Abstract
Marsupials are good experimental animals for developmental studies as their offspring are born at a stage comparable to embryonic stages of eutherian species. The South American opossum, Monodelphis domestica, is particularly useful because of its small size and easy maintenance. This study was carried out to compare development of opossum fore- and hindlimbs during postnatal life, using light microscopy and whole mount alizarin staining. At birth, well-developed mobile forelimbs show cartilage models of bones and myotubular striated muscle fibres. However, hindlimbs are relatively underdeveloped paddle-like outgrowths. Two days later mesodermal condensations form models of the future hindlimb bones and mononucleate myoblast aggregates are present; by 6 days post partum (dpp) the hindlimb has reached a stage of development similar to that of the forelimb at birth. At this stage, periosteal buds have invaded forelimb long bones and nuclei in forelimb muscle fibres have become displaced to the periphery. The 16 dpp hindlimb shows long bones invaded by periosteal buds and closely packed, striated muscle fibres. Epiphyseal plates are now seen in the forelimb long bones and forelimb muscle fibres show mature characteristics. Musculoskeletal development is well correlated with the functional demands of the limbs during postnatal development in the opossum, which provides an excellent model for investigations into the genes and molecules controlling limb development.
Keywords: histology, marsupial, myogenesis, ossification, skeleton
Introduction
Despite a great deal of diversity in size and shape among mammalian species, vertebrate limb morphology follows a very similar organizational plan (Oster et al. 1988), designed to allow movement yet provide support and resistance to buckling and bending loads and the forces of gravity and impact. Though limb development in eutherian mammals has been the subject of much investigation, relatively little is known about the pattern of growth of limbs in marsupials. During prenatal development, the eutherian forelimb is slightly more advanced than the hindlimb (Bagnall et al. 1982), but by birth hindlimbs are larger and growing more rapidly than forelimbs. Marsupials are born at a very rudimentary stage of development compared with eutherians (Gemmell et al. 1988) resembling 11- or 12-day mouse embryos in overall development (Smith, 2001), yet they must be able to move to the mammae. The young are attached to the teats for a period after birth corresponding to the latter part of eutherian intra-uterine gestation (Sharman, 1970). A strikingly exaggerated difference is seen between the limbs at birth, with the forelimbs being more advanced (Russell, 1982). In general, marsupial neonates have well-developed, mobile forelimbs supported by a complete cartilaginous skeleton internally and ending in digits bearing pointed deciduous claws externally (Hughes & Hall, 1988). By contrast, neonatal marsupial hindlimbs are immobile, unrotated paddles showing varying degrees of footplate development, depending on the species.
Although skeletogenesis has been documented for the rat (Spark & Dawson, 1928) and the mouse (Patton & Kaufman, 1995) few studies have been carried out in marsupials. Nesslinger (1956) described skeletal development in the Virginia opossum, Didelphis virginiana; however, her study was limited by reliance on specimens of uncertain age. The timing of ossification in two marsupial species, the bandicoot Isoodon macrourus and the possum Trichosurus vulpecula, was investigated by Gemmell et al. (1988). Similarly, growth and development of marsupial limb muscle has also been poorly documented, though Bridge & Allbrook (1970) studied growth of striated muscle in the Australian quokka Setonix brachyurus.
The Brazilian grey short-tailed opossum, Monodelphis domestica, intermediate in size between a mouse and a rat, is a pouchless marsupial which breeds well in captivity and produces several young after only 14 days of gestation (Gilmore, 2002). It has been proposed as a model species for both genetic research (VandeBerg, 1990) and biomedical research (Xie et al. 1996; VandeBerg & Robinson, 1998). A timetable for prenatal development in Monodelphis domestica includes the appearance of paddle-shaped forelimbs on day 12 after video-recorded mating; digital buds are seen a day later when a club-like hindlimb first appears. By day 14 after mating claws appear on forelimb digits and digital ridges are seen on the hindlimb paddle (Mate et al. 1994). The newborn opossum performs alternate rhythmic movements with the forelimbs to crawl towards the nipple; at this stage the hindlimbs are little more than embryonic buds and only begin to move late in the second week of postnatal life (Pflieger et al. 1996). The purpose of this investigation is to build on these earlier studies and describe the comparative postnatal development of the fore- and hindlimbs in the Brazilian grey short-tailed opossum, Monodelphis domestica.
Materials and methods
A total of 35 opossum pups were used in this study, obtained from a colony bred at the Glasgow Royal Infirmary, University of Glasgow. The day of birth was designated as Day 0 post partum (0dpp). After removal from the teat each pup was killed by inhalation of CO2 or halothane and fixed in 4% formaldehyde in 0.1 m phosphate buffer for a minimum of 24 h.
Light microscopy
Specimens were rinsed in buffer, dehydrated through an ascending ethanol series and routinely embedded in paraffin wax. Care was taken with dissected limbs to ensure that specimens were embedded so that their plane of section would be perpendicular to the transverse axis. Serial sections were cut at 6 µm, incubated at 37 °C overnight and then subjected to one of the following four staining procedures: haematoxylin and eosin, Masson's trichrome technique (Masson, 1929), haematoxylin, eosin and Alcian blue, or Alcian blue and methyl green pyronin.
Whole mount Alcian blue and alizarin staining
Limbs were dissected and where pups were older than 6 dpp skin was removed. Whole mount preparations were made using a modification of the method of Dingerkus & Uhler (1977) which utilizes alizarin red S to stain bone red and Alcian blue to counterstain cartilage blue. Specimens are stored in glycerine. This double-staining technique allows very accurate localization of specific centres of ossification within cartilage models (Kaufman, 1994; Patton & Kaufman, 1995).
Results
Although eight different postnatal stages have been examined, the most significant development changes were seen at day 0, day 2, day 6, day 16.5 and day 56. Results have been obtained from two sources: light microscopy and analysis of whole mount preparations. Using this information the presence or absence of centres of ossification in developing bone, the location of nuclei in muscle fibres and the presence or absence of striations in muscle fibres were recorded at each postnatal age in the fore- and hindlimbs (see Table 1).
Table 1. Summary of results obtained from histology and whole mount preparations.
| Age (dpp) | Limb type F = forelimb H = hindlimb | Ossification centre | Muscle fibre with peripheral nuclei | Muscle fibre with striations |
|---|---|---|---|---|
| 0 | F | ✓ | ✗ | ✓ |
| H | ✗ | ✗ | ✗ | |
| 2 | F | ✓ | ✗ | ✓ |
| H | ✗ | ✗ | ✗ | |
| 6 | F | ✓ | ✓ | ✓ |
| H | ✓ | ✗ | ✓ | |
| 9 | F | ✓ | ✓ | ✓ |
| H | ✓ | ✗ | ✓ | |
| 11 | F | ✓ | ✓ | ✓ |
| onwards | H | ✓ | ✓ | ✓ |
Day 0 (day of birth)
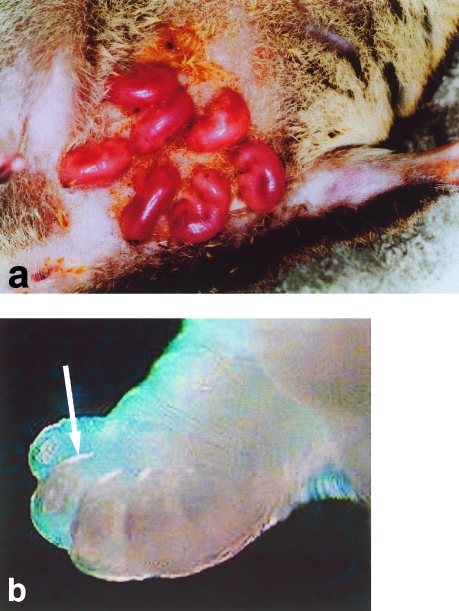
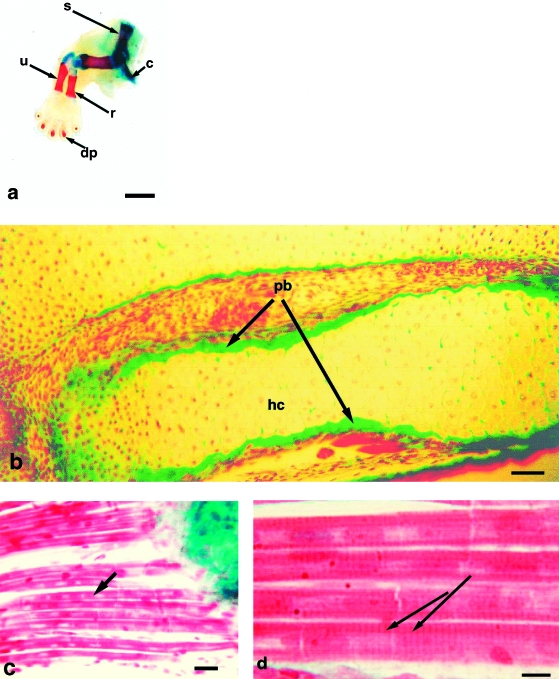
On the day of birth, each neonatal opossum pup is able to move from the birth canal to a nipple where it attaches (Fig. 1a). Claws, illustrated at 1dpp (Fig. 1b), are already present. At 0dpp, forelimb long bones and distal phalanges are represented by cartilage models, yet whole mount preparations show the presence of bone (Fig. 2a). Light microscopy shows this to be a periosteal collar of bone formed by intramembranous ossification around the cartilage model (Fig. 2b). Forelimb carpals, metacarpals and proximal and middle phalanges are in the form of cartilage models. All muscle groups are evident in the forelimb at 0dpp; numerous multinucleated myotubes with elongated nuclei are observed in longitudinal sections (Fig. 2c). Primary myotubes, distinguishable by their greater size, lie adjacent to immature myoblasts or secondary myotubes; myofilaments show striations (Fig. 2d).
Fig. 1.
(a) Neonatal opossums on the day of birth attached to the mammae. (b) Detail of opossum forepaw on 1dpp showing the presence of claws (arrow).
Fig. 2.
(a) Whole mount preparation of opossum forelimb on 0dpp. Note bone is stained red with alizarin and cartilage blue with Alcian blue. dp = distal phalanx, u = ulna, r = radius, s = scapula, c = clavicle. Humerus is seen articulating with scapula and radius and ulna. Scale bar = 1 mm. (b) Masson's trichrome histology of radius and ulna on 0dpp. hc = hypertrophied cartilage, pb = periosteal bone. Scale bar = 100 µm. (c) Forelimb muscle on 0dpp. Note myotubes with centrally placed nuclei (arrow). Scale bar = 5 µm. (d) At higher magnification myotubes show striations (arrows). Scale bar = 1.25 µm.
By contrast, the hindlimb at 0dpp is in the form of a mesenchymal core with prechondrogenic mesodermal condensations, showing characteristic closely packed cells in which the nuclei are almost in contact. No distinctive muscle groups are present.
Day 2 post partum
By 2dpp, the forelimb longbones and distal phalanges show an increase in the amount of calcified cartilage in the central hypertrophic region. The metacarpal cartilage models begin to show central hypertrophy, while the carpals, proximal and middle phalanges remain as cartilage models. Forelimb muscle at this stage remains myotubular and striations are evident.
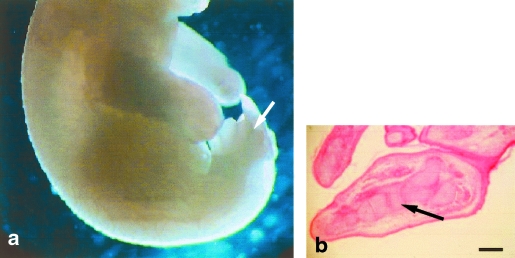
The hindlimb footplate now shows indentations between the developing toes (Fig. 3a). Mesodermal condensations that will become the future bones are clearly visible in the centre of the hindlimb (Fig. 3b). Mononucleate myoblast aggregates are present in the dorsal, ventral, proximal and distal regions of the limb bud and they are orientated parallel to the long axis of the hindlimb.
Fig. 3.
(a) Opossum at 2dpp; hindlimb shows indentations between digits (arrow). (b) Hindlimb at 2dpp shows prechondrogenic mesodermal condensations (arrow). Scale bar = 350 µm.
Day 6 post partum
In the forelimb, cartilage of the distal phalanges has continued to calcify, while cartilage models of the proximal phalanges and three middle metacarpals have begun to show central hypertrophy. The carpals remain as cartilage models, as seen at 9dpp (Fig. 4a). By 6dpp the hindlimb has reached a developmental stage similar to the forelimb at 0dpp: hypertrophic cartilage is evident in the centre of the femur and a collar of periosteal bone is present (Fig. 4b).
Fig. 4.
(a) Whole mount preparation of opossum forelimb at 9dpp; the carpus remains cartilaginous. Scale bar = 1 mm. (b) Whole mount preparation of hindlimb at 6dpp. Alizarin staining is seen in femur (large arrow). Note cartilage model of epipubic bone, a feature of the marsupial skeleton (small arrow). Scale bar = 0.25 mm. (c) Masson's trichrome histology shows endochondral ossification in centre of shaft of humerus; note endochondral bone (arrows). Scale bar = 100 µm.
Histological analysis of the 6dpp forelimb shows that vascular connective tissue sprouts derived from the periosteum, periosteal buds, have pierced the bone collar and entered the centre of the cartilage model of the humerus, radius and ulna (Fig. 4c). The first spicules of endochondral bone are now evident in the centre of these long bone shafts and the cavities between spicules are filled with fat cells, developing blood cells and osteogenic cells. Increasing myofibril density in each forelimb muscle fibre has begun to displace some of the previously centrally located nuclei to the periphery of the myotube. Individual hindlimb muscles have now become anatomically identifiable, though in the form of very loose bundles; the muscle fibres at this stage are myotubular with evident striations.
Day 16.5 post partum
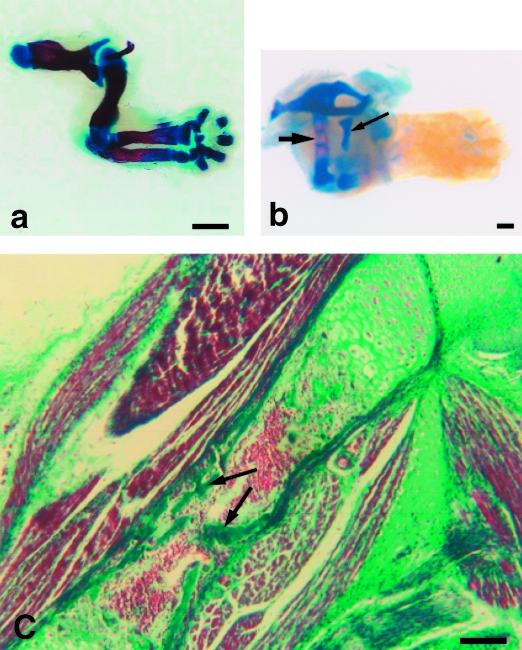
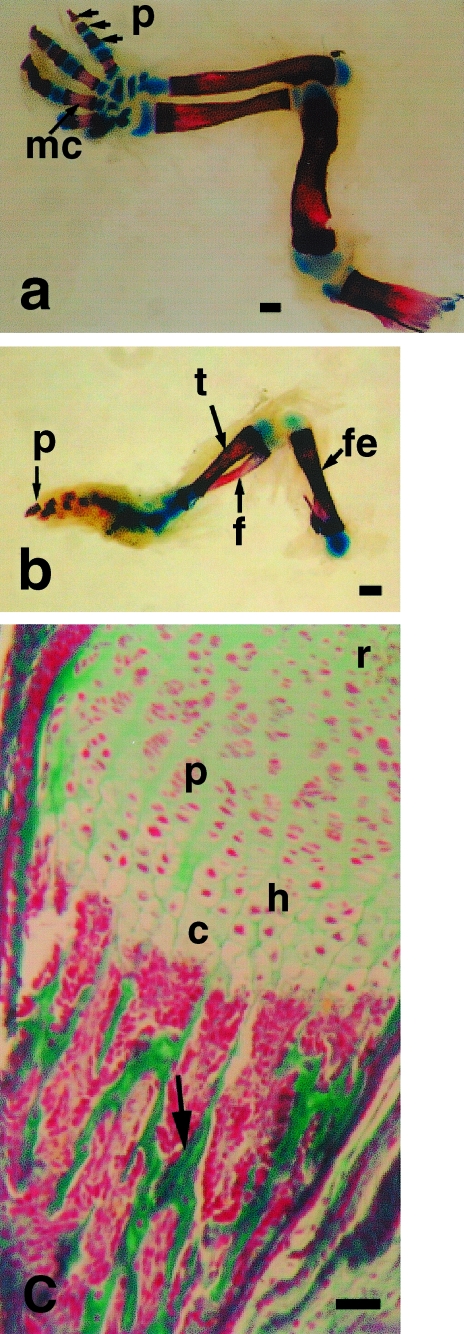
The forelimb distal and proximal phalanges and middle three metacarpals are continuing to ossify while the centres of the first and fifth metacarpals have begun to hypertrophy; carpal bones remain in the form of cartilage models (Fig. 5a). Periosteal buds have now invaded hindlimb long bones and bone spicules are evident centrally; distal phalanges have begun to ossify though the tarsals, metatarsals and proximal phalanges remain as cartilage models (Fig. 5b). Epiphyseal plates are now evident in forelimb longbones, with characteristic zones indicative of active growth of endochondral bone (Fig. 5c). Forelimb muscle at 16.5dpp consists of closely opposed fibres interlaced by dense capillary networks and nerve fibres. Individual muscle fibres are striated and have peripherally located nuclei. Hindlimb muscle fibres have become more densely packed together, display peripherally located nuclei and are striated.
Fig. 5.
(a) Ossification in forelimb seen by whole mount technique at 16.5dpp. Note ossification in phalanges (p) and metacarpals (mc). Carpus remains cartilaginous. Scale bar = 500 µm. (b) Opossum hindlimb at 16.5dpp. Note ossification in femur (fe), tibia (t), fibula (f) and phalanges (p). Scale bar = 500 µm. (c) Growth plate from forelimb long bone at 16.5dpp, stained by Masson's trichrome technique. r = resting zone of cartilage, p = zone of proliferation, h = zone of hypertrophy, c = zone of calcification of cartilage matrix. Note endochondral bone (arrow) in zone of ossification. Scale bar = 200 µm.
Day 56 post partum
By 8 weeks post partum long bones in both the fore- and hindlimbs consist of an epiphyseal plate at each end of the bone with lamellar bone at the periphery of the diaphysis. Secondary ossification centres are evident in the epiphyses. The phalanges in both the fore- and hindlimb are also composed of lamellar bone, as are the metatarsals and metacarpals. The tarsals and carpals continue to lay down endochondral bone. Muscle in both the fore- and the hindlimbs is arranged in long multinucleate units parallel with their neighbours. Nuclei lie at the periphery of the muscle fibre, just deep to the sarcolemma, as most of the sarcoplasm is densely occupied by contractile elements, producing a striated appearance.
Discussion
Patterns of growth of the musculoskeletal system in the forelimb and hindlimb of the opossum, Monodelphis domestica, are well correlated with the functional demands of the limbs during postnatal development. At birth the opossum has well developed forelimbs capable of digito-palmar prehension for grasping the mother's fur in order to move to the teat immediately after birth (Hughes & Hall, 1988), whilst the smaller, immobile hindlimbs are not required at this stage (Pflieger et al. 1996).
The pattern of the appearance of ossification centres in this study appears to be consistent with those observed in different marsupial species in previous studies (Nesslinger, 1956; Gemmell et al. 1988), though the time of appearance is slightly earlier. Gemmell and co-workers, using a similar whole mount technique with alizarin red and Alcian blue counter-staining, failed to find any ossification centres in either the bandicoot, Isoodon macrourus nor the possum Trichosurus vulpecula on the day of birth. Both species showed similar stages of ossification until about 10dpp, after which the development of the bandicoot was significantly more rapid than that of the possum, in line with its overall faster rate of development (Gemmell et al. 1988). In Monodelphis domestica, as in Didelphis virginiana, early ossification centres are present in the forelimb long bones and distal phalanges at birth. Howzever, by 6dpp the proximal phalanges and the middle three metacarpals have begun to ossify in Monodelphis whereas they do not appear until the second week in Didelphis. Ossification of the first and fifth metacarpals in Monodelphis lags behind those of the second to fourth digits, as in both Didelphis (Nesslinger, 1956) and the mouse (Patton & Kaufman, 1995). The appearance of ossification centres in the carpals of Monodelphis was not observed in this study until 56dpp. Furthermore, the appearance of ossification centres in the opossum hindlimb was found to be delayed by about a week compared to the forelimb. Barthélemy & Cabana (2001) reported that all hindlimb bones are still cartilaginous in Monodelphis at 4 weeks, when quadrupedal locomotion begins. However, these authors, who first observed ossification centres in the femur at 7 weeks, used Masson's trichrome histology but not the more sensitive whole mount technique utilized here which displays the periosteal collar of bone. In the mouse, ossification of the forelimb is initially in advance of the hindlimb prenatally, but by the time of birth the hindlimb and forelimb are at a similar stage (Patton & Kaufman, 1995).
This earlier stage of the appearance of ossification centres in Monodelphis could result from the fact that the Didelphis virginiana studied by Nesslinger (1956) and the Isodoon macrourus and Trichosurus vulpecula studied by Gemmell et al. (1988) are marsupials that possess pouches, whereas Monodelphis domestica is a pouchless marsupial. The earlier bone begins to develop the earlier the muscles are given a lever against which they can act; as a result the opossum is able to grasp the mother's fur and remain in close physical contact with her. For marsupials that do not possess a pouch this may represent an important survival technique, without which the young could become separated from the mother.
Bridge & Allbrook (1970) studied the growth of striated muscle in an Australian marsupial, the quokka (Setonix brachyurus), by comparing the development of one representative muscle from the forelimb and one from the hindlimb from 4 days before birth to adulthood. Like Monodelphis, the quokka is born with well-developed forelimbs and hindlimbs which are functionless buds, but by adulthood the limb proportions are reversed as the hindlimbs are then more well developed for locomotion. Bridge and Allbrook described three stages of muscle growth in the quokka: first, original myotubes grow rapidly in size and functional capacity; secondly, a new population of small fibres appears while growth of older fibres is temporarily suspended and, lastly, no new fibres form but all fibres grow together to reach the adult diameter.
The first stage of muscle growth involves the rapid development of a small number of fibres which is just sufficient to enable the newborn to climb to the mother's pouch. In this study, the opossum forelimb muscles were similarly found to be myotubular and arranged in loose anatomically identifiable groups on the day of birth, at which time only mesodermal condensations were evident in the hindlimb. When compared with the postnatal muscle development of the mouse (Capers, 1960; Cooper & Konigsberg, 1961; Platzer, 1978), Monodelphis domestica can be seen to develop at a slightly later stage, particularly in the hindlimb.
In a study of myogenesis in the mouse hindlimb, Platzer described four stages of development: immature myotube, mature myotube, young myofibre and mature myofibre. She reported that at 14–15 days gestation (E14–E15) the mouse embryo shows no movement, muscles are not anatomically distinguishable and the limb bud contains only isolated regions of immature myotubes. However, at E16 the embryo begins to move, muscles are identifiable and attached to bones and a few myotubes are mature. Towards the end of gestation the mouse embryo is active and young myofibres are numerous; by 2 weeks after birth the muscle fibre is mature and the animal shows good muscle co-ordination (Platzer, 1978). Therefore, it appears that forelimb skeletal muscle of Monodelphis domestica at birth is at a stage of development comparable to the later prenatal stages in the mouse, while the opossum hindlimb may pass through the same stages of development postnatally that are undergone prenatally in the mouse.
Wirsen & Larsson (1964) and Stickland (1982) also describe a similar sequence of events prenatally in the mouse as those which are seen postnatally in the opossum hindlimb. However, by the end of the second postnatal week, as in the mouse the forelimb muscles in Monodelphis domestica contain adult myofibres (with densely packed fibrils and peripherally located nuclei) with numerous nerve fibres and capillary networks between them. The hindlimb at this stage also consists of adult myofibres with mature characteristics; this finding confirms that of Barthélemy & Cabana (2001) who reported striations present in opossum hindlimb myofibres at 10dpp.
In a series of publications, Cabana and her group have attempted to correlate muscle functional development in Monodelphis with development of the nervous system. Though forelimbs are well developed in the newborn opossum, full flexion of the forearms at the elbow is not possible (Pflieger et al. 1996). The hindlimbs develop relatively rapidly late in the second week and begin to be active during the second to third week, when the ankle can be flexed. Hindlimb movements are alternate and rhythmic like the forelimb at birth, but have less strength and amplitude and are not co-ordinated with the forelimbs. Beginning in the fourth week the young can detach and walk, hindlimbs can at least partly support body weight and knee joints can be flexed. Linear locomotion is possible but co-ordination is poor. However, by the fifth and sixth weeks the hindlimbs are positioned less laterally and their activity begins to be co-ordinated with that of the forelimbs. By the time animals are weaned at 7 weeks, spontaneous locomotor movements like those of the adult occur. Synaptogenesis can be correlated with the appearance of motor behaviours, myelinogenesis with their maturation. As expected, synaptogenesis in the lumbosacral enlargement (serving the lower limb) lags behind that in the brachial enlargement (serving the upper limb) by a few days (Gingras & Cabana, 1999). Similarly, myelination of the dorsal and ventral roots occurs later at the lumbosacral level than at the cervical level (Leblond & Cabana, 1997).
Barthélemy & Cabana (2001) investigated the presence of motor axons in the newborn opossum hindlimb by looking at expression of acetylcholine, the motoneurone transmitter substance. Specifically, they examined expression of vesicular acetylcholine transporter (VAChT), which transports newly formed acetylcholine into vesicles where it is stored until release. The opossum hindlimb develops along a proximodistal gradient over several weeks and VAChT expression follows this proximodistal gradient of muscle development: motor axons grow into presumptive muscles as soon as the latter start to form (Barthélemy & Cabana, 2001).
It is not until 6dpp that the opossum hindlimb has reached a similar stage of development as the forelimb at day 0, thus indicating the differing rates of development of the fore- and hindlimbs in Monodelphis domestica. A comparison has been made of limb bone scaling during development in Monodelphis and Didelphis virginiana since although these two species are born at a similar size, Didelphis grows to be about 50 times larger. Although Didelphis grows at a higher rate of growth and for a longer period of time, few differences are seen in the scaling over time of length to width in limb bones. The hindlimbs in both species have higher rates of growth compared with the forelimbs, beginning at the time of birth, reflecting the eventual use of relatively larger hindlimbs in locomotion (Maunz & German, 1997). Differences between fore and hindlimbs in bone lengths at day 40 are more significant in Didelphis than in Monodelphis. The authors suggest that this may be attributed to the greater percentage of total growth achieved by Monodelphis at this time, about a week before they leave the mother, in order to meet the demands of locomotion; Didelphis does not leave the pouch until 100 days of age. Human fetuses also show different growth rates: during the first 8–16 weeks of development forelimb growth is accelerated relative to that of the hindlimb, but from about 18 weeks to birth, the hindlimbs are growing at higher rates (Bagnall et al. 1982).
In conclusion, on the day of birth and throughout the postnatal period studied the stages and rates of development of the opossum fore- and hindlimbs are different. It seems likely that these differences are due to the differing functional demands placed on the limbs at birth, as in Monodelphis domestica the forelimbs are used in locomotion to move from the birth canal to the mother's anterior abdominal wall on the day of birth. In contrast, the hindlimbs are required to display no sign of movement until the second week of postnatal development when they begin to support body weight (Pflieger et al. 1996).
Further work is necessary; a prenatal study would allow the exact timing of forelimb development to be mapped. Moreover, since the opossum fore- and hindlimbs develop at different times this study could form the baseline for an investigation into the molecular control of limb bud outgrowth. It is extremely difficult to culture mammalian fetuses because of the problem of substituting for the allantoic placenta in vitro; however, some success has been achieved with rodents where the yolk sac placenta is functional during organogenesis. Marsupial embryos rely almost entirely on the yolk sac and so it has been possible to develop a method for culturing whole Virginia opossum embryos. This has allowed the formation of digits on the forefoot and development of the hindlimb bud to the paddle stage to be achieved in vitro (New & Mizell, 1972). We propose that Monodelphis domestica provides an excellent mammalian experimental model for limb development since hindlimb development largely occurs postnatally and by applying a whole embryo culture technique, forelimb development could be observed and accessible to experimental manipulations in vitro.
Acknowledgments
We thank Mr Andrew Lockhart, Mr David Russell and Mr Gordon Reford for technical expertise and assistance. We also thank Dr Qingchun Xie for permission to use the photograph of newborn opossum pups. The colony of Monodelphis domestica at Glasgow is supported by the Biotechnology and Biological Sciences Research Council (17/S13021).
References
- Bagnall KM, Harris PF, Jones PRM. A radiographic study of the longitudinal growth of primary ossification centres in limb long bones of the human fetus. Anat. Rec. 1982;203:293–299. doi: 10.1002/ar.1092030211. [DOI] [PubMed] [Google Scholar]
- Barthélemy D, Cabana T. The development of vesicular acetycholine transporter immunoreactivity in the hindlimbs of the opossum Monodelphis domestica. Dev. Brain. Res. 2001;128:191–195. doi: 10.1016/s0165-3806(01)00176-6. [DOI] [PubMed] [Google Scholar]
- Bridge DT, Allbrook D. Growth of striated muscle in an Australian marsupial (Setonix brachyurus) J. Anat. 1970;106:285–295. [PMC free article] [PubMed] [Google Scholar]
- Capers CR. Multinucleation of skeletal muscle in vitro. J. Cell. Biol. 1960;7:559–567. doi: 10.1083/jcb.7.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper WG, Konigsberg IR. Dynamics of myogenesis in vitro. Anat. Rec. 1961;140:195–206. doi: 10.1002/ar.1091400305. [DOI] [PubMed] [Google Scholar]
- Dingerkus G, Uhler LD. Enzyme clearing of alcian blue stained whole small vertebrates for demonstration of cartilage. Stain Technol. 1977;52:229–231. doi: 10.3109/10520297709116780. [DOI] [PubMed] [Google Scholar]
- Gemmell RT, Johnson G, Bryden MM. Osteogenesis in two marsupial species, the bandicoot Isoodon macrourus and the possum Trichosurus vulpecula. J. Anat. 1988;159:155–164. [PMC free article] [PubMed] [Google Scholar]
- Gilmore DP. Sexual dimorphism in the central nervous system of marsupials. Int. Rev. Cytol. 2002;214:193–224. doi: 10.1016/s0074-7696(02)14006-x. [DOI] [PubMed] [Google Scholar]
- Gingras J, Cabana T. Synaptogenesis in the brachial and lumbosacral enlargements of the spinal cord in the postnatal opossum, Monodelphis domestica. J. Comp. Neurol. 1999;414:551–560. [PubMed] [Google Scholar]
- Hughes RL, Hall LS. Structural adaptations of the newborn marsupial. In: Tyndale-Biscoe CH, Janssens PA, editors. The Developing Marsupial. Berlin: Springer-Verlag; 1988. pp. 8–27. [Google Scholar]
- Kaufman MH. Atlas of Mouse Development. 2. New York: Academic Press; 1994. [Google Scholar]
- Leblond H, Cabana T. Myelination of the ventral and dorsal roots of the C8 and L4 segments of the spinal cord at different stages of development in the gray opossum, Monodelphis domestica. J. Comp. Neurol. 1997;386:203–216. [PubMed] [Google Scholar]
- Masson P. Some histological methods. Trichrome stainings and their preliminary technique. Bull Int. Assoc. Med. 1929;12:75. [Google Scholar]
- Mate KE, Robinson ES, Vandeberg JL, Pedersen RA. Timetable of in vivo embryonic development in the grey short-tailed opossum (Monodelphis domestica) Mol.Reprod Dev. 1994;39:365–374. doi: 10.1002/mrd.1080390404. [DOI] [PubMed] [Google Scholar]
- Maunz M, German RZ. Ontogeny and limb bone scaling in two new world marsupials, Monodelphis domestica and Didelphis virginiana. J. Morph. 1997;231:117–130. doi: 10.1002/(SICI)1097-4687(199702)231:2<117::AID-JMOR1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Nesslinger CL. Ossification centers and skeletal development in the postnatal Virginia opossum. J. Mammal. 1956;37:382–394. [Google Scholar]
- New DAT, Mizell M. Opossum fetuses grown in culture. Science. 1972;175:533–536. doi: 10.1126/science.175.4021.533. [DOI] [PubMed] [Google Scholar]
- Oster GF, Shubin W, Murray JD, Alberden C. Evolution and morphogenetic rules: the shape of the vertebrate limb in ontogeny and phylogeny. Evolution. 1988;42:862–884. doi: 10.1111/j.1558-5646.1988.tb02508.x. [DOI] [PubMed] [Google Scholar]
- Patton JT, Kaufman MH. The timing of ossification of the limb bones, and growth-rates of various longbones of the fore and hind limbs of the prenatal and early postnatal laboratory mouse. J. Anat. 1995;186:175–185. [PMC free article] [PubMed] [Google Scholar]
- Pflieger J-F, Cassidy G, Cabana T. Development of spontaneous locomotor behaviors in the opossum, Monodelphis domestica. Behav. Brain Res. 1996;80:137–143. doi: 10.1016/0166-4328(96)00028-9. [DOI] [PubMed] [Google Scholar]
- Platzer AC. The ultrastructure of normal myogenesis in the limb bud of the mouse. Anat. Rec. 1978;190:639–658. doi: 10.1002/ar.1091900303. [DOI] [PubMed] [Google Scholar]
- Russell EM. Patterns of parental care and parental investment in marsupials. Biol. Rev. 1982;57:423–486. doi: 10.1111/j.1469-185x.1982.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Sharman GB. Reproductive physiology of marsupials. Science. 1970;167:1221–1228. doi: 10.1126/science.167.3922.1221. [DOI] [PubMed] [Google Scholar]
- Smith KK. Early development of the neural plate, neural crest and facial region of marsupials. J. Anat. 2001;199:121–131. doi: 10.1046/j.1469-7580.2001.19910121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spark C, Dawson AB. The order and time of appearance of centers of ossification in the fore and hind limbs of the albino rat, with special reference to the possible influence of the sex factor. Am. J. Anat. 1928;41:411–445. [Google Scholar]
- Stickland NC. Scanning electron microscopy of prenatal muscle development in the mouse. Anat. Embryol. 1982;164:379–385. doi: 10.1007/BF00315759. [DOI] [PubMed] [Google Scholar]
- VandeBerg JL. The grey short-tailed opossum (Monodelphis domestica) as a model Didelphid species for genetic research. In: Marshall Graves JA, Hope RM, Cooper DW, editors. Mammals from Pouches and Eggs. Australia: CSIRO.; 1990. pp. 93–106. [Google Scholar]
- VandeBerg JL, Robinson ES. The laboratory opossum (Monodelphis domestica) in biomedical research. In: Saunders NR, Hinds LA, editors. Marsupial Biology Recent Perspectives’. Sydney: University of New. South Wales Press.; 1998. pp. 238–253. [Google Scholar]
- Wirsen C, Larsson KS. Histochemical differentiation of skeletal muscle in foetal and newborn mice. J. Embryol Exp. Morph. 1964;12:759–767. [PubMed] [Google Scholar]
- Xie Q, Mackay S, Ullmann SL, Gilmore DP, Payne AP. Testis development in the opossum Monodelphis domestica. J. Anat. 1996;189:393–406. [PMC free article] [PubMed] [Google Scholar]