Abstract
Sonic hedgehog, SHH, is required for patterning the limb. The array of skeletal elements that compose the hands and feet, and the ordered arrangement of these bones to form the pattern of fingers and toes are dependent on SHH. The mechanism of action of SHH in the limb is not fully understood; however, an aspect that appears to be important is the localized, asymmetric expression of Shh. Shh is expressed in the posterior margin of the limb bud in a region defined as the zone of polarizing activity (ZPA). Analysis of mouse mutants which have polydactyly (extra toes) shows that asymmetric expression of Shh is lost due to the appearance of an ectopic domain of expression in the anterior limb margin. One such polydactylous mouse mutant, sasquatch (Ssq), maps to the corresponding chromosomal region of the human condition pre-axial polydactyly (PPD) and thus represents a model for this condition. The mutation responsible for Ssq is located 1 Mb away from the Shh gene; however, the mutation disrupts a long-range cis-acting regulator of Shh expression. By inference, human pre-axial polydactyly results from a similar disruption of Shh expression. Other human congenital abnormalities also map near the pre-axial polydactyly locus, suggesting a major chromosomal region for limb dysmorphologies. The distinct phenotypes range from loss of all bones of the hands and feet to syndactyly of the soft tissue and fusion of the digits. We discuss the role played by Shh expression in mouse mutant phenotypes and the human limb dysmorphologies.
Keywords: acheiropodia, Lmbr1, pre-axial polydactyly, sasquatch, Shh
Introduction
During development graded concentration profiles of signalling molecules provide information across a field of cells. The concentration of the molecule informs the cells where they are and what they should do; such molecules are known as morphogens. In limb development a focal, regulatory domain was postulated by Saunders & Gasseling (1968), which fits the definition of a signalling centre responsible for the production of a graded morphogen signal (Wolpert, 1969). This posterior localized signalling centre is the zone of polarizing activity (ZPA), and is responsible for the pattern of digits in our hands and feet or the limbs of all tetrapods. The ZPA is a biologically distinct region of cells which has the experimental attribute that it can be manipulated and transplanted to different sites in the limbud. The addition of the ZPA to the anterior edge of the limb opposite the normal ZPA gives rise to extra digits. In the chick wingbud, in which all (three) digits are unique and distinguishable, an additional ZPA gives digit duplications in a mirror image of the normal digit pattern; the number and identity of the digits are directly dependent on the number of ZPA cells transplanted (Tickle, 1981; see also reviews by Panman & Zeller, and by Sanz-Ezquerro & Tickle, in this volume).
The search for the morphogen responsible for the polarizing activity produced by the ZPA settled on a molecule called sonic hedgehog (SHH) (Echelard et al. 1993; Krauss et al. 1993; Riddle et al. 1993; Roelink et al. 1994). SHH satisfied the predicted criteria for the limb morphogen. First, SHH, which is a homologue of the Drosophila morphogen hedgehog (HH), is a secreted factor. Secondly, the expression is highly restricted and co-localizes with the ZPA in vertebrate limb buds and thus is produced at a localized source. Both properties are essential for the initiation of a graded production of the putative morphogen.
Proof that the Shh gene participates in polarizing the limb came from the grafting of SHH-producing cells or beads impregnated with SHH protein into the anterior of limb buds. Both treatments were capable of producing mirror image digit duplications (Riddle et al. 1993; Chang et al. 1994; Lopez-Martinez et al. 1995). In addition, SHH production is ‘turned-on’ by retinoic acid, a known inducer of the ZPA (Riddle et al. 1993). The removal of Shh in knockout mice also reveals that SHH has a polarizing function (Chiang et al. 1996). Mice homozygous for the Shh knockout allele exhibit severe defects in many embryonic structures including the limb. Mutants have four limbs with recognizable A-P patterning in the stylopod (humerus/femur), but distal to the stylopod the A-P patterning is severely disrupted. The forelimb autopod is represented by a single distal cartilage element whereas the hindlimb autopod consists of a single digit (Chiang et al. 2001; Kraus et al. 2001). Thus SHH is vital for the activity of the ZPA, and for A-P patterning the zeugopod and autopod of the limb.
Shh misregulation leads to mouse limb abnormalities
What mechanisms control the restricted Shh expression in the posterior located ZPA of the limb bud? Tight control of transcription is required for the restricted ZPA production essential for a graded morphogen action. Indeed, realization of the restricted Shh expression led to investigations into the role of misregulation as the basis for some limb dysmorphologies. Work focused on a group of spontaneously derived mouse limb mutations which arose showing additional digits on the pre-axial (anterior) side of the limb: some mutants are affected on one set of limbs, some on both. These were classed as the hemimelic-luxate group of mutants (Table 1). In many of these mutants the extra-digit phenotype appeared as mirror-image duplications, reflecting the transplant experiments performed in the chick wing bud. Thus misdirected expression of Shh seemed a reasonable mechanism for generating polydactyly, and subsequently a number of these pre-axial polydactylous mice were shown to express Shh in the anterior margin of the limb.
Table 1. hemimelic-luxate mutations which mis-express Shh.
| Mutation | Chromosome | Gene | Reference |
|---|---|---|---|
| Dominant hemimelia (Dh) | 1 | ? | Lettice et al. (1999) |
| Luxate (lx) | 5 | ? | Masuya et al. (1997) |
| Rim4 | 6 | ? | Masuya et al. (1995) |
| Strong's luxoid (lst) | 2 | Alx4 | Masuya et al. (1997) |
| extra toes (Xt) | 13 | Gli3 | Buscher et al. (1997) |
| X-linked polydactyly (Xpl) | X | ? | Masuya et al. (1997) |
| Hemimelic extratoes (Hx) | 5 | Lmbr1 | Clark et al. (2000) |
| Sasquatch (Ssq) | 5 | Shh | Sharpe et al. (1999) |
The genetic bases for Shh misexpression for three of the hemimelic-luxate mutations are known, i.e. the genes responsible for Strong's luxoid (lst) and extra toes (Xt) have been identified (the third, sasquatch, is discussed below). There are three alleles of the lst mutation, all of which are genetic defects (one is a targeted mutation in ES cells) in the Alx4 gene (Qu et al. 1997, 1998; Takahashi et al. 1998). Alx4 is a paired-type homeobox-containing gene that is homologous to Drosophila aristaless. The lst phenotype is a result of loss of function of Alx4 and is dosage dependent; as a heterozygote the hindlimbs are polydactylous whereas as a homozygote all four limbs are affected as well as displaying a broader phenotype. The suggestions are that Alx4, perhaps through interactions with the related gene Cart1 (Qu et al. 1999), is important in repressing the anterior limb bud expression of the Shh gene, important in assuring asymmetric, posterior expression of the gene. Gli3, the kruppel-like zinc finger-containing gene, has been identified as the gene responsible for the Xt mutation (Hui & Joyner, 1993). As postulated for Alx4, Gli3 may be a repressor of anterior Shh (Masuya et al. 1995; Buscher et al. 1997) such that reduction in gene dosage results in anterior Shh expression. (Other roles for Gli3 in the limb are more fully discussed in this volume: see review by Panman & Zeller).
Ssq: an Shh regulatory mutant
We have focused our work on the analysis of the Ssq polydactylous mutation. The Ssq mutation arose as the result of the random insertion of a Hoxb1 human placental alkaline phosphatase (HPAP) reporter construct (Sharpe et al. 1999). In adults heterozygous for the Ssq mutation the forelimbs are normal, but the hindlimbs display pre-axial polydactyly. Homozygotes (Fig. 1A,C) exhibit more extensive limb abnormalities, the hindlimb polydactyly is more severe, and the zeugopod displays hemimelia (reduction in the length of the tibia). Homozygous forelimbs also display pre-axial polydactyly and slight hemimelia. The semidominant nature of the Ssq mutation and the observation that hindlimbs are more severely affected than forelimbs is consistent with other members of the hemimelia-luxate group of mutants. Analysis of Shh expression in the developing Ssq limbuds showed, like other hemimelia-luxate mutants, ectopic anterior expression (Fig. 2A,B). Unlike most of the hemimelia-luxate group no other defects are seen outside of the limb.
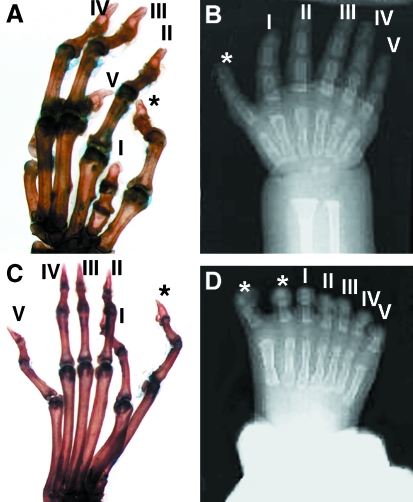
Fig. 1.
Comparison of limbs seen in mouse and human limb dysmorphologies. Panels A and C show the bones of left fore (A) and hind (C) limbs from a homozygous Ssq mouse. Panels B and D show a radiogram of the right hand (B) and foot (D) of a patient with PPD. The roman numerals mark the positions of the normal digits and the asterisks mark the supernumerary digits.
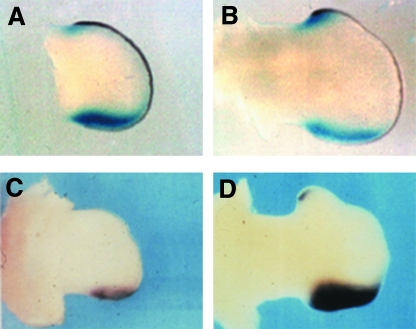
Fig. 2.
Comparison of the expression patterns of the Shh gene and the HPAP reporter gene in limbs of the Ssq mouse. Panels A and B show in situ hybridization analysis of Shh expression in the hindlimb bud of an E10.5 embryo (A) and an E12.5 (B) embryo. The limbs are double labelled; Fgf8 expression in the AER is shown by the dark brown label and Shh expression by the blue. In all panels the anterior margin of the limb is situated at the top and the posterior margin is at the bottom. The ZPA expression is shown by the large blue area at the bottom of the panel, and the smaller blue domain at the top of the panel represents the ectopic expression. Panels C and D show the alkaline phosphatase expression from the HPAP reporter gene that composes the Ssq transgene. The black areas show regions of alkaline phosphatase activity in an area that overlaps the ZPA at E10.5 (C) and E12.5 (D) and the ectopic anterior domain at E12.5.
Genetic analysis showed that the transgene insertion site on chr 5, which segregates with the polydactylous phenotype, was physically linked to the Shh gene but was situated almost 1 Mb away (Fig. 3A) (Sharpe et al. 1999). The transgenic insertion site was cloned and found to lie within an intron of a gene identified as Lmbr1 (Lettice et al. 2002). The insertion event led to duplication of the surrounding ∼20 kb of the intron.
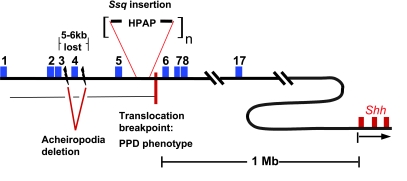
Fig. 3.
A composite representation of human and mouse Lmbr1/LMBR1 genes showing the relative positions of the limb mutations. The Lmbr1 gene is composed of 17 exons represented by the blue rectangles and is 1 MB away from the Shh (exons in red) gene. The position of the Ssq insertion site in intron 5 of mouse and the close relationship to the human PPD translocation breakpoint in the corresponding human intron are represented. The brackets around the HPAP suggest that the transgene has incorporated multiple times (n > 10). The acheiropodia deletion is shown around exon 4; most of the 5–6 kb of deletion is intronic DNA.
Human limb mutations mapped to 7q36
Preaxial polydactyly (PPD [MIM190605]), also referred to as pre-axial polydactyly type II (MIM174500), is one of the most frequently observed human congenital limb malformations. PPD is observed in sporadic cases, but most patients show an autosomal dominant mode of inheritance. The limb-specific phenotype varies markedly within families ranging from a simple addition of a phalanx in triphalangeal thumb to whole digit duplications and tibial aplasia. Genetic analysis of families showed that the PPD locus mapped to a 450-kb region on chromosome 7q36 (Heus et al. 1999) and all affected families described so far are linked to this locus (Heutink et al. 1994; Zguricas et al. 1994; Hing et al. 1995; Radhakrishna et al. 1997). PPD resides in a chromosomal region syntenic to the Lmbr1 region of mouse chromosome 5 and is limb specific. The similarity in phenotype (Fig. 1, compare A, C with B, D) and genomic location between Ssq and limb-specific PPD led us to suggest that Ssq is a mouse model for PPD. Interestingly, however, a thorough analysis of the LMBR1 structural gene failed to uncover any deleterious mutations (Lettice et al. 2002).
A major advance in investigating the genetic basis of PPD came from a young Japanese PPD patient who was found to carry a de novo reciprocal translocation t(5,7)(q11,q36) (Lettice et al. 2002). Fine mapping identified the location of the translocation breakpoint (Fig. 3) which lies within intron 5 of LMBR1, the corresponding mouse intron carrying the Ssq insertion.
The basis of the polydactylous mutations
Perturbations of the Lmbr1 gene in mouse and human were originally thought to play a role in generating the polydactyly phenotype, perhaps by acting as a regulator of the Shh gene. Lmbr1 encodes a 490 amino acid protein (LMBR1) (Clark et al. 2000) and possibly a 32 amino acid peptide by alternative splicing (LMBR1S). LMBR1 is hydrophobic and predicted to contain nine transmembrane domains. However, despite the efforts of several groups including our own, significant expression of Lmbr1 could not be detected in a developmentally relevant pattern in the mouse limb bud by in situ hybridization (unpublished observations), suggesting that Lmbr1 is expressed at low levels probably ubiquitously in the mouse. Secondly, and more importantly, no relevant mutations were found in the Lmbr1 structural gene in human or mouse.
As discussed above, the Ssq mutation was generated by the random insertion of a transgene that included a HPAP reporter. Intriguingly, unlike the other seven lines generated with the same construct, when Ssq embryos were assayed for HPAP activity, they exhibited HPAP expression in the limb in addition to the typical rhombomere-4 pattern mediated by Hoxb1 elements within the transgene (Sharpe et al. 1999). The limb pattern is first detected in the ZPA at E10.5 (Fig. 2C), and closely parallels that of endogenous Shh in the limb. Ssq heterozygous animals were also shown to exhibit HPAP activity in an overlapping anterior domain of the hindlimb buds that mirrors the anterior ectopic Shh (Fig. 2D). Homozygous embryos demonstrate anterior HPAP staining in forelimbs as well, consistent with the appearance of ectopic Shh. Thus, the HPAP reporter at the Ssq insertion site mirrors both normal and ectopic expression of Shh in the limb buds of Ssq heterozygotes and homozygotes.
These observations suggested a second hypothesis, that the HPAP transgene at the Ssq insertion has come under the influence of a cis-acting gene regulator that drives expression in the limb bud. A possible scenario is that the Ssq insertion event has revealed the presence of this regulator required for driving Shh expression in the limb, and in addition has disrupted the activity, such that Shh becomes anteriorly expressed in Ssq limb buds. This hypothesis is attractive as it does not require the limb mutations mentioned above to affect the expression of Lmbr1. Instead, the various genetic lesions mapped to human chr 7q36/mouse chr 5 could act to disrupt cis-acting regulatory elements of Shh.
Genetic test to distinguish hypotheses
To examine the basis for pre-axial polydactyly a cis-trans genetic test was devised. The prediction tested was that an Shh regulator would function in a cis-acting manner, i.e. the Ssq mutation would affect only chromosomally linked Shh. In contrast, disruption of the Lmbr1 structural gene which secondarily affected Shh expression would operate on both Shh alleles and therefore in trans. A mouse cross was devised to derive a recombinant chromosome 5 in which the Ssq mutation was located in cis to the targeted null allele of Shh. In the analysis of the phenotype, mice carrying the recombinant chromosome would exhibit extra pre-axial toes if Ssq is acting on Shh in trans and wildtype feet, i.e. suppression of the Ssq phenotype, if acting in cis.
Analysis of the limb phenotype in five recombinant mice showed no pre-axial polydactyly or other detectable limb phenotypes. Thus the data demonstrate that the Shh null allele inactivates the affects of the Ssq mutation when located in cis (but not in trans) on chromosome 5. It follows that the Ssq insertion is a dominant acting mutation that interferes with the limb-specific expression of Shh. The data are consistent with a long-range limb-specific regulator of Shh residing within or near the Lmbr1 gene. In addition, the data are consistent with human PPD resulting from similar disruption of a long-range SHH regulator.
Acheiropodia: congenital abnormalites without hands or feet
Human chromosome 7q36 region is becoming recognized as a major locus for limb defects. In addition to PPD, other defects including acheiropodia (Ianakiev et al. 2001), complex polysyndactyly (CPS) (Tsukurov et al. 1994) and acropectoral syndrome (distinct but related to F syndrome) (Dundar et al. 2001) map to the 7q36 region. In mouse two additional mutations map to the corresponding murine chromosome. The spontaneously derived mutation hemimelic extra toe (Hx) (Clark et al. 2000), a likely allele of Ssq, maps to the same locus but is distinct from hammertoe (Hm, a syndactyly phenotype) which may be the mouse counterpart of human CPS.
CPS in human occurs more rarely than PPD, and is typically bilateral, showing both pre- and post-axial polydactyly and syndactyly (fusion of the soft tissue between the digits) (Tsukurov et al. 1994). Acropectoral syndrome is similar, showing pre-axial polydactyly and syndactyly of the soft tissue: however, patients present with other skeletal defects such as a prominent upper sternum and blind-ending U-shaped sinus in the anterior chest wall. Compelling as the mapping relationship is, little is known about the molecular aetiology of either CPS or acropectoral syndrome. A recent report, however, showed that the autosomal recessive acheiropodia (Ianakiev et al. 2001) results from a small deletion within the LMBR1 gene. Acheiropodia is a severe limb-specific phenotype and, in contrast to PPD, patients present loss of all bones of the hands and feet, and the tibia is truncated distally. Analysis of five families with acheiropodia (Ianakiev et al. 2001) identified a critical region for the mutation on 7q36 and subsequently affected individuals showed deletions in both LMBR1 alleles that remove exon 4 and approximately 5–6 kb of surrounding genomic DNA (Fig. 3). These data were interpreted as suggesting that acheiropodia results from loss of function of the Lmbr1 gene.
Model for the role of Shh in limb defects
We predict that the inserted transgene in the Ssq genome, expression of which is activated in an Shh-like limb pattern, marks a proximate regulator which drives normal Shh expression. Thus the regulators responsible for normal as well as abnormal expression of Shh may reside in or near the Lmbr1 gene. The analysis so far reported does not distinguish between regulation dependent on chromatin or chromosome structure or a traditional enhancer-like element. Clearly, a number of distinct regulatory activities are required to promote the long-distance regulation of Shh (Fig. 4). First, the regulator must convey an early limb bud signal over a distance of 1 Mb to initiate and drive Shh expression. Secondly, since Lmbr1 is located within a cluster of tightly linked genes, none of which is expressed in the limb, the regulator must be able to control Shh expression without affecting that of the nearest genes. Thirdly, elements which drive Shh expression in the anterior margin of the limb bud exist but must normally be repressed.
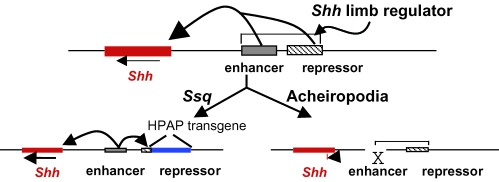
Fig. 4.
Shh regulatory model for the generation of limb abnormalities in mouse and human. Multiple elements for a major, limb-specific regulator of Shh are postulated. These elements lie in or near the Lmbr1 gene. At least two elements are necessary to generate the diverse limb phenotypes represented by Ssq (and PPD) and acheiropodia. First, an enhancer (grey box) is postulated which drives both the normal ZPA expression and the ectopic, anterior expression (the ectopic expression seen in Fig. 2A and B). Secondly, a repressor (cross hatched box) is present which down-regulates the ectopic, anterior expression. The Ssq transgene insertion (blue box) disrupts the repressor allowing anterior expression, and in the process the transgene has come under the influence of the enhancer (produces the pattern seen in Fig. 2D). The acheiropodia deletion removes and inactivates the enhancer such that there is no limb-specific expression of Shh.
Further predictions suggest that the long-distance Shh regulator is defective in the limb dysmorphologies that map to human chromosome 7q36. Disruption of different components of the regulator lead to the spectrum of dysmorphic phenotypes described. Pre-axial polydactyly, we submit, results from disruption of the proposed repressor of anterior limb bud expression. The location of the Ssq transgene insertion site and the PPD chromosomal translocation breakpoint suggests that the approximate genomic location of this repressor is in or near intron 5 of the Lmbr1 gene. This repressor normally acts to suppress anterior expression; the genetic mutations, however, disrupt this repressor and enable the misexpression of Shh. Since PPD is relatively common in the human population (Heutink et al. 1994), it seems likely that a number of independent mutations interrupt the repressor element.
Furthermore we hypothesize that LMBR1 is also incidental to the acheiropodia phenotype and propose an alternative rationale (Fig. 4). The ∼5-kb acheiropodia deletion from within the LMBR1 gene removes exon 4 and surrounding intronic sequences. Consistent with the proposal for generating polydactyly, we suggest that the acheiropodia mutation disrupts an SHH regulatory element; however, the mechanism is distinct from PPD in that normal ZPA expression of SHH is disrupted. These regulatory elements responsible for the phenotype may lie in the deleted region surrounding exon 4. Comparison of the acheiropodia phenotype with the mouse Shh targeted deletion (Chiang et al. 1996) is suggestive that loss of Shh plays a key role. In Shh−/– mutant mice the limbs show loss of all bones of the feet and truncations of the long bones, similar to that seen in the acheiropodia patients (Chiang et al. 2001; Kraus et al. 2001). We suggest that, in contrast to Ssq and PPD, acheiropodia results from a limb-specific loss of Shh expression. Thus, depending on the type of mutation, distinct phenotypes can arise. At present there is no hypothesis as to how CPS and acropectoral syndromes are caused. Molecular analysis of the proposed Shh regulators will be required for a clearer understanding of normal regulation and the relationship to these limb dysmorphologies.
Conclusions
The door is now open for the molecular analysis of limb-specific regulation of Shh to help answer some of the outstanding questions presented by the genetics of these limb abnormalities. Foremost is the issue surrounding the nature of regulatory elements disrupted by the mouse and human mutations. These elements are located ∼1 Mb away from the target gene. Thus are these elements the basis for moulding chromatin or chromosomal structure pertinent to Shh transcriptional activity? Alternatively, can enhancer elements act over such a distance of the chromosome to regulate a gene? It is difficult to understand why regulatory elements would reside at such a distance from the gene they regulate, and in the complicating circumstances of lying closer to unaffected genes. Such an arrangement of putative regulators along the chromosome may aid in pinpointing both transcriptional activators and silencers and the extreme distance may help in uncovering the chromatin interactions which presumably occur.
A second conundrum suggested by the analysis discussed above is the anterior ectopic expression of Shh. Speculatively, a molecular consequence of normal expression may be the ectopic anterior expression in the limb bud. To achieve the normal asymmetric pattern, the anterior expression must therefore be repressed. Interestingly, early tetrapods, such as Acanthostega and Ichthyostega, had large footplates composed of 7–8 digits (Kardong, 1998), perhaps a product of a primitive, unrepressed pattern of Shh expression. Candidate molecules involved in anterior repression of Shh are suggested by other mouse mutants of the hemimelia-luxate group, and presently the best candidate is Alx4. Further studies will possibly shed more light on both the normal regulation of Shh and the mechanisms for generating the disease phenotypes.
References
- Buscher D, Bosse B, Heymer J, Ruther U. Evidence for genetic control of Sonic hedgehog by Gli3 in mouse limb development. Mech. Dev. 1997;62:175–182. doi: 10.1016/s0925-4773(97)00656-4. [DOI] [PubMed] [Google Scholar]
- Chang DT, Lopez A, von Kessler DP, Chiang C, Simandl BK, Zhao R, et al. Products, genetic linkage and limb patterning activity of a murine hedgehog gene. Development. 1994;120:3339–3353. doi: 10.1242/dev.120.11.3339. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Harris MP, Simandl BK, Li Y, Beachy PA, et al. Manifestation of the limb prepattern: limb development in the absence of sonic hedgehog function. Dev. Biol. 2001;236:421–435. doi: 10.1006/dbio.2001.0346. [DOI] [PubMed] [Google Scholar]
- Clark RM, Marker PC, Kingsley DM. A novel candidate gene for mouse and human preaxial polydactyly with altered expression in limbs of Hemimelic extra-toes mutant mice. Genomics. 2000;67:19–27. doi: 10.1006/geno.2000.6225. [DOI] [PubMed] [Google Scholar]
- Dundar M, Gordon TM, Ozyazgan I, Oguzkaya F, Ozkul Y, Cooke A, et al. A novel acropectoral syndrome maps to chromosome 7q36. J. Med. Genet. 2001;38:304–309. doi: 10.1136/jmg.38.5.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- Heus HC, Hing A, van Baren MJ, Joosse M, Breedveld GJ, Wang JC, et al. A physical and transcriptional map of the preaxial polydactyly locus on chromosome 7q36. Genomics. 1999;57:342–351. doi: 10.1006/geno.1999.5796. [DOI] [PubMed] [Google Scholar]
- Heutink P, Zguricas J, van Oosterhout L, Breedveld GJ, Testers L, Sandkuijl LA, et al. The gene for triphalangeal thumb maps to the subtelomeric region of chromosome 7q. Nat. Genet. 1994;6:287–292. doi: 10.1038/ng0394-287. [DOI] [PubMed] [Google Scholar]
- Hing AV, Helms C, Slaugh R, Burgess A, Wang JC, Herman T, et al. Linkage of preaxial polydactyly type 2–7q36. Am. J. Med. Genet. 1995;58:128–135. doi: 10.1002/ajmg.1320580208. [DOI] [PubMed] [Google Scholar]
- Hui CC, Joyner AL. A mouse model of greig cephalopolysyndactyly syndrome: the extra-toesJ mutation contains an intragenic deletion of the Gli3 gene. Nat. Genet. 1993;3:241–246. doi: 10.1038/ng0393-241. [DOI] [PubMed] [Google Scholar]
- Ianakiev P, van Baren MJ, Daly MJ, Toledo SP, Cavalcanti MG, Neto JC, et al. Acheiropodia is caused by a genomic deletion in C7orf2, the human orthologue of the Lmbr1 gene. Am. J. Hum. Genet. 2001;68:38–45. doi: 10.1086/316955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardong K. Vertebrates: Comparative Anatomy, Function, Evolution. Boston MA: McGraw-Hill.; 1998. [Google Scholar]
- Kraus P, Fraidenraich D, Loomis CA. Some distal limb structures develop in mice lacking Sonic hedgehog signaling. Mech. Dev. 2001;100:45–58. doi: 10.1016/s0925-4773(00)00492-5. [DOI] [PubMed] [Google Scholar]
- Krauss S, Concordet JP, Ingham PW. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75:1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- Lettice L, Hecksher-Sorensen J, Hill RE. The dominant hemimelia mutation uncouples epithelial–mesenchymal interactions and disrupts anterior mesenchyme formation in mouse hindlimbs. Development. 1999;126:4729–4736. doi: 10.1242/dev.126.21.4729. [DOI] [PubMed] [Google Scholar]
- Lettice LA, Horikoshi T, Heaney SJ, van Baren MJ, van der Linde HC, Breedveld GJ, et al. Disruption of a long-range cis-acting regulator for Shh causes preaxial polydactyly. Proc. Natl Acad. Sci. USA. 2002;99:7548–7553. doi: 10.1073/pnas.112212199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Martinez A, Chang DT, Chiang C, Porter JA, Ros MA, Simandl BK, et al. Limb-patterning activity and restricted posterior localization of the amino-terminal product of Sonic hedgehog cleavage. CurrBiol. 1995;5:791–796. doi: 10.1016/s0960-9822(95)00156-4. [DOI] [PubMed] [Google Scholar]
- Masuya H, Sagai T, Wakana S, Moriwaki K, Shiroishi T. A duplicated zone of polarizing activity in polydactylous mouse mutants. Genes Dev. 1995;9:1645–1653. doi: 10.1101/gad.9.13.1645. [DOI] [PubMed] [Google Scholar]
- Masuya H, Sagai T, Moriwaki K, Shiroishi T. Multigenic control of the localization of the zone of polarizing activity in limb morphogenesis in the mouse. DevBiol. 1997;182:42–51. doi: 10.1006/dbio.1996.8457. [DOI] [PubMed] [Google Scholar]
- Qu S, Niswender KD, Ji Q, van der Meer R, Keeney D, Magnuson MA, et al. Polydactyly and ectopic ZPA formation in Alx-4 mutant mice. Development. 1997;124:3999–4008. doi: 10.1242/dev.124.20.3999. [DOI] [PubMed] [Google Scholar]
- Qu S, Tucker SC, Ehrlich JS, Levorse JM, Flaherty LA, Wisdom R, et al. Mutations in mouse Aristaless-like4 cause Strong's luxoid polydactyly. Development. 1998;125:2711–2721. doi: 10.1242/dev.125.14.2711. [DOI] [PubMed] [Google Scholar]
- Qu S, Tucker SC, Zhao Q, deCrombrugghe B, Wisdom R. Physical and genetic interactions between Alx4 and Cart1. Development. 1999;126:359–369. doi: 10.1242/dev.126.2.359. [DOI] [PubMed] [Google Scholar]
- Radhakrishna U, Blouin JL, Mehenni H, Patel UC, Patel MN, Solanki JV, et al. Mapping one form of autosomal dominant postaxial polydactyly type A to chromosome 7p15-q11.23 by linkage analysis. Am. J. Hum. Genet. 1997;60:597–604. [PMC free article] [PubMed] [Google Scholar]
- Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75:1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- Roelink H, Augsburger A, Heemskerk J, Korzh V, Norlin S, Ruiz i Altaba A, et al. Floor plate and motor neuron induction by vhh-1, a vertebrate homolog of hedgehog expressed by the notochord. Cell. 1994;76:761–775. doi: 10.1016/0092-8674(94)90514-2. [DOI] [PubMed] [Google Scholar]
- Saunders JW, Gasseling MT. Ectoderm–mesenchymal interactions in the origins of wing symmetry. In: Fleishman R, Billingham RE, editors. Epithelial–Mesenchymal Interactions. Baltimore: Williams & Wilkins; 1968. pp. 78–97. [Google Scholar]
- Sharpe J, Lettice L, Hecksher-Sorensen J, Fox M, Hill R, Krumlauf R. Identification of sonic hedgehog as a candidate gene responsible for the polydactylous mouse mutant Sasquatch. Curr. Biol. 1999;9:97–100. doi: 10.1016/s0960-9822(99)80022-0. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Tamura K, Buscher D, Masuya H, Yonei-Tamura S, Matsumoto K, et al. The role of Alx-4 in the establishment of anteroposterior polarity during vertebrate limb development. Development. 1998;125:4417–4425. doi: 10.1242/dev.125.22.4417. [DOI] [PubMed] [Google Scholar]
- Tickle C. The number of polarizing region cells required to specify additional digits in the developing chick wing. Nature. 1981;289:295–298. doi: 10.1038/289295a0. [DOI] [PubMed] [Google Scholar]
- Tsukurov O, Boehmer A, Flynn J, Nicolai JP, Hamel BC, Traill S, et al. A complex bilateral polysyndactyly disease locus maps to chromosome 7q36. Nat. Genet. 1994;6:282–286. doi: 10.1038/ng0394-282. [DOI] [PubMed] [Google Scholar]
- Wolpert L. Positional information and the spatial pattern of cellular differentiation. J. Theor. Biol. 1969;25:1–47. doi: 10.1016/s0022-5193(69)80016-0. [DOI] [PubMed] [Google Scholar]
- Zguricas J, Snijders PJ, Hovius SE, Heutink P, Oostra BA, Lindhout D. Phenotypic analysis of triphalangeal thumb and associated hand malformations. J. Med. Genet. 1994;31:462–467. doi: 10.1136/jmg.31.6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]