Abstract
Published levels of apoptosis in developing rat kidney (˜2.5%) seem large for a tissue with no obvious need for continual cell death. This paper examines the levels and patterns of apoptosis and mitosis in the cortical region of the developing metanephros of the mouse, the standard mammalian model embryo. Using confocal microscopy on specimens stained with propidium iodide to highlight nuclear morphology, optical sections of wholemount kidneys to a depth of ˜50 mm were analysed and mitotic, apoptotic and interphase nuclei counted in the various compartments.Of the ˜200 000 cells examined over E11.5–16.5, 2–3% were mitotic, confirming observations based on cryosections; the mitotic index peaked at E14.5, dropping to ˜ 0.5 % by P14. The mean apoptotic index during this period was 0.28% this figure from wholemounts was ˜10 % of that earlier reported in cryosectioned rat kidneys. One possible explanation for the difference is that cryosectioning turns out to create small nuclear fragments that can stain strongly with propidium. Such fragments are not seen in wholemounts and do not stain with TUNEL. Wholemount mouse E11.5 tails and E16.5 lungs were also analysed and both their mitotic and their apoptotic indexes were similar to those in wholemount developing kidneys. These results show that the level of apoptosis in wholemount embryonic mouse kidney cortex is far less than previously reported in cryosectioned rat embryonic kidneys, and typical of that in other mouse embryonic tissues whose development seems not to require apoptosis.
Keywords: apoptosis, confocal microscopy, kidney development, mouse development, TUNEL
Introduction
Mouse metanephrogenesis begins just before E11 when the ureteric bud grows from the nephric duct and invades the metanephrogenic mesenchyme (MM), a small, dense group of cells within the intermediate mesoderm at the level of the hind limb bud. A few hours later, reciprocal inductive interactions begin between the epithelium of the ureteric bud and the MM, with signalling from the former first inhibiting the MM from undergoing programmed cell death and then inducing it to become stem cells that will form nephrons in the cortex (Kreidberg et al. 1993; Camp & Martin, 1996; Barasch et al. 1999). The developing kidney then grows roughly exponentially until near birth, its volume doubling every 9–10 h or so between E11 and E16 (Davies & Bard, 1998). Despite the wealth of knowledge about the molecular mechanisms responsible for this rapid growth (Bard, 2002), relatively little is known about the dynamics of proliferation and apoptosis within the developing kidney. Coles et al. (1993) did, however, measure the proportion of mitotic and apoptotic figures in the E14.5 rat kidney cortex, and found them to be similar. On the basis of kinetic analysis (see Discussion),they suggested that one of every 2–4 cells born will die.
Although these studies establish apoptosis as an intrinsic part of metanephric development beyond that derived from any nutritional insufficiency (Welham et al. 2002), cell death on such a scale is unexpected, given the rapid growth rate of the kidney and the lack of any obvious structural need for apoptosis. We have therefore examined apoptotic and mitotic indexes in the developing mouse kidney, the standard model mammal, using fluorescent nuclear markers and confocal microscopy to measure the amounts of mitosis (MI, mitotic index) and apoptosis (AI, apoptotic index) in the various compartments of the developing kidney cortex. In particular, we report that the mean AI was 0.28% in the renal cortex as a whole during embryogenesis, only one tenth of that given by Coles et al. (1993), and was fairly consistent in each compartment and throughout the ages examined. We also find that this value of the AI was very similar to those in other developing tissues for which apoptosis plays no obvious role. These findings thus imply that apoptosis is considerably less significant for kidney development than previously reported.
Materials and methods
Tissue preparation
Pregnant (CD57BLxCBA/Ca)F1 mice were killed by cervical dislocation at the appropriate embryonic age, and their kidneys and other control tissues dissected out. Tissues were fixed overnight in 4% paraformaldehyde in 0.01 m phosphate buffered saline solution (PBS) which was washed out with PBS at 4 °C. For cryosections, tissues were frozen in OCT (Sakura), 12-µm sections were cut on a Leica CM1900 cryostat and stored at −80°C until required. It should be noted that mouse development is about 24 h advanced over that for the rat.
Tissue labelling
Fixed and washed whole kidneys were placed in 1% Triton-X PBS with 0.015 m sodium azide (PBST) and experimental samples were incubated with antilaminin rabbit antibody (Sigma, 1: 100 in PBST), on a shaker at room temperature for 48 h; controls were incubated without the antibody. Both were then washed with fresh PBST to remove unbound antibody. Samples were then incubated with whole-conjugate FITC–anti-rabbit IgG antibody (Sigma; 1 : 200 in PBST) on a shaker at room temperature for 48 h. The samples were again washed with fresh 1% PBST to remove any unbound antibody, then incubated with 10 µg ribonuclease A (Sigma) per mL of PBST for 2 h at 37 °C to remove any RNA. They were left overnight at 4 °C with 2.4 µg propidium iodide (Sigma) per mL PBST. The specimens were finally washed with fresh PBS, and mounted on a slide in 1 : 1 PBS/glycerol.
TUNEL staining
Frozen sections were brought to room temperature, fixed in fresh paraformaldehyde for 20 min, and stained for TUNEL using the kit instructions (Boehringer Mannheim). Experimental slides were incubated with terminal deoxynucleotidyl transferase and FITC-labelled nucleotides. Control slides incubated with nucleotides alone gave no stained nuclei.
Confocal microscopy
Tissue specimens were viewed using a Leica NT confocal system (Leica Microsystems, Heidelberg, Germany, GmbH), with a ×63 water-immersion or ×40 dry lens. The FITC-labelled basal laminae of nephrons and collecting ducts appeared green, and the propidium iodide-labelled nuclei appeared red (Fig. 1). Sets of serial optical sections were taken through 9–13 different kidneys at each age, starting at the kidney periphery. Between 4 and 8 serial sections were taken per set at approximately 7-µm intervals so as to avoid showing the same nuclei in successive sections. When more than one set of serial sections was taken per kidney, the field was moved to ensure that no structures from the previous field were visible.
Fig. 1.
Confocal micrographs of nuclei within a wholemount E14.5 mouse kidney stained with propidium iodide.(A,B) Fragmented apoptotic nuclei showing their intense staining compared to normal nuclei. (C) Prometaphase (white arrow) and late anaphase (yellow arrow) nuclei. (D) Early anaphase nucleus. Scale bar = 10 μm.
Images were analysed in Image Tool 2.00 (http://ddsdx.uthscsa.edu/dig/itdesc.html): apoptotic and mitotic nuclei in a field were first identified and counted, and remaining nuclei then individually marked. This procedure allowed the program to keep a count of total nuclei numbers. Data are given in Tables 1 and 2
Table 1.
Comparison of apoptosis and mitosis in the various compartments of developing kidneys
| Age | No. of kidneys | Data type | Stem cells | Collecting duct | Stroma | Tubules | Glomeruli |
|---|---|---|---|---|---|---|---|
| E11.5 | 10 | Apoptotic cells | 8 | 0 | 64 | * | * |
| Mitotic cells | 8 | 4 | 89 | ||||
| Total cell no | 1937 | 605 | 8926 | ||||
| Mean AI (%) | 0.41 ± 0.49 | 0 | 0.72 ± 0.63 | ||||
| Mean MI (%) | 0.41 ± 0.35 | 0.66 ± 0.24 | 1.00 ± 0.45 | ||||
| E12.5 | 9 | Apoptotic cells | 2 | 1 | 12 | * | * |
| Mitotic cells | 35 | 17 | 107 | ||||
| Total cell no | 2575 | 1176 | 9637 | ||||
| Mean AI (%) | 0.08 ± 0.09 | 0.09 ± 0.05 | 0.12 ± 0.11 | ||||
| Mean MI (%) | 1.36 ± 0.71 | 1.45 ± 0.78 | 1.11 ± 0.35 | ||||
| E13.5 | 12 | Apoptotic cells | 8 | 5 | 36 | 2 | 0 |
| Mitotic cells | 23 | 54 | 210 | 27 | 9 | ||
| Total cell no | 2605 | 4534 | 18 456 | 1548 | 573 | ||
| Mean AI (%) | 0.31 ± 0.45 | 0.11 ± 0.07 | 0.20 ± 0.08 | 0.13 ± 0.09 | 0.00 | ||
| Mean MI (%) | 0.88 ± 0.38 | 1.19 ± 0.41 | 1.14 ± 0.31 | 1.74 ± 1.04 | 1.68 ± 0.24 | ||
| E14.5 | 10 | Apoptotic cells | 16 | 1 | 51 | 11 | 0 |
| Mitotic cells | 76 | 96 | 337 | 114 | 3 | ||
| Total cell no | 3488 | 4643 | 18 406 | 5520 | 163 | ||
| Mean AI (%) | 0.46 ± 0.22 | 0.02 ± 0.03 | 0.28 ± 0.18 | 0.20 ± 0.12 | 0.00 | ||
| Mean MI (%) | 2.18 ± 0.44 | 2.07 ± 0.45 | 1.83 ± 0.29 | 2.07 ± 0.43 | 1.84 ± 2.45 | ||
| E15.5 | 13 | Apoptotic cells | 8 | 7 | 115 | 10 | 2 |
| Mitotic cells | 52 | 145 | 582 | 152 | 11 | ||
| Total cell no | 6175 | 7807 | 38 717 | 9921 | 855 | ||
| Mean AI (%) | 0.13 ± 0.12 | 0.09 ± 0.09 | 0.30 ± 0.10 | 0.10 ± 0.08 | 0.23 ± 0.27 | ||
| Mean MI (%) | 0.84 ± 0.28 | 1.86 ± 0.42 | 1.50 ± 0.24 | 1.53 ± 0.32 | 1.29 ± 1.87 | ||
| E16.5 | 10 | Apoptotic cells | 2 | 3 | 74 | 13 | 0 |
| Mitotic cells | 43 | 65 | 279 | 94 | 1 | ||
| Total cell no | 4322 | 4768 | 27 811 | 8071 | 351 | ||
| Mean AI (%) | 0.05 ± 0.12 | 0.06 ± 0.18 | 0.27 ± 0.09 | 0.16 ± 0.09 | 0.00 | ||
| Mean MI (%) | 0.99 ± 0.44 | 1.36 ± 0.41 | 1.00 ± 0.25 | 1.16 ± 0.29 | 0.28 ± 1.24 |
These structures do not form until E13.
Table 2.
Comparison of apoptosis and mitosis in embryonic tissues and postnatal kidneys
| E11.5–E16.5 kidneys | P14 kidneys | E11.5 tails | E16.5 lungs | |
|---|---|---|---|---|
| Apoptotic cells | 541 | 5 | 31 | 88 |
| Mitotic cells | 2633 | 26 | 142 | 275 |
| Total cell no | 193 590 | 8318 | 13 015 | 25 613 |
| No. of samples | 64 | 4 | 6 | 9 |
| Mean AI (%) | 0.28 ± 0.09 | 0.06 ± 0.03 | 0.24 ± 0.23 | 0.34 ± 0.12 |
| Mean MI (%) | 1.36 ± 0.15 | 0.31 ± 0.08 | 1.09 ± 0.29 | 1.07 ± 0.12 |
Recognizing apoptosis and mitosis
The cell nuclei of whole-mount tissues stained with propidium iodide are easily analysable using confocal microscopy (Davis & Ryan, 1998). Apoptotic nuclei are smaller and more brightly stained than normal nuclei, and sometimes fragmented (Fig. 1A,B). Clusters of fragments lying within one normal nuclear diameter of each other were counted as a single pyknotic nucleus (Wyllie et al. 1980). Mitotic figures were only counted if they lacked a nuclear membrane, and were therefore only identifiable for 60–70% of the duration of mitosis, i.e. between prometaphase and late anaphase (Fig. 1C,D). The measured MIs (Tables 1 and 2) are therefore underestimates by ∼35%, so the values in each graph have been corrected (for review, see Alberts et al. 2001).
Results
Wholemount kidneys
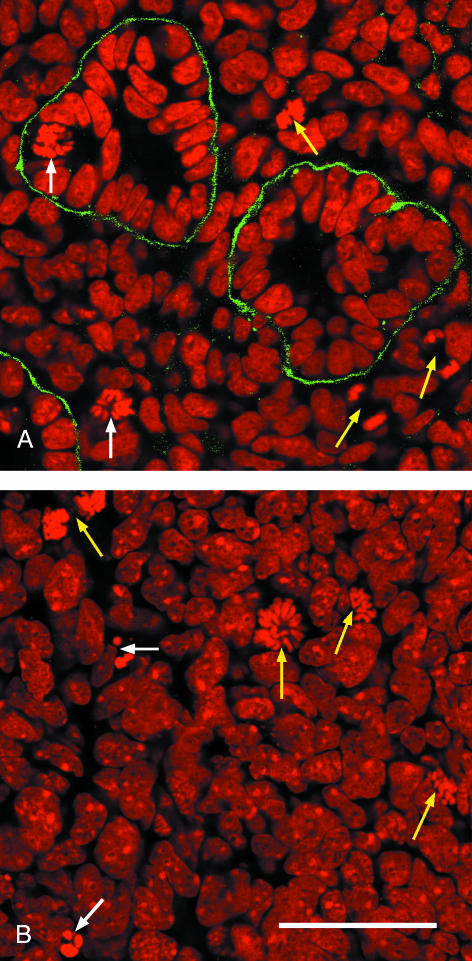
Confocal microscopy allowed images of the stained kidneys to be captured to a depth of about 50 µm, a depth that contains several structures. Laminin labelling of basal laminae made it easy to identify epithelial tubules, with the collecting ducts (trunk and tip combined), nephrons and glomeruli being distinguishable by their 3D morphology as seen in serial optical sections. Stem cells could be identified by their nuclear density, location at the very periphery of the kidney, and absence of a basal lamina (McLaren et al. 2000), while the stromal cells lie between the ducts (Fig.2). Within cells, mitotic nuclei from prometaphase to late anaphase, when there is no nuclear envelope, could readily be recognized, while apoptotic cells were identified on the basis of the intense staining of their small or fragmented nuclei (Figs 1 and 2).
Fig. 2.
Optical sections through an E14.5 mouse kidney stained with propidium iodide (red) and anti-laminin antibody (green). (A) Nephric tubules embedded in stromal cells with two cells in metaphase (white arrows) and three cells in anaphase (yellow arrows). (B) Stem cells at the kidney periphery with two cells undergoing apoptosis (white arrows) and four cells in different stages of mitosis (yellow arrows). Scale bar = 40 μm.
The raw measurements on mitosis and apoptosis in the cortex of embryonic kidneys are given in Table 1. Of the 193 590 cells counted in the embryonic kidney cortex over the period E11.5–16.5, 2633 were mitotic and 541 were apoptotic, giving an MI and AI of 1.36% and 0.28%, respectively (Table 1). When these mitotic values are corrected for their ∼35% underestimate (see Materials and methods), the mean MI is about 2.1%.
The MI (corrected, Fig. 3) for all kidney compartments combined (Table 1) increased from a value of 1.4% at E11.5, to 1.8% by E12.5 and E13.5, to a significantly high (P < 0.001, Mann–Whitney) peak of 3.0% at E14.5. It then fell steadily to 1.6% at E16.5 and to 0.5 ± 0.08% at P14 (P <0.01, Mann–Whitney). ANOVAR analysis showed no other significant differences in mitotic indexes across the various compartments during embryogenesis.
Fig. 3.
Bar charts showing apoptotic and mitotic rates. (A) Variation of mitotic and apoptotic indexes with kidney age for all compartments in the mouse cortex combined. (B) Variation of mitotic and apoptotic indexes across compartments (averaged for age). (C) Comparison of mitotic and apoptotic indexes in embryonic (E, average E11.5–E16.5) and postnatal (P) kidneys with those for E11.5 tails and E16.5 lungs. The raw data are from Tables 1(A, B) and 2(C), but note that the mitotic indexes have been increased to account for the fact that a nuclear membrane is only present for about ˜65% of mitosis.
In contrast, overall values of AI remained essentially constant (0.28% ± 0.09) during embryogenesis, though this too dropped after birth (0.06 ± 0.03%). anovar analysis showed that the only significant differences were that the AIs of stromal and stem cells were greater than those for the nephric tubules and collecting ducts (P < 0.05; Fig. 3B).
Frozen sections
Although our corrected MIs of ∼2.1% were similar to the value of 2.4% given by Coles et al. (1993), our mean apoptotic index of 0.28% was only about 10% of their value of 2.7% that came from frozen sections rather than wholemount kidneys. In order to see if preparative techniques might be responsible for the difference, we have repeated their protocol on frozen sections of E14.5 mouse kidneys, an age with the highest apoptotic indexes in both studies.
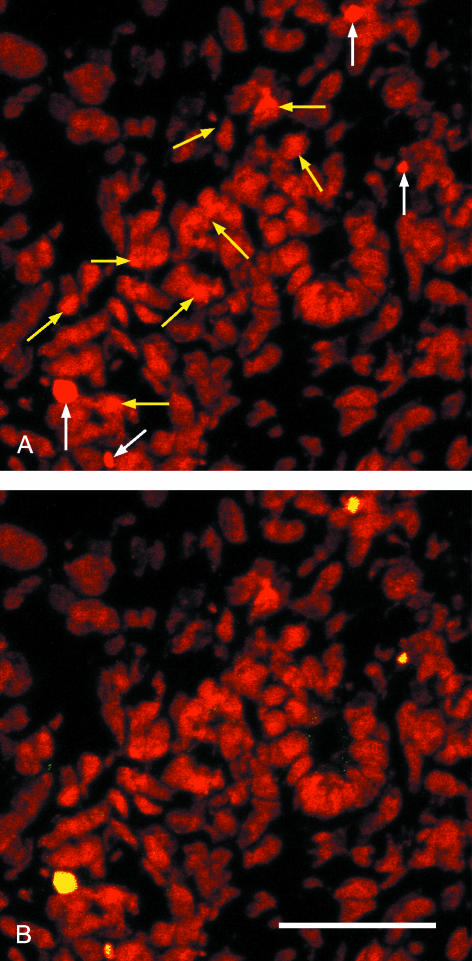
Comparisons of images of frozen sections stained with propidium iodide with those from wholemount kidneys pointed to an unexpected difference: while all nuclei in wholemount specimens showed the expected oval morphology (Figs 1 and 2), nuclei seen in frozen sections were of far worse quality (Fig. 4). Nuclear membranes were less smooth in outline and many nuclear fragments were visible, and it was not always obvious whether such fragments were remnants of apoptotic nuclei or cryosectioning artefacts. FITC-labelled TUNEL and confocal microscopy were used to clarify the situation.
Fig. 4.
Confocal micrographs of a cryosectioned E14.5 kidney cortex stained with propidium iodide (red, A,B) and FITCTUNEL (yellow, B). (A) Propidium staining alone shows four intensely stained nuclei (white arrows) and several fragmented nuclei whose staining is a little more intense than the background (yellow arrows). (B) The FITC–TUNEL confirms that the four intense nuclei (A) are undergoing apoptosis but that none of the fragmented nuclei is apoptotic. Scale bar = 40 μm.
For TUNEL-treated frozen sections viewed in the confocal microscope, the FITC (TUNEL) channel was first switched off so that only propidium staining was visible, and fields selected for their relatively large numbers of apoptotic nuclei and ‘non-apoptotic’ nuclear fragments. The FITC channel was then turned on and, of the 4967 nuclei examined, all 16 nuclei previously identified as apoptotic on the basis of morphology and staining intensity were TUNEL-positive. In contrast, of the 68 fragmented nuclei whose propidium staining was below apoptotic levels, only six were TUNEL-positive (even in sections selected for obvious apoptosis, the total AI was thus only ∼0.44%). It thus seems that most of the nuclear fragments visible in cryosectioned kidneys are not apoptotic, but, on the basis of morphology and staining alone, could be misidentified. As a whole, these figures suggest that there are some four times as many non-apoptotic nuclear fragments as there are condensed apoptotic nuclei in our frozen sections.
Non-kidney material
In order to see whether growth in the kidney is abnormally high as compared with other tissues in the developing mouse embryo, we have compared mitotic and apoptotic indexes in embryonic (grouping E11.5–E16.5 data from Table 1) and postnatal kidneys with those in two other tissues, E11.5 tails and E16.5 lungs. These were chosen as examples of rapidly growing tissues having no obvious requirement for apoptosis, with the former containing a mix of epithelial, mesenchymal and neuronal cells while the latter is mainly epithelial ducts.
The data (Table 2) show that the raw MI for developing kidneys (1.36 ±0.15%) is not significantly higher than those for the tail (1.09 ± 0.29) and lung (1.07 ± 0.12). Furthermore, the apoptotic index for developing kidneys (0.28 ± 0.09%) is indistinguishable from those for the tail (0.24 ± 0.23) and lung (0.34 ± 0.12%). The only significant differences in AI are between the P14 kidney and each of the embryonic tissues (P < 0.05, Mann–Whitney, Fig. 3C).
Discussion
This paper reports the use of confocal microscopy and nuclear staining to measure mitotic and apoptotic indexes in the developing kidney cortex (a depth of ∼50 µm) for all the major metanephric compartments, apart from vascular and neuronal cells, over a range of ages (E11.5–16.5). The results lead us to make two main points: that the amount of apoptosis in the developing kidney cortex is far less than previously reported, and that this amount is in line with that of other developing tissues. Here we consider the validity of these points, the reasons for the differences between our observations and the earlier report, and the significance of the findings as a whole for kidney growth.
Estimates of levels of apoptosis and mitosis in the developing kidney
The key observation presented here is that, on the basis of confocal analysis of stained nuclei from almost 200 000 cortical cells with well-defined nuclear preservation, levels of apoptosis in the developing mouse kidney vary from about 0.2 to 0.4%, depending on age and compartment, with a mean of 0.28%. This figure is about 10% of the 2.7% reported by Coles et al. (1993) who analysed nuclear morphology in frozen sections of ∼E14.5 rat kidneys, although there is little difference between the E14.5 mitotic indexes reported here (∼2.1%, corrected) and those of the Coles group (2.4%). Even allowing for the fact that we may have missed some TUNEL-positive cells, the AI is most unlikely to be more than about 0.4%.
It is not clear why the two sets of AI measurements are so different when the MI indexes are so similar. One possibility is that there are substantial differences between rat and mouse kidney morphogenesis in that the former needs much more apoptosis than the other. As development rates for the two do not seem markedly different, there is no evidence that points to this being a likely explanation. A likelier explanation is that, as cryosectioning, the technique used by Coles et al. (1993), causes a degree of nuclear fragmentation (Fig. 4), at least in our hands, it is not always easy to distinguish between sectioning artefacts and apoptotic condensations on the basis of propidium staining. Indeed, our counts suggest that, by comparing propidium-stained frozen sections stained with and without TUNEL, it is possible to overestimate the amount of apoptosis by a factor of three or more, although TUNEL can give false positives (Jerome et al. 2000). Even with this caveat, it is not clear why Coles et al. (1993) obtained values for apoptosis in embryonic kidneys that were so much greater than ours. Unfortunately, they do not report absolute counts so statistical analysis is not possible here.
Coles et al. (1993) also reported, and we have confirmed, that levels of apoptosis dropped significantly in older kidneys. Indeed, our AI value of 0.06% for P14 mice is much closer to the value of 0.15% reported by Coles et al. (1993) than those for younger embryos, while their AI value of 0.2 for P7 thymus where apoptosis occurs (Egerton et al. 1990) is far closer to our values for developing tissues (Fig. 3C). One possible explanation for the differences between embryonic and postnatal tissues is that, because older kidneys grow more slowly, they may have nuclei that are more robust than embryonic ones and less likely to be disrupted by cryosectioning. Such an explanation cannot, however, account for the 75% drop in the apoptotic index that Coles et al. (1993) measured after EGF treatment, and our results have no bearing on this effect.
Kidney growth and development
Detailed examination of nuclei in the cortical area of developing mouse kidneys shows that mitotic indexes in kidneys as a whole peak at E14.5, an age after which the rate of growth of the developing kidney slows (Davies & Bard, 1998). Apoptotic levels similarly decline after birth, but remain constant during embryogenesis, at least up to E16.5. It is, however, worth noting that levels of apoptosis are higher in the stromal and stem cells than in the epithelial tubules of the collecting ducts and early nephrons.
There are two possible explanations for this increase. First, both stem and stromal cells are transitional tissues: the former are lost once all the nephrons have formed while most of the latter disappear from the medulla, presumably through apoptosis, as the loops of Henle start to form (Sainio et al. 1994). They may therefore have a higher likelihood of entering apoptosis than other cells. Second, an increased AI in these cells is compatible with the suggestion of Koseki et al. (1992) that stromal cell death in the immediate vicinity of early nephrons facilitates their morphogenesis, events rather easier to see in sectioned tissue than in wholemounts. If so, the extra apoptosis required for the relatively few forming nephrons could account for the increase in AI in these cells.
An independent, albeit rough test of the validity of our results comes from the predictions that they make about the rate of increase in kidney size with time. Here, the key figures are the rates of mitosis (m = MI/h) and apoptosis (a = AI/h). Given that these are essentially uniform across the kidney, the basic equation for kidney growth mass M is
provided we assume that relative changes in such factors as cell size, extracellular matrix and luminal space are second order. For the E14.5 mouse embryo, the average values of MI and AI are 2.9% and 0.25%. Assuming that mitosis takes about 30 min in vivo, while apoptosis lasts about an hour (Coles et al. 1993), this becomes
This standard equation of exponential growth gives a kidney doubling time of [(In 2)/0.0555]h, or about 12 h. As measurement shows that the kidney doubling time is about 9–10 h at E14.5 (Davies & Bard, 1998), the estimate is not unreasonable. In contrast, a value of the apoptotic index of 2.4% (Coles et al. 1993) for the E14.5 embryo increases the doubling time to ∼21 h, a figure that is high because apoptosis is almost halving the effect of proliferation. These figures do of course depend on the estimate of the time of mitosis and it is worth noting that, were this is as long as 1 h, then the figure for apoptosis given by Coles et al. (1993) would give no growth at all as mitosis would be exactly balanced by apoptosis.
The results as a whole suggest that apoptosis is not abnormally high during kidney formation, but is typical of that in other tissues whose development does not require cell death. Nevertheless, these results remain compatible with the suggestion of Koseki et al. (1992) that local cell death in the stroma of the cortex may facilitate nephron morphogenesis.
Acknowledgments
We thank Grace Grant for making the frozen sections, Linda Sharp for running the confocal microscope which was funded by the Wellcome Trust, and Jamie Davies for commenting on the manuscript.
References
- Alberts B, Bray D, Lewis J, Raff R, Roberts K, Watson DJ. Molecular Biology of the Cell. 3. New York: Garland Science Publishing; 2001. [Google Scholar]
- Barasch J, Yang J, Ware CB, Taga T, Yoshida K, Erdjument-Bromage H, et al. Mesenchymal to epithelial conversion in rat metanephros is induced by LIF. Cell. 1999;99:377–386. doi: 10.1016/s0092-8674(00)81524-x. [DOI] [PubMed] [Google Scholar]
- Bard JBL. Growth and death in the developing mouse kidney: signals receptors and conversations. Bioessays. 2002;24:72–82. doi: 10.1002/bies.10024. [DOI] [PubMed] [Google Scholar]
- Camp V, Martin P. Programmed cell death and its clearance in the developing mammalian kidney. Exp. Nephrol. 1996;4:105–111. [PubMed] [Google Scholar]
- Coles HSR, Burne JF, Raff MC. Large scale normal cell death in the developing rat kidney and its reduction by epidermal growth factor. Development. 1993;118:777–784. doi: 10.1242/dev.118.3.777. [DOI] [PubMed] [Google Scholar]
- Davies JA, Bard JBL. The development of the kidney. Curr. Top. Dev. Biol. 1998;39:245–301. doi: 10.1016/S0070-2153(08)60458-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Ryan DH. Apoptosis in the kidney. Toxicol. Pathol. 1998;26:810–825. doi: 10.1177/019262339802600615. [DOI] [PubMed] [Google Scholar]
- Egerton M, Scollay R, Shortman K. Kinetics of mature T-cell development in the thymus. Proc. Natl Acad. Sci. USA. 1990;8:2579–2582. doi: 10.1073/pnas.87.7.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerome KR, Vallan C, Jaggi R. The tunel assay in the diagnosis of graft-versus-host disease: caveats for interpretation. Pathology. 2000;32:186–190. [PubMed] [Google Scholar]
- Koseki C, Herzlinger D, Al-Awqati Q. Apoptosis in metanephric development. J. Cell Biol. 1992;119:1327–1333. doi: 10.1083/jcb.119.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, et al. WT–1 is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- McLaren L, Boyle S, Mason JO, Bard JBL. Mouse protein phosphatase inhibitor-1: genetic characterization and significance in mesothelial development. Mech. Dev. 2000;96:237–241. doi: 10.1016/s0925-4773(00)00388-9. [DOI] [PubMed] [Google Scholar]
- Sainio K, Nonclercq D, Saarma M, Palgi J, Saxen L, Sariola H. Neuronal characteristics in embryonic renal stroma. Int. J. Dev. Biol. 1994;38:77–84. [PubMed] [Google Scholar]
- Welham SJ, Wade A, Woolf AS. Protein restriction in pregnancy is associated with increased apoptosis of mesenchymal cells at the start of rat metanephrogenesis. Kidney Int. 2002;61:1231–1242. doi: 10.1046/j.1523-1755.2002.00264.x. [DOI] [PubMed] [Google Scholar]
- Wyllie AH, Kerr JF, Currie AR. Cell death: the significance of apoptosis. Int. Rev. Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]