicted that there would also be problems with swallowing if the three-phase oscillator was no longer properly co-ordinated. Our data also indicate that during hypoxia post-inspiratory activity is reduced and there is paradoxical glottic constriction during neural inspiration. This might have implications for understanding certain pathophysiological syndromes such as cot death and sleep apnoea/snoring when systemic hypoxia is prevalent.
Conclusions
We wish to propose that central post-inspiratory activity and laryngeal adduction during early expiration are important criteria for defining eupnea. We suggest that central glycinergic inhibition is essential for co-ordination of the three-phase respiratory rhythm, reflexly induced inspiratory off-switching and upper airway protection during the diving response.
Acknowledgments
The British Heart Foundation and DFG funded the present studies.
References
- Barillot JC, Grélot L, Bianchi AL. Discharge patterns of laryngeal motoneurones in the cat: an intracellular study. Brain Res. 1990;509:99–106. doi: 10.1016/0006-8993(90)90314-2. [DOI] [PubMed] [Google Scholar]
- Bartlett DJ. Upper airway motor systems. In: Cherniack NS, Widdicombe JG, editors. Handbook for Physiology, Sec 3, the Respiratory System. II. Bethesda, MD: The American Physiological Society; 1986. pp. 223–245. [Google Scholar]
- Becker CM, Hoch W, Betz H. Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO J. 1988;7:3717–3726. doi: 10.1002/j.1460-2075.1988.tb03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubié M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane potentials and neurotransmitters. Physiol. Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Bonham AC. Neurotransmitters in the CNS control of breathing. Resp. Physiol. 1995;101:219–230. doi: 10.1016/0034-5687(95)00045-f. [DOI] [PubMed] [Google Scholar]
- Bryant TH, Shigetaka Y, De Castro D, Lipski J. Expiratory neurons of the Bötzinger complex in the rat: a morphological study following intracellular labeling with biocytin. J. Comp. Neurol. 1993;335:267–282. doi: 10.1002/cne.903350210. [DOI] [PubMed] [Google Scholar]
- Büsselberg D, Bischoff AM, Paton JFR, Richter DW. Loss of glycinergic inhibition reveals two modes of respiratory rhythm generation. Eur. J. Physiol. 2001;441:444–449. doi: 10.1007/s004240000453. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H. The Kölliker-Fuse nucleus mediates the trigeminally induced apnea in the rat. Neuroreport. 1996;7:1432–1436. doi: 10.1097/00001756-199605310-00022. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Herbert H. NMDA- and GABAA-receptors in the rat Kölliker-Fuse nucleus control cardio-respiratory responses evoked by trigeminal ethmoidal nerve stimulation. J. Physiol. (Lond.) 1998;510:793–804. doi: 10.1111/j.1469-7793.1998.793bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Wilson RJA, Paton JFR. Respiratory activity in neonatal rats. Auton. Neurosci. 2000;84:19–29. doi: 10.1016/S1566-0702(00)00177-6. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Paton JFR. Trigeminal reflex regulation of the glottis depends on central glycinergic inhibition in the rat. Am. J. Physiol. Integrative Comp. Physiol. 2002;282:R999–R1005. doi: 10.1152/ajpregu.00502.2001. [DOI] [PubMed] [Google Scholar]
- Gestreau C, Grelot L, Bianchi AL. Activity of respiratory laryngeal motoneurons during fictive coughing and swallowing. Exp. Brain Res. 2000;130:27–34. doi: 10.1007/s002210050003. [DOI] [PubMed] [Google Scholar]
- Grélot L, Milano S, Portillo F, Miller AD, Bianchi AL. Membrane potential changes of phrenic motoneurons during fictive vomiting, coughing, and swallowing in the decerebrate cat. J. Neurophysiol. 1992;68:2110–2119. doi: 10.1152/jn.1992.68.6.2110. [DOI] [PubMed] [Google Scholar]
- Haji A, Remmers JE, Connelly C, Takeda R. Effects of glycine and GABA on bulbar respiratory neurons of cat. J. Neurophysiol. 1990;63:955–965. doi: 10.1152/jn.1990.63.5.955. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat. Neurosci. 2000;3:600–607. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- Paton JFR. A working heart-brainstem preparation of the mouse. J. Neurosci. Meth. 1996;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Paton JFR, Li Y-W, Kasparov S. Reflex response and convergence of pharyngoesophageal and peripheral chemo- receptors in the nucleus of the solitary tract. Neuroscience. 1999;93:143–154. doi: 10.1016/s0306-4522(99)00098-6. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Quellmalz UJ, Wilken B, Richter DW. The hypoxic response of neurones within the in vitro mammalian respiratory network. J. Physiol. (Lond.) 1998;507:571–582. doi: 10.1111/j.1469-7793.1998.571bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW. Neural regulation of respiration: Rhythmogenesis and afferent control. In: Greger R, Windhorst U, editors. Comprehensive Human Physiology. Berlin: Springer Verlag; 1996. pp. 2079–2095. [Google Scholar]
- Richter DW, Spyer KM. Studying rhythmogenesis of breathing: comparisons of in vivo and in vitro models. TINS. 2001;24:464–472. doi: 10.1016/s0166-2236(00)01867-1. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Paton JFR, Schwaber JS. Modelling neural mechanisms for genesis of respiratory rhythm and pattern. II. Network models of the central respiratory pattern generator. J. Neurophysiol. 1997;77:2007–2026. doi: 10.1152/jn.1997.77.4.2007. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Nonaka S, Katada A. Control of respiratory muscles during speech and vocalization. In: Miller AD, Bianchi AL, Bishop BP, editors. Neuronal Control of the Respiratory Muscles. Boca Raton, FL: CRC Press; 1996. pp. 249–258. [Google Scholar]
- Schmid K, Bohmer G, Gebauer K. Glycine receptor-mediated fast synaptic inhibition in the brainstem respiratory system. Respir. Physiol. 1991;4:351–361. doi: 10.1016/0034-5687(91)90129-7. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Bellingham MC, Richter DW. Adenosinergic modulation of respiratory neurones and hypoxic responses in the anaesthetized cat. J. Physiol. (Lond.) 1995;483:769–781. doi: 10.1113/jphysiol.1995.sp020621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba K, Satoh I, Kobayashi N, Hayashi F. Multifunctional laryngeal motoneurons: an intracellular study in the cat. J. Neurosci. 1999;19:2717–2727. doi: 10.1523/JNEUROSCI.19-07-02717.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St.-John WM. Neurogenesis of patterns of automatic ventilatory activity. Prog. Neurobiol. 1998;56:97–117. doi: 10.1016/s0301-0082(98)00031-8. [DOI] [PubMed] [Google Scholar]
- Widdicombe JG. Reflexes from the upper respiratory tract. In: Cherniack NS, Widdicombe JG, editors. Handbook for Physiology, Sec 3, the Respiratory System. II. Bethesda, MD: The American Physiological Society; 1986. pp. 223–245. [Google Scholar]
- Zhou D, Huang Q, St.-John WM, Bartlett D., Jr Respiratory activities of intralaryngeal branches of the recurrent laryngeal nerve. Am. J. Physiol. 1989;67:1171–1178. doi: 10.1152/jappl.1989.67.3.1171. [DOI] [PubMed] [Google Scholar]