Abstract
The vertebrate limb is one of the most relevant experimental models for analysing cell–cell signalling during patterning of embryonic fields and organogenesis. Recently, the combination of molecular and genetic studies with experimental manipulation of developing limb buds has significantly advanced our understanding of the complex molecular interactions co-ordinating limb bud outgrowth and patterning. Some of these studies have shown that there is a need to revise some of the textbook views of vertebrate limb development. In this review, we discuss how signalling by the polarizing region is established and how limb bud morphogenesis is controlled by both long-range and signal relay mechanisms. We also discuss recent results showing that differential mesenchymal responsiveness to SHH signalling is established prior to its expression by the polarizing region.
Keywords: apical ectodermal ridge, ALX4, BMP, dHAND, GLI3, Gremlin, limb bud, SHH, ZPA
Introduction: a history of the limb bud mesenchymal organizer called the polarizing region or ZPA
About half a century ago the seminal experiments by Saunders (1948) led to the discovery of two major signalling centres controlling vertebrate limb pattern formation. The first is a group of cells located in the posterior limb bud mesenchyme, the so-called polarizing region or zone of polarizing activity (ZPA, reviewed by Johnson & Tabin, 1997). The second signalling centre is a ridge of cells running along the distal margin of the limb bud ectoderm called the apical ectodermal ridge. When this is removed during the early development of a chicken limb bud, only proximal limb skeletal elements develop; removal at a later stage of development, on the other hand, ablates the more distal skeletal elements.
These studies showed that the apical ectodermal ridge is necessary to maintain limb bud morphogenesis, while apical ridge removal causes elimination of the underlying mesenchyme by apoptotic cell death (see review by Duboule, 2002). The polarizing region is a classical organizer as defined by Spemann & Mangold (2001; reprinted), which instructs limb bud mesenchymal cells with respect to their proliferation potential and fate. This was established by transplantation of polarizing region cells from the posterior mesenchyme to ectopic anterior locations, which induces complete mirror image duplications of the distal-most limb skeletal elements (digits, Saunders & Gasseling, 1968). In addition, it was shown that several other embryonic organizing centres such as the notochord and floorplate also possess polarizing activity upon grafting to chicken limb buds. In trying to develop a molecular model of polarizing region signalling, Wolpert (1969) proposed that these cells produce a small diffusible molecule, termed morphogen. This morphogen would form a diffusion gradient across the limb bud, and the polarity and fate of digit precursor cells, for example, would be determined by their response to specific thresholds (Fig. 1A, also known as the ‘French flag’ model, see Wolpert, 1969). Wolpert's model provided a straightforward explanation for most, if not all, experimental manipulations of the polarizing region in chicken limb buds and the theoretical basis to search for this mysterious morphogen signal.
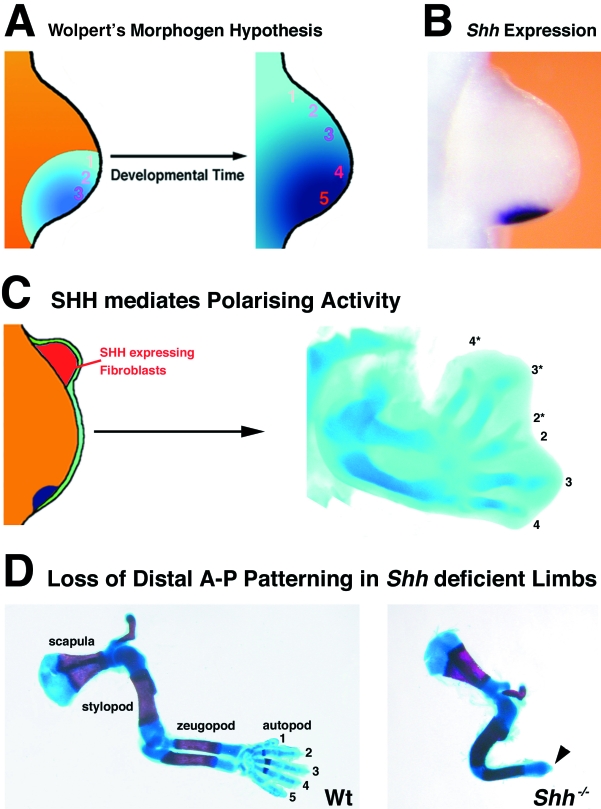
Fig. 1.
The polarizing region and SHH signalling. (A) Wolpert morphogen hypothesis (or French flag model): the posterior mesenchymal cells of the polarizing region produce a small diffusible molecule, which over time forms a gradient and patterns the mesenchyme according to threshold values. The hypothetical thresholds that pattern digits are indicated by numbers 1–5 according to the five digits of mouse and human limbs (1, most anterior digit – thumb; 5, most posterior digit – little finger). (B) Shh expression as revealed by whole mount in situ hybridization (purple colour) in a mouse forelimb bud of embryonic day 10.5. (C) Anterior grafts of fibroblasts expressing SHH (red in scheme) into an early chicken wing bud induces mirror-image duplications of the digit pattern. 2–4 indicate normal digits (2, most anterior; 4, most posterior), 2*−4* indicate duplicated digits. Note reversal of antero-posterior polarity in induced anterior ectopic digits. (D) Loss of distal limb structures and the digit arch in Shh−/– limb buds. Wt, Wild-type forelimb skeleton at embryonic day 15; stylopod, humerus; zeugopod, ulna and radius; autopod, metacarpals and digits. Shh−/–, Shh-deficient mouse forelimb. Note: only one fused zeugopodal bone forms and the digit arch is completely absent, with the exception of a terminal phalange (arrowhead). In contrast, one complete digit forms in hind limbs (Chiang et al. 2001; Kraus et al. 2001). All limb buds are orientated with anterior to the top and posterior to the bottom.
Tickle et al. (1982) achieved a new breakthrough by establishing that implantation of an inert bead soaked in retinoic acid into a chicken limb bud mimics transplants of polarizing region cells perfectly. Retinoic acid is indeed present in the limb bud mesenchyme (Thaller & Eichele, 1987), but its specific production by polarizing region cells could not been shown (see also below). The continued search for Wolpert's morphogen led Tabin and colleagues (Riddle et al. 1993) to identify Sonic hedgehog (SHH), a homologue of the Drosophila hedgehog gene, as the signal expressed by the polarizing region in vertebrate limb buds (Fig. 1B). Furthermore, SHH expression in fibroblasts is sufficient to endow them with polarizing activity (as assessed by the induction of mirror image digit duplications; Fig. 1C). Inactivation of the Shh gene in the mouse results in a pleiotropic phenotype (for details see Chiang et al. 1996) including complete disruption of distal limb development and digit arch formation (Fig. 1D; Chiang et al. 2001; Kraus et al. 2001). Detailed genetic analysis provides evidence for long-range SHH signalling and threshold effects (see below), all in agreement with its proposed function as a morphogen. However, recent studies also showed that the SHH signal is relayed by activation of secondary signals in responding mesenchymal cells.
Furthermore, many regulator genes defined as SHH targets by experimental manipulation of chicken limb buds are in fact activated prior to and/or independent of SHH signalling (see below). In agreement, te Welscher et al. (2002a) provided evidence that the limb bud mesenchyme is not nascent at the time when it receives the SHH signal, but prepatterned by an upstream acting mechanism. These results show that SHH functions to maintain and modulate gene expression rather than acting as an inducer. Therefore, these recent insights are discussed in more detail in the following sections.
Current views on how the polarizing region is established
During the formation and patterning of the primary body axis, the positions of fore- and hindlimb fields are determined and polarized by molecular mechanisms that are still largely unknown. Hornbruch & Wolpert (1991), and more recently Tanaka et al. (2000), established that the potential to form limbs is initially spread throughout the flank. Prior to initiation of limb bud outgrowth, flank tissue possesses weak polarizing activity and the ability to activate Shh expression. During the initiation of limb bud outgrowth, polarizing activity is restricted to the posterior mesenchyme and up-regulated concurrent with activation of SHH signalling.
Molecular analysis of limbless-ness in snake embryos (Cohn & Tickle, 1999) and ectopic expression of Hox genes in mouse limb buds (Charité et al. 1994; Knezevic et al. 1997) indicates that the nested expression of Hox genes along the primary body axis may both position and polarize the limb field along its antero-posterior axis. In particular, anterior ectopic expression of the Hoxb8 gene in forelimb buds results in establishment of an ectopic anterior SHH signalling centre and mirror image duplications of digits similar to polarizing region grafts (Charité et al. 1994).
However, loss-of-function analysis of this and many other Hox genes alone or in combination has to date not revealed any essential functions for the so-called Hox code in positioning and/or polarization of the limb field and polarizing region (see, e.g. van den Akker et al. 2001). In contrast, retinoic acid seems essential during these early stages as inactivation of RALDH2, an enzyme necessary for retinoic acid synthesis, completely disrupts limb bud formation and activation of SHH signalling (Niederreither et al. 1999).
Forelimb bud development of Raldh2-deficient mouse embryos can be rescued by administration of retinoic acid during limb field stages and early limb bud outgrowth (Niederreither et al. 2002). These studies establish that retinoic acid is critically required to induce SHH signalling during initiation of forelimb bud outgrowth. Niederreither et al. (2002) propose that retinoic acid and the bHLH transcription factor dHAND together activate Shh expression in the posterior mesenchyme (Fig. 2). This proposal is based on the fact that inactivation of the dHAND gene in mice and zebrafish disrupts SHH activation and severely impairs paired appendage (limb/fin) bud development (Charite et al. 2000; Yelon et al. 2000). Ectopic expression of dHAND in limb buds in turn induces ectopic anterior Shh expression and digit polydactyly (Fernandez-Teran et al. 2000; McFadden et al. 2002). Interestingly, dHAND is initially expressed throughout the flank mesenchyme (reviewed by Cohn, 2000) as may be expected from the widespread competence to induce polarizing activity (Tanaka et al. 2000; see above).
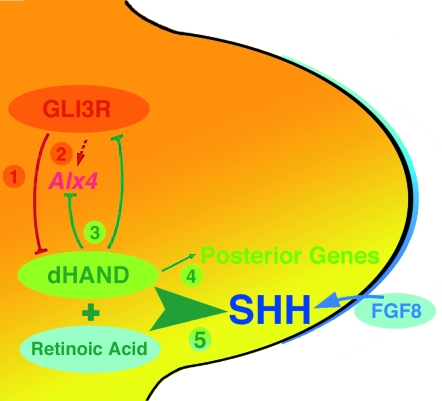
Fig. 2.
Mutual antagonistic interaction of Gli3R and dHAND prepatterns the mesenchyme and controls establishment of the polarizing region. (1) GLI3R restricts dHAND expression to the posterior mesenchyme during initiation of limb bud outgrowth. (2) GLI3R acts upstream of other anterior genes such as ALX4 and is necessary for temporal up-regulation of Alx4 expression. (3) dHAND in turn keeps Gli3 and Alx4 expression anteriorly restricted. (4) The genetic interaction of Gli3 with dHAND controls activation and spatial expression of posterior genes such as 5′Hoxd, Gremlin, apical ectodermal ridge Fgfs (Fgf4, –9, –17). (5) Last but not least, dHAND interacts with retinoic acid (and FGF8 expressed by the apical ectodermal ridge) to establish SHH signalling and thereby the polarizing region in the posterior limb bud mesenchyme (scheme modified from te Welscher et al. 2002a).
During initiation of limb bud formation, dHAND expression is rapidly restricted to the posterior limb bud mesenchyme, which may be crucial to restrict polarizing activity to the posterior mesenchyme. Interestingly, expression of the Gli3 transcriptional repressor (GLI3R, Wang et al. 2000) is activated in anterior mesenchyme concurrent with posterior restriction of dHAND (te Welscher et al. 2002a). These authors showed that posterior restriction of dHAND is disrupted in Gli3-deficient mouse limb buds and further genetic analysis implicated the GLI3 repressor in positioning of the Shh expression domain (Zuniga & Zeller, 1999; see also below). In Gli3-deficient mouse limb buds, dHAND expression is maintained in the anterior mesenchyme and expression of ‘posterior’ genes is anteriorly expanded much prior to detecting ectopic anterior SHH signalling. This early loss of posterior restriction in Gli3-deficient limb buds (and not late ectopic anterior SHH signalling as previously assumed; Masuya et al. 1995; Buescher et al. 1997) is the most likely cause of the digit polydactyly with associated loss of identity (Zuniga & Zeller, 1999).
In dHAND-deficient mouse limb buds, expression of Gli3 and another ‘anterior’ gene, Alx4, is conversely expanded posteriorly (te Welscher et al. 2002a). These studies establish that a mutual genetic antagonism between GLI3 and dHAND polarizes the nascent limb bud mesenchyme upstream of positioning and activating SHH signalling (Fig. 2).
This genetic interaction of GLI3 with dHAND uncovers essential components of the molecular mechanism, which positions the polarizing region at the posterior limb bud margin (Fig. 2). Previous work has also implicated FGF8 signalling from the apical ectodermal ridge in the activation of SHH signalling (Lewandoski et al. 2000). These results show that repression by anterior factors such as GLI3 in combination with retinoic acid and dHAND is crucial to polarize the limb bud and establish the polarizing region. In fact, the studies by te Welscher et al. (2002a) indicate that polarization of the early limb bud mesenchyme is triggered by GLI3-mediated anterior repression of dHAND expression (step 1, Fig. 2). Therefore, it will be important to identify the gene products regulating Gli3 expression in the anterior limb field mesenchyme.
Many ‘posterior’ genes originally identified as transcriptional targets of SHH signalling are expressed considerably prior to Shh and their distribution is regulated by GLI3 repressor activity (Zuniga & Zeller, 1999; te Welscher et al. 2002a). In contrast to Gli1 and Patched (Ptc), which are bona vide SHH targets, expression of mesenchymal SHH mediators such as, e.g. 5′Hoxd genes, Bmp2, Gremlin and Fgfs in the posterior apical ectodermal ridge (see below), is activated independent of and prior to SHH signalling (Zuniga et al. 1999; Sun et al. 2000; Chiang et al. 2001; Panman et al. unpublished results). In Gli3-deficient mouse limb buds, the expression of these genes is anteriorly expanded independent of ectopic SHH signalling (Zuniga & Zeller, 1999; te Welscher et al. 2002a,b). Therefore, it is likely that these genes are activated in response to retinoic acid and/or dHAND (see above) and excluded from anterior mesenchyme by GLI3-mediated repression. Taken together, these studies show that responsiveness to SHH signalling is established in the nascent mesenchyme by a prepatterning mechanism acting upstream of SHH signalling (Fig. 2). Rapid down-regulation of these genes in Shh-deficient limb buds (Zuniga et al. 1999; Sun et al. 2000; Chiang et al. 2001) shows that SHH signalling is essential to maintain and elaborate expression during progression of limb bud morphogenesis as discussed below.
Long- and short-range SHH signalling
Long-range
Analysis of SHH signalling in chicken limb buds provided clear evidence that SHH can act as a long-range morphogenetic signal and elicits a dose-dependent response as postulated by Wolpert's morphogen hypothesis (Yang et al. 1997). However, biochemical studies showed that the full-length SHH protein undergoes auto-proteolytic cleavage yielding a C-terminal peptide and the active N-terminal SHH peptide, which is covalently linked to cholesterol (Lee et al. 1994; Porter et al. 1995, 1996). Such cholesterol attachment results in retention of the active SHH peptide by plasma membranes, which poses an apparent paradox with respect to the proposed long-range signalling during limb bud development.
To shed light on this rather puzzling biochemical finding, Lewis et al. (2001) generated a mouse mutant able to express a modified SHH peptide that can no longer be cholesterol modified. Analysis of mouse embryos expressing this modified SHH peptide during limb bud development showed that formation of anterior digits is disrupted, while posterior digits are normal. Therefore, the cholesterol modification is essential for full-range activity of SHH signalling. Furthermore, analysis of target gene expression and protein distribution shows that the signalling range of the mutant, non-cholesterated SHH peptide, but not polarizing activity, is more restricted in comparison with the wild-type protein (Lewis et al. 2001).
Analysis of Drosophila Hedgehog (Hh) signalling showed that the Dispatched gene product is required to release cholesterol-bound Hh protein from cells to enable long-range signalling (Burke et al. 1999). In the absence of Dispatched, Hh protein accumulates in cells, suggesting that Dispatched is crucial for release and long-range signalling activity of Hh proteins (Burke et al. 1999). Therefore, it will be important to analyse genetically the potential requirement of vertebrate Dispatched homologues for long-range SHH signalling in mouse limb buds (Lewis et al. 2001).
Furthermore, Zeng et al. (2001) have shed light on a possible cellular mechanism that permits SHH to act in the long range. Experimental evidence reveals the existence of a multimeric, cholesterol-modified SHH peptide that is freely diffusible and seems to form a gradient across the limb bud. These authors propose that multiple cholesterol-modified SHH peptides accumulate in lipid rafts to form a soluble and freely diffusible complex. Alternatively, cholesterated SHH peptides may traffic across the limb bud mesenchyme through cytonemes, which are long actin-based cellular processes extended by mesenchymal cells in response to FGF signalling (Ramírez-Weber & Kornberg, 1999; see Fig. 3A). While it is clear that long-range SHH signalling is necessary during limb bud development, the underlying molecular and cellular mechanisms enabling such morphogenetic signalling remain in need of further investigation.
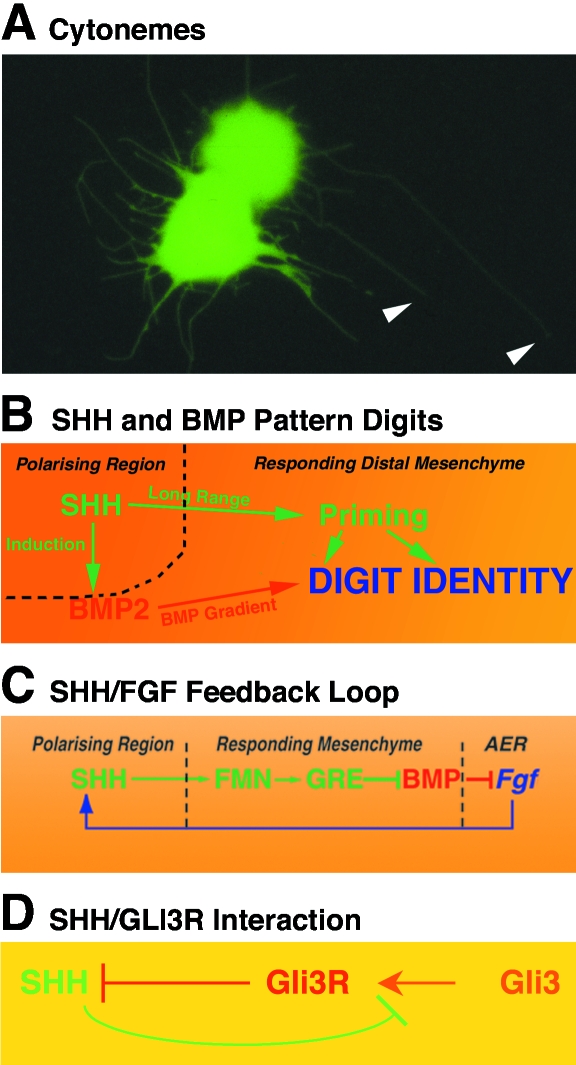
Fig. 3.
SHH signalling interactions in vertebrate limb buds. (A) Posterior mouse limb bud mesenchymal cells form long actin-based cellular processes, called cytonemes. GFP-expressing limb bud mesenchymal cells (green) were sandwiched with wild-type cells (non-fluorescent) and cultured for 1 h in the presence of 10 ng mL−1 FGF2 as described by Ramírez-Weber & Kornberg (1999). Arrowheads point to select cytonemes extending from two GPF-positive cells on a layer of non-fluorescent wild-type cells. (B) SHH and BMP interact to pattern digits. SHH signalling primes mesenchymal cells through long-range signalling and induces BMP2 in a fraction of posterior mesenchymal cells. Graded BMP signalling provides primed mesenchymal cells with positional identity (modified from Drossopoulou et al. 2000). (C) Establishment and maintenance of the SHH/FGF feedback loop. SHH up-regulates and maintains expression of the secreted BMP antagonist Gremlin in a Formin-dependent manner in posterior distal mesenchyme. Gremlin-mediated BMP antagonism enables expression of Fgfs such as Fgf4, Fgf9 and Fgf17 in the posterior apical ectodermal ridge. FGF signalling by the apical ridge in turn maintains and propagates SHH signalling by the polarizing region in the posterior–distal limb bud mesenchyme (modified from Zuniga et al. 1999). (D) Antagonistic interaction of SHH with GLI3. SHH signalling by the posterior polarizing region inhibits processing of the full-length GLI3 protein into an active repressor (GLI3R). Graded SHH signalling results in establishment of a GLI3R gradient with its highpoint in the anterior mesenchyme. GLI3R activity in turn participates in keeping Shh expression posteriorly restricted (modified from Wang et al. 2000).
Short-range
Several mechanisms limit the range of Shh signalling during limb bud development to prevent ectopic activation of Shh target genes. The most direct of these mechanisms involves transcriptional up-regulation of the SHH receptor PTC in response to SHH signalling (Goodrich et al. 1996). In the absence of SHH signalling, PTC inhibits the trans-membrane protein Smoothened (SMO) and thereby signal transduction and transcriptional activation of Shh target genes. One of these transcriptional targets is Ptc itself, which results in up-regulation of PTC activity in cells receiving the SHH signal. Genetic analysis in the mouse shows that SHH targets are ectopically activated in Ptc1-deficient embryos (Goodrich et al. 1997). Reduction of PTC1 levels in mouse limb buds also results in ectopic anterior Shh expression and digit polydactyly (Milenkovic et al. 1999). In addition, PTC1 is also able to sequester the cholesterol-free SHH peptide, as reduction of PTC1 levels in mouse limbs expressing only the non-cholesterated SHH peptide also expands the SHH signalling range anteriorly (Lewis et al. 2001).
In vertebrates, the SHH signalling range is further restrained by the Hedgehog-interacting protein, HIP (Chuang & McMahon, 1999). Hip expression is activated in limb bud mesenchymal cells in response to SHH signalling. The HIP protein generates an additional negative feedback loop by sequestering the ligand and thereby limiting the Hedgehog activity range (Chuang & McMahon, 1999).
Signal relay
A fraction of SHH responsive limb bud mesenchymal cells activate the secondary signal BMP2 (Fig. 3B, Drossopoulou et al. 2000). BMP2 is a vertebrate homologue of Dpp, which is activated in Drosophila wing discs in response to Hh signalling (Basler & Struhl, 1994). However, BMP2 is not able to mimic all morphogenetic functions of SHH during vertebrate limb development. Ectopic expression of BMP2 in chicken limb buds only duplicates anterior-most digits, in contrast to the complete digit duplications induced by ectopic SHH signalling (Duprez et al. 1996). Although BMP2 does not have the full morphogenetic potential of SHH, an important role for BMP2 in determining digit identities has been proposed.
Experimental manipulation of chicken limb buds led Drossopoulou et al. (2000) to propose that mesenchymal cells are primed by long-range SHH signalling and that induction of Bmp2 expression by SHH signalling results in graded BMP activity, which together with the priming effect of SHH specifies digit identity (Fig. 3B). Even during advanced limb development, when the digit primordia have long been determined, changes in interdigital BMP levels cause transformations of digit identities (Dahn & Fallon, 2000). This suggests that positional information is initially set by long-range SHH in combination with graded BMP signalling. However, identities of particular digits may only be specified much later under the influence of BMP signalling from the interdigital mesenchyme, just prior to the regression of this tissue by apoptosis.
SHH not only signals to the limb bud mesenchyme, but maintains and propagates its own expression through establishment of a positive feedback loop between polarizing region and apical ectodermal ridge (Fig. 3C; SHH/FGF feedback loop, reviewed by Capdevila & Izpisua Belmonte, 2001). Rather than acting long-range, the SHH signal is relayed to the apical ectodermal ridge through Formin-dependent activation of the BMP antagonist Gremlin in responding mesenchymal cells (Fig. 3C). Gremlin-mediated BMP antagonism enables expression of several Fgfs (Fgf4, –9, –17) in the posterior apical ectodermal ridge (Zuniga et al. 1999; L. Panman et al. unpublished). FGF signalling by the posterior apical ridge in turn maintains and propagates SHH signalling distally through the SHH/FGF4 feedback loop (Niswander et al. 1993; Laufer et al. 1994), thereby co-ordinating outgrowth with patterning.
The mouse limb deformity (ld) mutation disrupts the functioning of both Formin and Gremlin, which prevents establishment of the SHH/FGF feedback loop (Haramis et al. 1995; Zuniga et al. 1999). The limbs of ld mutant mice exhibit fusions of ulna and radius and digit syndactyly (reviewed by Zeller et al. 1999), indicating that antero-posterior patterning and to some extent outgrowth are affected.
Shh-deficient mouse limbs display a much more dramatic limb phenotype as only one zeugopod bone forms and the digit arch is absent (Chiang et al. 2001; Kraus et al. 2001; see also Fig. 1D). The phenotypic differences between ld and Shh mutant limbs indicate that SHH patterns the mesenchyme (Fig. 3B) largely independent of the SHH/FGF feedback loop (Fig. 3C).
GLI proteins and SHH signal transduction
In the Drosophila imaginal discs, nuclear response to Hh signalling is mediated by the Cubitus interruptus (Ci) transcription factor. In the absence of Hh signalling, the Ci protein is processed to generate a repressor of Hh target genes (Aza-Blanc et al. 1997). Hh signalling inhibits PKA-mediated Ci cleavage and the full-length Ci protein in turn acts as a transcriptional activator in the presence of Hh signalling (Methot & Basler, 1999). Several vertebrate Ci homologues, called GLI proteins, have been identified. In particular, Gli1 is a direct target of SHH signalling (Lee et al. 1997) and has been suggested to mediate response to SHH signalling (Ruiz i Altaba, 1998). However, the lack of a phenotype in Gli1-deficient mice indicates that GLI1 alone is not sufficient to mediate nuclear response to SHH signal reception (Park et al. 2000). The neural tube phenotype of Gli2-deficient mouse embryos suggests that GLI2 mediates aspects of SHH signalling during ventral neural tube patterning (Ding et al. 1998; Matize et al. 1998). However, neither Gli2-deficient nor Gli1; Gli2 double homozygous mouse embryos display striking limb phenotypes (Park et al. 2000), suggesting that another GLI protein regulates the expression of SHH target genes during limb bud morphogenesis. As discussed below, the GLI3 protein is a good candidate for regulating expression of genes in response to SHH signalling.
In the absence of SHH signalling, the GLI3 protein is constitutively processed to a repressor similar to Ci in Drosophila (Wang et al. 2000). SHH signalling inhibits GLI3 processing and experimental evidence indicates that SHH signalling in the limb bud generates an antero-posterior GLI3 protein repressor gradient with highest GLI3 repressor levels in the anterior mesenchyme (Fig. 3D; Wang et al. 2000). The balance between SHH-mediated activation and GLI3-mediated repression of target genes might provide limb bud mesenchymal cells with the necessary positional cues to enable antero-posterior patterning. Indeed, analysis of Shh−/–; Gli3−/– double mutant mouse embryos has revealed that antagonistic interactions between SHH and GLI3 control pattern formation. Despite the fact that the direct SHH transcriptional targets such as Gli1 are not activated in double mutant embryos (Litingtung & Chiang, 2000; te Welscher et al. 2002b), expression of many ‘posterior’ genes (e.g. 5′Hoxd, Hoxa13, Gremlin) and distal limb patterning are successively restored and anteriorly expanded by removing one or both Gli3 alleles in the context of a Shh-deficient limb bud (te Welscher et al. 2002b). These results show that SHH normally functions to overcome GLI3-mediated repression and down-regulation of posterior genes to enable distal progression of limb morphogenesis. As limbs of Gli3−/–, Shh−/– double mutant embryos are polydactylous and indistinguishable from Gli3 single mutant limbs, these results confirm that GLI3 acts initially upstream of SHH. Subsequent balancing of graded SHH (and BMP, Fig. 3B; Drossopoulou et al. 2000) signalling with GLI3 repressor activity (Fig. 3D) enables co-ordinated progression of limb bud outgrowth and patterning to determine numbers and identity of digits.
In summary, SHH signalling prevents GLI3R formation in the posterior–distal mesenchyme (Fig. 3D) and thereby enables maintenance and propagation of the expression of posterior–distal genes (5′Hoxd, Hoxa13, Gremlin, etc.). Their expression is induced and positively regulated by factors acting upstream of SHH such as dHAND, most likely in combination with cellular response to retinoic acid (Fig. 2). Further research will be needed to shed light on the molecular cascades by which SHH inhibits processing of GLI3 to its active repressor form (GLI3R) and on the interactions by which GLI3R keeps SHH posteriorly restricted.
Acknowledgments
We apologise to all colleagues whose work could not be cited due to space limitations and briefness of this review. We thank Pascal te Welscher for providing the SHH graft shown in Fig. 1(C) and our group members, Rosanna Dono and Aimée Zuniga, for helpful discussions and suggestions. The research of the Department of Developmental Biology is supported by NWO, KNAW and the Faculty of Biology, Utrecht University.
References
- van den Akker E, Fromental-Ramain C, de Graaff W, Le Mouellic H, Brulet P, Chambon P, et al. Axial skeletal patterning in mice lacking all paralogous group 8 Hox genes. Development. 2001;128:1911–1921. doi: 10.1242/dev.128.10.1911. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Aza-Blanc P, Ramirez-Weber F, Laget M, Schwartz C, Kornberg T. Proteolysis that is inhibited by hedgehog targets Cubitus interruptus protein to the nucleus and converts it to a repressor. Cell. 1997;89:1043–1053. doi: 10.1016/s0092-8674(00)80292-5. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Basler K, Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Nature. 1994;368:208–214. doi: 10.1038/368208a0. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Buescher D, Bosse B, Heymer J, Ruether U. Evidence for genetic control of Sonic hedgehog by Gli3 in mouse limb development. Mech. Dev. 1997;1997:175–182. doi: 10.1016/s0925-4773(97)00656-4. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Burke R, Nellen D, Bellotto M, Hafen E, Senti K-A, Dickson BJ, et al. Dispatched, a novel sterol sensing domain protein dedicated to the release of cholesterol-modified hedgehog from signaling cells. Cell. 1999;99:803–815. doi: 10.1016/s0092-8674(00)81677-3. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Izpisua Belmonte JC. Patterning mechanisms controlling vertebrate limb development. Annu. Rev. Cell Dev. Biol. 2001;17:87–132. doi: 10.1146/annurev.cellbio.17.1.87. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Charité J, de Graaff W, Shen S, Deschamps J. Ectopic expression of Hox-8 causes duplication of the ZPA in the forelimb and homeotic transformation of axial structures. Cell. 1994;78:589–601. doi: 10.1016/0092-8674(94)90524-x. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Charite J, McFadden DG, Olson EN. The bHLH transcription factor dHAND controls Sonic hedgehog expression and establishment of the zone of polarizing activity during limb development. Development. 2000;127:2461–2470. doi: 10.1242/dev.127.11.2461. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Harris MP, Simandl BK, Li Y, Beachy PA, et al. Manifestation of the limb prepattern: limb development in the absence sonic hedgehog function. Dev. Biol. 2001;236:421–435. doi: 10.1006/dbio.2001.0346. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Chuang P-T, McMahon AP. Vertebrate hedgehog sig-nalling modulated by induction of a hedgehog-binding protein. Nature. 1999;987:617–621. doi: 10.1038/17611. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Cohn MJ, Tickle C. Developmental basis of limblessness and axial patterning in snakes. Nature. 1999;399:474–479. doi: 10.1038/20944. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Cohn MJ. Giving limbs a hand. Nature. 2000;406:953–954. doi: 10.1038/35023216. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Dahn RD, Fallon JF. Interdigital regulation of digit identity and homeotic transformation by modulated BMP signaling. Science. 2000;289:438–441. doi: 10.1126/science.289.5478.438. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Ding Q, Motoyama J, Gasca S, Mo R, Sasaki H, Rossant J, et al. Diminished Sonic hedgehog signaling and lack of floor plate differentiation in Gli2 mutant mice. Development. 1998;125:2533–2543. doi: 10.1242/dev.125.14.2533. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Drossopoulou G, Lewis KE, Sanz-Ezquerro JJ, Nikbakht N, McMahon AP, Hofmann C, et al. A model for anteroposterior patterning of the vertebrate limb based on sequential long- and short-range Shh signalling and Bmp signalling. Development. 2000;127:1337–1348. doi: 10.1242/dev.127.7.1337. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Duboule D. Making progress with limb models. Nature. 2002;418:492–493. doi: 10.1038/418492a. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Duprez DM, Kostakopoulou K, Francis-West PH, Tickle C, Brickell PM. Activation of Fgf-4 and HoxD gene expression by BMP-2 expressing cells in the developing chick limb. Development. 1996;122:1821–1828. doi: 10.1242/dev.122.6.1821. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Teran M, Piedra ME, Kathiriya IS, Srivastava D, Rodriguez-Rey JC, Ros MA. Role of dHAND in the anterior-posterior polarization of the limb bud: implications for the Sonic hedgehog pathway. Development. 2000;127:2133–2142. doi: 10.1242/dev.127.10.2133. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, Scott MP. Conservation of the hedgehog/patched signaling pathway from flies to mice: induction of a mouse patched gene by Hedgehog. Genes Dev. 1996;10:301–312. doi: 10.1101/gad.10.3.301. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Haramis AG, Brown JM, Zeller R. The limb deformity mutation disrupts the SHH/FGF-4 feedback loop and regulation of 5′HoxD genes during limb pattern formation. Development. 1995;121:4237–4245. doi: 10.1242/dev.121.12.4237. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Hornbruch A, Wolpert L. The spatial and temporal distribution of polarizing activity in the flank of the pre-limb-bud stages in the chick embryo. Development. 1991;111:725–731. doi: 10.1242/dev.111.3.725. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Johnson RL, Tabin CJ. Molecular Models for Vertebrate Limb Development. Cell. 1997;90:979–990. doi: 10.1016/s0092-8674(00)80364-5. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Knezevic V, De Santo R, Schughart K, Huffstadt U, Chiang C, Mahon KA, et al. Hoxd-12 differentially affects preaxial and postaxial chondrogenic branches in the limb and regulates Sonic hedgehog in a positive feedback loop. Development. 1997;124:4523–4536. doi: 10.1242/dev.124.22.4523. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Kraus P, Fraidenraich D, Loomis CA. Some distal limb structures develop in mice lacking Sonic hedgehog signaling. Mech. Dev. 2001;100:45–58. doi: 10.1016/s0925-4773(00)00492-5. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Laufer E, Nelson CE, Johnson RL, Morgan BA, Tabin C. Sonic hedgehog and Fgf-4 act through a signalling cascade and feedback loop to integrate growth and patterning of the development limb bud. Cell. 1994;79:993–1003. doi: 10.1016/0092-8674(94)90030-2. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Ekker SC, von Kessler DP, Porter JA, Sun BI, Beachy PA. Autoproteolysis in hedgehog protein biogenesis. Science. 1994;266:1528–1537. doi: 10.1126/science.7985023. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Platt KA, Censullo P, RuiZ. i Altaba A. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development. 1997;124:2537–2552. doi: 10.1242/dev.124.13.2537. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Lewandoski M, Sun X, Martin GR. Fgf8 signalling from the AER is essential for normal limb development. Nat. Genet. 2000;26:460–463. doi: 10.1038/82609. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Lewis PM, Dunn MP, McMahon JA, Logan M, Martin JF, St-Jacques B, et al. Cholesterol modification of sonic hedgehog is required for long-range signaling activity and effective modulation of signaling by Ptc1. Cell. 2001;105:599–612. doi: 10.1016/s0092-8674(01)00369-5. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Litingtung Y, Chiang C. Specification of ventral neuron types is mediated by antagonistic interaction between Shh and Gli3. Nat. Neurosci. 2000;3:979–985. doi: 10.1038/79916. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Masuya H, Tomoko S, Wakana S, Moriwaki K, Shiroishi T. A duplicated zone of polarizing activity in polydactylous mouse mutants. Genes Dev. 1995;9:1645–1653. doi: 10.1101/gad.9.13.1645. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Matise MP, Epstein DJ, Park HL, Platt KA, Joyner AL. Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development. 1998;125:2759–2770. doi: 10.1242/dev.125.15.2759. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- McFadden DG, McAnally J, Richardson JA, Charite J, Olson EN. Misexpression of dHAND induces ectopic digits in the developing limb bud in the absence of direct DNA binding. Development. 2002;129:3077–3088. doi: 10.1242/dev.129.13.3077. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Methot N, Basler K. Hedgehog controls limb development by regulating the activities of distinct transcriptional activator and repressor forms of Cubitus interruptus. Cell. 1999;96:819–831. doi: 10.1016/s0092-8674(00)80592-9. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Milenkovic L, Goodrich LV, Higgins KM, Scott MP. Mouse patched1 controls body size determination and limb patterning. Development. 1999;126:4431–4440. doi: 10.1242/dev.126.20.4431. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 1999;21:444–448. doi: 10.1038/7788. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dolle P. Embryonic retinoic acid synthesis is required for forelimb growth and anteroposterior patterning in the mouse. Development. 2002;129:3563–3574. doi: 10.1242/dev.129.15.3563. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Niswander L, Tickle C, Vogel A, Booth I, Martin GR. FGF-4 replaces the apical ectodermal ridge and directs outgrowth and patterning of the limb. Cell. 1993;75:579–587. doi: 10.1016/0092-8674(93)90391-3. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Park HL, Bai C, Platt KA, Matise MP, Beeghly A, Hui CC, et al. Mouse Gli1 mutants are viable but have defects in SHH signaling in combination with a Gli2 mutation. Development. 2000;127:1593–1605. doi: 10.1242/dev.127.8.1593. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Porter JA, von Kessler DP, Ekker SC, Young KE, Lee JJ, Moses K, et al. The product of hedgehog autoproteolytic cleavage active in local and long-range signalling. Nature. 1995;374:363–366. doi: 10.1038/374363a0. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Porter JA, Young KE, Beachy PA. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–259. doi: 10.1126/science.274.5285.255. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Ramírez-Weber FA, Kornberg TB. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97:599–607. doi: 10.1016/s0092-8674(00)80771-0. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Ruiz i Altaba A. Combinatorial Gli gene function in floor plate and neuronal inductions by Sonic hedgehog. Development. 1998;125:2203–2212. doi: 10.1242/dev.125.12.2203. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Saunders JWJ. The proximo-distal sequence of origin of limb parts of the chick wing and the role of the ectoderm. J. Exp. Zool. 1948:363–404. doi: 10.1002/jez.1401080304. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Saunders JWJ, Gasseling MT. Ectodermal–mesenchymal interactions in the origin of limb symmetry. In: Fleischmajer R, Billingham RE, editors. Epithelial–Mesenchymal Interactions. Baltimore: Williams & Wilkins; 1968. pp. 78–97. 10.1046/j.1469-7580.2003.00138.x. [Google Scholar]
- Spemann H, Mangold H. reprinted. Induction of Embryonic Primordia by Implantation of Organizers from a Different Species. Int. J. Dev. Biol. 2001;45:13–38. 10.1046/j.1469-7580.2003.00138.x. [PubMed] [Google Scholar]
- Sun X, Lewandoski M, Meyers EN, Liu YH, Maxson RE, Jr, Martin GR. Conditional inactivation of Fgf4 reveals complexity of signalling during limb bud development. Nat. Genet. 2000;25:83–86. doi: 10.1038/75644. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Cohn MJ, Ashby P, Davey M, Martin P, Tickle C. Distribution of polarizing activity and potential for limb formation in mouse and chick embryos and possible relationships to polydactyly. Development. 2000;127:4011–4021. doi: 10.1242/dev.127.18.4011. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Thaller C, Eichele G. Identification and spatial distribution of retinoids in the developing chick limb bud. Nature. 1987;327:625–628. doi: 10.1038/327625a0. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Tickle C, Alberts BM, Wolpert L, Lee J. Local application of retinoic acid in the limb bud mimics the action of the polarizing region. Nature. 1982;296:564–565. doi: 10.1038/296564a0. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Wang B, Fallon JF, Beachy PA. Hedgehog-Regulated Processing of Gli3 Produces an Anterior/Posterior Repressor gradient in the Developing Vertebrate Limb. Cell. 2000;100:423–434. doi: 10.1016/s0092-8674(00)80678-9. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- te Welscher P, Fernandez-Teran M, Ros MA, Zeller R. Mutual genetic antagonism involving GLI3 and dHAND prepatterns the vertebrate limb bud mesenchyme prior to SHH signaling. Genes Dev. 2002a;16:421–426. doi: 10.1101/gad.219202. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Welscher P, Zuniga A, Kuijper S, Drenth T, Goedemans HJ, Meijlink F, et al. Progression of Vertebrate Limb Development through SHH-Mediated Counteraction of GLI3. Science. 2002b;298:827–830. doi: 10.1126/science.1075620. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Positional information and the spatial pattern of cellular differentiation. J. Theor. Biol. 1969;25:1–47. doi: 10.1016/s0022-5193(69)80016-0. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Yang Y, Drossopoulou G, Chuang PT, Duprez D, Marti E, Bumcrot D, et al. Relationship between does, distance and time in Sonic Hedgehog-mediated regulation of anteroposterior polarity in the chick limb. Development. 1997;124:4393–4404. doi: 10.1242/dev.124.21.4393. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Yelon D, Ticho B, Halpern ME, Ruvinsky I, Ho RK, Silver LM, et al. The bHLH transcription factor hand2 plays parallel roles in zebrafish heart and pectoral fin development. Development. 2000;127:2573–2582. doi: 10.1242/dev.127.12.2573. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Zeller R, Haramis A, Zuniga A, McGuigan C, Dono R, Davidson G, et al. Formin defines a large family of morphoregul-atory genes and functions in establishment of the polarizing region. Cell Tissue Res. 1999;296:85–93. doi: 10.1007/s004410051269. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Zeng X, Goetz JA, Suber LM, Scott WJ, Jr, Schreiner CM, Robbins DJ. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature. 2001;411:716–720. doi: 10.1038/35079648. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Zuniga A, Haramis AP, McMahon AP, Zeller R. Signal relay by BMP antagonism controls the SHH/FGF4 feedback loop in vertebrate limb buds. Nature. 1999;401:598–602. doi: 10.1038/44157. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]
- Zuniga A, Zeller R. Gli3 (Xt) and formin (ld) participate in the positioning of the polarizing region and control of posterior limb-bud identity. Development. 1999;126:13–21. doi: 10.1242/dev.126.1.13. 10.1046/j.1469-7580.2003.00138.x. [DOI] [PubMed] [Google Scholar]