Abstract
Lactating animals are particularly susceptible to mastitis during the early stages of mammary gland involution following weaning. In this study we compared the phagocytic capacity of cells collected from sheep mammary secretions at different stages of involution. The ability of neutrophils and macrophages to ingest latex beads in an in vitro phagocytosis assay was found to be dependent on how heavily the phagocytes were loaded with milk constituents. There was a decline in the phagocytic capacity of neutrophils from 1 to 2 days after weaning, while macrophages collected from fully involuted glands were more effective phagocytes compared with earlier stages (7–15 days) of involution. In addition, dendritic cells present in fully involuted mammary gland secretions (30 days after weaning) were highly phagocytic. These studies demonstrate that neutrophils and macrophages in sheep mammary secretions at early stages of involution are incapacitated, and as such may compromise the immune status of the mammary gland.
Keywords: dendritic cells, lactation, mammary involution, phagocytes, phagocytosis assay
Introduction
The mammary gland is endowed with a significant local immune system that comprises specific and innate factors and plays an important role in the local defence of the gland throughout the lactation cycle (Sheldrake et al. 1985; Lee et al. 1992; Sordillo et al. 1997). Regression or involution of the mammary gland takes place either at the end of lactation or following weaning, during which time the morphology of the gland alters dramatically. Studies in our laboratory (Lee & Outteridge, 1981; Lee et al. 1983; Tatarczuch et al. 1997, 2000) have shown that the tissue changes associated with involution have a major influence on the local immune cells and the proportion of the different leucocyte subpopulations present in the mammary gland. During involution of the mammary gland, neutrophils, macrophages and T lymphocytes are present in the glandular tissues and mammary involution secretions (Tatarczuch et al. 1997, 2000). Our previous studies have shown that phagocytes in early involution secretions contain a large amount of engulfed material, suggesting that they may be incapacitated (Tatarczuch et al. 2000). This is consistent with other studies which showed that milk cells are less effective at phagocytosis than blood leucocytes (Sordillo et al. 1997). Our findings may partially account for the reports that the mammary gland is more susceptible to intramammary infections during the early period of involution (Nickerson, 1989; Oliver & Sordillo, 1989). However, it has not been demonstrated whether the phagocytic capacity of neutrophils and macrophages is compromised in early compared to fully involuted glands.
As an extension of previous studies we employed an in vitro phagocytosis assay to investigate changes in the phagocytic capacity of neutrophils and macrophages collected from mammary secretions at different stages of mammary gland involution.
Materials and methods
Collection and preparation of cells from milk and mammary involution secretions
All experimental animal procedures and the collection of cells were approved by the Animal Experimentation Ethics Committee of the University of Melbourne, following guidelines set by the National Health and Medical Research Council (NH & MRC) of Australia.
Pregnant ewes were randomly allocated to seven groups of three, and mammary secretions were collected at 1, 2, 4, 7, 15, 30 and 60 days after lambs were weaned 5 days after birth. These time points were chosen on the basis of our previous report (Tatarczuch et al. 2000) reflecting the appearance of phagocytic cells (neutrophils and macrophages) in the mammary secretions after weaning. Approximately 40 mL of milk/involution secretions at early stages of involution (1–7 days) were collected from each gland. Each sample was then mixed with equal volumes of phosphate-buffered saline (PBS) before centrifugation. For glands at more advanced stages (15–60 days) of involution, 15 mL of sterile PBS was infused into the glands to harvest the cells.
Cells from milk and mammary secretions were centrifuged (100 g at 4 °C for 10 min), washed twice in 10 mL PBS and resuspended in 1–2 mL PBS/2% bovine serum albumin (BSA). Cells were enumerated using a Coulter counter® (Coulter Electronics, Luton, UK) and resuspended at 4 × 106 cells mL−1. Some cells were used for preparing cytospots for immunostaining and some were fixed for electron microscopy and immunoelectron microscopy. Cytospots were prepared using a plastic well mounted to a glass slide, as described previously (Tatarczuch et al. 2000), and stained with 10% Giemsa for differential cell counts. A total of 200 cells were counted for each sample with a ×100 oil-immersion objective and classified as neutrophils, monocytes/macrophages, lymphocytes or other cell types that may be present (eosinophils, epithelial cells).
Immunostaining of cytospots
Cytospot preparations were stained by the indirect immunoperoxidase method, as described previously (Tatarczuch et al. 2000), using an anti-CD45 (leucocyte common antigen) monoclonal antibody (mAb) to determine the total percentage of leucocytes in the cell preparation. A total of 20 microscopic fields for each sample were counted with a ×100 oil-immersion objective. Direct immunofluorescence staining of cytospots was performed using an anti-MHC Class II mAb conjugated to FITC. Sections were examined using a fluorescence microscope (Leica, Heerbrugg, Switzerland).
Electron microscopy and immunoelectron microscopy
Cells collected from milk and mammary secretions were cytocentrifuged (8 × 105 cells; 500 g for 5 min), and the cell pellets were fixed in 2.5% glutaraldehyde at RT then post-fixed in 1% osmium tetroxide in PBS for 1 h. After washing in dH2O, the cell pellets were dehydrated in acetone and embedded in Procure-Araldite resin (ProSciTech, Thuringowa, Australia). During dehydration, the cells were stained en bloc with 2% uranyl acetate in 70% acetone. Semithin (1 µm) sections were cut through the maximum thickness of each pellet and stained with a solution of 1% methylene blue and 1% sodium tetraborate. Ultrathin sections were stained with uranyl acetate and Reynold's lead citrate, and examined using a Phillips 300 transmission electron microscope at 60 kV.
The immunogold–silver staining of cells in mammary involution secretions was performed as previously described (Tatarczuch et al. 2000). Briefly, cells were fixed in 0.05% glutaraldehyde for 20 min at 4 °C then washed and incubated with anti-CD45 overnight at 4 °C. Cells were washed and incubated with goat antimouse IgG conjugated with 5-nm gold particles (Amersham International, Poole, UK) for 4 h at 4 °C, then fixed in 2.5% glutaraldehyde and post-fixed in 0.5% osmium tetroxide, each for 1 h at RT, followed by incubation in silver enhancement developer (Amersham) for 7 min. The cell pellets were embedded and ultrathin sections prepared as described above.
Phagocytosis assay
For the in vitro phagocytosis assay, cells collected from milk and mammary secretions were resuspended in a glucose medium (Santos et al. 1995) containing 2% BSA and adjusted to 2 × 107 mL−1. The fluorescent latex Fluorospheres (L-5282 carboxylate-modified, 1.0-µm diameter, 2% solids; Molecular Probes, Eugene, USA) were first precoated with 1% BSA before 1 mL of BSA-coated beads (10 × 108) was added to 2 × 107 cells (final cell/bead ratio of 1 : 50). The cells were then incubated with the fluorescent latex beads for 60 min in a water bath at 37 °C in plastic tubes, with regular agitation. Control cultures with no addition of latex beads or incubation at 4 °C were also included. Following incubation, cells were washed three times with 0.5% Tween 20/PBS to remove uningested latex spheres attached to the cell surface, and fixed with 2.5% glutaraldehyde in PBS at 4 °C overnight. Cell pellets were centrifuged and resuspended in PBS, then processed for electron and immunoelectron microscopy as outlined above or analysed by flow cytometry on a FACSCalibur® instrument (Becton-Dickinson, CA, USA) using Cellquest® software (Becton-Dickinson).
Evaluation of phagocytic activity of leucocytes in mammary involution secretions
The phagocytic activity of leucocytes was evaluated by counting phagocytic cells (neutrophils and macrophages) after their identification on semithin sections (1 µm) stained with 1% methylene blue and 1% tetraborate (Esteban & Meseguer, 1997). For each assay, one embedded pellet was cut at three different levels. A minimum of 100 phagocytic cells were counted in each section, together with the number of ingested beads, using a ×100 oil-immersion objective. The percentage of neutrophils and macrophages showing phagocytic activity and the number of beads contained within cells was determined.
Phagocytic activity was expressed as phagocytic index (PI), calculated using the following formula (Barbuddhe et al. 1998):
PI = (% phagocytic cells containing ≥ 1 bead) × (mean number of beads/phagocytic cell containing beads).
At 1 day and 2 days after weaning, macrophages were rarely present and were tabulated with neutrophils, which constituted the majority of all phagocytic cells (> 98%). From 7 days, macrophages were the major phagocytic cell type (> 98%).
Statistical analyses
Statistical analysis was carried out using the Kruskall–Wallis non-parametric test to assess if there was any significant (P < 0.05) difference between means of the sample groups.
Results
Cellular composition of mammary secretions
The quantitative study revealed that the mean total somatic cell count in mammary secretions showed little variation over the first 4 days after weaning (5–6 × 105 cells mL−1); however, the proportion of leucocytes recovered over this period declined significantly (P < 0.05) from 5.0 × 105 cells mL−1 at 2 days to 0.4 × 105 cells mL−1 at 4 days after weaning (Table 1). At day 7, there was a six-fold increase in the somatic cell count and 90–95% of these cells were leucocytes (Table 1).
Table 1.
Percentages of different leucocyte subpopulations in mammary involution secretionsa
| Leucocyte subpopulationb | |||||
|---|---|---|---|---|---|
| Days after weaning | Total cells (×105 mL−) | Neutrophils % | Macrophages % | Lymphocytes % | Eosinophils % |
| 1 | 5.0 ± 0.1 | 94.5 ± 2.8 | 2.9 ± 1.2 | 0.7 ± 0.5 | 0 |
| 2 | 3.8 ± 0.5 | 94.3 ± 2.6 | 3.5 ± 1.3 | 0.6 ± 0.4 | 0 |
| 4 | 0.4 ± 0.0 | 90.7 ± 7.6 | 4.1 ± 1.1 | 1.2 ± 1.2 | 0.4 ± 0 |
| 7 | 32.0 ± 0.5 | 4.2 ± 2.8 | 80.2 ± 8.1 | 12.7 ± 2.1 | 0 |
| 15 | 120.0 ± 1.9* | 1.1 ± 0.1 | 86.1 ± 2.2 | 10.7 ± 1.3 | 0 |
| 30 | 40.7 ± 0.4* | 1.3 ± 0.6 | 74.3 ± 8.9 | 20.8 ± 10.8 | 1.3 ± 0.8 |
| 60 | 19.1 ± 0.5* | 1.4 ± 0.5 | 59.1 ± 21.2 | 36.1 ± 19.8 | 0.6 ± 0.4 |
Cells were collected from mammary involution secretions 1–60 days following weaning and cytospots stained for differential cell counts as described in Materials and Methods.
Values are mean percentages ± SD for separate groups of three sheep.
At 15, 30 and 60 days post-weaning cells were collected by first infusing 15 mL of PBS into the mammary gland.
Somatic differential cell counts showed that neutrophils were the predominant leucocytes (> 90% of cells) in mammary secretions during early involution (up to 4 days after weaning), as previously noted (Tatarczuch et al. 2000), but declined dramatically at day 7, from which point macrophages represented 50–90% of all leucocytes recovered. Lymphocytes were also present in the involution secretions and were often seen in close contact with macrophages.
Evaluation of phagocytic capacity in vitro
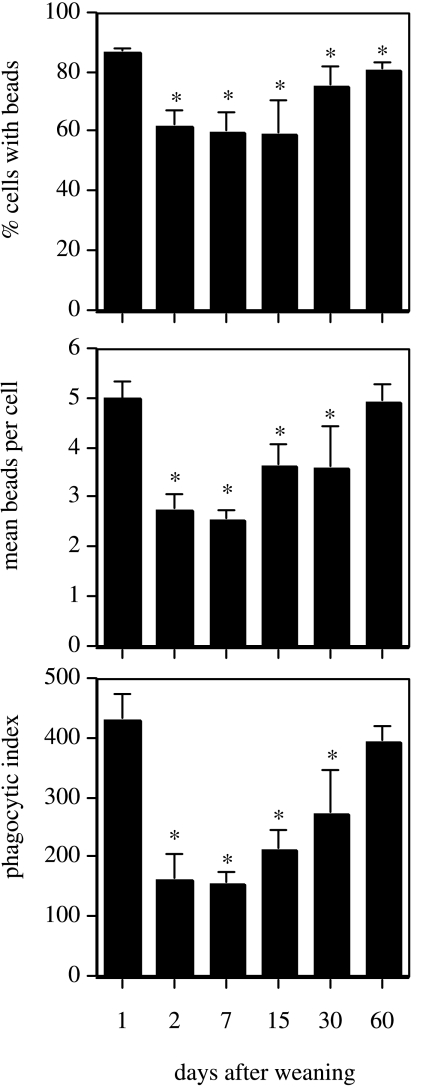
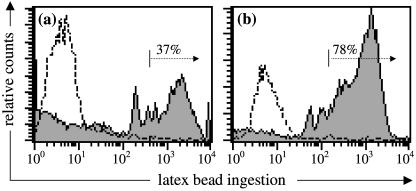
The leucocytes collected at 1–60 days after weaning were used in an in vitro assay of phagocytosis. As shown in Fig. 1, immediately following weaning when neutrophils were predominant, the phagocytic index (PI) was at its peak at 1 day post-weaning, reflecting both the highest percentage of active cells and the largest mean number of ingested beads. The PI of neutrophils at 2 days after weaning was significantly (P < 0.05) lower, resulting from a significant (P < 0.05) decrease in the percentage of active cells and a significant (P < 0.05) drop in the mean number of ingested beads. Analysis by flow cytometry confirmed this, showing that at 2 days after weaning less than 40% of neutrophils contained three or more beads (Fig. 2a). At 4 days after weaning, the absolute concentration of neutrophils was so low that there were insufficient cells to perform an assay. The PI of macrophages collected at days 7 and 15 was significantly lower (P < 0.05) than neutrophils isolated at day 1 but was comparable to that seen at day 2 for neutrophils. At 30 days there was an increase both in the percentage of active cells and in the mean number beads/cell, but the PI of macrophages was still significantly lower (P < 0.05) than neutrophils isolated at day 1. By 60 days after weaning the PI of macrophages had increased to a level similar to that seen in the neutrophil-rich population at 1 day. Flow cytometry revealed that a major proportion of the macrophages (75–85%) in involuted gland secretions were capable in vitro of ingesting more than three beads (per cell) (Fig. 2b).
Fig. 1.
Phagocytic activity of leucocytes in mammary secretions at different stages of involution. Cells were incubated in vitro with fluorescent latex beads, then fixed and processed for microscopic analysis. The percentage of phagocytes containing beads and the mean number of beads per cell was etermined, and phagocytic index calculated (see Materials and Methods). Values represent mean percentages ± SD (n = 3 sheep/group); *P < 0.05, significant difference compared to alue at 1 day post-weaning.
Fig. 2.
Flow cytometric analysis of the in vitro phagocytic activity of (a) neutrophils collected from mammary involution secretions 2 days after weaning, and (b) macrophages collected 30 days after weaning. Cells were incubated with (closed histogram) or without (open histogram) fluorescent latex beads and phagocytosis measured by flow cytometry (see Materials and Methods). Distinct peaks in the closed histogram correspond to cells containing 1, 2, 3, etc., phagocytosed beads; the percentage of phagocytes containing three or more ingested beads/cell is indicated. Data presented are from two representative sheep.
Electron microscopic examination of phagocytic leucocytes
(a) Neutrophils
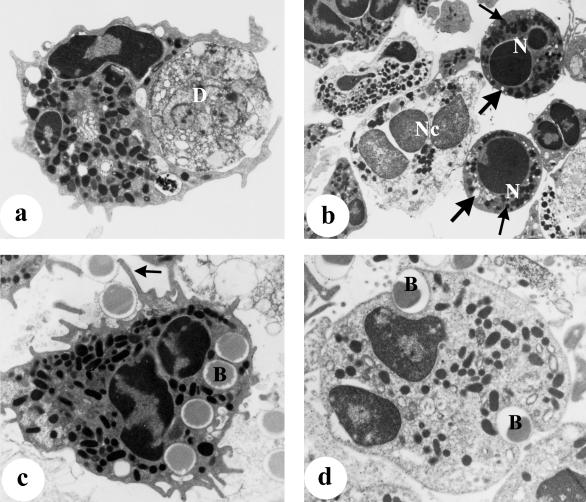
Neutrophils collected from mammary secretions 1–2 days after weaning contained phagocytosed casein material (membrane-bound), lipid vacuoles, cellular debris and apoptotic bodies (electron-dense material, presumably nuclei of apoptotic cells) and some were seen with pseudopodia extending around cellular debris (Fig. 3a). In addition, some neutrophils displayed ultrastructural features of apoptosis (markedly compacted electron-dense nuclei) and necrosis (degeneration of cellular organelles without apoptosis of the nucleus) (Fig. 3b). In the in vitro phagocytosis assay, electron microscopy confirmed that most neutrophils isolated 1 day after weaning contained ingested latex beads. Most of the neutrophils were seen with more than one bead but some contained up to 10 beads within the cytoplasm. That these cells were activated was evidenced by the presence of variable numbers of primary granules, mitochondria and glycogen particles in their cytoplasm. Many of these cells also displayed numerous pseudopodia, which occasionally surrounded attached beads (Fig. 3c). However, neutrophils isolated 2 days after weaning showed a great variation in phagocytic activity. In agreement with the analysis by flow cytometry (Fig. 2a), a smaller proportion of these cells appeared to contain numerous ingested beads (35–42%; cells containing three or more beads), while the majority (∼60%) were seen to contain no beads or only a few beads (Figs 2a and 3d). In contrast to neutrophils isolated 1 day after weaning, many of the neutrophils collected at 2 days appeared rounded and without pseudopodia, and glycogen particles were rarely observed in their cytoplasm.
Fig. 3.
Neutrophils collected from mammary secretions at 1–2 days after weaning (a,b) for routine electron microscopy and (b,c) for the in vitro phagocytosis assay, as described in Materials and Methods. (a) Neutrophil seen engulfing cellular debris (D), ×10 700. (b) Two apoptotic neutrophils (N) containing numerous intact granules (small arrows) and empty vacuoles (large arrows), and a neutrophil undergoing necrosis (Nc) characterized by an electron-lucent nucleus and degenerating organelles, ×6003. (c) Neutrophil with numerous granules, ingested beads (B) and pseudopodia surrounding attached beads (arrow), ×10 700. (d) A rounded neutrophil containing two beads (B) and lacking pseudopodia, ×13 050.
(b) Macrophages
As shown in Table 1, macrophages were the predominant cell type in mammary secretions from 7 days after weaning, and electron microscopy indicated that these cells displayed various ultrastructural features depending upon the stage of involution. In early involution (7 days after weaning), many macrophages were heavily laden with ingested lipid, casein and cellular debris including engulfed apoptotic bodies or neutrophils (Fig. 4a). These cells typically displayed a highly regular cell surface with very few pseudopodia. In contrast, macrophages from glands at more advanced stages of involution (15–30 days) contained less ingested material; these cells were mainly filled with lipid vacuoles and displayed numerous pseudopodia. Staining for CD45 (leucocyte common antigen) was more intense on the macrophages that displayed numerous pseudopodia (Fig. 4b).
Fig. 4.
Macrophages isolated from mammary secretions at different stages after weaning (a) for routine electron microscopy and (b–d) for the in vitro phagocytosis assay as described in Materials and Methods. (a) Macrophage containing an engulfed condensed neutrophil (N), an apoptotic body (Ab) and a nuclear remnant (Nr), 4 days after weaning, ×6003. (b) Macrophage with numerous pseudopodia showing intense staining with anti-CD45 antigen, 30 days after weaning, immunogold-silver method, ×6394. (c) Macrophage seen heavily laden with lipid (L) and devoid of any beads, 7 days after weaning, ×8874. (d) A non-vacuolated macrophage seen with pseudopodia (arrows) and containing numerous ingested beads (B), 60 days after weaning, ×5089.
Similar to neutrophils, macrophages also showed a great variation in phagocytic activity in vitro, depending both on the stage of involution and on the amount of ingested material. A large proportion of macrophages collected 7–15 days after weaning were devoid of any beads (Fig. 4c). In contrast, most of the macrophages isolated from involuted glands (30–60 days after weaning) contained ingested beads. Non-vacuolated macrophages with a well-developed Golgi complex, containing numerous beads and displaying numerous pseudopodia (Fig. 4d), were typically observed.
(c) Dendritic cells
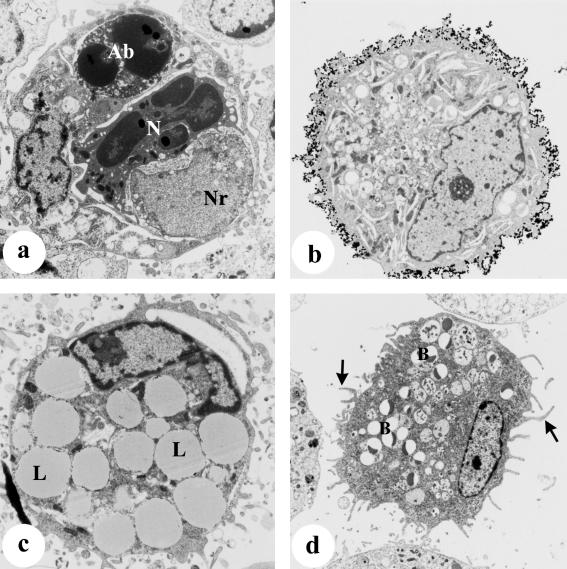
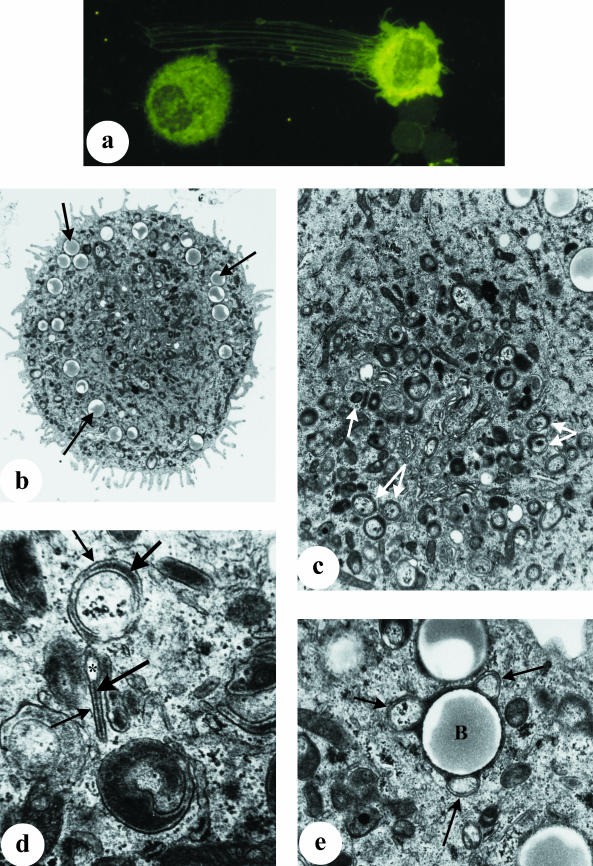
As reported previously (Tatarczuch et al. 2000), a small but distinct subpopulation of leucocytes (< 1%) present in involuted secretions of the mammary gland (from 15 days after weaning) displayed phenotypic characteristics resembling dendritic cells, including extensive cytoplasmic processes and high MHC Class II expression (Fig. 5a). Electron microscopy confirmed the presence of cells with distinctive ultrastructural features; these cells had numerous cytoplasmic processes of varying shape, length and thickness (Fig. 5b), and while they also contained numerous ingested beads, they lacked lysosomes, phagosomes or lipid droplets, as is frequently observed in macrophages. Their cytoplasm contained some rough-endoplasmic reticulum, free ribosomes, a well-developed Golgi complex and randomly dispersed fine filaments. The most prominent ultrastructural feature of these cells was the presence of numerous cytoplasmic granules located mainly in the perinuclear region (Fig. 5c). The size and shape of these granules varied but usually comprised two parallel limiting membranes and a central electron-dense lamina. Some granules appeared with one of the limiting membranes forming a vesicular portion at one edge, whilst others appeared to be oval-shaped structures containing an electron-dense core (Fig. 5d). These structures appeared similar to Birbeck granules as described in human epidermal Langerhans cells (Birbeck et al. 1961; Wolff, 1967; Sagebiel & Reed, 1968). Although these granules appeared to assume rod, disc and cup shapes, the majority appeared to be cup-shaped. Occasionally, these granules were seen to enclose ingested beads (Fig. 5e).
Fig. 5.
Dendritic cells collected from involuted mammary secretions at 30 days after weaning. (a) Cytospot preparation of cells stained with an anti-MHC Class II mAb, showing an intensely stained dendritic cell displaying extensive processes and in close contact with a lymphocyte, ×400. (b–e) Electron microscopy of dendritic cells following incubation with latex beads in an in vitro phagocytosis assay, as described in Materials and Methods. (b) Dendritic cell with numerous pseudopodia and containing ingested beads (arrows) but devoid of lysosomes and fat droplets, ×5070. (c) Higher magnification of (b) showing that the cytoplasm of this cell contains numerous Birbeck-like granules (arrows) which assume different shapes, ×13 000. (d) Birbeck-like granules under higher magnification (×24 000), seen with two limiting parallel membranes (small arrows) and a central electron-dense lamina (large arrows), and in some granules a limiting membrane forms a vesicular portion (*). (e) Higher magnification of (b) showing Birbeck-like granules (arrows) seen to enclose ingested bead (B), ×24 000.
Discussion
The present studies confirm earlier observations (Lee et al. 1969, 1983; Lee & Outteridge, 1981) that during involution of the mammary gland numerous leucocytes, including neutrophils, macrophages and lymphocytes, infiltrate into the mammary secretions within the lumina of alveoli and ducts. There are two waves of migration of phagocytic cells into the mammary gland during mammary involution, the first involving the recruitment of neutrophils over the first 2 days after weaning. Neutrophils have a short half-life (Cotter et al. 1994) and by 4 days most of these cells were seen to have degenerated, mainly by apoptosis, as evidenced by electron microscopy. Similar dynamics of neutrophil migration followed by apoptosis were observed during induced influx of neutrophils into the juvenile bovine mammary gland (Sladek & Rysanek, 2000). The current studies showed that in early involution secretions, the presence of macrophages containing numerous apoptotic bodies at various stages of degradation, and occasionally an intact neutrophil, indicated that apoptotic neutrophils were removed by these cells. Indeed, apoptosis of ageing but otherwise intact neutrophils and their phagocytosis by macrophages represents an important neutrophil disposal mechanism which limits the potential toxicity of this leucocyte (Savill, 1992).
At 2 days after weaning, numerous neutrophils had infiltrated into the mammary secretions and most of them had taken up casein, lipid and cellular debris. Therefore, these cells loaded with engulfed material would be expected to be less phagocytic than those neutrophils freshly infiltrated into the gland (1 day after weaning). This was confirmed in the in vitro studies where the phagocytic index of neutrophils at 2 days after weaning was significantly lower than those isolated 1 day after weaning. Electron microscopy indicated that the impairment of neutrophil phagocytic activity may have been due to the loss of cytoplasmic granules, or the reduced glycogen reserves resulting from the ingestion of lipid droplets, casein and cellular debris. The previous ingestion of large amounts of material would cause rounding of the cell, as seen in these studies and previously (Lintner & Eberhart, 1990), thereby eliminating pseudopodia required for phagocytosis.
After 4 days, the second wave of phagocytic cell migration was that of macrophages which from this point were seen as the predominant cell type in the mammary secretions right through to complete involution. Similar to that seen for neutrophils, macrophages collected at early involution (7–15 days) were heavily laden with ingested casein, lipid and cellular debris and they lacked pseudopodia. These macrophages were shown in vitro to be less phagocytic than those obtained from fully involuted glands. Possible reasons for this reduced phagocytic function of mammary macrophages could be that they are replete with engulfed material, the reduction of cell membrane (Cannon & Swanson, 1992) or the blocking of Fc receptors by factors such as immune complexes present in involuting mammary secretion (Targowski & Niemialtowski, 1986).
Another likely role for macrophages in the involution secretions is that of antigen processing and presentation as evidenced by the frequent appearance of macrophages attached to lymphocytes. The interaction of macrophages with lymphocytes has previously been described in human colostrum (Crago et al. 1979) and it has been shown that bovine mammary macrophages respond to bacteria as antigen-presenting cells to induce antigen-specific T-cell proliferation (Politis et al. 1992).
Following weaning, neutrophils and macrophages traffic into mammary secretions where they participate in the removal of casein, lipid droplets and cellular debris, an activity vital to the involution process. As far as we are aware the present studies are the first to demonstrate that neutrophils and macrophages heavily laden with ingested material during early involution display a diminished capacity for phagocytosis. That these cells are replete with engulfed material, and are incapacitated, may partly account for the observation that the mammary gland is more susceptible to infection during the early stages of involution (Nickerson, 1989; Oliver & Sordillo, 1989). In addition, it is likely that the local intramammary environment, including the presence of milk proteins (Cooray, 1996), humoral factors such as lactoferrin (Smith & Schanbacher, 1977) and the cytokine milieu may promote some degree of protection of the mammary gland against infection.
We recently reported the presence of a small subpopulation of leucocytes in the involution secretions collected at 15–60 days after weaning that displayed similar morphological characteristics to dendritic cells, including extensive cytoplasmic processes and the expression of MHC class II and CD1 (Tatarczuch et al. 2000). In the present study we have further characterized this population of dendritic cells by electron microscopy, revealing the presence of specific granules resembling the Birbeck granules reported in Langerhans cells (Sagebiel & Reed, 1968) and vascular dendritic cells present in human arterosclerotic aorta (Bobryshev et al. 1997). The role of Birbeck granules is still to be defined, but it has been suggested than these granules are involved in the antigen-presenting function of Langerhans cells (Hanau et al. 1987). In the present studies, Birbeck granules were occasionally seen to contain ingested beads, thus implicating that these cells are capable of processing phagocytosed particulate antigen. Dendritic cells in peripheral tissues, particularly at mucosal surfaces, play a central role in sampling and processing particulate and soluble antigens for presentation to T cells and induction of an immune response (Stumbles, 1999). Phagocytosis is one mechanism employed in the uptake of exogenous antigen, including large particles such as bacteria, and this activity appears to be closely associated with immature or freshly isolated tissue dendritic cells (Stumbles, 1999; Watts & Amigorena, 2001). It is not known whether the dendritic cells present in the secretion of fully involuted mammary glands, but absent during early involution, are derived from mammary monocytes or recruited directly as dendritic cells from the circulation. Interestingly, transforming growth factor-β (TGF-β), implicated in the differentiation of monocytes into dendritic cells (Geissmann et al. 1998), is present in mammary gland secretions and its levels increase dramatically with the progression of mammary gland involution (Ayoub & Yang, 1997). One may speculate that the TGF-β-rich microenvironment provided by the involution secretion at this time is sufficient to stimulate cells of the monocyte/macrophage lineage in the involution secretion to differentiate into dendritic cells.
In summary, these studies showed that while the mammary gland is endowed with a broad spectrum of immune cells, the incapacitation of phagocytic neutrophils and macrophages in the mammary secretions, together with other possible contributing factors, results in the immune status of the gland being compromised during early involution. In fully involuted mammary gland secretions, the presence of dendritic cells and a prominent macrophage population with a high capacity for phagocytosis may partly account for the observation that non-lactating mammary glands are more resistant to infection than lactating glands or glands at early stages of involution.
Acknowledgments
We would like to thank Brendan Kehoe for technical assistance. This work was partially supported by the Australian Research Council.
References
- Ayoub IA, Yang TJ. The regulatory role of transforming growth factor-beta in activation of milk mononuclear cells. Am. J. Reprod. Immunol. 1997;38:121–128. doi: 10.1111/j.1600-0897.1997.tb00286.x. [DOI] [PubMed] [Google Scholar]
- Barbuddhe SB, Malik SV, Gupta LK. Effect of in vitro monocyte activation by Listeria monocytogenes antigens on phagocytosis and production of reactive oxygen and nitrogen radicals in bovines. Vet. Immunol. Immunopathol. 1998;64:149–159. doi: 10.1016/s0165-2427(98)00129-9. [DOI] [PubMed] [Google Scholar]
- Birbeck MSL, Breathnatch AS, Everall JD. An electron microscopic study of basal melanocytes and high-level clear cells (Langerhans cells) in vitiligo. J. Invest. Dermatol. 1961;37:51–64. [Google Scholar]
- Bobryshev YV, Ikezawa T, Watanabe T. Formation of Birbeck granule-like structures in vascular dendritic cells in human atherosclerotic aorta. Lag-antibody to epidermal Langerhans cells recognizes cells in the aortic wall. Atherosclerosis. 1997;133:193–202. doi: 10.1016/s0021-9150(97)00129-9. [DOI] [PubMed] [Google Scholar]
- Cannon GJ, Swanson JA. The macrophage capacity for phagocytosis. J. Cell Sci. 1992;101:907–913. doi: 10.1242/jcs.101.4.907. [DOI] [PubMed] [Google Scholar]
- Cooray R. Casein effects on the myeloperoxidase-mediated oxygen-dependent bactericidal activity of bovine neutrophils. Vet. Immunol. Immunopathol. 1996;51:55–65. doi: 10.1016/0165-2427(95)05496-0. [DOI] [PubMed] [Google Scholar]
- Cotter TG, Fernandes RS, Verhaegen S, McCarthy JV. Cell death in the myeloid lineage. Immunol. Rev. 1994;142:93–112. doi: 10.1111/j.1600-065x.1994.tb00884.x. [DOI] [PubMed] [Google Scholar]
- Crago SS, Prince SJ, Pretlow TG, McGhee JR, Mestecky J. Human colostral cells. I. Separation and characterization. Clin. Exp. Immunol. 1979;38:585–597. [PMC free article] [PubMed] [Google Scholar]
- Esteban MA, Meseguer J. Factors influencing phagocytic response of macrophages from the sea bass (Dicentrarchus labrax L.): an ultrastructural and quantitative study. Anat. Rec. 1997;248:533–541. doi: 10.1002/(SICI)1097-0185(199708)248:4<533::AID-AR5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Prost C, Monnet JP, Dy M, Brousse N, Hermine O. Transforming growth factor beta1, in the presence of granulocyte/macrophage colony-stimulating factor and interleukin 4, induces differentiation of human peripheral blood monocytes into dendritic Langerhans cells. J. Exp. Med. 1998;187:961–966. doi: 10.1084/jem.187.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanau D, Fabre M, Schmitt DA, Stampf JL, Garaud JC, Bieber T, et al. Human epidermal Langerhans cells internalize by receptor-mediated endocytosis T6 (CD1 ‘NA1/34’) surface antigen. Birbeck granules are involved in the intracellular traffic of the T6 antigen. J. Invest. Dermatol. 1987;89:172–177. doi: 10.1111/1523-1747.ep12470555. [DOI] [PubMed] [Google Scholar]
- Lee CS, McDowell GH, Lascelles AK. The importance of macrophages in the removal of fat from the involuting mammary gland. Res. Vet. Sci. 1969;10:34–38. [PubMed] [Google Scholar]
- Lee CS, Outteridge PM. Leucocytes of sheep colostrum, milk and involution secretion, with particular reference to ultrastructure and lymphocyte sub-populations. J. Dairy Res. 1981;48:225–237. doi: 10.1017/s0022029900021646. [DOI] [PubMed] [Google Scholar]
- Lee CS, McCauley I, Hartman PE. Light and electron microscopy of cells in pig colostrum, milk and involution secretion. Acta Anat. 1983;117:270–280. doi: 10.1159/000145798. [DOI] [PubMed] [Google Scholar]
- Lee CS, Meeusen E, Brandon MR. Local immunity in the mammary gland. Vet. Immunol. Immunopathol. 1992;32:1–11. doi: 10.1016/0165-2427(92)90064-w. [DOI] [PubMed] [Google Scholar]
- Lintner TJ, Eberhart RJ. Effects of bovine mammary secretion during the early nonlactating period and antibiotics on polymorphonuclear neutrophil function and morphology. Am. J. Vet Res. 1990;51:524–532. [PubMed] [Google Scholar]
- Nickerson SC. Immunological aspects of mammary involution. J. Dairy Sci. 1989;72:1665–1678. doi: 10.3168/jds.S0022-0302(89)79278-X. [DOI] [PubMed] [Google Scholar]
- Oliver SP, Sordillo LM. Approaches to the manipulation of mammary involution. J. Dairy Sci. 1989;72:1647–1664. doi: 10.3168/jds.S0022-0302(89)79277-8. [DOI] [PubMed] [Google Scholar]
- Politis I, Zhao X, McBride BW, Burton JH. Function of bovine mammary macrophages as antigen-presenting cells. Vet. Immunol. Immunopathol. 1992;30:399–410. doi: 10.1016/0165-2427(92)90108-3. [DOI] [PubMed] [Google Scholar]
- Sagebiel RW, Reed TH. Serial reconstruction of the characteristic granule of the Langerhans cell. J. Cell Biol. 1968;36:595–602. doi: 10.1083/jcb.36.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JL, Montes MJ, GutierreZ F, RuiZ C. Evaluation of phagocytic capacity with a modified flow cytometry technique. Immunol. Lett. 1995;45:1–4. doi: 10.1016/0165-2478(94)00180-y. [DOI] [PubMed] [Google Scholar]
- Savill J. Macrophage recognition of senescent neutrophils. Clin. Sci. (Lond.) 1992;83:649–655. doi: 10.1042/cs0830649. [DOI] [PubMed] [Google Scholar]
- Sheldrake RF, Husband AJ, Watson DL. Specific antibody-containing cells in the mammary gland of non-lactating sheep after intraperitoneal and intramammary immunisation. Res. Vet. Sci. 1985;38:312–316. [PubMed] [Google Scholar]
- Sladek Z, Rysanek D. Apoptosis of polymorphonuclear leukocytes of the juvenile bovine mammary gland during induced influx. Vet. Res. 2000;31:553–563. doi: 10.1051/vetres:2000139. [DOI] [PubMed] [Google Scholar]
- Smith KL, Schanbacher FL. Lactoferrin as a factor of resistance to infection of the bovine mammary gland. J. Am. Vet. Med. Assoc. 1977;170:1224–1227. [PubMed] [Google Scholar]
- Sordillo LM, Shafer-Weaver K, DeRosa D. Immunobiology of the mammary gland. J. Dairy Sci. 1997;80:1851–1865. doi: 10.3168/jds.S0022-0302(97)76121-6. [DOI] [PubMed] [Google Scholar]
- Stumbles PA. Regulation of T helper cell differentiation by respiratory tract dendritic cells. Immunol. Cell Biol. 1999;77:428–433. doi: 10.1046/j.1440-1711.1999.00850.x. [DOI] [PubMed] [Google Scholar]
- Targowski SP, Niemialtowski M. Appearance of Fc receptors on polymorphonuclear leukocytes after migration and their role in phagocytosis. Infect. Immun. 1986;52:798–802. doi: 10.1128/iai.52.3.798-802.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatarczuch L, Philip C, Lee CS. Involution of the sheep mammary gland. J. Anat. 1997;190:405–416. doi: 10.1046/j.1469-7580.1997.19030405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatarczuch L, Philip C, Bischof R, Lee CS. Leucocyte phenotypes in involuting and fully involuted mammary glandular tissues and secretions of sheep. J. Anat. 2000;196:313–326. doi: 10.1046/j.1469-7580.2000.19630313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts C, Amigorena S. Phagocytosis and antigen presentation. Semin. Immunol. 2001;13:373–379. doi: 10.1006/smim.2001.0334. [DOI] [PubMed] [Google Scholar]
- Wolff K. The fine structure of the Langerhans cell granule. J. Cell Biol. 1967;35:468–473. doi: 10.1083/jcb.35.2.468. [DOI] [PMC free article] [PubMed] [Google Scholar]