Abstract
Purified mast cells (MC), isolated from the rat peritoneum, were stimulated in vitro with recombinant human IFN-α 2a (rhIFN-α 2a) and studied ultrastructurally. Quantitative determination of histamine release was also performed. The following ultrastructural features were observed: (1) dilation of single granules, leading to the formation of cytoplasmic cavities filled with dissolved or eroded matrices; (2) induction of partially empty, non-fused granule containers close to unaltered resting granules, a process very suggestive of piecemeal degranulation; (3) focal exocytosis, characterized by opening of single granules to the cell exterior and/or fusion of a few granules into small secretory channels. Histamine release was slightly increased in rhIFN-α 2a-treated MC, although not to significant levels. These results indicate that rhIFN-α 2a induces a characteristic pattern of degranulation in rat peritoneal MC and that a small proportion of rhIFN-α 2a-stimulated MC shows ultrastructural features suggestive for piecemeal degranulation.
Keywords: atypical degranulation, interferon, mast cell, piecemeal degranulation, rat
Introduction
The interferons (IFN) are a family of cytokines of 17–20 kDa with several functions. IFN-α and -β are induced in virally infected cells and share an antiviral activity, while IFN-γ is produced by natural killer cells and Th-1 cells in response to antigen or cytokines and has an immunological activity (Pestka et al. 1987; De Mayer & De Mayer-Guignard, 1988).
Mast cells (MC) are secretory cells storing synthetic products in large granules filling their cytoplasm. They show nearly ubiquitous distribution and participate in many types of immune and inflammatory responses (Dvorak, 1991). IFNs exert several regulatory effects on MC activity (Swieter et al. 1989; Bissonnette & Befus, 1990; Coleman et al. 1991; Holliday et al. 1994). In rodent models, IFNs exert an overall down-regulatory effect on MC activation, mediator release, growth and differentiation (Nafziger et al. 1990; Takagi et al. 1990).
As IFN-α exerts many opiate/endorphin-like effects, which can be blocked by the opiate antagonist naloxone (Weigent & Blalock, 1987), and endorphins stimulate histamine release from MC (Shanahan et al. 1984), we asked whether IFN-α may act as secretagogues in MC. To answer this question, rat peritoneal MC were incubated with recombinant human IFN-α 2a (rhIFN-α 2a) in vitro and studied for both quantitative determination of histamine release and ultrastructural morphology. Results indicate that rhIFN-α 2a induces an atypical pattern of MC exocytosis with ultrastructural features suggestive for piecemeal degranulation.
Materials and methods
MC were isolated from Wistar rats (Charles River, Como, Italy) as previously described (Candussio et al. 1999) and purified according to Lagunoff & Richard (1987). Their purity in the final preparation, as determined by staining with toluidine blue, was more than 90% (about 95–97% in some experiments). The contaminating cell population observed at the electron microscopy consisted of middle-sized or large non-granular lymphocytes and, to a lesser degree, macrophages.
Purified MC were suspended in sterile RPMI-1640 (5 × 105 cells mL−1), with an osmolality of 300 ± 8 mosm kg−1. The medium was buffered to pH 7.3 with 10 mM HEPES, supplemented with 10% fetal calf serum and 1% penicillin/streptomycin, and admixed with various amounts of rhIFN-α 2a (Roferon-A, Roche, Grenzach, Wyhlen, Germany) 100, 250, 500, 1000 U mL−1. Experiments were done in triplicate. Cells were maintained in conditioned medium for 24 h at 37 °C. Cell controls were incubated in RPMI-1640 medium without rhIFN-α 2a.
For determination of the effects of rhIFN-α 2a on histamine release, quadruplicate 1-mL aliquots of MC at a cell density of 5 × 105 mL−1 were incubated with RPMI-1640 containing 100, 250, 500 or 1000 U mL−1 rhIFN-α 2a or with the RPMI-1640 medium alone, for 24 h at 37 °C. MC were separated from supernatants by centrifugation at 150 g for 5 min at 4 °C and the cell pellets were resuspended in 1 mL of 5% trichloracetic acid to release residual histamine; 0.5 mL of 10% trichloracetic acid was added to 0.5 mL of the supernatants of controls. All samples were assayed for histamine using the fluorimetric methods of Shore et al. (1959). The amount of histamine released was expressed as a percentage of the total histamine present in the control suspension. Averages ± SE of the means were calculated for data from four samples from a typical experiment; statistical evaluation of results was carried out using Student's t-test for independent samples. Values of P < 0.05 were considered significant.
For assessment of ultrastructural changes induced by rhIFN-α 2a, purified MCs were incubated with 100, 250, 500 or 1000 U mL−1 rhIFN-α 2a or with the medium alone for 24 h, as described above. They were then processed for electron microscopic examination as described previously (Candussio et al. 1999) and observed in a Philips CM 12 electron microscope at 80 kW. To facilitate determination of whether granule containers or cytoplasmic channels were open to the cell exterior, aliquots of MC suspensions were incubated with cationized ferritin (Sigma Aldrich, Italy) according to Dvorak (1991).
For quantitative evaluation of ultrastructural changes induced by rhIFN-α 2a, randomly sectioned grids taken from each block of uniformly distributed cells were examined. MC secretory activity was evaluated as follows: (1) focal exocytosis (1–3 secretory granules in the cell); (2) mild exocytosis (less than 30% of secretory granules); (3) moderate exocytosis (30–60% of the granules); (4) diffuse exocytosis (more than 60% of the granules); (5) atypical degranulation (one or two dilated, non-fused granular cavities with partially eroded or dissolved granular matrices); (6) piecemeal degranulation (numerous non-fused granular chambers filled with altered matrices, nearby normal resting granules).
Results
Spontaneous histamine release, evaluated immediately after MC isolation (time 0), was 5.8 ± 2.1%. After MC incubation for 24 h with RPMI-1640 medium alone, histamine release was 8.5 ± 0.9% (time 1). The difference between histamine release at time 0 and 1 was not statistically significant. Determination of histamine release after MC incubation with 100, 250, 500 or 1000 U mL−1 rhIFN-α 2a for 24 h showed that histamine secretion was slightly increased in comparison to control samples, although not to significant levels (Fig. 1).
Fig. 1.
Percentages of histamine release from rat peritoneal MC after 24 h incubation with different rhIFN-α 2 concentrations. Each bar represents mean ± SE of data from four determinations from a typical experiment. Comparisons with control values are statistically not significant.
After 24 h incubation with rhIFN-α 2a, a proportion of 40–60% MC presented the ultrastructural features of resting, unstimulated cells (Fig. 2a and Table 1). This percentage varied according to the concentration of rhIFN-α 2a used. MC showed round to oval compact shape, smooth surface outline and a full complement of uniformly electron-dense secretory granules. Between 30% and 40% of rhIFN-α 2a-treated MC exhibited an atypical degranulation pattern, which consisted in dilation of a few cytoplasmic granules; this led in some cases to the formation of enormous cytoplasmic containers filled by partially dissolved or eroded granular matrices (Fig. 2b). These very enlarged granular cavities could fuse with neighbouring granules but they did not open to the cell exterior. In fact, experiments performed with cationized ferritin, an electron-dense tracer that binds to accessible negatively charged membranes, did not show any decoration of the inner lining of such dilated granular structures, suggesting no continuity with the plasma membrane (Fig.3). Ultrastructural features indistinguishable from piecemeal degranulation could be appreciated in a proportion of 3–5% MC (Fig. 4a). In these cells, partially emptied non-fused granules were retained within the cytoplasm of the activated MC. Many of these granules contained an altered, moderately dense matrix pattern, and maintained a round-to-oval shape, but were often enlarged. These granules coexisted with normally electron-dense granules. Some MC undergoing piecemeal degranulation presented a limited number of cytoplasmic secretory granules containing dense, irregular threads or very thick circular condensations (Fig. 4a,b). Five to 10% MC exhibited focal or mild exocytosis, characterized by membrane fusion, formation of small secretory channels and granule opening to the cell exterior (Fig. 4c). Exocytosis involving the whole cell granular repertoire was never observed in MC incubated with rhIFN-α 2a.
Fig. 2.
Rat peritoneal MCs treated with 500 (a) and 1000 (b) U mL−1 rhIFN-α 2 for 24 h. In (a), MC shows the ultrastructural morphology of a resting cell, with smooth contour and a full complement of cytoplasmic secretory granules. In (b), MC shows dilation of a few secretory granules, whose altered matrices appear dissolved or eroded. ×5000.
Table 1.
Morphometric quantitative data of exocytosis in mast cells incubated with 100, 250, 500 and 1000 U mL−1 rhIFN-α 2a for 24 h
| Morphological features | 100 U mL−1 (152 cells) | 250 U mL−1 (138 cells) | 500 U mL−1 (136 cells) | 1000 U mL−1 (127 cells) | Control (194 cells) |
| Resting cells | 93 (61.2) * | 78 (56.5) | 66 (48.5) | 51 (40.1) | 184 (94.8) |
| Focal exocytosis | 8 (5.3) | 10 (7.2) | 14 (10.3) | 14 (11) | – |
| Mild exocytosis | 1 (0.6) | 3 (2.2) | 3 (2.2) | 4 (3.1) | 2 (1.0) |
| Moderate exocytosis | – | – | – | 1 (0.8) | 7 (3.6) |
| Diffuse exocytosis | – | – | – | – | 1 (0.5) |
| Atypical degranulation | 45 (29.6) | 42 (30.4) | 47 (34.5) | 50 (39.4) | – |
| Piecemeal degranulation | 5 (3.3) | 5 (3.6) | 6 (4.4) | 7 (5.5) | – |
Numbers in parentheses represent percentages.
Fig. 3.
Rat peritoneal MC incubated with 250 U mL−1 rhIFN-α 2a, fixed and exposed to cationized ferritin. A giant secretory container is observable near the nucleus. Cationized ferritin decorates the cell surface but not the inner lining of the secretory container. ×5000.
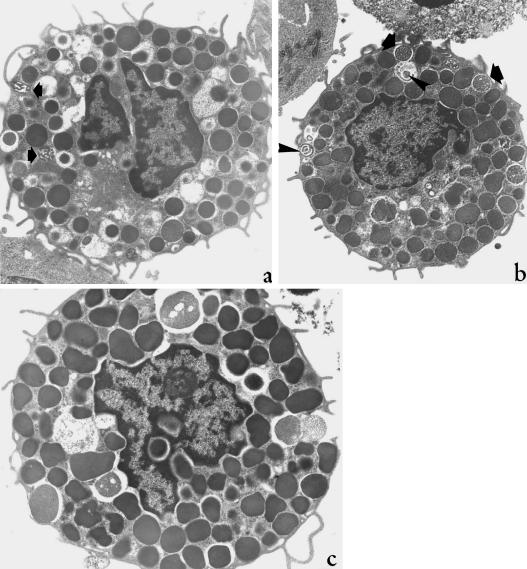
Fig. 4.
Rat peritoneal MCs, incubated with 500 (a,c) and 1000 (b) U mL−1 rhIFN-α 2a. In (a), MC shows many slightly dilated, nonfused, partially empty granule containers, all features highly suggestive for piecemeal degranulation. The arrows point to condensing granule material. In (b), MC presents some granules containing dense irregular threads (arrow) or circular thick condensed lamellae (arrowhead). These ultrastructural features are suggestive for granule refilling after slow losses of matrix materials. In (c), MC shows focal exocytosis, characterized by limited granule fusion, small secretory channel formation and opening of plasma membrane pores. a, c, ×6300; b, ×4400.
In control samples, 95% MC appeared as resting, unstimulated cells. The remaining 5% displayed ultrastructural features compatible with mild to moderate exocytosis. These MC exhibited the common findings of intracytoplasmic channel formation, opening of plasma membrane pores and discharge of altered granular matrices to the cell exterior.
Discussion
In this study we show that rat peritoneal MC exposed to various concentrations of rhIFN-α 2a undergo a secretory process characterized by: (a) dilation of single granules, leading at time to the formation of enormous cytoplasmic cavities filled with dissolved or eroded matrices; (b) induction of partially empty, slightly enlarged, non-fused granule containers nearby unaltered resting granules, a process indistinguishable from piecemeal degranulation in vivo; (c) focal exocytosis, characterized by opening of single granules to the cell exterior and/or fusion of a few granules into small secretory channels. Taken together, all these ultrastructural features suggest a slow mechanism of MC secretion in response to rhIFN-α 2a treatment.
Moreover, we have found that a small proportion (3–5%) of MC exhibits the ultrastructural features of piecemeal degranulation, i.e. partially empty, non-fused granule chambers filled with altered matrices nearby normal resting granules. The dense irregular threads or circular thick condensed lamellae observed inside certain granules suggest a process of matrix recovery after slow material losses (Dvorak & Kissel, 1991). Moreover, Dvorak et al. (1994) reported piecemeal degranulation of MC in vivo in inflammatory lesions of interleukin-4 overexpressing transgenic mice and our results suggest that also rhIFN-α 2a stimulates MC to undergo piecemeal degranulation.
Dvorak (1991) proposed that anaphylactic exocytosis and piecemeal degranulation in MC should be considered two steps of a generalized degranulation model, in which a ‘continuum’ of MC secretory events between these two basic degranulation pathways exists. In fact, intermediate or mixed secretory patterns have been observed in in situ MC from various human clinical settings (Dvorak, 1991; Dvorak et al. 1992) as well as in our experimental model where MC proceed from dilation and emptying of a few granules to slow emptying of many, non-fused granules (piecemeal degranulation) and, finally, to focal or mild exocytosis.
In conclusion, this study shows that rhIFN-α 2a promotes a unique degranulation pattern in rat peritoneal MC, suggesting a slow release of granule-stored contents and indicate that a small proportion of rhIFN-α 2a-treated MC undergo a secretive process indistinguishable from piecemeal degranulation. Finally, we would consider rhIFN-α 2a-mediated MC secretion as a putative new experimental model for studying in vitro piecemeal degranulation in MC.
Acknowledgments
This work was supported in part by grants of Fondazione Italiana per la Lotta al Neuroblastoma, Ministero dell’Istruzione e della Ricerca Scientifica, Rome, Italy, and Lega Italiana per la Lotta contro i Tumori, Sezione Friulana ‘Elio ed Enrico Morpurgo’.
References
- Bissonnette EY, Befus AD. Inhibition of mast cell-mediated cytotoxicity by IFN-α/β and −γ. J. Immunol. 1990;145:3385–3390. [PubMed] [Google Scholar]
- Candussio L, Crivellato E, Decorti G, Bartoli-Klugmann F, Mallardi F. Effect of low temperature on mast cell exocytosis. Early cytoplasmic vesicle formation and the role of cytoskeleton. J. Submicrosc. Cytol. Pathol. 1999;31:477–485. [PubMed] [Google Scholar]
- Coleman JW, Buckley MG, Holliday MR, Morris AG. Interferon−γ inhibits serotonin release from mouse peritoneal mast cells. Eur. J. Immunol. 1991;21:2559–2564. doi: 10.1002/eji.1830211037. [DOI] [PubMed] [Google Scholar]
- De Mayer E, De Mayer-Guignard J. Interferons and Other Regulatory Cytokines. New York: Wiley & Sons; 1988. [Google Scholar]
- Dvorak AM. Basophil and mast cell degranulation and recovery. In: Harris JD, editor. Blood Cell Biochemistry. Vol. 4. New York: Plenum Press; 1991. [Google Scholar]
- Dvorak AM, Kissel S. Granule changes of human skin mast cells characteristic of piecemeal degranulation and associated with recovery during wound healing in situ. J. Leukoc. biol. 1991;49:197–210. doi: 10.1002/jlb.49.2.197. [DOI] [PubMed] [Google Scholar]
- Dvorak AM, McLeod RS, Onderdonk A, Monahan-Earley RA, Cullen JB, Antonioli DA, et al. Ultrastructural evidence for piecemeal and anaphylactic degranulation of human gut mucosal mast cells in vivo. Int. Arch. Allergy Immunol. 1992;99:74–83. doi: 10.1159/000236338. [DOI] [PubMed] [Google Scholar]
- Dvorak AM, Tepper RI, Weller PF, Morgan ES, Esterella P, Monahan RA, Galli SJ. Piecemeal degranulation of mast cells in the inflammatory eyelid lesions of interleukin-4 transgenic mice. Evidence of mast cell histamine release in vivo by diamine oxidase-gold enzyme-affinity ultrastructural cytochemistry. Blood. 1994;83:3600–3612. [PubMed] [Google Scholar]
- Holliday RM, Banks EMS, Dearman RJ, Kimber I, Coleman JW. Interactions of IFN-γ with IL-3 and IL-4 in the regulation of serotonin and arachidonate release from mouse peritoneal mast cells. Immunology. 1994;82:70–74. [PMC free article] [PubMed] [Google Scholar]
- Lagunoff D, Richard A. Methods for the study of rat peritoneal mast cell secretion. In: Poisner AM, Trifarò JM, editors. In Vitro Methods for Studying Secretion. The Secretory Process. Amsterdam: Elsevier Scientific Publishers; 1987. pp. 13–28. [Google Scholar]
- Nafziger J, Arock M, Guillosson JJ, Wietzerbin J. Specific high-affinity receptors for interferon-γ on mouse bone marrow-derived mast cells: inhibitory effect of interferon-γ on mast cell precursors. Eur. J. Immunol. 1990;20:113–117. doi: 10.1002/eji.1830200117. [DOI] [PubMed] [Google Scholar]
- Pestka S, Langer JA, Zoon KC, Samuel CE. Interferons and their actions. Annu. Rev. Biochem. 1987;56:727–777. doi: 10.1146/annurev.bi.56.070187.003455. [DOI] [PubMed] [Google Scholar]
- Shanahan F, Lee TDG, Bienenstock J, Befus AD. The influence of endorphins on peritoneal and mucosal mast cell secretion. J. Allergy Clin. Immunol. 1984;74:499–504. doi: 10.1016/0091-6749(84)90385-3. [DOI] [PubMed] [Google Scholar]
- Shore PA, Burkhalter A, Cohn VH. A method for the fluorometric assay of histamine in tissues. J. Pharmacol. Exp. Ther. 1959;127:182–186. [PubMed] [Google Scholar]
- Swieter M, Ghali WA, Rimmer C, Befus AD. Interferon α-β inhibits IgE-dependent histamine release from rat mast cells. Immunology. 1989;66:606–610. [PMC free article] [PubMed] [Google Scholar]
- Takagi M, Koike K, Nakahata T. Antiproliferative effect of IFN-γ on proliferation of mouse connective tissue-type mast-cells. J. Immunol. 1990;145:1880–1884. [PubMed] [Google Scholar]
- Weigent DA, Blalock JE. Interactions between the neuroendocrine and immune system: common hormones and receptors. Immunol. Rev. 1987;100:79–108. doi: 10.1111/j.1600-065x.1987.tb00528.x. [DOI] [PubMed] [Google Scholar]