Abstract
This paper reports on the presence of the conus arteriosus in the heart of the adult gilthead seabream, Sparus auratus (Perciformes, Teleostei). The junctional region between the single ventricle and the bulbus arteriosus has been studied by conventional light microscopy, and by scanning and transmission electron microscopy. In addition, fluorescent phalloidin and antibodies against the muscle myosin heavy chains, laminin and collagen type IV have been used. The conus arteriosus is a distinct muscular segment interposed between the ventricle and the bulbus arteriosus. It is clearly different from the bulbus arteriosus due to its myocardial nature. It can also be distinguished from the ventricular myocardium because: (1) it has a conus shape; (2) it is formed by compact, well-vascularized myocardium; (3) it is surrounded on its inner and outer faces by fibrous layers rich in collagen and elastin; (4) it constitutes the anatomical support of the so-termed conus valves; (5) it shows intense staining for laminin and type-IV collagen; and (6) the myocardial cells located close to the inner fibrous layer are helicoidally arranged. By contrast, the ventricular myocardium is highly trabecular, lacks a compacta, shows no vessels, and presents barely detectable amounts of laminin and collagen type IV. The presence of a distinct conus arteriosus in the heart of an evolutionary advanced teleost species indicates that the conus is not a vestigial segment from the evolutionary or embryological points of view. The characteristic spatial arrangement of the conus myocytes strongly suggests that the conus is implicated in the mechanical performance of the conus valves.
Keywords: conus arteriosus, gilthead seabream, heart, myocardium, teleost
Introduction
The gross anatomy and the structural characteristics of the heart outflow tract chamber vary considerably among different fish groups. In elasmobranchs, for instance, the outflow tract is a contractile chamber of variable length endowed with a muscular layer, an inner elastic coat and several rows of valves. In the nomenclature proposed by Gegenbaur (1866), this chamber is commonly named conus arteriosus (Santer, 1985; Zummo & Farina, 1989; Sans-Coma et al. 1995), although terms such as bulbus aortae and bulbus cordis have also been used (Goodrich, 1930; Parsons, 1930; De la Cruz et al. 1999). A well-developed conus arteriosus also exists in lungfishes (Lankester, 1879; Boas, 1880a; Robertson, 1914), coelacanths (Anthony et al. 1965; Millot et al. 1978), and primitive actinopterygians such as the polypteriformes (Boas, 1880a), chondrosteans (Parsons, 1930; Icardo et al. 2002a, b) and holosteans (Stöhr, 1876; Boas, 1880b; Parsons, 1930; Bertin, 1958). In addition, the holostean Amia calva possesses a short bulbus arteriosus of non-myocardial nature connecting the conus arteriosus with the ventral aorta (Parsons, 1930).
In teleosts, the main component of the cardiac outflow tract is the bulbus arteriosus. This is an elastic chamber organized into layers containing myofibrogblasts, smooth muscle cells, collagen and elastin (Priede, 1976; Santer, 1985; Satchell, 1991), mostly distributed in species-specific patterns (Icardo et al. 1999a, b, 2000a, 2000b). Primitive teleost species belonging to the genera Albula, Pterothrissus, Megalops and Tarpon (Stannius, 1846; Boas, 1880b; Senior, 1907a, b, c; Satchell, 1991) show, in addition to the bulbus arteriosus, a distinct conus arteriosus with two transverse tiers of valves interposed between the ventricle and the bulbus arteriosus. However, the conus is considered to be a primitive heart chamber which has been progressively lost in evolution (Satchell, 1991). Consequently, the conus arteriosus is considered to be very reduced in size, or even absent, in most osteichthyans (Smith, 1918; Santer, 1985; Satchell, 1991; Farrell & Jones, 1992), and there are no data available on its histological organization or structure.
In a study of the heart of the adult gilthead seabream (Sparus auratus), an evolutionarily advanced teleost species commonly used for cardiovascular research in our laboratory, we detected the presence of a distinct cardiac segment interposed between the ventricle and the bulbus arteriosus. We have identified this segment of the heart as the conus arteriosus and have examined its histological and ultrastructural characteristics.
Materials and methods
Twenty-two adult gilthead seabream (Sparus auratus, Perciformes, Teleostei) (20 male, aged 1–2 years, and two female, older than 3 years) were either purchased at the local fish market or obtained alive at the fish farm CUPIMAR (San Fernando, Spain). Live specimens were over-anaesthetized with MS–222 (tricaine methane sulphonate, Sigma Chemical Co., UK). The hearts were removed from the pericardial cavity, transferred to an ice-cold phosphate-buffered saline (pH 7.4), and lightly perfused with the same buffer. Twelve specimens were examined by means of histological, histochemical and/or immunohistochemical techniques for light microscopy. Four hearts were processed for laser confocal microscopy. Another four were studied by scanning electron microscopy (SEM), and the remaining two by means of semithin sections and transmission electron microscopy (TEM).
Histology and histochemistry
Whole hearts were fixed by immersion in Bouin's solution for 24 h or in MAW (methanol–acetone–water 2:2:1) for 6–8 h (fixative-to-tissue-volume ratio=80:1), embedded in Paraplast and serially cut at 10µm. Four specimens were sectioned longitudinally, three sagitally and five transversely. The sections were then stained with haematoxylin and eosin, with Mallory's trichrome stain for the detection of collagen, or with Weigert's resorcin–fuchsin method in combination with van Gieson's stain for the detection of elastin.
Immunohistochemistry
The hearts fixed in MAW and embedded in Paraplast were transversely, longitudinally or sagitally cut at 10 µm. Dewaxed sections were washed in Tris-phosphate buffered saline (TPBS, pH 7.8) and stained with the monoclonal antibody MF20 (Developmental Studies Hibridoma Bank, University of Iowa, UK), which recognizes the myosin heavy chains of the striated and cardiac muscle, with the polyclonal antibody L9393 (Sigma Chemical Co.) against laminin, or with the polyclonal antibody AB748 (Chemicon, USA) against type IV collagen. For the detection of laminin and type IV collagen, the sections were previously digested with 0.5% papain in 0.02M phosphate buffer (pH 4.7) for 10 min at 37°C.
Endogenous peroxidase activity was blocked by incubation with 6% hydrogen peroxide in TPBS for 30 min. Non-specific binding sites were saturated for 1 h with 3% bovine serum albumin in TPBS (SB) for staining with both L9393 and AB748, or with the same solution plus 0.5% Triton X-100 (SBT) for staining with MF20. In all cases, endogenous biotin was subsequently blocked with the avidin–biotin blocking kit (Vector, Burlingame, USA).
The primary antibodies were allowed to bind overnight at 4°C, using the following concentrations: 1:1000 in SB for L9393; 1:500 in SB for AB748; or 1:100 in SBT for MF20. Control slides were incubated in SB or SBT only, and were routinely negative. In addition, an aliquot of L9393 was incubated at 4°C for 1h with MatriGel (Becton Dickinson Labware, MA, USA), and then applied to positive control slides. MatriGel is a solubilized basement membrane preparation, extracted from Engelbreth–Holm–Swarm (EHS) mouse sarcoma, the major component of which is laminin.
After washing with TPBS, the slides were incubated for 1h at room temperature in biotin-conjugated anti-mouse goat IgG (Sigma) diluted 1:100 in SB for MF20, or in biotin-conjugated anti-rabbit goat IgG (Sigma) diluted 1:100 in SB for both L9393 and AB748. The slides were washed again with TPBS and then incubated for 1h in avidin-peroxidase complex (Sigma) diluted 1:150 in TPBS. Peroxidase activity was developed with Sigma Fast® 3,3′-diaminobenzidine tablets according to the indications of the supplier. The slides were counterstained with Delafield's haematoxylin.
Laser confocal microscopy
The hearts were fixed in 4% paraformaldehyde for 7h, washed in PBS and stained with FITC-conjugated phalloidin (Sigma) for actin, as previously described (Germroth et al. 1995). Briefly, the conus was embedded in 10% acrylamide and sectioned at 200 µm with a FTB Vibracut vibratome along the longitudinal and transverse axes. The sections were then washed in PBS and stained in-gel overnight with a 1:40 solution of fluorescent phalloidin. The sections were washed in PBS, mounted with Vectashield H-1000 (Vector) and observed with a laser confocal Bio-Rad MC 1024 microscope.
Scanning electron microscopy (SEM)
The hearts were immersion-fixed in 3% glutaraldehyde in PBS for 3h. The samples were then dissected under a stereomicroscope, dehydrated in graded acetone, dried by the critical point method and gold-sputter-coated. Observations were made using a Philips SEM 501.
Semithin sections and transmission electron microscopy (TEM)
Selected heart fragments were fixed by immersion in 3% glutaraldehyde in PBS for 3h, post-fixed with 1% osmium tetroxide for 2 h, dehydrated in graded acetone and propylene oxide, and embedded in Araldite (Fluka). Semithin sections were cut with a LKB III ultratome, stained with 1% toluidine blue, and observed with a Zeiss III photomicroscope. Ultrathin sections were cut with a Leica Ultracut UCT ultratome, stained with uranyl acetate and lead citrate, and examined with a Zeiss ME 10C microscope.
Results
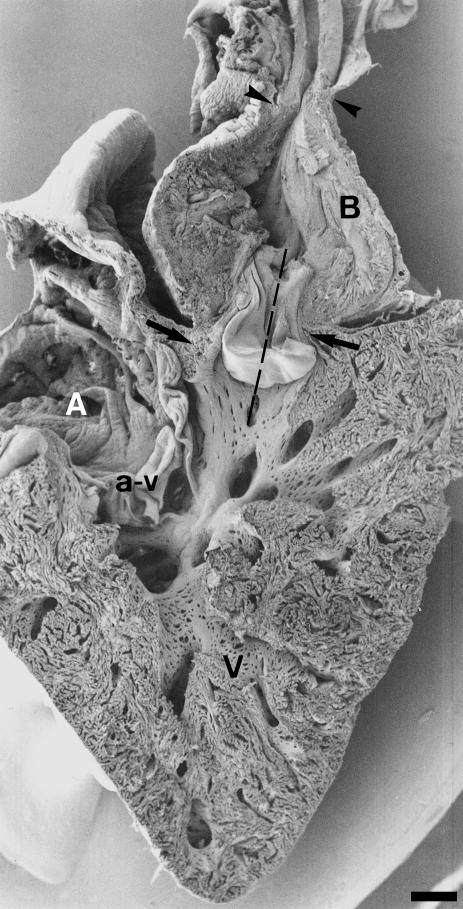
The heart of the adult gilthead seabream displays four chambers arranged in series: the sinus venosus, the atrium, the ventricle and the bulbus arteriosus (Fig. 1). All four are contained within the pericardial cavity. The sinus venosus and the atrium occupy a dorsal position. The ventricle displays the typical pyramidal shape of the active teleosts. The bulbus arteriosus lies above the ventricle and connects with the ventral aorta (Fig. 1). The junction between the ventricle and the bulbus arteriosus is clearly visible when the inner heart surface is exposed. Careful dissection of this region reveals the existence of a muscular ring around the outflow tract valves (Fig. 2a). This muscular ring consists of compact, well-vascularized myocardium (Fig. 2b). Longitudinal sections of the heart (Fig. 3) show that this myocardial ring has a conus shape tapering at its distal end. It can be distinguished clearly from both the non-myocardial bulbus arteriosus and from the trabecular ventricular myocardium. This cardiac segment, which we tentatively term conus arteriosus, constitutes the main support of the outflow tract valves (Figs 1 and 3).
Fig. 1.
Mid-sagittal section of the adult gilthead seabream heart. Left side of the heart. The ventricle (V) is pyramidal. The atrium (A) Is located in a dorsal position, being separated from the ventricle by the atrioventricular (a–v) valve. The bulbus arteriosus (B) is located above the ventricle and shows an enlarged proximal portion. Arrows indicate the conus region and the left conus valve. The broken line through the conus valve indicates the approximate planes of section in Fig. 3. Arrowheads mark the bulbus—ventral aorta junction and the insertion of the pericardium. Scale bar = 50 μm.
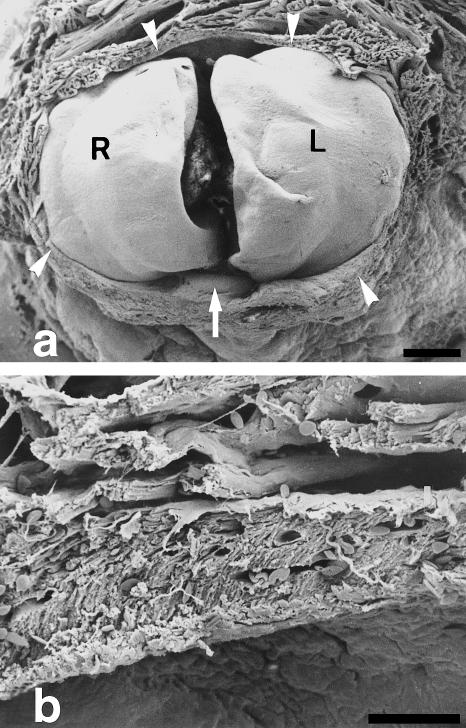
Fig. 2.
SEM micrographs depicting the junctional area between the ventricle and the bulbus arteriosus. (a) The ventricle has been dissected out and the caudal (ventricular) aspect of the left (L) and right (R) conus valves are exposed. Note the presence of a muscular ring (arrowheads) surrounding the valve leaflets. Arrow points to a posterior, supernumerary leaflet. (b) Detail of the anterior part of the muscular ring. It is formed of compact, vascularized myocardium. Myocytes are arranged in parallel and appear obliquely orientated. Vessels are more apparent on the outer side of the muscular ring. Myocardial trabeculae appear on the upper half of the micrograph. Scale bars = a, 200 μm; b, 30 μm.
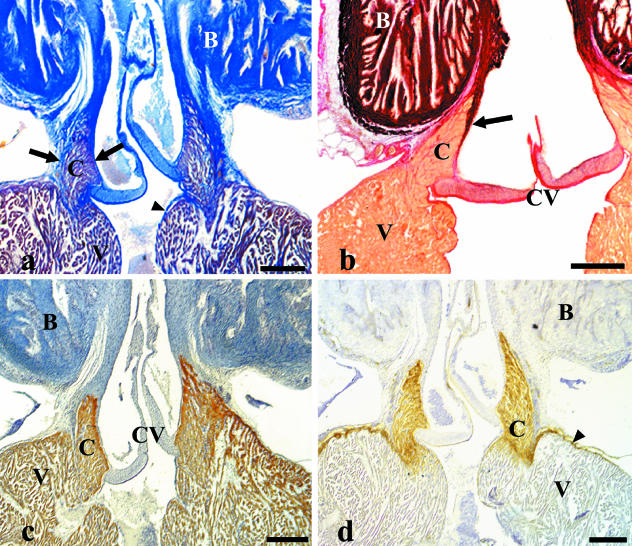
Fig. 3.
Longitudinal sections of the heart through the ventricle–bulbus junction. A ring of compact myocardium is interposed between the trabecular ventricle and the bulbus arteriosus. This myocardial segment has a conus shape which tapers at its distal end. Its base blends with the ventricular myocardium. B, bulbus arteriosus; C, conus; CV, conus valves; V, ventricle. (a) Mallory's trichrome stain. The conus myocardium is surrounded on its inner and outer sides by collagenous fibrous layers (arrows) which merge at the tapered end. The outer fibrous layer separates the conus from the bulbus distally, and merges with the subepicardium proximally. The inner fibrous layer continues proximally with the ventricular subendocardium (arrowhead). Collagen bundles separate the conus myocardium into fascicles. (b) Weigert—van Gieson stain. The inner fibrous layer (arrow) is very rich in elastin. Note the differences in staining with the outer fibrous layer. The bulbus also stains intensely. (c) MF20 immunostaining. Both the conus and the ventricular myocardium stains intensely, demonstrating the presence of myosin heavy chains. (d) L9393 immunostaining. Intense staining for laminin is specific to the conus. Laminin is barely detectable in the ventricular myocardium, except at the outer ventricular surface (arrowhead). Scale bars = a–c, 250 μm; d, 200 μm.
The inner and outer faces of the conus myocardium are covered by fibrous layers which merge at the tapered, distal end. The inner fibrous layer is rich in collagen and elastin, and separates the myocardium from the endocardial endothelium (Fig. 3a, b). The outer fibrous layer is very rich in collagen, poor in elastin, and separates the conus myocardium from the bulbus arteriosus and from the subepicardium (Fig. 3a, b). Collagen bundles coming from the two fibrous layers enter the conus and divide the myocardium into fascicles or bundles (Fig. 3a). This division is irregular and becomes more evident at the conus base. The inner and outer fibrous layers are thicker near the tapered end of the conus, becoming thinner toward the base. At the conus base, the two fibrous layers are continuous with the ventricle subendocardium and subepicardium, respectively, while the conus myocardium merges with the trabecular ventricular myocardium. The conus myocardium displays positive immunoreactivity to the MF20 antibody, similar to that expressed by the ventricular myocardium (Fig. 3c). The conus myocardium also displays positive immunoreactivity to the anti-laminin antibody used in this study (Fig. 3d) and, to a lesser extent, to the anti-type IV collagen antibody (not shown). In contrast, laminin (Fig. 3d) and type IV collagen are barely detectable in the ventricular myocardium.
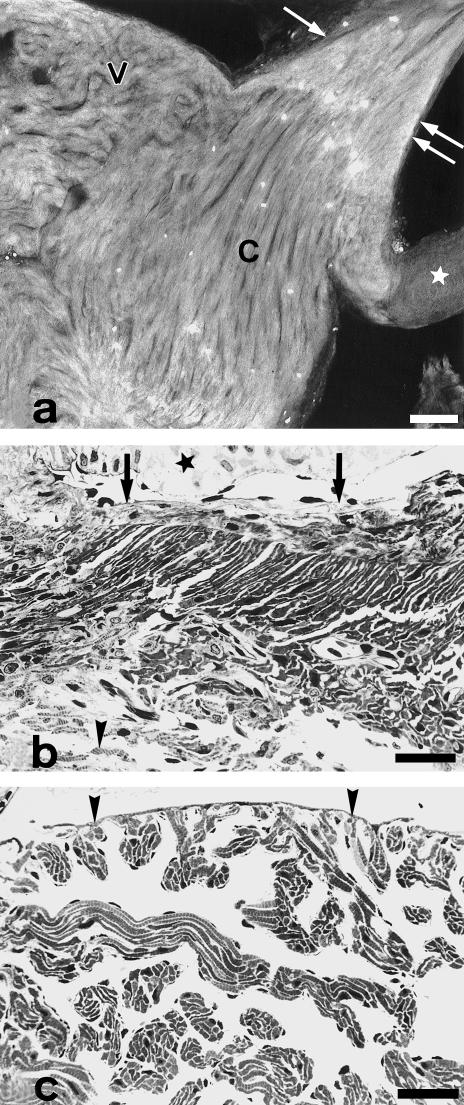
Laser confocal microscopy (Fig. 4a) shows that conus myocardial cells form a compact tissue, keep an overall spiralling or helicoidal orientation, and attach to the inner and outer fibrous layers. In semithin sections, the luminal face of the conus arteriosus is lined by a single layer of flattened endothelial cells which show discretely bulging nuclei (Fig. 4b). Myocardial cells in the vicinity of the subendocardial fibrous layer are elongated, appear in parallel to each other and display an oblique orientation with respect to the main heart axis. This oblique orientation is observed in both longitudinal (Fig. 4b) and transverse sections (Fig. 2b), further indicating an overall spiralling organization. Blood vessels are more abundant in the peripheral portion of the conus myocardium (Figs 2b and 4b), but small vascular profiles can be also observed in the inner fibrous layer. In contrast, the ventricular myocardium shows neither compacta nor vessels (Fig. 4c).
Fig. 4.
(a) Confocal microscope image of the conus after FITC-conjugated phalloidin staining. Longitudinal section. Conus myocardial cells (C) are helicoidally arranged and form a compact tissue which differentiates clearly from the ventricular (V) myocardium. Single and double arrow indicate the outer and inner fibrous layers, respectively. Star, valve leaflet. The bright spots correspond to autofluorescent erythrocytes. (b) Longitudinal, semithin section of the conus. Similar area to that indicated by double arrow in (a). Myocytes are arranged in parallel keeping an oblique orientation with respect to the main heart axis. Orientated myocardial cells extend between the dense connective tissue which forms the inner fibrous layer (arrows) and the rest of the conus myocardium. Note the presence of vessels and of individual myofibrils (arrowhead). The endocardium covers the inner side of the conus and is formed of flattened cells. A valve leaflet (star) appears in the upper part of the photograph. (c) Semithin section of the ventricle. Myocardial cells in the ventricle form an outer monolayer to which myocardial trabeculae are attached. Striated myofibrils become apparent when the trabeculae are sectioned longitudinally. Arrowheads indicate the epicardial side. Scale bars = a, 100 μm; b–c, 25 μm.
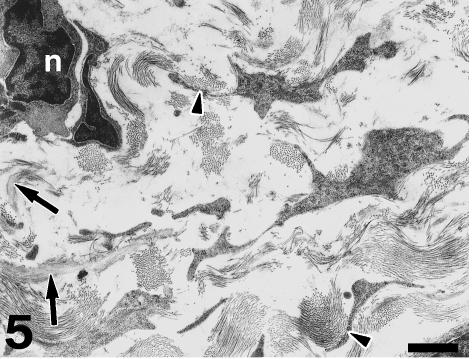
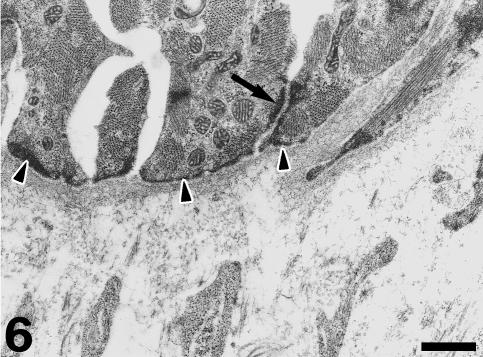
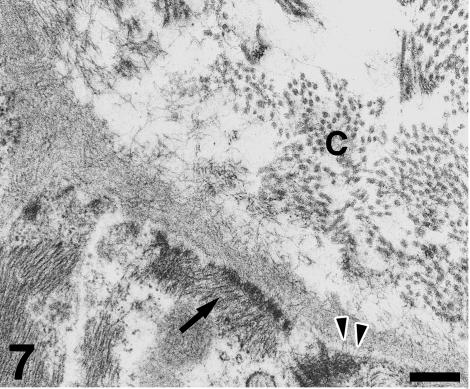
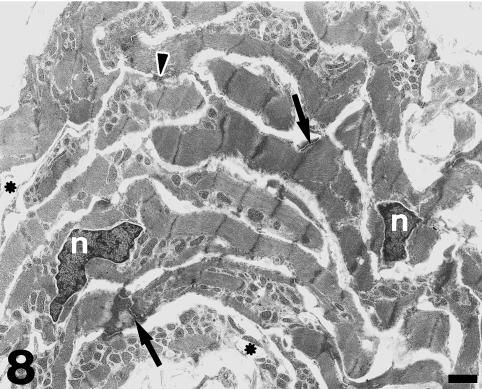
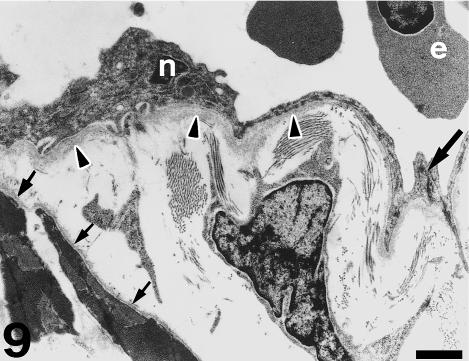
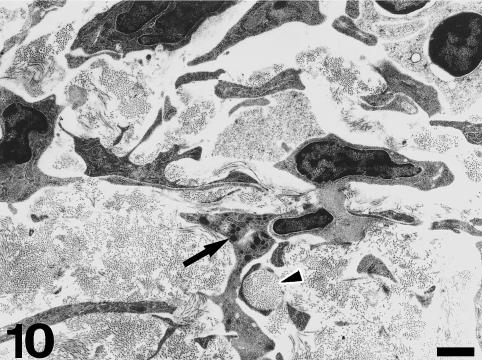
Under TEM, the subendocardial fibrous layer contains numerous irregularly shaped fibroblasts embedded in a dense extracellular matrix rich in collagen and elastin fibres (Fig. 5). The fibroblasts display dark nuclei, cisternae of the rough endoplasmic reticulum, small mitochondria, secretory vacuoles and microfilaments. Fibroblasts send out long, thin cell prolongations which traverse the matrix and contact collagen fibres and bundles. Myocardial cells close to the inner fibrous layer are elongated, lie in parallel to each other and extend from the inner fibrous layer to the rest of the conus myocardium (Fig. 6). These cells contain numerous myofibrils and mitochondria, and are separated by clear intercellular spaces often bridged by lateral desmosomes. More interestingly, the inner end of these myocytes attaches to a very thick basement membrane formed by a lamina rara, a lamina densa, and a pars fibroreticularis that blends with the subendocardial fibrous layer (Figs 6 and 7). Numerous hemidesmosomes appear at the points of attachment. Myocardial cells in the rest of the conus show an overall circumferential organization in transverse sections (Fig. 8). These cells show a centrally located nucleus, junctional complexes mostly formed by long fascia adherens, lateral desmosomes and basement membranes of regular thickness. The conus myocardium is supplied by numerous coronary arterioles and capillaries. Blood vessels are lined by endothelial cells with dark nuclei, small mitochondria and microvilli, and are surrounded by a thick basement membrane and pericytes (Fig. 9). The base of the conus myocardium blends with the spongy ventricular myocardium (Fig. 3). Myocardial ventricular cells display similar ultrastructural features to those of the conus (not shown), being organized into a superficial cell monolayer and the inner myocardial trabeculae (Fig. 4c). The outer conus fibrous layer is a connective tissue containing collagen fibres and bundles orientated in several directions, fibroblasts and blood vessels (Fig. 10). Its structural appearance is similar to that of the inner fibrous layer, showing irregularly shaped fibroblasts and numerous collagen bundles often surrounded by the fibroblast prolongations. However, elastin fibres are very scarce. This fibrous layer separates the conus from the bulbus cordis distally, and is continuous with the looser connective tissue of the subepicardium proximally (Fig. 3).
Fig. 5.
TEM micrograph depicting the structural appearance of the inner fibrous layer. Fibroblasts show dark nuclei (n), endoplasmic reticulum profiles, microfilaments and numerous cell prolongations extended through the extracellular space. Most of this space is occupied by collagen fibres and bundles orientated in multiple directions. Collagen fibres often contact the fibroblast cell surface (arrowheads), and collagen bundles may appear surrounded by the cell prolongations. Elastin fibres (arrows) are associated with collagen fibres and with the fibroblast surface. Scale bar = 1 μm.
Fig. 6.
Close to the inner fibrous layer the conus myocardial cells arrange in parallel, are rich in myofilaments and mitochondriae, and may show lateral desmosomes (arrow). The inner end of these myocytes attaches to a thick basement membrane. Numerous hemidesmosomes (arrowheads) appear at the areas of attachment. Scale bar = 600 nm.
Fig. 7.
Detail of the attachment area. The myocardial basement membrane shows a lamina lucida traversed by small filaments (arrowheads), a thick lamina densa and an extended lamina fibroreticularis. The latter is formed by numerous matrix filaments which contact collagen (C) fibres in the inner fibrous layer. Myofilaments (arrow) attach to a hemidesmosome. Scale bar = 300 μm.
Fig. 8.
The outer part of the conus myocardium shows an overall circumferential organization in transverse sections. Note the presence of centrally located nuclei (n) and the richness in myofibrils and mitochondriae. The intercalated discs (arrows) are mostly formed by long fascia adherens and show a variable amount of dense material. Lateral desmosomes can also be observed(arrowhead). Blood vessels are also indicated (asterisks). Scale bar = 1 μm.
Fig. 9.
Detail of a myocardial vessel. Endothelial cells show dark nuclei (n), microvilli and a thick basement membrane (arrowheads). Tight junctions appear at the points of contact (large arrow). An interstitial cell appears in the subendocardium contacting abundant collagenous material. Myocytes show a well-developed basement membrane (small arrows). Erythrocytes (e) appear in the vessel lumen. Scale bar = 1 μm.
Fig. 10.
The outer fibrous layer is mostly formed by irregularly shaped fibroblasts and collagen bundles. Cell prolongations may enclose collagen bundles (arrowhead). Some cells show dark secretory granules (arrow). Note the absence of elastin fibres in this field. Scale bar = 1 μm.
Discussion
The present observations indicate that the adult gilthead seabream has a distinct cardiac segment interposed between the ventricle and the bulbus arteriosus. This segment is obviously different from the bulbus arteriosus since it is myocardial in nature. It is also different from the entirely trabecular ventricle in that: (1) it has a conus shape; (2) it is surrounded by thick inner and outer fibrous layers rich in collagen and elastin; (3) it is formed by compact myocardium divided into fascicles or bundles; (4) it contains numerous blood vessels; (5) it stains strongly with the anti-laminin and anti-collagen antibodies, indicating a better development of the myocardial basement membrane than in the rest of the heart myocardium; and (6) it supports the valvular system of the outflow tract. In addition, the conus myocardial cells show an overall spiralling organization which does not appear anywhere in the ventricle.
We have used the term conus arteriosus to designate the distinct cardiac segment interposed between the ventricle and the bulbus arteriosus of the adult gilthead seabream. It should be stressed, however, that a considerable confusion persists in the nomenclature of the outflow tract region of the vertebrate heart. The term conus arteriosus was introduced more than a century ago (Gegenbaur, 1866) to designate the caudal (closest to the ventricle) myocardial region of the outflow tract of the adult selachian heart, in order to distinguish it from the most cranial (closest to the ventral aorta), non-myocardial cardiac region, which was called bulbus arteriosus. More recently, the term conus arteriosus was used to designate the entire outflow tract region of the primitive cardiac tube in actinopterygians, including both the myocardial and the non-myocardial segments (Parsons, 1930). It was also argued that the name conus arteriosus should be applied to designate the muscular segment of the adult outflow tract in vertebrates, while the embryonic cardiac segment from which the conus is derived should be named bulbus cordis (Goodrich, 1930). Even more recently, exactly the opposite has been proposed based on developmental studies carried out in chick embryos (De la Cruz et al. 1999). These changes in nomenclature arise from different interpretations of both gross anatomy and embryology.
We believe that there is no reason to discontinue the use of the term conus arteriosus to designate the adult myocardial outflow tract of the elasmobranch heart, which displays the most primitive structural condition among the living vertebrates. The term was created specifically to name this cardiac segment, and any change in the nomenclature should be based on empirical data and not on hypothetical homologies. Also, there is no reason to use bulbus cordis to refer to the embryonic segment from which the conus arteriosus develops in elasmobranchs. A myocardial conus arteriosus occurs in the elasmobranch embryo from the earliest developmental stages (Muñoz-Chápuli et al. 1994), and it is reasonable to use the same term for the embryonic segment and its corresponding mature structure. In addition, we believe that there is sufficient morphological evidence for the term conus arteriosus to be applied to teleosts. However, this term should never be applied to the entire outflow tract. As shown here, the teleost conus arteriosus is a distinct heart segment. In addition, it supports the outflow tract valves which should therefore be named conus valves (see Sans-Coma et al. 1995).
It is unclear why a distinct conus arteriosus has not been properly identified and studied in other teleosts. This may, at least in part, be a conceptual problem. It has been presumed that the conus disappears by intussuspection into the ventricle (Smith, 1918). This statement has often cited in the literature, although there is no empirical evidence to support it. The remaining conus, if any at all (see Smith, 1918), is considered to be vestigial (Satchell, 1991; Farrell & Jones, 1992) and has been overlooked. Another reason may lie in the fact that the gilthead seabream has a spongy ventricle, which brings out the differences in myocardial architecture. We cannot claim at this time that a distinct myocardial conus arteriosus exists in all the adult teleosts, but preliminary observations made in other species such as Serranus cabrilla, Sparus pagrus, Sardina pilchardus and Trachurus trachurus reveal a distinct conus. On the other hand, an embryonic conus arteriosus occurs in the developing gilthead seabream (unpublished observations) and is most probably present in all telosts (see Hu et al. 2000), at least as a transient segment during cardiac development.
The present observations indicate not only that the conus arteriosus is a distinct segment of the adult gilthead seabream heart, but that it probably plays an important functional role. The conus muscle is organized into bundles and isolated for the most part by the inner and outer fibrous layers. This concentrates the contractile forces. The presence of vessels and the richness in mitochondria indicate high energy requirements. The thickness of the basement membrane and the existence of hemidesmosomes indicate the presence of considerable tensile stress. Furthermore, tension must be applied helicoidally due to the spiral arrangement of the conus myocytes. This would facilitate closure of the conus lumen and valve apposition. Thus all the morphological data strongly suggest that the conus myocardium is actively involved in the mechanical functioning of the conus valves. In this context, myocardial cells have been found to display specific orientation patterns at the level of the cardiac outflow tract valves in several teleost species (Sánchez-Quintana & Hurlé, 1987; Farrel & Jones, 1992; Sánchez-Quintana et al. 1996). Although these papers do not mention the presence or absence of a conus arteriosus, the changes in myocardial orientation at the most anterior part of the ventricle have been viewed as specific morphological traits (Sánchez-Quintana & Hurlé, 1987; Sánchez-Quintana et al. 1996) that suggest active participation of this myocardium in the closure of the valvular system.
Acknowledgments
We thank R. Garcia-Ceballos and M. Mier for expert technical assistance. The study was supported by grants PB98–1418–C02–01, PB98–1418–C02–02 and BMC2000–0118–CO2–01 from the Ministerio de Ciencia y Tecnología.
References
- Anthony J, Millot J, Robineau D. Le coeur et l’aorte ventrale de Latimeria chalumnae (Poisson, Coelocanthidae) CR Acad. Sci. 1965;261:223–226. [PubMed] [Google Scholar]
- Bertin L. Appareil circulatoire. In: Grassé PP, editor. Traité de Zoologie. XIII/II. Paris: Masson; 1958. pp. 1399–1458. [Google Scholar]
- Boas JEV. Über HertZ. und Arterienbogen bei Ceratodus und Protopterus. Morph. Jahrbücher. 1880a;6:321–354. [Google Scholar]
- Boas JEV. Über den Conus arteriosus bei Butirinus und bei anderen Knochenfischen. Morph. Jahrbücher. 1880b;6:527–534. [Google Scholar]
- De la Cruz MT, Moreno-Rodriguez R, Angelini P. Phylogeny of the coronary arteries. In: Angelini P, editor. Coronary Artery Anomalies: a Comprehensive Approach. Philadelphia: Lippincot & Wilkins; 1999. pp. 1–9. [Google Scholar]
- Farrell AP, Jones DR. The heart The Cardiovascular System, Part a. In: Hoar WS, Randall DJ, Farrell AP, editors. Fish Physiology. XII. San Diego: Academic Press; 1992. pp. 1–87. [Google Scholar]
- Gegenbaur C. Zur vergleichenden Anatomie des Herzens. Jenaische Z. Naturwissenschaft. 1866;2:365–383. [Google Scholar]
- Germroth PG, Gourdie RG, Thompson RP. Confocal microscopy of thick sections from acrylamide gel embedded embryos. Microsc. Res. Technique. 1995;30:513–520. doi: 10.1002/jemt.1070300608. [DOI] [PubMed] [Google Scholar]
- Goodrich ES. Studies on the Structure and Development of Vertebrates, Chapter X. London: Macmillan; 1930. Vascular system and heart; pp. 506–577. [Google Scholar]
- Hu N, Sedmera D, Yost HJ, Clark EB. Structure and function of the developing zebrafish heart. Anat. Record. 2000;160:148–157. doi: 10.1002/1097-0185(20001001)260:2<148::AID-AR50>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Icardo JM, Colvée E, Cerra MC, Tota B. Bulbus arteriosus of Antarctic teleosts. I. The white-blooded Chionodraco hamatus. Anat. Record. 1999a;254:396–407. doi: 10.1002/(SICI)1097-0185(19990301)254:3<396::AID-AR11>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Icardo JM, Colvée E, Cerra MC, Tota B. Bulbus arteriosus of Antarctic teleosts. II. The red-blooded Trematomus bernacchii. Anat. Record. 1999b;256:116–126. doi: 10.1002/(SICI)1097-0185(19991001)256:2<116::AID-AR2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Icardo JM, Colvée E, Cerra MC, Tota B. Light and electron microscopy of the bulbus arteriosus of the European eel (Anguilla anguilla) Cells Tissues Organs. 2000a;157:184–198. doi: 10.1159/000016781. [DOI] [PubMed] [Google Scholar]
- Icardo JM, Colvée E, Cerra MC, Tota B. Light and electron microscopy of the bulbus arteriosus. J. Fish Biol. 2000b;57(Suppl. A):121–135. doi: 10.1159/000016781. [DOI] [PubMed] [Google Scholar]
- Icardo JM, Colvée E, Cerra MC, Tota B. The structure of the conus arteriosus of the sturgeon (Acipenser naccarii) heart. I. The conus valves and the subendocardium. Anat. Record. 2002a;267:17–27. doi: 10.1002/ar.10080. [DOI] [PubMed] [Google Scholar]
- Icardo JM, Colvée E, Cerra MC, Tota B. The structure of the conus arteriosus of the sturgeon (Acipenser naccarii) heart. II. The myocardium, the subepicardium and the conus-aorta transition. Anat. Record. 2002b doi: 10.1002/ar.10170. in press. [DOI] [PubMed] [Google Scholar]
- Lankester ER. On the hearts of Ceratodus, Protopterus and Chimaera. Trans. Zool. Soc. London. 1879;10:493–506. [Google Scholar]
- Millot J, Anthony J, Robineau D. Anatomie de Latimeria Chalumnae, Tome 3. Paris: Centre National de la Recherche Scientifique; 1978. [Google Scholar]
- Muñoz-Chápuli R, Macías D, Ramos C, De Andrés V, Gallego A, Navarro P. Cardiac development in the dogfish (Scyliorhinus canicula): a model for the study of vertebrate cardiogenesis. Cardioscience. 1994;5:245–253. [PubMed] [Google Scholar]
- Parsons CW. The conus arteriosus in fishes. Q. J. Microsc. Sci. 1930;73:145–176. [Google Scholar]
- Priede IG. Functional morphology of the bulbus arteriosus of rainbow trout (Salmo gairdneri Richardson) J. Fish Biol. 1976;9:209–216. [Google Scholar]
- Robertson J. The development of the heart and vascular system of Lepidosiren paradoxa. Q. J. Microsc. Sci. 1914;59:53–132. [Google Scholar]
- Sánchez-Quintana D, Hurlé JM. Ventricular myocardial architecture in marine fishes. Anat. Record. 1987;217:263–273. doi: 10.1002/ar.1092170307. [DOI] [PubMed] [Google Scholar]
- Sánchez-Quintana D, García-Martínez V, Climent V, Hurlé JM. Myocardial fiber and connective tissue architecture in the fish heart ventricle. J. Exp. Zool. 1996;275:112–124. doi: 10.1016/S0940-9602(11)80198-6. [DOI] [PubMed] [Google Scholar]
- Sans-Coma V, Gallego A, Muñoz-Chápuli R, De Andrés AV, Durán AC, Fernández B. Anatomy and histology of the cardiac conal valves of the adult dogfish (Scyliorhinus canicula) Anat. Record. 1995;241:496–504. doi: 10.1002/ar.1092410407. [DOI] [PubMed] [Google Scholar]
- Santer RM. Morphology and innervation of the fish heart. Adv. Anat. Embryol. 1985;89:1–102. doi: 10.1007/978-3-642-70135-1. [DOI] [PubMed] [Google Scholar]
- Satchell GH. Physiology and Form of Fish Circulation. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- Senior HD. The conus arteriosus in Tarpon atlanticus (Cuvier & Valenciennes) Biol. Bull. 1907a;12:146–151. [Google Scholar]
- Senior HD. Teleosts with a conus having more than one row of valves. Anat. Record. 1907b;4:83–84. [Google Scholar]
- Senior HD. Note on the conus of Megalops cyprinoides (Broussonet) Biol. Bull. 1907c;12:378–379. [Google Scholar]
- Smith WC. On the process of disappearance of the conus arteriosus in teleosts. Anat. Record. 1918;15:65–71. [Google Scholar]
- Stannius H. Bermerkugen über das Verhältniss der Ganoiden zu den Clupeiden insbesondere zu Butirinus Rostock. 1846.
- Stöhr P. Über den Klappenapparat im Conus arteriosus der Selachier und Ganoiden. Morph. Jahrbücher. 1876;2:197–228. [Google Scholar]
- Zummo G, Farina F. Ultrastructure of the conus arteriosus of Scyliorhinus stellaris. J. Exp. Zool. 1989;2:158–164. [Google Scholar]