Abstract
Leptin, a 16-kDa hormone, plays an important role in the control of food intake and in energy homeostasis both in rodents and in man. Leptin is mainly produced and secreted by adipocytes, but other tissues and gastric glands have also recently been shown to produce it in a dual (endocrine and exocrine) mode. In addition, a leptin receptor has been detected in taste cells of mouse circumvallate papillae and in rat intestinal epithelium. These data prompted us to carry out a detailed study of human salivary glands as potential leptin-producing organs. Biopsies of salivary glands (submandibular and parotid) obtained from male and female patients during surgery for different clinical indications were subjected to immunohistochemical study for the presence of leptin, its functional receptor, insulin and glucagon. The presence and cellular distribution of glucocorticoid receptor in leptin-secreting cells were also investigated. Double immunohistochemical staining (silver–gold intensification and avidin–biotin–peroxidase) was used for the visualization of glucocorticoid receptor and leptin labelling, respectively. The results show that intralobular duct cells of submandibular and parotid glands are immunoreactive for leptin, leptin receptor and glucagon but not for insulin. Leptin was also detected in some microglobules in whole saliva obtained from four healthy volunteers. Co-localization for leptin, leptin receptor and glucocorticoid receptor in the same cell type suggested a functional relationship between glucocorticoid hormone and leptin secretion also at the level of the salivary glands.
Keywords: glucocorticoid receptor, human salivary gland, immunohistochemistry, leptin
Introduction
Leptin, the leptin gene product, is a 16-kDa hormone first reported to be produced by white adipocytes (Zhang et al. 1994). It acts mainly on the hypothalamus, where it exerts a strong neuroendocrine effect on food intake and energy metabolism in both rodents (Campfield et al. 1995; Pelleymount et al. 1995) and man (Friedman & Halaas, 1998), as well as on several peripheral organs (De Matteis et al. 1998). Leptin is also expressed by other cell types (i.e. mammary gland and placenta) (Casabiell et al. 1997; Masuzaki et al. 1997) and, interestingly, by chief cells of rat and human stomach glands. In man, leptin is localized in gastric exocrine granules. In both species, food ingestion reduces leptin expression (Bado et al. 1998; Cinti et al. 2000). Together with the evidence for a leptin receptor in the intestinal epithelium (Buyse et al. 2001a; Stan et al. 2001), and for a role for leptin in lipid (Morton et al. 1998; Stan et al. 2001), galactose (Barrenetxe et al. 2001) and peptide (Buyse et al. 2001a) intestinal absorption, these data suggest a possible exocrine function for the hormone. In addition, the demonstration that a subset of taste receptor cells in tongue epithelium was affected by leptin administration and expressed functional leptin receptor (lepR) (Kawai et al. 2000) indicates that taste cells are a further peripheral site of leptin action in the gastrointestinal tract.
These data prompted us to investigate whether the human salivary glands could be a source of leptin. Since adipocytes, which produce leptin, are abundant in the salivary gland parenchyma, we used immunohistochemistry, which allows cell-specific phenotypical detection of antigen localization, to study its presence. Previous studies suggest that the major salivary glands produce and secrete peptides with similarities to pancreatic insulin (Lawrence et al. 1976; Murakami et al. 1982; Taouis et al. 1995; Égèa et al. 2000) and glucagon (Lawrence et al. 1976a, b; Smith et al. 1979; Pérez-Castillo & Blázquez, 1980), but their cytolocalization has never been studied. We therefore also studied these proteins with immunohistochemistry. Since adipocytes express lepR together with lep, and glucocorticoids are known to regulate leptin expression in adipose tissue (De Vos et al. 1995; Buyse et al. 2001b), we investigated the localization of these receptors in human salivary glands.
Materials and methods
Patients and tissue preparation
Human parotid and submandibular gland samples obtained from 10 patients of both sexes (aged 37–76 years BMI in the range of 21–30; glycaemia values in the normal range: 76–120 mg dL−1) for different clinical indications, were collected according to the guidelines of the Ethical Committee of the University of Cagliari. The glands were immediately cut into small pieces and fixed in 4% paraformaldehyde in 0.1 m sodium phosphate buffer (PB), pH 7.2, for 12 h at 4 °C. Tissue specimens were dehydrated in a graded ethanol series and embedded in paraffin blocks for light microscopy and immunohistochemistry.
Whole saliva from four unstimulated (resting) healthy subjects of both sexes (aged 27–40 years) was collected with a micropipette, spread as a one-cell-thick layer on a slide, air-dried, and fixed in acetone for 10 min at room temperature. Smears and tissue samples were processed for immunolocalization according to the avidin–biotin–peroxidase technique (ABC method; Hsu et al. 1981).
Antibodies
The following primary antibodies were used: rabbit polyclonal antiserum raised against a peptide corresponding to amino acids 137–156 at the carboxy terminus of the leptin gene product of human origin (Santa Cruz Biotech, CA, USA; diluted 1 : 150 v/v); goat polyclonal antiserum recognizing the amino terminus of all the alternatively spliced forms of human lepR, i.e. lepRs and lepRL (amino acids 32–51) (Santa Cruz Biotech; diluted 1 : 400 v/v); goat polyclonal antiserum directed against the peptide corresponding to amino acids 1146–1165 at the carboxy terminus of human leptin receptor, thus recognizing only the long form of LepR (Santa Cruz Biotech; diluted 1 : 300 v/v); guinea-pig antiserum to insulin, prediluted and optimized for use on human tissues (Dako, CA, USA); rabbit antiserum to glucagon, prediluted and optimized for use on human tissues (Dako); mouse monoclonal antiserum raised against a peptide corresponding to the amino terminus of the glucocorticoid receptor (Novocastra, UK; diluted 1 : 20 v/v). A high-temperature antigen unmasking technique was applied, as recommended by the manufacturer.
Immunohistochemistry
Serial sections 3 µm in thickness were processed for immunolocalization by the ABC method and incubated according to the following incubation steps: 2% aqueous hydrogen peroxide for 5 min at room temperature (r.t.) to block endogenous peroxidase; two washes in 0.015 m phosphate-buffered saline (PBS), pH 7.5; normal goat serum diluted 1 : 75 in PBS for 20 min at r.t. to reduce non-specific background staining (Vector Laboratories, CA, USA); incubation in primary antibodies, overnight at high humidity at 4 °C. After thorough washing, the sections were incubated in biotinylated secondary antibodies (Vector Laboratories) at 1 : 200 dilution in PBS for 30 min at r.t., then washed again and incubated in avidin–biotin–peroxidase complex, using the Vectastain ABC kit (Vector Laboratories). After further washes, peroxidase activity was detected by incubation with 0.075% diaminobenzidine hydrochloride and 0.02% hydrogen peroxide in 0.05 m Tris buffer, pH 7.6, for 4 min in a dark room. Sections were then rinsed in tap water, counterstained with haematoxylin, dehydrated and finally mounted in Eukitt (Kindler GmbH & Co., Germany).
For double immunohistochemical staining, a silver–gold intensification procedure, modified from the technique described by Danscher & Nörgaard (1983), was used for the visualization of glucocorticoid receptor antibody–antigen binding (first immunostaining). For the second immunostaining, leptin was labelled with the ABC method as described above.
Hydrated sections (3 µm thick) of human salivary glands were processed with glucocorticoid receptor primary antibody (see above), thoroughly washed with PBS, then incubated with goat antimouse IgG labelled with 10-nm gold particles (British Biocell International, UK) diluted 1 : 100 in 1% bovine serum albumin, 0.5% Tween 20 and 0.1% TritonX-100 in PBS for 40 min at r.t. Sections were repeatedly washed in PBS and processed for the second immunostaining (leptin). After the visualization of the leptin immunoreaction, the silver–gold intensification procedure was performed. Sections were counterstained with haematoxylin, dehydrated and finally mounted in Eukitt.
Antibody specificity was tested by parallel demonstration of specific antigens in several human tissues processed exactly like the experimental samples: leptin in human stomach, placenta and white adipose tissue; leptin receptor in pancreas, placenta and white adipose tissue; insulin and glucagon in specific cell types of Langerhans islets; glucocorticoid receptor in skin, lung, prostate and liver.
Method specificity was tested: (a) by omitting the primary antibody; (b) by replacing the primary antibody with non-immune specific serum; and (c) by incubating sections with the antiserum saturated with the homologous antigen. Each antibody was incubated with a 10-fold excess concentration of the homologous peptide (15 µg mL−1 for 48 h); (d) using high-molar (0.5 m) PBS as rinsing solution between the various steps of the immunohistochemical protocol (Grube, 1980).
Results
The major salivary glands are tubuloacinar structures made of tubules capped by acini. The human ductal system is mainly composed of intralobular ducts (consisting of a proximal intercalated part and a distal striated one) and interlobular (excretory) ducts. A variable amount of white adipocytes was observed in the parenchyma of the salivary glands.
Leptin immunoreactivity
Both the intercalated and the striated tracts of submandibular and parotid salivary gland intralobular ducts stained for leptin (Fig. 1a–c). White adipocytes in the parenchyma were consistently positive in all the samples we studied (Fig. 1a). The interlobular ducts and the other cell types in the tubuloacinar parenchyma never stained for leptin (Fig. 1b).
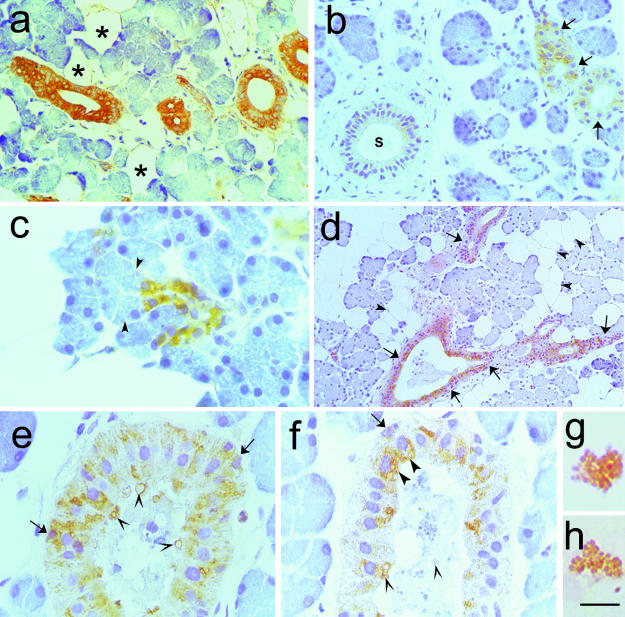
Fig. 1.
Immunohistochemistry of human major salivary glands. The primary antibodies were a polyclonal antileptin antiserum (A-20), diluted 1 : 100 (a—c and e—h) and a prediluted polyclonal antiglucagon antiserum (d). Paraffin-embedded tissue, ABC method. (a,b) Intralobular duct epithelial cells showing cytoplasmic staining in both the parotid (a) and the submandibular (b) gland; some completely negative cells were closely apposed to leptin-positive ones (arrows in b). A leptin-negative excretory duct is also visible (S in b). (c) High magnification of leptin-positive parotid tissue. Strongly leptin-stained cuboid cells of a sprouting intercalated duct. The tubuloacinar cells (arrowheads) were not labelled. (d) Glucagon immunoreaction was detected exclusively in the intralobular ducts of a parotid gland. Most epithelial cells were labelled, but negative ones were also visible (arrows). Adipocytes were not labelled (arrowheads). (e,f) At high magnification, leptin immunoreactive granules were evident in the cytoplasm of the epithelial cells in a striated duct (e) and in a distal intercalated (f) duct. Sometimes these leptin-positive granules were in the apical portion of the cell (large arrowheads) or mixed to salivary material (small arrowheads). Some basal cells also stained for leptin (arrows). (g,h) Leptin-labelled microglobules in saliva were observed at high magnification. Scale bars: a,b 7 = 44 μm; c = 17 μm; d = 88 mgr;m; e,f = 20 μm; g,h = 9 μm
Leptin immunoreactivity was extremely variable and in three of the 10 subjects both glands were completely negative, although the white adipocytes (internal control) were positive. This lack of immunoreactivity suggested a possible metabolic correlation (BMI and glycaemia) which we tested but did not find (data not shown). Cytoplasmic staining was observed in epithelial cells of varying shape and morphology around duct lumina as well as in some cells of the basal portion of striated ducts (Fig. 1e). Sometimes, completely negative epithelial cells were seen closely apposed to intensely leptin-immunoreactive cells.
High-magnification microscopy demonstrated positive granules in the apical regions of duct cells; immunoreactive material was at times observed in the lumen (Fig. 1e,f).
The saliva smears from the four volunteers showed leptin-positive microglobules, whereas the seromucous material and flaking epithelial cells were negative (Fig. 1g,h).
Insulin and glucagon immunoreactivities
The samples analysed for leptin expression were processed with anti-insulin and antiglucagon antibodies. The salivary glands stained for glucagon but were insulin-negative. Glucagon immunoreaction was detected in the intralobular salivary ducts (Fig. 1d) in the same cell types that stained for leptin in serial sections from both types of glands. Again, staining intensity was quite variable, and almost identical to leptin staining even though one of three leptin-negative patients was weakly glucagon-positive.
Antibody specificity was tested by demonstration of glucagon and insulin in pancreas endocrine cells (isles of Langerhans): specific cell types (α cells for glucagon and β cells for insulin) were used as positive controls (not shown).
Leptin receptor (lepR) immunoreactivity
All samples stained for both anti-lepR antisera, indicating that the functional lepR is localized in the major salivary glands. LepR staining exhibited variable intensity that was unrelated to leptin and glucagon expression. Diffuse cytoplasmic staining was observed in most epithelial cells of the intralobular (both intercalated and striated) ducts but never in interlobular ducts (Fig. 2a). Whereas the tubuloacinar parenchyma was negative, all adipocytes were intensely lepR-positive.
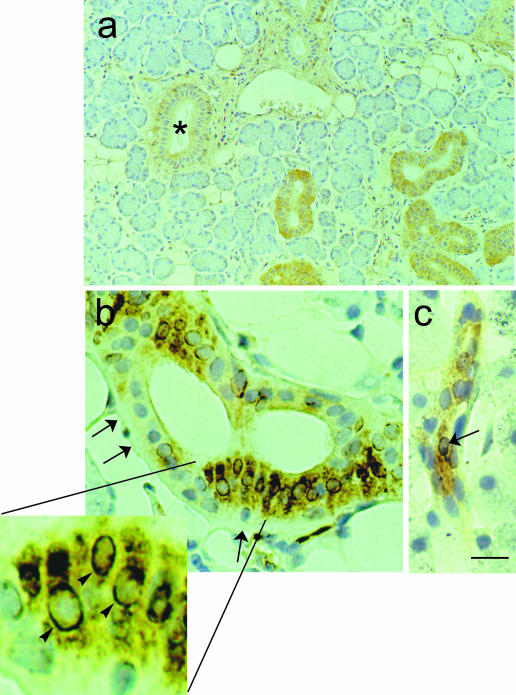
Fig. 2.
Immunohistochemistry of human major salivary gland (submandibular). The primary antibody is a polyclonal anti-lepR antiserum (C-20), diluted 1 : 300. Paraffin-embedded tissue, ABC method. The intralobular ducts showed diffuse cytoplasmic staining, whereas the interlobular ducts were not labelled (*). Adipocytes were also lepR-positive. (b,c) Double staining of human major salivary gland (parotid). A silver—gold intensification procedure, modified from the technique described by Danscher et al. (1983), was used for the histochemical visualization of glucocorticoid receptor (GR) antibody—antigen binding (first immunostaining; black). In the second immunostaining, leptin was labelled (yellowish brown) as described above (ABC method). A monoclonal anti-GR antiserum, diluted 1: 20 and a polyclonal antileptin antiserum, diluted 1 : 120, were used. Paraffinembedded tissue. (b) Numerous intralobularduct cells exhibited double (leptin and GR) staining: the cytoplasm, nuclei, or both stained for GR, but the cytoplasm wasalso leptin-positive. Completely negative cells were also present (arrows). High-power inset of the area in (b): some cells showed clear GR staining around the nuclear outline (arrowheads); their cytoplasm was intensely leptin-immunoreactive. (c) Proximal intercalated duct in a different field of the same sample exhibiting leptin-positive cells, one of which also stains for GR (arrow). Scale bars: a = 60 μm; b,c = 12 μm; inset of b = 4.6 μm.
Glucocorticoid receptor (GR) immunoreactivity
We used a mouse monoclonal antibody prepared against a peptide mapping at the GR N-terminus which does not discriminate between the α and β isoforms. All the tissues used as positive controls stained for GR, as described in the literature (Adcock et al. 1996; Mohler et al. 1996; Serres et al. 1996; Oakley et al. 1997).
GR was similarly distributed in submandibular and parotid glands in many though not all intralobular duct epithelial cells. Intense nuclear staining was observed in some tubuloacinar cells (c 10%) as well as in some myoepithelial, interstitial and endothelial cells of some vessels.
GR cytoplasmic staining was observed in the intralobular ducts, in agreement with the subcellular distribution of GR reported in several target tissues (Antakly & Eisen, 1984; Wikstrom et al. 1987).
In double-labelling experiments, GR colocalized with leptin (Fig. 2b,c).
Discussion
Saliva, which is mainly produced by the secretory acini in the major salivary glands, is modified by duct cells through the secretion and reabsorption of electrolytes (Schneyer et al. 1972; Young & Van Lennep, 1979; Young & Schneyer, 1981) and proteins (Hand, 1979; Rutberg, 1961; Riva et al. 1976; Lima et al. 1977; Testa-Riva, 1977).
Many of the organic products of the human salivary glands are produced by tubuloacinar cells and enter in the composition of the saliva by exocytosis. However, some normal organic salivary components are produced by ductal cells in both mouse (Barka, 1980) and man (Lantini & Cossu, 1998; Tandler & Phillips, 2000).
In this work, we demonstrate that human major salivary glands contain leptin. The technique adopted allowed us to demonstrate that leptin immunostaining is localized exclusively in the epithelial cells of intralobular ducts and that the same cells are positive for glucagon as well as for glucocorticoid and leptin receptors. Although glucagon was already known to be synthesized by human salivary glands (Lawrence, 1976; Smith, 1979; Pérez-Castillo & Blázquez, 1980), the secreting cell type had not yet been identified. Here we show, for the first time, that intralobular ducts are the salivary gland site of its production. As we were unable to detect any insulin immunoreactivity, we cannot confirm the findings of other authors (Lawrence et al. 1976; Murakami et al. 1982; Taouis et al. 1995; Égèa et al. 2000).
We demonstrated that human major salivary glands have cell types producing peptides such as leptin that correspond to specialized epithelial cells, the granular convoluted tubular cells, found in a large intralobular portion of the tubules of rat salivary glands: these secrete a number of polypeptide hormones (Antakly et al. 1991). The detection of leptin in saliva suggests that it is an exocrine secretory product.
Several data support leptin's exocrine role in the human gastroenteric tract: leptin is synthesized and stored in the exocrine granules of gastric chief cells and released after food ingestion (Cinti et al. 2000) and after pharmacological stimulus (Sobhani et al. 2000). Furthermore, the peptide is not proteolytically degraded (Sobhani et al. 2000), it reaches the intestine in an active form and could thus have biological effects on lipid (Morton et al. 1998; Stan et al. 2001), galactose (Barrenetxe et al. 2001) and peptide absorption (Buyse et al. 2001a).
Leptin secretion by the salivary glands could have a role in gastrointestinal function. This hypothesis is consistent with the recent demonstration that leptin suppresses taste nerve responses to sweet stimuli in mice, and with the detection of functional leptin receptors in tongue epithelium (Kawai et al. 2000).
The variable leptin expression observed in our subjects could be ascribed to their different endocrine and metabolic parameters (Dubuc et al. 1998), although we found no correlation with BMI and glycaemia.
The expression of functional leptin receptor in leptin-producing cells suggests that leptin may exert an autocrine regulatory control of its own synthesis as in other target organs (Zhang et al. 1997). Nevertheless, the hypothesis that the adipocytes found in salivary gland parenchyma play an active role in the regulation of salivary leptin expression cannot be ruled out with certainty, especially as a considerable number of leptin-producing adipocytes are consistently observed in salivary gland parenchyma, and leptin expression by them is known to be regulated by food intake (Saladin et al. 1995; Kolaczynski et al. 1996).
While writing the final draft of the present manuscript, Gröschl et al. (2001) reported the presence of leptin in human saliva and leptin mRNA expression in salivary glands. On the whole, our data are in line with theirs. However, the different methodological approach (ABC method on paraffin-embedded tissue) and the high-resolution study of our samples allowed us to demonstrate that the tubuloacinar parenchyma and excretory ducts are totally leptin-negative.
Detection of the glucocorticoid receptors around the nuclear outline of cells that stained intensely for leptin suggested to us that glucocorticoids could regulate leptin salivary secretion in human intralobular ducts, in line with their regulatory action on the synthesis of parotid secretory proteins in rat (Johnson et al. 1987) and HSG cells, a human neoplastic duct epithelial cell line (Kurokawa et al. 1988). Finally, since glucocorticoids are known to regulate leptin expression in adipose tissue (De Vos et al. 1995; Buyse et al. 2001b), they might also be involved in the expression of salivary leptin.
Acknowledgments
This work was supported by grants from the Italian Ministry of University and Scientific Research (MIUR) and the University of Ancona to S. Cinti.
References
- Adcock IM, Gilbey T, Gelder CM, Chung KM, Barnes PJ. Glucocorticoids receptor localization in normal and asthmatic lung. Am. J. Respir. Crit. Care Med. 1996;154(3 Part 1):771–782. doi: 10.1164/ajrccm.154.3.8810618. [DOI] [PubMed] [Google Scholar]
- Antakly T, Eisen HJ. Immunocytochemical localization of glucocorticoid receptor in target cells. Endocrinology. 1984;115:1984–1989. doi: 10.1210/endo-115-5-1984. [DOI] [PubMed] [Google Scholar]
- Antakly T, Zhang C, Sarrieau A, Raquidan D. Cell-specific expression of the glucocorticoid receptor within granular convoluted tubules of the rat submaxillary gland. Endocrinology. 1991;128:617–622. doi: 10.1210/endo-128-1-617. [DOI] [PubMed] [Google Scholar]
- Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, et al. The stomach is a source af leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- Barka T. Biologically active polypeptides in submandibular glands. J. Histochem. Cytochem. 1980;28:836–859. doi: 10.1177/28.8.7003006. [DOI] [PubMed] [Google Scholar]
- Barrenetxe J, Barber A, Lostao MP. Leptin effect on galactose absorption in mice jejunum. J. Physiol. Biochem. 2001;57:345–346. doi: 10.1007/BF03179829. [DOI] [PubMed] [Google Scholar]
- Buyse M, Berlioz F, Guilmeau S, Tsocas A, Voisin T, Peranzi G, et al. PepT1-mediated epithelial transport of dipeptides and cephalexin is enhanced by luminal leptin in the small intestine. J. Clin. Invest. 2001a;108:1483–1494. doi: 10.1172/JCI13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buyse M, Viengchareun S, Bado A, Lombes M. Insulin and glucocorticoids differentially regulate leptin transcription and secretion in brown adipocytes. FASEB J. 2001b;15:1357–1366. doi: 10.1096/fj.00-0669com. [DOI] [PubMed] [Google Scholar]
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse ob protein: Evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- Casabiell X, Pineiro V, Tomé MA, Peino R, DiegueZ. C, Casanueva FF. Presence of leptin in colostrum and/or breast milk from lactating mothers: a potential role in the regulation of neonatal food intake. J. Clin. Endocrinol. Metabol. 1997;82:4270–4273. doi: 10.1210/jcem.82.12.4590. [DOI] [PubMed] [Google Scholar]
- Cinti S, De Matteis R, Pico C, Ceresi E, Obrador A, Maffeis C, et al. Secretory granules of endocrine and chief cells of human stomach mucosa contain leptin. Int. J. Obesity. 2000;24:789–793. doi: 10.1038/sj.ijo.0801228. [DOI] [PubMed] [Google Scholar]
- Danscher G, Nörgaard R. Light microscopic visualization of colloidal gold on resin-embedded tissue. J. Histochem. Cytochem. 1983;31:1394–1398. doi: 10.1177/31.12.6631001. [DOI] [PubMed] [Google Scholar]
- De Matteis R, Dashtipour K, Ognibene A, Cinti S. Localization of leptin receptor splice variants in mouse peripheral tissues by immunohistochemistry. Proc. Nutr. Soc. 1998;57:441–448. doi: 10.1079/pns19980063. [DOI] [PubMed] [Google Scholar]
- De Vos P, Saladin R, Auwerx J, Staels B. Induction of ob gene expression by corticosteroid is accompanied by body weight loss and reduced food intake. J. Biol. Chem. 1995;270:15958–15961. doi: 10.1074/jbc.270.27.15958. [DOI] [PubMed] [Google Scholar]
- Dubuc GR, Phinney SD, Stern JS, Havel PJ. Changes of serum leptin and endocrine and metabolic parameters after 7 days of energy restriction in men and women. Metabolism. 1998;47:429–434. doi: 10.1016/s0026-0495(98)90055-5. [DOI] [PubMed] [Google Scholar]
- Égèa J-C, HirtZ C, Gross R, Lajoix A-D, Traskawka E, Ribes G, deville de Périère D. preproinsulin I and II mRNA expression in adult rat submandibular glands. Eur. J. Oral. Sci. 2000;108:292–296. doi: 10.1034/j.1600-0722.2000.108004292.x. [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Gröschl M, Rauh M, Wagner R, Neuhuber W, Metzler M, Tamgüney G, et al. Identification of leptin in human saliva. J. Clin. Endocrinol. Metab. 2001;86:5234–5239. doi: 10.1210/jcem.86.11.7998. [DOI] [PubMed] [Google Scholar]
- Grube D. Immunoreactivities of gastrin (G-) cells. II. Non-specific binding of immunoglobulins to G–cells by ionic interactions. Histochemistry. 1980;66:149–167. doi: 10.1007/BF00494642. [DOI] [PubMed] [Google Scholar]
- Hand AR. Synthesis of secretory and plasma membrane glycoprotein by striated duct cells of rat salivary glands as visualized by radioautography after 3H-fucose injection. Anat. Rec. 1979;195:317–340. doi: 10.1002/ar.1091950207. [DOI] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase (ABC) in immunoperoxidase technique: a comparison between ABC and unlabelled antibody (PAP procedure) J. Histochem. Cytochem. 1981;2:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Johnson DA, Etzel KR, Alvares OF, CorteZ JE. Regulation of parotid salivary proteins by glucocorticoids. J. Dent. Res. 1987;66:1563–1568. doi: 10.1177/00220345870660101001. [DOI] [PubMed] [Google Scholar]
- Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc. Natl. Acad. Sci. 2000;97:11044–11049. doi: 10.1073/pnas.190066697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaczynski JW, Ohannesian JP, Cousidine RV, Marco CC, Caro JF. Response of leptin to short-term and prolonged overfeeding in humans. J. Clin. Endocrinol. Metab. 1996;81:4162–4165. doi: 10.1210/jcem.81.11.8923877. [DOI] [PubMed] [Google Scholar]
- Kurokawa R, Kyakumoto S, Ota M. Glucocorticoid regulates secretion of epidermal growth factor in the human salivary gland adenocarcinoma cell line. J. Endocr. 1988;116:451–455. doi: 10.1677/joe.0.1160451. [DOI] [PubMed] [Google Scholar]
- Lantini MS, Cossu M. Immunocytochemical investigation of the subcellular distribution of some secretory products in human salivary glands. Eur. J. Morph. 1998;36:230–234. [PubMed] [Google Scholar]
- Lawrence AM, Kirsteins L, Mitton J. Parotid gland insulin: an extrapancreatic source of insulin in rats. Diabetes. 1976a;25(Suppl. 1):328. [Google Scholar]
- Lawrence AM, Tan S, Hojvat S, Kirsteins L. Salivary gland hyperglycemic factor: an extrapancreatic source of glucagon-like material. Science. 1976b;195:70–72. doi: 10.1126/science.63992. [DOI] [PubMed] [Google Scholar]
- Lima TG, Haddad A, Fava -d, e-Moraes F. Glycoprotein secretion in the mouse submandibular gland as revealed by radioautography after 3H-fucose injection. Cell. Tissue Res. 1977;183:519–530. doi: 10.1007/BF00225664. [DOI] [PubMed] [Google Scholar]
- Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, et al. Nonadipose tissue production of leptin. Leptin as a novel placenta-derived hormone in humans. Nat. Med. 1997;3:1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- Mohler JL, Chen Y, Hamil K, Hall SH, Cidlowski JA, Wilson EM, et al. Androgen and glucocorticoid receptors in the stroma and epithelium of prostatic hyperplasia and carcinoma. Clin. Cancer Res. 1996;2:889–895. [PubMed] [Google Scholar]
- Morton NM, Emilsson V, Liu YL, Cawthorne MA. Leptin action in intestinal cells. J. Biol. Chem. 1998;273:26194–26201. doi: 10.1074/jbc.273.40.26194. [DOI] [PubMed] [Google Scholar]
- Murakami K, Taniguchi H, Baba S. Presence of insulin-like immunoreactivity and its biosynthesis in rat and human parotid gland. Diabetologia. 1982;22:358–361. doi: 10.1007/BF00253582. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Webster JC, Sar M, Parker CR, Cidlowski JA. Expression and subcellular distribution of the β-isoform of the human glucocorticoid receptor. Endocrinology. 1997;138:5028–5038. doi: 10.1210/endo.138.11.5501. [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Pérez-Castillo A, Blázquez E. Syntesis and release of glucagon by human salivary glands. Diabetologia. 1980;19:123–129. doi: 10.1007/BF00421858. [DOI] [PubMed] [Google Scholar]
- Riva A, Testa-Riva F, Del Fiacco M, Lantini MS. Fine structure and cytochemistry of the intralobular ducts of the human parotid gland. J. Anat. 1976;122:627–640. [PMC free article] [PubMed] [Google Scholar]
- Rutberg U. Ultrastructure and secretory mechanism of the parotid gland. Acta Odont. Scand. 1961;19(Suppl. 30):11–69. [Google Scholar]
- Saladin R, De Vos P, Guerre-Millo M, Leturque A, Girard J, Staels B, et al. Transient increase of obese gene expression after food intake or insulin administration. Nature. 1995;377:527–529. doi: 10.1038/377527a0. [DOI] [PubMed] [Google Scholar]
- Schneyer LH, Young JA, Schneyer CA. Salivary secretion of electrolytes. Physiol. Rev. 1972;52:720–777. doi: 10.1152/physrev.1972.52.3.720. [DOI] [PubMed] [Google Scholar]
- Serres M, Viac J, Schmitt D. Glucocorticoid receptor localization in human epidermal cells. Arch. Dermatol. Res. 1996;288:140–146. doi: 10.1007/BF02505823. [DOI] [PubMed] [Google Scholar]
- Smith S, Mazur A, Voyles N, Bhathena S, Recant L. Submaxillary gland immunoreactive glucagon important in carbohydrate homeostasis? Metabolism. 1979;28:343–347. doi: 10.1016/0026-0495(79)90105-7. [DOI] [PubMed] [Google Scholar]
- Sobhani I, Bado A, Vissuzaine C, Buyse M, Kermorgant S, Laigneau J-P, et al. Leptin secretion and leptin receptor in the human stomach. Gut. 2000;47:178–183. doi: 10.1136/gut.47.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stan S, Levy E, Bendayan M, Zoltowska M, Lambert M, Michaud J, et al. Effect of human recombinant leptin on lipid handling by fully differentiated Caco-2 cells. FEBS Lett. 2001;508:80–84. doi: 10.1016/s0014-5793(01)03032-0. [DOI] [PubMed] [Google Scholar]
- Tandler B, Phillips CJ. Organic secretion by striated ducts. Eur. J. Morph. 2000;38:233–236. doi: 10.1076/0924-3860(200010)38:4;1-o;ft233. [DOI] [PubMed] [Google Scholar]
- Taouis M, Deville de Periere D, Hillaire-Buys D, Derouet M, Gross R, Simon J, et al. Biological activity of immunoreactive insulin-like activity extracted from rat submandibular gland. Am. J. Physiol. 1995;269:E277–E282. doi: 10.1152/ajpendo.1995.269.2.E277. [DOI] [PubMed] [Google Scholar]
- Testa-Riva F. Ultrastructure of human submandibular gland. J. Submicr. Cytol. 1977;9:251–266. [Google Scholar]
- Wikstrom A-N, Vekke O, Okret S, Bronnegard M, Gustafsson J-A. Intracellular localization of the glucocorticoid receptor: evidence for cytoplasmic and nuclear localization. Endocrinology. 1987;120:1232–1242. doi: 10.1210/endo-120-4-1232. [DOI] [PubMed] [Google Scholar]
- Young JA, Schneyer CA. Composition of saliva in Mammalia. Aust. J. Exp. Biol. Med. Sci. 1981;59:1–53. doi: 10.1038/icb.1981.1. [DOI] [PubMed] [Google Scholar]
- Young JA, Van Lennep EW. Transport of salt in salivary glands. In: Giebisch G, Tosteson DC, Ussing HH, editors. Membrane Transport in Biology. 4B. Berlin: Springer-Verlag; 1979. pp. 563–674. [Google Scholar]
- Zhang Y, Olbort M, Schwarzer K, Nuesslein-Hildesheim B, Nicolson M, Murphy E, et al. The leptin receptor mediates apparent autocrine regulation of leptin gene expression. Biochem. Biophys. Res. Commun. 1997;240:492–495. doi: 10.1006/bbrc.1997.7622. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]