Abstract
The limb myogenic precursors arise by delamination from the lateral dermomyotome in response to signals from the lateral plate mesoderm. They subsequently migrate into the developing limb bud where they switch on the expression of the myogenic regulatory factors, MyoD and Myf5, and coalese to form the dorsal and ventral muscle masses. The myogenic cells subsequently undergo terminal differentiation into slow or fast fibres which have distinct contractile properties determining how a muscle will function. In general, fast fibres contract rapidly with high force and are characterized by the expression of fast myosin heavy chains (MyHC). These fibres are needed for movement. In contrast, slow fibres express slow MyHC, contract slowly and are required for maintenance of posture. This review focuses on the molecular signals that control limb myogenic development from the initial delamination and migration of the premyogenic cells to the ultimate formation of the complex muscle pattern and differentiation of slow and fast fibres.
Keywords: Bmp, fibretype, muscle, Shh, Wnt
Introduction
The myogenic cells of the limb arise from the somites, epithelial balls of cells which form from the paraxial mesodem. As a result of inductive interactions from surrounding tissues, the ventromedial part of the somite undergoes an epithelial–mesenchymal transformation and gives rise to the sclerotome, the precursors of the ribs and axial skeleton. In contrast, the dorso-lateral region of the somite keeps its epithelial character and forms the dermomyotome, which will generate the musculature and dermis. The dorso-medial lip of the dermomyotome forms the epaxial muscle, ultimately the body wall muscles whilst the dorso-lateral lip gives rise to the hypaxial muscle – the limb, tongue, diaphragm and ventral wall musculature (Fig. 1). The somite also gives rise to endothelial precursors (Chevallier et al. 1977; Beddington & Martin, 1989; Ordahl & Le Douarin, 1992; Wilting et al. 1995; Kardon et al. 2002).
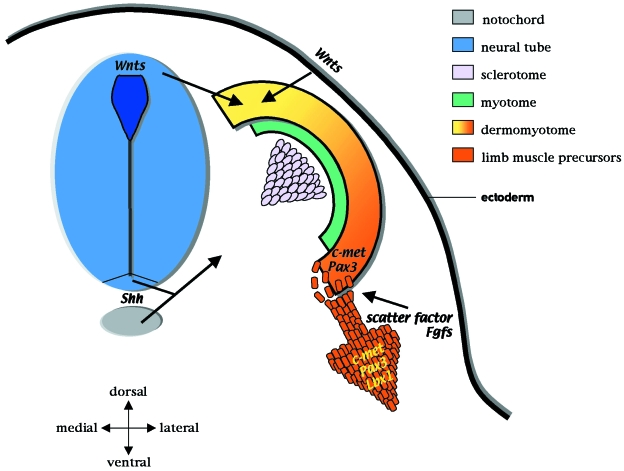
Fig. 1.
Factors that control myogenic induction in the somite. The somite is initially specified into two regions – the sclerotome (pink) and dermomyotome (orange–yellow). The sclerotome forms the vertebrae and ribs whilst the dermomyotome gives rise to the myogenic precursors and the dermis. The dorso-medial edge of the dermomyotome (yellow) forms the epaxial muscles. This region involutes to give rise to the myotome (green), consisting of committed myogenic cells (i.e. expressing Myf5) which will form the back musculature. The dorso-lateral region of the dermomyotome (orange) gives rise to the hypaxial muscles. At the limb level, the premyogenic limb precursors delaminate and migrate distally into the developing limb bud (large orange arrow). The molecular signals that control these events are well characterized. Simplistically, Shh, produced by the notochord (grey) and floor plate of the neural tube (blue), induces the sclerotome. Wnt proteins are expressed in the dorsal neural tube and the dorsal ectoderm and, together with Shh, signalling promote myogenesis. The limb premyogenic cells are induced to delaminate by scatter factor and FGFs. These factors are also thought to control migration. The premyogenic cells express Pax3, Lbx1 and the scatter factor receptor, c-met, and are not committed to myogenic differentiation due to repressive signals from the lateral plate mesoderm (Bmp-4). Pax3 and c-met are needed for delamination whilst Lbx1 is required for migration.
The limb myogenic progenitors arise by delamination from the lateral dermomyotome in response to signals from the adjacent lateral plate mesoderm (Chevallier et al. 1977; Christ et al. 1977; Jacob et al. 1978; Solursh et al. 1987; Hayashi & Ozawa, 1995). The premyogenic cells then migrate distally towards the tip of the limb bud and begin to switch on the expression of the myogenic determination helix–loop–helix transcription factors, MyoD and/or Myf5, which mark myogenic commitment (Figs 2 and 3).
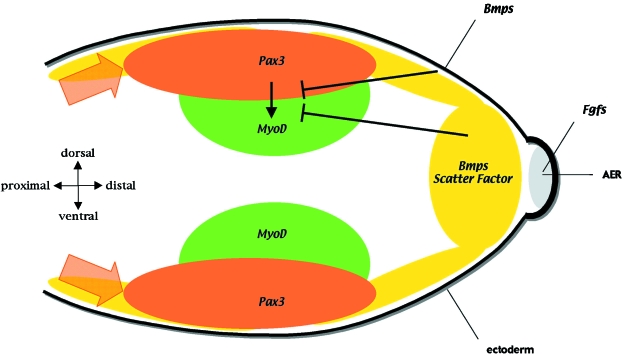
Fig. 2.
Signals that control migration and differentiation of the limb muscle precursors. The premyogenic cells (expressing Pax3 and shown in orange) migrate distally towards the AER (grey) which expresses FGFs and regulates scatter factor expression in the underlying mesenchyme (yellow). Once within the limb bud a subpopulation of premyogenic cells in the proximal limb bud start to differentiate, switching on the expression of the myogenic regulatory genes, Myf5 and MyoD (green). Cells committed to myogenesis are found towards the centre of the limb bud whilst the proliferative Pax3-expressing cells are found closer to the ectoderm. Bmp signalling from the ectoderm and underlying mesenchyme, together with scatter factor in the mesenchyme (yellow) and FGFs in the AER, repress myogenic differentiation.
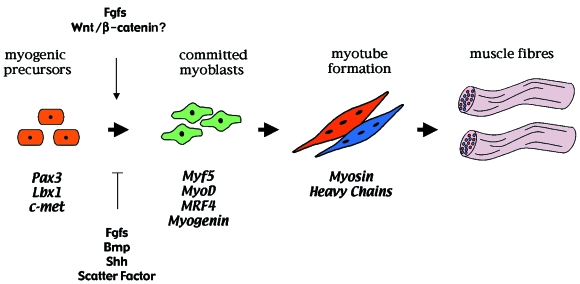
Fig. 3.
The regulation of primary myogenesis in the developing limb. The muscle precursors of the limb initially express the transcription factors Pax3 and Lbx1 together with the c-met tyrosine kinase receptor for scatter factor. Within the limb field, commitment to myogenic differentiation is marked by the expression of the myogenic regulatory genes (MRFs): Myf5 is detected first and is soon followed by MyoD. MRF4 is then transiently expressed and this is followed by myogenin expression. The onset of MRF expression is regulated by growth factors produced by the limb bud (also see Fig. 2). Subsequently, myoblasts terminally differentiate and express either slow or fast myosin heavy chain (MyHC) isoforms which determine the muscle fibretype. The MyHC-expressing myoblasts fuse into multinucleate myotubes and assemble to form the muscle fibres.
Myoblasts subsequently coalesce to form the dorsal and ventral muscle masses, the template of the future muscles (Fig. 2; Schramm & Solursh, 1990). At stage 25 in the chick the myogenic cells start to terminally differentiate, switching on the expression of the terminal differentiation factors, including myosin heavy chains (MyHC), and fuse to form the multinucleated fibres which are able to contract (Fig. 3; Hilfer et al. 1973; Sweeney et al. 1989). This primary fibre development is followed by a wave of secondary fibre formation, which encapsulates the primary fibres, starting at day 7 in the chick embryo (Duxson et al. 1989; Fredette & Landmesser, 1991; Wigmore & Evans, 2002). The secondary fibres have distinct biochemical and morphological characteristics and constitute the bulk of skeletal muscle at birth (Fredette & Landmesser, 1991).
Factors that control delamination and migration
Scatter factor (also known as hepatocyte growth factor) and members of the Fibroblast Growth Factor (FGF) family have been shown to be the major players controlling delamination and migration (Fig. 1). Both FGF and scatter factor can evoke delamination of the lateral dermomyotome when applied ectopically into the interlimb flank mesenchyme (Brand-Saberi et al. 1996; Heymann et al. 1996). Furthermore, genetic inactivation of c-met, the tyrosine kinase receptor for scatter factor, prevents delamination of the lateral dermomyotome in mice. FGF signalling appears to be upstream of scatter factor, which is expressed in the lateral plate mesoderm at the limb levels, as judged by its ability to induce ectopic scatter factor expression. However, scatter factor is not the sole mediator of FGF's effects and other targets of FGF signalling must exist. For example, FGF (but not scatter factor) can induce Lbx1 expression in the migrating myogenic precursors (Mennerich et al. 1998). In addition to inducing delamination, both scatter factor and FGFs act as a chemotactic source promoting migration towards the distal tip of the limb bud (Fig. 1; Itoh et al. 1996; Takayama et al. 1996; Webb et al. 1997; Lee et al. 1999; Scaal et al. 1999).
The two transcription factors that have been shown to be essential for delamination and migration are the homeobox genes, Pax3 and Lbx1, respectively. Both of these are initially expressed in the lateral dermomyotome. Pax3 is necessary for the epithelial–mesenchymal transformation of the lateral dermomyotome and appears to lie upstream of c-met expression. In Splotch mice, which are defective in Pax3 function, c-met expression is significantly reduced or absent in the lateral dermomyotome, which is disorganized, and the limb myogenic cells do not migrate (Daston et al. 1996; Epstein et al. 1996; Yang et al. 1996; Mennerich et al. 1998; Tremblay et al. 1998). In contrast, Lbx1 is needed for migration but like c-met is downstream of Pax3 function (Mennerich et al. 1998; Gross et al. 2000). Following gene inactivation of Lbx1, the premyogenic cells delaminate appropriately but do not migrate correctly. The majority, if not all, of the myogenic hindlimb precursors remain near the dermomyotome and appear to be unable to migrate. In contrast, the forelimb premyogenic cells can migrate but some mismigrate ventrally. This ultimately leads to an almost total absence of hindlimb musculature whilst, in the forelimb, the extensors (the dorsal muscles) are missing (Schäfer & Braun, 1999; Brohmann et al. 2000; Gross et al. 2000). This phenotype resembles that following gene inactivation of gab1, a docking protein involved in the transduction of the c-met signal, and has been suggested to be due to the inability of the cells to respond to limb migratory cues (Schäfer & Braun, 1999; Brohmann et al. 2000; Gross et al. 2000; Sachs et al. 2000). This assumes that the cues that govern ventral vs. dorsal migration, or that the precursors that give rise to these premyogenic muscle masses, are distinct in the forelimb. However, it does not explain why some of the Lbx1−/– cells appear to be competent to migrate along another route into the diaphragm. Hence, another possibility is that following delamination the myogenic cells can migrate but migrate too slowly, ultimately losing their chemotactic cues from the developing limb bud (Schäfer & Braun, 1999; Brohmann et al. 2000; Gross et al. 2000). By default, some premyogenic cells would then subsequently migrate into the competing pathway, which gives rise to the diaphragm musculature.
The premyogenic cells migrate along a fibrillar network and migration is dependent on the membrane molecules, N-cadherin and integrins, together with the extracellular matrix components, fibronectin and hyaluronan (Jacob et al. 1978; Jaffredo et al. 1988; Brand-Saberi et al. 1993, 1996; Swartz et al. 2001). In addition, the membrane receptor, ephA4, which is expressed by the muscle precursors, controls migration through inhibitory interactions. EphA4 interacts with the ligand ephrinA5, which is initially expressed at higher levels distally in regions where myogenic cells are not found. Furthermore, overexpression of ephrinA5 prevents migration of the premyogenic cells, suggesting that inhibitory ephA4–ephrinA5 interactions control the localization of the muscle cells within the limb bud (Swartz et al. 2001).
Differentiation of premyogenic cells
During early development and migration, signals from the lateral plate mesoderm inhibit differentiation. The growth factor Bone morphogenetic protein-4 (Bmp-4) has been shown to be one key inhibitory molecule, but FGFs and scatter factor probably also play a role (Pourquie et al. 1996). Once within the limb bud the muscle cells switch off Pax3 and Lbx1 expression and start to express the myogenic regulatory transcription factors (MRFs) (Fig. 2; Gross et al. 2000; Uchiyama et al. 2000). These factors comprise a family of four genes –Myf5, MyoD, MRF4 and myogenin– which are expressed sequentially during myogenic differentiation (reviewed by Pownall et al. 2002). Myf5 and MyoD mark the initial onset to myogenic commitment whereas MRF4 and myogenin are expressed later. Forced expression of MRFs is sufficient to drive the myogenic pathway. Conversely, loss of function of MRFs in mice has clearly demonstrated that they are crucial for myogenic differentiation. For example, in the double Myf5/MyoD knockout myogenesis is ablated (Rudnicki et al. 1993). Similarly, the myogenin null mouse has severe muscle defects. In this case, the myoblasts form but are unable to undergo terminal differentiation (Hasty et al. 1993; Nabeshima et al. 1993).
Differentiation starts in the proximal mesenchyme and then progresses distally as the limb bud develops and elongates. This proximal to distal wave of differentiation correlates with the proximity of the cells to the apical ectodermal ridge (AER), a region of thickened ectoderm at the tip of the limb bud which controls outgrowth (Fig. 2). The AER expresses a number of FGFs including Fgf2, 4 and 8, which can inhibit myogenic differentiation (Fig. 2; Robson & Hughes, 1996). FGF signalling also maintains the expression of another inhibitory molecule, scatter factor, in the underlying mesenchyme (Fig. 2; Scaal et al. 1999). Indeed, myogenic cells placed in the mesenchyme underlying the AER will not differentiate, in contrast to those placed in the proximal region of the limb bud (Robson & Hughes, 1996). There are many studies showing that FGF signalling represses myogenic differentiation and it is generally assumed that this also occurs in the developing limb bud. Fitting with this overexpression of FGF4 or -5, or loss of FGF function by misexpression of a dominant-negative FGFR1 receptor, have been shown to reduce the number of terminally differentiated myogenic cells (Clase et al. 2000; Flanagan-Steet et al. 2000; Edom-Vovard et al. 2001). However, other interpretations of these studies are possible and whether FGF signalling simply blocks myogenic differentiation has recently been challenged. In these recent studies, misexpression of soluble FGFR4 (previously known as FREK) but not FGFR1, both of which will block FGF signalling, in the developing limb bud was shown to decrease Myf5 and MyoD expression and ultimately the number of terminally differentiated myoblasts (Marics et al. 2002). Pax3 expression was unaffected and no change in the apoptosis or proliferation was observed, suggesting that FGF signalling is required for the transition from a Pax3 (uncommitted) to myogenic committed state. Thus, it has been proposed that FGFs are a critical step for progression along the myogenic differentiation pathway (Marics et al. 2002). This would fit with previous in vitro observations that FGF signalling cannot only repress myogenic differentiation but is also needed to permit differentiation of a subpopulation of myogenic cells from the limb bud (Seed & Hauschka, 1988). Exactly how FGF signalling regulates myogenic differentiation is presently unclear but the situation is likely to be very complex, probably being dependent on the distinct effects of different FGF ligands, other growth factors and the stage of myogenic differentiation. Indeed, FGFs are not only expressed in the surrounding limb bud tissues, which are thought to repress differentiation, but several ligands (FGF2, -4, -5 and -6) are expressed in the myogenic cells themselves (Haub & Goldfarb, 1991; Niswander & Martin, 1992).
In addition to the AER, dorsal signals from the ectoderm also inhibit differentiation. Thus, removal of the ectoderm accelerates myogenic differentiation. This is illustrated by the down-regulation of Pax3 expression, which marks the early myogenic population, and the up-regulation of MyoD (Amthor et al. 1998). As the pool of proliferative premyogenic cells is prematurely depleted in the absence of the ectoderm, this procedure ultimately results in smaller muscles. Bmp-2, which is expressed in the limb ectoderm, has been shown to substitute for the ectodermal signal (Fig. 2). However, it is likely that Bmp-2, together with other Bmps (-4 and -7) expressed in the mesenchyme are also involved (Fig. 2). In addition, other members of the TGF-β family, including the founding member TGF-β itself, can inhibit myogenic differentiation. Notable amongst these is myostatin (or Bmp-8), which is mutated in the double-muscled cattle breeds, Belgium Blue and Piedmontese (Grobet et al. 1997; Kambadur et al. 1997; McPherron & Lee, 1997). Similarly, gene-inactivation of myostatin in mice results in a large muscled mouse (Thomas et al. 2000). Myostatin is expressed by the developing muscles themselves and negatively regulates myogenic proliferation (Thomas et al. 2000). Myostatin function is antagonized by the secreted molecule, follistatin, which is again expressed by the developing myogenic cells (Brand-Saberi et al. 1996). Thus, misexpression of follistatin increases muscle mass whilst loss of function of follistatin reduces the amount of muscle that develops (Matzuk et al. 1995; Lee & McPherron, 2001). An intracellular modulator of TGF-β, and presumably myostatin signalling is the transcription factor, Ski, which inhibits the function of smad3 (Luo et al. 1999). Smad3, when activated by TGF-β signalling, can bind to MyoD and inhibit its function in myogenic cell lines (Liu et al. 2001). Knockout of Ski in mice, which would presumably result in excess smad3 activity, i.e. TGF-β signalling results in smaller muscles (Berk et al. 1997). Conversely, gain of Ski function results in increased muscle mass (Sutrave et al. 2000).
Sonic hedgehog (Shh) has also been shown to repress myogenic differentiation at least in part by the maintenance of Bmp expression (Amthor et al. 1998; Krüger et al. 2001). Therefore, overexpression in the chick limb expands the Pax3 domain and increases myoblast proliferation, ultimately resulting in muscle hyperplasia (Duprez et al. 1998; Bren-Mattison & Olwin, 2002). Confirmation of the endogenous role of Shh signalling during muscle development has been obtained in the mouse knockout where the ventral muscles are absent, and by blocking Shh signalling in the chick limb (Krüger et al. 2001; Bren-Mattison & Olwin, 2002). In the latter case, there was a reduction in the number of terminally differentiated myogenic cells (Bren-Mattison & Olwin, 2002). Interestingly, in the Shh knockout studies a slight delay in the onset of MyoD expression was observed, suggesting that, as in the somite, Shh signalling forms part of the regulatory network that initiates myogenic differentiation (Münsterberg et al. 1995).
Finally, notch signalling has been implicated in the regulation of myogenic differentiation. Ectopic activation of the notch pathway in the developing chick limb decreases MyoD expression whilst Pax3 and Myf5 are unaffected, suggesting that notch signalling may control the transition from a Myf5 to MyoD expressing state in the myogenic pathway (Delfini et al. 2000).
This cohort of inhibitory signals prevents myogenic differentiation and allows the expansion of the premyogenic pool with only a subpopulation of premyogenic cells becoming committed at any one time. This undifferentiated state appears to be maintained by the expression of the transcriptional repressor, Msx1, which is expressed in at least a subset of the premyogenic population (Bendall et al. 1999; Houzelstein et al. 1999). Evidence for this is that forced expression of Msx1 in vivo in the developing chick limb and cell lines in vitro block myogenic differentiation, in part mediated by direct binding to the MyoD enhancer (Song et al. 1992; Woloshin et al. 1995; Bendall et al. 1999). Indeed, Msx1 has been shown to bind and inhibit Pax3, which can induce myogenic differentiation not only when ectopically expressed within the limb bud but within neural tube explants (Maroto et al. 1997; Bendall et al. 1999). Therefore, one model could be that following loss of Msx1 expression, Pax3 induces myogenic commitment. Having said this, Pax3 is not essential for myogenic differentiation as presumptive limb myogenic cells from Splotch mice, which lack Pax3 function, can differentiate when placed in the limb environment (Daston et al. 1996). However, it is still possible that Pax7, which is also expressed by limb myogenic precursors, compensates for the loss of Pax3.
This raises the question as to whether myogenic differentiation is just a passive default state following the loss of these inhibitory signals. We would propose it is not and that positive signals are required. As mentioned before, this is supported by recent work which has shown that FGF signalling is necessary for myogenic commitment within the developing limb bud (Fig. 3; Marics et al. 2002). Other factors that may be involved include members of the Wnt gene family. Support for this hypothesis is that in the pluripotent embryonic carcinoma P19 line, β-catenin, a component of the canonical Wnt pathway, is sufficient and necessary for myogenic differentiation (Fig. 3; Petropoulos & Skerjanc, 2002). Furthermore, although not conclusive evidence, misexpression of the Wnt antagonist, Sfrp3, in developing somites can block myogenic differentiation upstream of MyoD but downstream of Pax3 expression (Borello et al. 1999). Finally, two components of the Wnt pathway, the transcription factor, Lef-1 and its partner, β-catenin, can be induced in the developing myotome in response to Wnt and Shh signalling prior to the onset of MyoD expression (Schmidt et al. 2000). As promoter analysis has shown that a 258-bp MyoD enhancer can recapitulate MyoD expression in both the somite and limb, it is likely that many of the regulatory elements that control MyoD expression in somite and limb development are conserved (Faerman et al. 1995; Goldhamer et al. 1995; Kablar et al. 1999). Therefore, by extrapolation from the data obtained in the somites and the P19 cell line, we would propose that the Wnt pathway might be crucial for myogenic commitment in the developing limb. Indeed, we have found that misexpression of either Wnt3a or its downstream mediator β-catenin or that by blocking this pathway, we can change the number of terminally differentiated myogenic cells consistent with this possibility. However, at present other explanations are also possible. For example, Wnt signalling could have the converse effect to that proposed above and inhibit differentiation. Alternatively, Wnt signalling may change cell survival and/or proliferation.
Terminal differentiation
Each muscle is characterized by a highly specific and unique arrangement of slow and fast fibretypes specialized for its particular function (Fig. 3; Miller & Stockdale, 1986a,b). The different fibres are characterized by the specific expression of myosin heavy chains (MyHC), and distinct metabolic activities. Fast fibres express one of the fast MyHC isoforms and generate high force. They can be further subdivided into three groups: the small fast oxidative fibres (IIA), the fast intermediate fibres (IIX) and fast glycolytic fibres (IIB). The last of these are the fastest and as a consequence of the glycolytic metabolism they fatigue easily. In contrast, slow fibres express the slow isoform of the MyHC, contract slowly, use oxidative metabolism and are able to maintain a contraction for longer without fatigue (Hughes & Salinas, 1999; Wigmore & Evans, 2002). Uniquely, in the chick, muscle fibres can simultaneously express both fast and slow MyHCs. The highly complex arrangement of the fast and slow fibres, which not only differs between each muscle but can vary along the proximo-distal axis of a muscle, raises the challenging question as to how this pattern is specified (Zhang & McLennan, 1998). In the fetus and postnatally, exercise, hormones and neuronal activity are major players (reviewed by Hughes & Salinas, 1999). However, in the embryo the fast and slow fibres are initially assembled prior to innervation and hormonal influence and must be specified by different molecular and/or cellular interactions.
When and where slow and fast fibres are specified has been a matter of continuing debate. Studies in which clones of quail premyogenic cells have been grafted into a chick host have shown that embryonic myogenic cells are committed to terminal fates by embryonic day 4 (DiMario et al. 1993). Limb myogenic cells isolated in culture also appear to be biased or prediposed to either a slow or fast fate, showing that they are a heterogeneous population (Miller & Stockdale, 1986a,b; Cossu & Molinaro, 1987; Stockdale, 1990; DiMario et al. 1993; DiMario & Stockdale, 1995; Pin & Merrifield, 1997). Furthermore, these distinct fast/slow properties can be inherited in successive generations (reviewed by Stockdale, 1992; Robson & Hughes, 1999). However, these studies have not answered when and where this fibretype commitment takes place. Chimeric grafting studies by Van Swearingen & Lance-Jones (1995) showed that in the hindlimb the first premyogenic cells to enter the limb bud form the proximal slow muscles whilst the fast muscles are formed by the slightly later migratory wave. From this it was proposed that the first myoblasts to enter the limb are specified to form slow fibres and that they become determined in the somite and/or during migration, i.e. they are precommitted (Fig. 4). This early determination has been supported by a recent elegant study in which the somitic precursors of the quail pectoralis muscle, a hypaxial-derived muscle that connects the shoulder and thorax, were grafted into the equivalent position in a chick host, and it was suggested that commitment occurs within the somite (Fig. 4; Nikovits et al. 2001). Thus, the pectoralis muscle consisted of both slow and fast fibres characteristic of quail, and not chick muscle, which is predominantly fast. In contrast, other studies have shown that when clones of fetal or satellite myogenic cells are grafted into a new host, they differentiate or modify their fate according to the new environmental cues, suggesting that environmental signals within the limb bud determine terminal myogenic fate (Fig. 4; Hughes & Blau, 1992; DiMario & Stockdale, 1997; Robson & Hughes, 1999). Furthermore, recent retroviral labelling studies in which groups of presumptive myogenic cells have been labelled within the somite have shown that they can contribute to a number of muscles in the limb bud and, therefore, the final destination of the presumptive muscle cells is not predetermined (Kardon et al. 2002; Rees et al. 2003). In addition, the studies by Kardon et al. (2002) in which each presumptive myogenic cell was labelled with a distinct nucleotide tag have shown that the progeny of individual myogenic cells contribute to both slow and fast fibres clearly arguing that environmental signals within the limb bud control fibretype fate (Kardon et al. 2002). The apparent discrepancy between some of these studies may be due to differences between the myogenic cell populations analysed. Embryonic myoblasts, for example, generate the primary myotubes, fetal myoblasts are responsible for the formation of the secondary myotubes and growth of primary fibres whilst satellite cells are required for postnatal muscle growth (Seed & Hauschka, 1984; Evans et al. 1994).
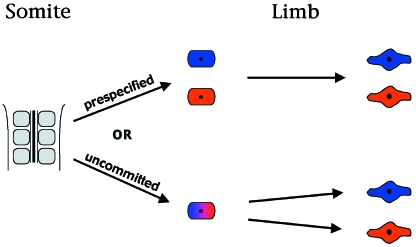
Fig. 4.
Specification of slow and fast myoblasts. There is debate as to when the distinct fibretypes are specified. One model proposes that slow and fast fibretypes are already specified within the somite. The other proposes that premyogenic cells are unspecified as they leave the somite and are competent to generate both slow and fast fibretypes under the influence of environmental factors in the limb bud. See text for further details.
An alternative unification of these data is that the myogenic cells may be biased towards one fate as they leave the somite. However, as occurs in other developmental systems, such as the neuroectoderm and its derivatives, this bias can be over-ruled by local environmental signals. This occurs in cranial neural crest progeny when transplanted ectopically in the head. In this scenario, ‘true commitment’ to a fast vs. slow fibretype would be a relatively late event occurring within the limb bud. This plasticity of cell fate is seen in the myogenic cells of the developing somite. In the u-boot zebrafish mutant the somitic myogenic cells cannot respond appropriately to Shh signalling and fail to form slow fibres (Roy et al. 2001). Yet, when given excess Shh signalling, the myogenic cells initially differentiate as slow myoblasts but later transdifferentiate to form fast fibres (Roy et al. 2001). Therefore, slow and fast fibre development should probably not be viewed as a one-step commitment process but a reversible acquisition of competence/specification which requires continued reinforcement signals as seen in adult myoblasts. This would particularly make sense as skeletal muscle is an adaptable tissue which must respond to changing environmental cues to function correctly.
The issue may be further clouded by potential differential responses and mechanisms of muscle development along the proximo-distal axis. The limb has been proposed to have evolved in two parts – the proximal structures develop independently of Shh and are homologous to the fin whereas the distal structures are dependent on Shh and are evolutionary additions. Therefore, it is possible that the development/maintenance of the proximal and distal limb muscles is differentially controlled, as has recently been shown for the tendons (Kardon, 1998). Fast and slow myoblasts, which give rise to the proximal muscles, may be prepatterned in the somite as suggested by the studies of Nikovits et al. (2001) whilst those distally may be patterned by the environment. Whatever is true, it is clear that environmental signals within the limb bud must control the number and distribution of slow and fast fibres, as highlighted by the recent studies of Kardon et al. (2002).
Furthermore, following duplication of the anterior–posterior axis the presumptive anterior muscles are respecified to form posterior muscles with the appropriate distribution of fast and slow fibres, again supporting the notion that environmental signals control the distribution and fate of myogenic cells (Robson et al. 1994).
Molecular regulation of slow/fast differentiation
Factors that specify limb myogenic fibretype differentiation are unknown. In chick somites and zebrafish adaxial musculature, Shh or hedgehog signalling has been shown to promote slow fibretype formation. Thus, loss of Shh signalling ablates slow fibre development, whilst excess Shh promotes slow fibre formation (Currie & Ingham, 1996; Blagden et al. 1997; Du et al. 1997; Cann et al. 1999; Lewis et al. 1999; Barresi et al. 2000). In zebrafish adaxial musculature, the promotion of slow fibre formation in vivo and in vitro appears to be at the expense of fast fibre formation, suggesting that hedgehog signalling may act as an instructive binary switch between the two differentiation states, the ‘default’ state in the absence of hedgehog signalling being fast (Norris et al. 2000). However, within the limb bud itself, Shh does not appear to determine myogenic cell fate but does prevent differentiation of a subpopulation of the presumptive slow muscle precursors, hence maintaining them in a proliferative state and, ultimately, increasing the number of slow fibres (Bren-Mattison & Olwin, 2002). Our recent data have also suggested that the Wnt family of growth factors may influence fibretype differentiation. Wnt5a which is initially expressed throughout the mesenchyme and later around the chondrogenic core, where the majority of slow fibres are found, promotes slow fibre development seemingly at the expense of fast. In contrast, Wnt11, which is expressed in the subectodermal mesenchyme where the majority of fast fibres develop, has the converse effect promoting fast fibre development again seemingly at the expense of slow (K. Anakwe et al. submitted).
In the adult the MRFs have been strongly implicated in the regulation of slow/fast fibres. Postnatally, but not during development, MyoD has been shown to be differentially expressed in fast and slow fibres in rats, being found at high levels in fast and intermediate fibres (IIB and IIX) in fast muscles and at lower levels in type I fibres in slow, but not fast, muscles (Hughes et al. 1997). In the MyoD knockout mice there are more IIA and slow type I fibres but fewer type IIB fibres in some of the fast muscles (Hughes et al. 1997). Hence some fast muscles acquire slower characteristics. The activity of the catalase enzyme, which is higher in slow fibres, is also increased (Tiidus et al. 1996). Somewhat paradoxically and emphasizing the complexity of the problem, the slow muscles gain a faster phenotype (Hughes et al. 1997). Likewise, myogenin controls fibretype development, and following misexpression of myogenin, the levels/activity of glycolytic enzymes decrease whilst oxidative metabolism increases in the fast muscles (Hughes et al. 1999). However, in this case metabolic changes are not associated with changes in MyHC expression (Hughes et al. 1999).
Finally, slow fibre development and maintenance in the adult has been linked to activation of calcium signalling via the serine–threonine phosphatase, calcineurin (Chin et al. 1998; Dunn et al. 1999; Bigard et al. 2000; Naya et al. 2000; Serrano et al. 2001). This is consistent with the high levels of intracellular calcium found in slow fibres compared with the short brief calcium fluxes that occur in fast muscles during contraction. Recently, the transcriptional co-factor, PGC-1α, involved in oxidative metabolism has been shown to be a downstream target of calcineurin signalling. Furthermore, calcineurin is sufficient to promote slow fibre formation when misexpressed in fast fibres (Lin et al. 2002). Increases in calcium signalling may also be responsible for slow fibre formation in the embryonic limb, and in our studies we found that Wnt5a, which activates calcium signalling in Xenopus and zebrafish embryos, promoted slow fibre formation (Slusarski et al. 1997a,b; Kühl et al. 2000; K. Anakwe et al. submitted). In support of this, misexpression of activated calmodulin kinase, a downstream target of calcium signalling, in developing limb myogenic cells also increased the number of slow myocytes (K. Anakwe et al. submitted). However, activation of calcineurin cannot be totally responsible for slow fibre formation. Following IGF stimulation of muscle cells, calcineurin is activated – yet fast fibres, and not slow, are formed (Semsarian et al. 1999). Fast fibre formation in adult muscle has also been linked to activation of the mitogen-activated protein kinase kinase 6 (MKK6) (Delling et al. 2000).
Patterning of the musculature
The arrangement of muscles in the limb is extremely complex and is established very early with the future orientation of the fibres being apparent as the myotubes form in the chick (Kardon, 1998). What determines this intricate arrangement is presently unclear. However, the overall pattern is clearly related to the skeletal structures. For example, following duplication of the anterior–posterior axis, where the anterior mesenchyme gives rise to posterior structures, posterior muscles develop from the anterior region (Robson et al. 1994). Likewise, respecification of the dorso–ventral axis, such as occurs following loss of Wnt7a function or as a result of ectoderm rotation experiments, also results in the respecification of the musculature almost in complete accordance with the new skeleton (Parr & McMahon, 1995; Akita, 1996). At a molecular level the spatial localization of the muscles has been in part linked to the differential expression of Hoxa13 within the premuscle masses (Yamamoto et al. 1998).
Gene knock-out experiments are starting to indicate that the development of each muscle may be dependent on a specific combination of factors. As mentioned earlier, the Lbx1 knockout lacks the dorsal muscles in the forelimb whilst, following gene inactivation of Mox2, a homeobox gene expressed in the migratory premyogenic cells and in the distal limb mesenchyme, a subset of forelimb muscles are affected (Mankoo et al. 1999). In the latter case, these muscles are either reduced in size or absent. In contrast, all the hindlimb muscles are present but are smaller and not all are correctly patterned (Mankoo et al. 1999). Therefore, it can easily be envisaged that the gain or loss of genes such as Mox2 in different species will determine the final arrangement of muscles. This is similar to what has been proposed for many other regions of the body where the differential expression of genes – usually homeobox genes – specify patterning.
Acknowledgments
We are grateful to Darrell Evans and Lesley Robson for comments and helpful discussions on the manuscript. The authors work described in this review was done in collaboration with Darrell Evans and Lesley Robson with reagents generously provided by Tsutomu Nohno, Anthony Brown and Cliff Tabin and was funded by a Medical Research Studentship to Kelly Anakwe and the BBSRC.
References
- Akita K. The effect of the ectoderm on the dorsoventral pattern of epidermis, muscles and joints in the developing chick leg: a new model. Anat. Embryol. (Berl.) 1996;193:377–386. doi: 10.1007/BF00186694. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Amthor H, Christ Weil M, Patel K. The importance of timing differentiation during limb muscle development. Curr. Biol. 1998;8:642–652. doi: 10.1016/s0960-9822(98)70251-9. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Barresi MJ, Stickney H, Devoto SH. The zebrafish slow-muscle-omitted gene product is required for Hedgehog signal transduction and the development of slow muscle identity. Development. 2000;127:2189–2199. doi: 10.1242/dev.127.10.2189. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Beddington RS, Martin P. An in situ transgenic enzyme marker to monitor migration of cells in the mid-gestation mouse embryo. Somite contribution to the early forelimb bud. Mol. Biol. Med. 1989;6:263–274. 10.1046/j.1469-7580.2003.00136.x. [PubMed] [Google Scholar]
- Bendall AJ, Ding J, Hu G, Shen MM, Abate-Shen C. Msx1 antagonizes the myogenic activity of Pax3 in migrating limb muscle precursors. Development. 1999;126:4965–4976. doi: 10.1242/dev.126.22.4965. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Berk M, Desai SY, Heyman HC, Colmenares C. Mice lacking the ski proto-oncogene have defects in neurulation, craniofacial, patterning, and skeletal muscle development. Genes Dev. 1997;11:2029–2039. doi: 10.1101/gad.11.16.2029. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigard X, Sanchez H, Zoll J, Mateo P, Rousseau V, Veksler V, et al. Calcineurin co-regulates contractile and metabolic components of slow muscle phenotype. J. Biol. Chem. 2000;275:19653–19660. doi: 10.1074/jbc.M000430200. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Blagden CS, Currie PD, Ingham PW, Hughes SM. Notochord induction of zebrafish slow muscle mediated by Sonic hedgehog. Genes Dev. 1997;11:2163–2175. doi: 10.1101/gad.11.17.2163. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borello U, Coletta M, Tajbakhsh S, Leyns L, De Robertis EM, Buckingham M, et al. Transplacental delivery of the Wnt antagonist Frzb1 inhibits development of caudal paraxial mesoderm and skeletal myogenesis in mouse embryos. Development. 1999;126:4247–4255. doi: 10.1242/dev.126.19.4247. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Brand-Saberi B, Krenn V, Grim M, Christ B. Differences in the fibronectin-dependence of migrating cell populations. Anat. Embryol. (Berl.) 1993;187:17–26. doi: 10.1007/BF00208193. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Brand-Saberi B, Wilting J, Ebensperger C, Christ B. The formation of somite compartments in the avian embryo. Int. J. Dev. Biol. 1996;40:411–420. 10.1046/j.1469-7580.2003.00136.x. [PubMed] [Google Scholar]
- Bren-Mattison Y, Olwin BB. Sonic hedgehog inhibits the terminal differentiation of limb myoblasts committed to the slow muscle lineage. Dev. Biol. 2002;242:130–148. doi: 10.1006/dbio.2001.0528. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Brohmann H, Jagla K, Birchmeier C. The role of Lbx1 in migration of muscle precursor cells. Development. 2000;127:437–445. doi: 10.1242/dev.127.2.437. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Cann GM, Lee JW, Stockdale FE. Sonic hedgehog enhances somite cell viability and formation of primary slow muscle fibers in avian segmented mesoderm. Anat. Embryol. (Berl.) 1999;200:239–252. doi: 10.1007/s004290050276. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Chevallier A, Kieny M, Mauger A. Limb-somite relationship: origin of the limb musculature. J. Embryol. Exp. Morph. 1977;41:245–258. 10.1046/j.1469-7580.2003.00136.x. [PubMed] [Google Scholar]
- Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, et al. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 1998;12:2499–2509. doi: 10.1101/gad.12.16.2499. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ B, Jacob HJ, Jacob M. [Experimental findings on muscle development in the limbs of the chick embryo] Verh. Anat. Ges. 1977;71:1231–1237. 10.1046/j.1469-7580.2003.00136.x. [PubMed] [Google Scholar]
- Clase KL, Mitchell PJ, Ward PJ, Dorman CM, Johnson SE, Hannon K. FGF5 stimulates expansion of connective tissue fibroblasts and inhibits skeletal muscle development in the limb. Dev. Dyn. 2000;219:368–380. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1056>3.0.CO;2-8. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Cossu G, Molinaro M. Cell heterogeneity in the myogenic lineage. Curr. Top. Dev. Biol. 1987;23:185–208. doi: 10.1016/s0070-2153(08)60625-0. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Currie PD, Ingham PW. Induction of a specific muscle cell type by a hedgehog-like protein in zebrafish. Nature. 1996;382:452–455. doi: 10.1038/382452a0. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Daston G, Lamar E, Olivier M, Goulding M. Pax-3 is necessary for migration but not differentiation of limb muscle precursors in the mouse. Development. 1996;122:1017–1027. doi: 10.1242/dev.122.3.1017. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Delfini M, Hirsinger E, Pourquie O, Duprez D. Delta 1-activated notch inhibits muscle differentiation without affecting Myf5 and Pax3 expression in chick limb myogenesis. Development. 2000;127:5213–5224. doi: 10.1242/dev.127.23.5213. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Delling U, Tureckova J, Lim HW, De Windt LJ, Rotwein P, Molkentin JD. A calcineurin-NFATc3-dependent pathway regulates skeletal muscle differentiation and slow myosin heavy-chain expression. Mol. Cell Biol. 2000;20:6600–6611. doi: 10.1128/mcb.20.17.6600-6611.2000. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMario JX, Fernyak SE, Stockdale FE. Myoblasts transferred to the limbs of embryos are committed to specific fibre fates. Nature. 1993;362:165–167. doi: 10.1038/362165a0. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- DiMario JX, Stockdale FE. Differences in the developmental fate of cultured and noncultured myoblasts when transplanted into embryonic limbs. Exp. Cell Res. 1995;216:431–442. doi: 10.1006/excr.1995.1054. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- DiMario JX, Stockdale FE. Both myoblast lineage and innervation determine fiber type and are required for expression of the slow myosin heavy chain 2 gene. Dev. Biol. 1997;188:167–180. doi: 10.1006/dbio.1997.8619. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Du SJ, Devoto SH, Westerfield M, Moon RT. Positive and negative regulation of muscle cell identity by members of the hedgehog and TGF-beta gene families. J. Cell Biol. 1997;139:145–156. doi: 10.1083/jcb.139.1.145. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn SE, Burns JL, Michel RN. Calcineurin is required for skeletal muscle hypertrophy. J. Biol. Chem. 1999;274:21908–21912. doi: 10.1074/jbc.274.31.21908. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Duprez D, Fournier-Thibault C, Le Douarin N. Sonic Hedgehog induces proliferation of committed skeletal muscle cells in the chick limb. Development. 1998;125:495–505. doi: 10.1242/dev.125.3.495. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Duxson MJ, Usson Y, Harris AJ. The origin of secondary myotubes in mammalian skeletal muscles: ultrastructural studies. Development. 1989;107:743–750. doi: 10.1242/dev.107.4.743. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Edom-Vovard F, Bonnin MA, Duprez D. Misexpression of Fgf-4 in the chick limb inhibits myogenesis by down-regulating Frek expression. Dev. Biol. 2001;233:56–71. doi: 10.1006/dbio.2001.0221. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Epstein JA, Shapiro DN, Cheng J, Lam PY, Maas RL. Pax3 modulates expression of the c-Met receptor during limb muscle development. Proc. Natl Acad. Sci. USA. 1996;93:4213–4218. doi: 10.1073/pnas.93.9.4213. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D, Baillie H, Caswell A, Wigmore P. During fetal muscle development, clones of cells contribute to both primary and secondary fibers. Dev. Biol. 1994;162(2):348–353. doi: 10.1006/dbio.1994.1092. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Faerman A, Goldhamer DJ, Puzis R, Emerson CP, Jr, Shani M. The distal human myoD enhancer sequences direct unique muscle-specific patterns of lacZ. expression during mouse development. Dev. Biol. 1995;171:27–38. doi: 10.1006/dbio.1995.1257. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Flanagan-Steet H, Hannon K, McAvoy MJ, Hullinger R, Olwin BB. Loss of FGF receptor 1 signaling reduces skeletal muscle mass and disrupts myofiber organization in the developing limb. Dev. Biol. 2000;218:21–37. doi: 10.1006/dbio.1999.9535. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Fredette BJ, Landmesser LT. Relationship of primary and secondary myogenesis to fiber type development in embryonic chick muscle. Dev. Biol. 1991;143:1–18. doi: 10.1016/0012-1606(91)90050-d. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Goldhamer DJ, Brunk BP, Faerman A, King A, Shani M, Emerson CP., Jr Embryonic activation of the myoD gene is regulated by a highly conserved distal control element. Development. 1995;121:637–649. doi: 10.1242/dev.121.3.637. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Grobet L, Martin LJ, Poncelet D, Pirottin D, Brouwers B, Riquet J, et al. A deletion in the bovine myostatin gene causes the double-muscled phenotype in cattle. Nature Genet. 1997;17:71–74. doi: 10.1038/ng0997-71. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Gross MK, Moran-Rivard L, Velasquez T, Nakatsu MN, Jagla K, Goulding M. Lbx1 is required for muscle precursor migration along a lateral pathway into the limb. Development. 2000;127:413–424. doi: 10.1242/dev.127.2.413. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Hasty P, Bradley A, Morris JH, Edmondson DG, Venuti JM, Olson EN, et al. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993;364:501–506. doi: 10.1038/364501a0. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Haub O, Goldfarb M. Expression of the fibroblast growth factor-5 gene in the mouse embryo. Development. 1991;112:397–406. doi: 10.1242/dev.112.2.397. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ozawa E. Myogenic cell migration from somites is induced by tissue contact with medial region of the presumptive limb mesoderm in chick embryos. Development. 1995;121:661–669. doi: 10.1242/dev.121.3.661. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Heymann S, Koudrova M, Arnold H, Koster M, Braun T. Regulation and function of SF/HGF during migration of limb muscle precursor cells in chicken. Dev. Biol. 1996;180:566–578. doi: 10.1006/dbio.1996.0329. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Hilfer SR, Searls RL, Fonte VG. An ultrastructural study of early myogenesis in the chick wing bud. Dev. Biol. 1973;30:374–391. doi: 10.1016/0012-1606(73)90095-x. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Houzelstein D, Auda-Boucher G, Cheraud Y, Rouaud T, Blanc I, Tajbakhsh S, et al. The homeobox gene Msx1 is expressed in a subset of somites, and in muscle progenitor cells migrating into the forelimb. Development. 1999;126:2689–2701. doi: 10.1242/dev.126.12.2689. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Blau HM. Muscle fiber pattern is independent of cell lineage in postnatal rodent development. Cell. 1992;68:659–671. doi: 10.1016/0092-8674(92)90142-y. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Koishi K, Rudnicki M, Maggs AM. MyoD protein is differentially accumulated in fast and slow skeletal muscle fibres and required for normal fibre type balance in rodents. Mech. Dev. 1997;61:151–163. doi: 10.1016/s0925-4773(96)00631-4. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Chi MM, Lowry OH, Gundersen K. Myogenin induces a shift of enzyme activity from glycolytic to oxidative metabolism in muscles of transgenic mice. J. Cell Biol. 1999;145:633–642. doi: 10.1083/jcb.145.3.633. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SM, Salinas PC. Control of muscle fibre and motoneuron diversification. Curr. Opin. Neurobiol. 1999;9:54–64. doi: 10.1016/s0959-4388(99)80007-5. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Itoh N, Mima T, Mikawa T. Loss of fibroblast growth factor receptors is necessary for terminal differentiation of embryonic limb muscle. Development. 1996;122:291–300. doi: 10.1242/dev.122.1.291. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Jacob M, Christ B, Jacob HJ. On the migration of myogenic stem cells into the prospective wing region of chick embryos. A scanning and transmission electron microscope study. Anat. Embryol. (Berl.) 1978;153:179–193. doi: 10.1007/BF00343373. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Jaffredo T, Horwitz AF, Buck CA, Rong PM, Dieterlen-Lievre F. Myoblast migration specifically inhibited in the chick embryo by grafted CSAT hybridoma cells secreting an anti-integrin antibody. Development. 1988;103:431–446. doi: 10.1242/dev.103.3.431. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Kablar B, Krastel K, Ying C, Tapscott SJ, Goldhamer DJ, Rudnicki MA. Myogenic determination occurs independently in somites and limb buds. Dev. Biol. 1999;206:219–231. doi: 10.1006/dbio.1998.9126. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Kambadur R, Sharma M, Smith TP, Bass JJ. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res. 1997;7:910–916. doi: 10.1101/gr.7.9.910. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Kardon G. Muscle and tendon morphogenesis in the avian hind limb. Development. 1998;125:4019–4032. doi: 10.1242/dev.125.20.4019. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Kardon G, Campbell JK, Tabin CJ. Local extrinsic signals determine muscle and endothelial cell fate and patterning in the vertebrate limb. Dev. Cell. 2002;3:533–545. doi: 10.1016/s1534-5807(02)00291-5. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Krüger M, Mennerich D, Fees S, Schafer R, Mundlos S, Braun T. Sonic hedgehog is a survival factor for hypaxial muscles during mouse development. Development. 2001;128:743–752. doi: 10.1242/dev.128.5.743. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Kühl M, Sheldahl LC, Malbon CC, Moon RT. Ca(2+)/calmodulin-dependent protein kinase II is stimulated by Wnt and Frizzled homologs and promotes ventral cell fates in Xenopus. J. Biol. Chem. 2000;275:12701–12711. doi: 10.1074/jbc.275.17.12701. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc. Natl Acad. Sci. USA. 2001;98:9306–9311. doi: 10.1073/pnas.151270098. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KKH, Wong CC, Webb SE, Tang MK, Leung AKC, Kwok PF, et al. Hepatocyte growth factor stimulates chemotactic response in mouse embryonic limb myogenic cells in vitro. J. Exp. Zool. 1999;283:170–180. doi: 10.1002/(sici)1097-010x(19990201)283:2<170::aid-jez7>3.0.co;2-p. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Lewis KE, Currie PD, Roy S, Schauerte H, Haffter P, Ingham PW. Control of muscle cell-type specification in the zebrafish embryo by Hedgehog signalling. Dev. Biol. 1999;216:469–480. doi: 10.1006/dbio.1999.9519. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, et al. Transcriptional co-activator PGC-1alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Liu D, Black BL, Derynck R. TGF-beta inhibits muscle differentiation through functional repression of myogenic transcription factors by Smad3. Genes Dev. 2001;15:2950–2966. 10.1046/j.1469-7580.2003.00136.x. [Google Scholar]
- Luo K, Stroschein SL, Wang W, Chen D, Martens E, Zhou S, et al. The Ski oncoprotein interacts with the Smad proteins to repress TGFbeta signaling. Genes Dev. 1999;13:2196–2206. doi: 10.1101/gad.13.17.2196. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankoo BS, Collins NS, Ashby P, Grigorieva E, Pevny LH, Candia A, et al. Mox2 is a component of the genetic hierarchy controlling limb muscle development. Nature. 1999;400:69–73. doi: 10.1038/21892. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Marics I, Padilla F, Guillemot J-F, Scaal M, Marcelle C. FGFR4 signaling is a necessary step in limb muscle differentiation. Development. 2002;129:4559–4569. doi: 10.1242/dev.129.19.4559. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Maroto M, Reshef R, Münsterberg AE, Koester S, Goulding M, Lassar AB. Ectopic Pax-3 activates MyoD and Myf-5 expression in embryonic mesoderm and neural tissue. Cell. 1997;89:139–148. doi: 10.1016/s0092-8674(00)80190-7. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lu N, Vogel H, Sellheyer K, Roop DR, Bradley A. Multiple defects and perinatal death in mice deficient in follistatin. Nature. 1995;374:360–363. doi: 10.1038/374360a0. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl Acad. Sci. USA. 1997;94:12457–12461. doi: 10.1073/pnas.94.23.12457. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennerich D, Schafer K, Braun T. Pax-3 is necessary but not sufficient for lbx1 expression in myogenic precursor cells of the limb. Mech. Dev. 1998;73:147–158. doi: 10.1016/s0925-4773(98)00046-x. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Miller JB, Stockdale FE. Developmental origins of skeletal muscle fibers: clonal analysis of myogenic cell lineages based on expression of fast and slow myosin heavy chains. Proc. Natl Acad. Sci. USA. 1986a;83:3860–3864. doi: 10.1073/pnas.83.11.3860. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JB, Stockdale FE. Developmental regulation of the multiple myogenic cell lineages of the avian embryo. J. Cell Biol. 1986b;103:2197–2208. doi: 10.1083/jcb.103.6.2197. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münsterberg AE, Kitajewski J, Bumcrot DA, McMahon AP, Lassar AB. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev. 1995;9:2911–2922. doi: 10.1101/gad.9.23.2911. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y, Hanaoka K, Hayasaka M, Esumi E, Li S, Nonaka I. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature. 1993;364:532–535. doi: 10.1038/364532a0. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Naya FJ, Mercer B, Shelton J, Richardson JA, Williams RS, Olson EN. Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J. Biol. Chem. 2000;275:4545–4548. doi: 10.1074/jbc.275.7.4545. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Nikovits W, Jr, Cann GM, Huang R, Christ B, Stockdale FE. Patterning of fast and slow fibers within embryonic muscles is established independently of signals from the surrounding mesenchyme. Development. 2001;128:2537–2544. doi: 10.1242/dev.128.13.2537. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Niswander L, Martin GR. Fgf-4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development. 1992;114:755–768. doi: 10.1242/dev.114.3.755. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Norris W, Neyt C, Ingham PW, Currie PD. Slow muscle induction by Hedgehog signalling in vitro. J. Cell Sci. 2000;113:2695–2703. doi: 10.1242/jcs.113.15.2695. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Ordahl CP, Le Douarin NM. Two myogenic lineages within the developing somite. Development. 1992;114:339–353. doi: 10.1242/dev.114.2.339. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Parr BA, McMahon AP. Dorsalizing signal Wnt-7a required for normal polarity of D-V and A-P axes of mouse limb. Nature. 1995;374:350–353. doi: 10.1038/374350a0. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Petropoulos H, Skerjanc IS. Beta-catenin is essential and sufficient for skeletal myogenesis in P19 cells. J. Biol. Chem. 2002;277:15393–15399. doi: 10.1074/jbc.M112141200. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Pin CL, Merrifield PA. Regionalized expression of myosin isoforms in heterotypic myotubes formed from embryonic and fetal rat myoblasts in vitro. Dev. Dyn. 1997;208:420–431. doi: 10.1002/(SICI)1097-0177(199703)208:3<420::AID-AJA12>3.0.CO;2-3. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Pourquie O, Fan CM, Coltey M, Hirsinger E, Watanabe Y, Breant C, et al. Lateral and axial signals involved in avian somite patterning: a role for BMP4. Cell. 1996;84:461–471. doi: 10.1016/s0092-8674(00)81291-x. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Pownall ME, Gustafsson MK, Emerson CP., Jr Myogenic Regulatory Factors and the Specification of Muscle Progenitors in Vertebrate Embryos. Annu. Rev. Cell Dev. Biol. 2002;18:18. doi: 10.1146/annurev.cellbio.18.012502.105758. [DOI] [PubMed] [Google Scholar]
- Rees E, Young RD, Evans DJR. Spatial and temporal contribution of somitic myoblasts to avian limb muscles. Dev. Biol. 2003 doi: 10.1016/s0012-1606(02)00028-3. in press. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Robson LG, Hughes SM. The distal limb environment regulates MyoD accumulation and muscle differentiation in mouse-chick chimaeric limbs. Development. 1996;122:3899–3910. doi: 10.1242/dev.122.12.3899. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Robson LG, Hughes SM. Local signals in the chick limb bud can override myoblast lineage commitment: induction of slow myosin heavy chain in fast myoblasts. Mech. Dev. 1999;85:59–71. doi: 10.1016/s0925-4773(99)00060-x. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Robson LG, Kara T, Crawley A, Tickle C. Tissue and cellular patterning of the musculature in chick wings. Development. 1994;120:1265–1276. doi: 10.1242/dev.120.5.1265. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Roy S, Wolff C, Ingham PW. The u-boot mutation identifies a Hedgehog-regulated myogenic switch for fiber-type diversification in the zebrafish embryo. Genes Dev. 2001;15:1563–1576. doi: 10.1101/gad.195801. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnicki MA, Schnegelsberg PN, Stead RH, Braun T, Arnold HH, Jaenisch R. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell. 1993;75:1351–1359. doi: 10.1016/0092-8674(93)90621-v. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Sachs M, Brohmann H, Zechner D, Muller T, Hulsken J, Walther I, et al. Essential role of Gab1 for signaling by the c-Met receptor in vivo. J. Cell Biol. 2000;150:1375–1384. doi: 10.1083/jcb.150.6.1375. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaal M, Bonafede A, Dathe V, Sachs M, Cann G, Christ B, Brand-Saberi B. SF/HGF is a mediator between limb patterning and muscle development. Development. 1999;126:4885–4893. doi: 10.1242/dev.126.21.4885. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Schäfer K, Braun T. Early specification of limb muscle precursor cells by the homeobox gene Lbx1h. Nat. Genet. 1999;23:213–216. doi: 10.1038/13843. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Tanaka M, Munsterberg A. Expression of (beta)-catenin in the developing chick myotome is regulated by myogenic signals. Development. 2000;127:4105–4113. doi: 10.1242/dev.127.19.4105. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Schramm C, Solursh M. The formation of premuscle masses during chick wing bud development. Anat. Embryol. 1990;182:235–247. doi: 10.1007/BF00185517. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Seed J, Hauschka SD. Temporal separation of the migration of distinct myogenic precursor populations into the developing chick wing bud. Dev. Biol. 1984;106:389–393. doi: 10.1016/0012-1606(84)90237-9. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Seed J, Hauschka SD. Clonal analysis of vertebrate myogenesis. VIII. Fibroblasts growth factor (FGF)-dependent and FGF-independent muscle colony types during chick wing development. Dev. Biol. 1988;128:40–49. doi: 10.1016/0012-1606(88)90264-3. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Semsarian C, Wu MJ, Ju YK, Marciniec T, Yeoh T, Allen DG, et al. Skeletal muscle hypertrophy is mediated by a Ca2+-dependent calcineurin signalling pathway. Nature. 1999;400:576–581. doi: 10.1038/23054. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Serrano AL, Murgia M, Pallafacchina G, Calabria E, Coniglio P, Lomo T, et al. Calcineurin controls nerve activity-dependent specification of slow skeletal muscle fibers but not muscle growth. Proc. Natl Acad. Sci. USA. 2001;98:13108–13113. doi: 10.1073/pnas.231148598. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997a;390:410–413. doi: 10.1038/37138. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Slusarski DC, Yang-Snyder J, Busa WB, Moon RT. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev. Biol. 1997b;182:114–120. doi: 10.1006/dbio.1996.8463. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Solursh M, Drake C, Meier S. The migration of myogenic cells from the somites at the wing level in avian embryos. Dev. Biol. 1987;121:389–396. doi: 10.1016/0012-1606(87)90175-8. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Song K, Wang Y, Sassoon D. Expression of Hox-7.1 in myoblasts inhibits terminal differentiation and induces cell transformation. Nature. 1992;360:477–481. doi: 10.1038/360477a0. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Stockdale FE. Myoblast diversity and the formation of the early limb musculature. Ann. NY Acad. Sci. 1990;599:111–118. doi: 10.1111/j.1749-6632.1990.tb42369.x. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Stockdale FE. Myogenic cell lineages. Dev. Biol. 1992;154:284–298. doi: 10.1016/0012-1606(92)90068-r. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Sutrave P, Leferovich JM, Kelly AM, Hughes SH. The induction of skeletal muscle hypertrophy by a ski transgene is promoter-dependent. Gene. 2000;241:107–116. doi: 10.1016/s0378-1119(99)00461-8. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Swartz ME, Eberhart J, Pasquale EB, Krull CE. EphA4/ephrin–A5 interactions in muscle precursor cell migration in the avian forelimb. Development. 2001;128:4669–4680. doi: 10.1242/dev.128.23.4669. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Sweeney LJ, Kennedy JM, Zak R, Kokjohn K, Kelley SW. Evidence for expression of a common myosin heavy chain phenotype in future fast and slow skeletal muscle during initial stages of avian embryogenesis. Dev. Biol. 1989;133:361–374. doi: 10.1016/0012-1606(89)90040-7. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Takayama H, LaRochelle WJ, Anver M, Bockman DE, Merlino G. Scatter factor/hepatocyte growth factor as a regulator of skeletal muscle and neural crest development. Proc. Natl Acad. Sci. USA. 1996;93:5866–5871. doi: 10.1073/pnas.93.12.5866. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J. Biol. Chem. 2000;275:40235–40243. doi: 10.1074/jbc.M004356200. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Tiidus PM, Bombardier E, Xeni J, Bestic NM, Vandenboom R, Rudnicki MA, et al. Elevated catalase activity in red and white muscles of MyoD gene- inactivated mice. Biochem. Mol. Biol. Int. 1996;39:1029–1035. doi: 10.1080/15216549600201192. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Tremblay P, Dietrich S, Mericskay M, Schubert FR, Li Z, Paulin D. A crucial role for Pax3 in the development of the hypaxial musculature and the long-range migration of muscle precursors. Dev. Biol. 1998;203:49–61. doi: 10.1006/dbio.1998.9041. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Uchiyama K, Ishikawa A, Hanaoka K. Expression of lbx1 involved in the hypaxial musculature formation of the mouse embryo. J. Exp.Zool. 2000;286:270–279. 10.1046/j.1469-7580.2003.00136.x. [PubMed] [Google Scholar]
- Van Swearingen J, Lance-Jones C. Slow and fast muscle fibers are preferentially derived from myoblasts migrating into the chick limb bud at different developmental times. Dev. Biol. 1995;170:321–337. doi: 10.1006/dbio.1995.1218. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Webb SE, Lee KK, Tang MK, Ede DA. Fibroblast growth factors 2 and 4 stimulate migration of mouse embryonic limb myogenic cells. Dev. Dyn. 1997;209:206–216. doi: 10.1002/(SICI)1097-0177(199706)209:2<206::AID-AJA6>3.0.CO;2-M. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Wigmore PM, Evans DJ. Molecular and cellular mechanisms involved in the generation of fiber diversity during myogenesis. Int. Rev. Cytol. 2002;216:175–232. doi: 10.1016/s0074-7696(02)16006-2. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Wilting J, Brand-Saberi B, Huang R, Zhi Q, Kontges G, Ordahl CP, et al. Angiogenic potential of the avian somite. Dev. Dyn. 1995;202:165–171. doi: 10.1002/aja.1002020208. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Woloshin P, Song K, Degnin C, Killary AM, Goldhamer DJ, Sassoon D, et al. MSX1 inhibits myoD expression in fibroblast x 10T1/2 cell hybrids. Cell. 1995;82:611–620. doi: 10.1016/0092-8674(95)90033-0. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Gotoh Y, Tamura K, Tanaka M, Kawakami A, Ide H, et al. Coordinated expression of Hoxa-11 and Hoxa-13 during limb muscle patterning. Development. 1998;125:1325–1335. doi: 10.1242/dev.125.7.1325. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Yang XM, Vogan K, Gros P, Park M. Expression of the met receptor tyrosine kinase in muscle progenitor cells in somites and limbs is absent in Splotch mice. Development. 1996;122:2163–2171. doi: 10.1242/dev.122.7.2163. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]
- Zhang M, McLennan IS. Primary myotubes preferentially mature into either the fastest or slowest muscle fibers. Dev. Dyn. 1998;213:147–157. doi: 10.1002/(SICI)1097-0177(199809)213:1<147::AID-AJA15>3.0.CO;2-#. 10.1046/j.1469-7580.2003.00136.x. [DOI] [PubMed] [Google Scholar]