Abstract
Motoneurones that supply the vertebrate limb innervate their muscle targets in a highly reproducible manner. As development proceeds, these limb-specific motoneurones send out axons, which grow towards the developing limb and then congregate at its base to form the plexus. In the plexus, in response to unknown positional cues, these axons rearrange, often changing their original spatial relationships, before sorting out to emerge in the defined nerve trunks that innervate the limb. Several proposals have been put forward to explain how this reproducible innervation pattern is achieved. These include (1) that early differences in the motoneurone identity dictate their future axonal trajectories, (2) that axons actively respond to attractive or repulsive positional cues provided by the limb bud itself, or (3) that motor axons are passively deployed, following pathways of least mechanical resistance. We have addressed the question of the relative roles of motoneurone identity and the signals that the axons encounter on their journey towards the limb bud. Using the developing chick embryo as our experimental model we tested the effect of providing an additional limb target for motor axons leaving the flank level of the spinal cord. To do this we placed FGF-soaked beads in the presumptive flank of 2-day-old chick embryos. This treatment induces an additional limb containing muscles. We investigated whether such additional limbs are innervated and by which neurones. We show that rather than the additional limbs being solely supplied by axons diverted from the two existing limb plexuses, motoneurones that normally supply the flank alter their trajectories to enter the induced limb. Once in the limb, axons respond to positional cues within the bud to generate the stereotypical innervation pattern. Our results show that the tendency of ‘flank’ motoneurones to innervate flank can be overcome by the presence of an additional limb.
Keywords: chick wing bud, innervation
Introduction
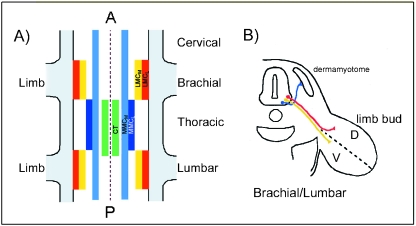
The muscles of the limb must be innervated in an accurate, precise and reproducible fashion to ensure co-ordinated movement. The motoneurones that innervate the limb muscles are spatially arranged in the embryonic spinal cord. After they are born, motoneurones settle into motor columns within the cord, which occupy defined locations along the anterior–posterior (AP) and transverse axis of the embryo (Levi-Montalcini, 1950; Landmesser, 1978; Fig. 1). Motoneurones that lie within the Medial Motor Column (MMC) innervate dorsal and ventral muscle targets in the body wall and flank, whilst those within the Column of Terni (CT), a motor column extending over thoracic and rostral lumbar levels (Levi-Montalcini, 1950), project into the sympathetic chain. Motoneurones that innervate muscles in the paired limb buds lie in the Lateral Motor Columns (LMC) which are only present at brachial and lumbar regions (limb bud levels) of the embryo. Detailed mapping experiments by Landmesser and colleagues showed that there is a topographical relationship between the location of motoneurone cell bodies within the LMC and the limb muscles that they innervate (Landmesser, 1978; Lance-Jones & Landmesser, 1981a; Fig. 1). Motoneurones in the medial part of the LMC (LMCM) innervate ventral limb muscle targets, whereas those within the lateral LMC (LMCL) innervate dorsal targets. Further, motoneurones that innervate the same limb muscle are clustered into motor pools, which occupy characteristic locations along the anterio-posterior axis of the LMC (Landmesser, 1978; Hollyday, 1980). The LMC motoneurones send out axons that grow out through the anterior portion of the adjacent somites forming discrete spinal nerves (Fig. 2; Keynes & Stern, 1984). These nerves enter the somatopleure (comprising ectoderm and underlying lateral plate mesoderm) and congregate at the base of the limb forming the plexus (brachial or lumbar), then undergo a ‘waiting period’ during which time undefined changes occur in the limb bud necessary to permit them to enter (Wang & Scott, 2000). Within the plexus the motor axons rearrange, in response to unknown positional cues, before emerging in defined nerve trunks that innervate the limb (Landmesser, 1978; Lance-Jones & Landmesser, 1981a; Tosney & Landmesser, 1985; Fig. 2).
Fig. 1.
The topographical relationship between the position of motoneurone cell bodies in the spinal cord and their muscle targets in the developing limb bud. (A) Schematic diagram showing the organization of the motor columns (depicted as coloured boxes) in the vertebrate spinal cord. Motoneurones located in the Medial Motor Column (MMC) innervate dorsal and ventral targets in the flank and body wall. This column is subdivided into medial (MMCM; light blue) and lateral (MMCL; dark blue) portions. Motoneurones in the Column of Terni (CT; green) innervate visceral targets. At limb bud levels (brachial and lumbar) the lateral motor column (LMC) contains the motoneurones that innervate the limb bud. It is subdivided into the medial part (LMCM) which sends out axons to ventral muscle targets (shown in yellow), and the lateral LMC (LMCL) which houses motoneurones that innervate dorsal muscle targets (shown in red). (B) Transverse section through the embryo at limb bud levels (brachial or lumbar) showing the axonal trajectories originating from motoneurones in the MMCM (blue), LMCM (yellow) and LMCL (red).
Fig. 2.
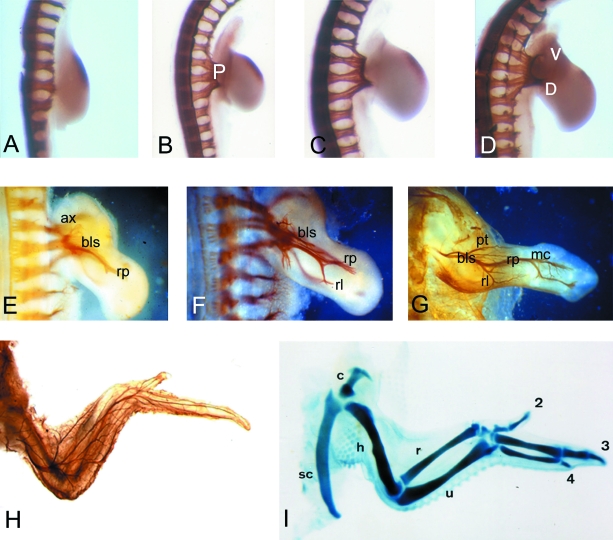
Normal innervation pattern of the developing chick wing bud. The innervation pattern has been detected by whole-mount immunohistochemistry with the 3A10 antibody. (A) stage 20; (B) stage 22; (C) stage 24, showing the location of the plexus (P); D) stage 25 showing dorsal (D) and ventral (V) trunks; (E) stage 26; (F) stage 28; (G) stage 34; (H) stage 36; (I) whole-mount cartilage stain with Alcian green to show the cartilage skeleton of the stage 36 chick embryo wing. In all cases the dorsal view of the wing bud is shown, and in the older stages (E–H) the ventral pattern of nerves is not visible in these preparations. In A–D, there is a small patch of non-specific staining in the anterior-distal wing mesenchyme. ax = axillaris; bls = brachialis longus superior; rp = radialis profundus; rl = radialis lateralis; pt = patagiales; mc = metacarpales; sc = scapula; c = coracoid; h = humerus; = radius; u = ulna and 2,3,4 refer to the three wing digits.
A combination of embryo and molecular manipulation experiments has supported a series of hypotheses to explain how the highly reproducible innervation pattern of the limb is achieved. One possibility is that early differences in the specification of motoneurone identity, as defined by differential gene expression, govern their future trajectories (Tsuchida et al. 1994; Lin et al. 1998; Liu et al. 2001). In the chick embryo, a critical period for motoneurone specification has been defined as being just after neural tube closure at stage 14–15 (embryonic day 2; Hamburger & Hamilton, 1951; Matise & Lance-Jones, 1996). This coincides with the stabilization of differential LIM homeodomain protein expression in each motor column subtype (Tsuchida et al. 1994). Undefined signals from the flanking paraxial mesoderm regulate LIM protein expression (Ensini et al. 1998), which may co-ordinate motoneurone specification in the cord with their peripheral dorsoventral (DV) targets. LIM proteins are also required for accurate dorsal/ventral pathway choices at the limb bud base (Kania et al. 2000). Recent progress has been made in understanding the basis of the arrangement of the motor pools within the LMC. Experiments in the mouse and chick show that changing Hox gene expression disrupts axonal projections from defined motor pools (Tiret et al. 1998; Bell et al. 1999; Liu et al. 2001). In addition, perturbing the expression of MN-cad, a type II cadherin, affects how the motor pools that normally express this protein segregate (Price et al. 2002).
Experimental evidence also shows that the emerging axons are exposed to a series of positional cues that are encountered along their route through the paraxial mesoderm, somatopleure and then within the limb bud itself. Chick grafting experiments exclude limb bud-level paraxial (somitic) mesoderm as the source of specific guidance information, because if the somites are removed by γ-irradiation, the major nerve pathways remain unaffected and still form in the muscleless limbs. However, local nerve-muscle branches are absent, thus implicating muscles in short-range nerve/target recognition (Lewis et al. 1981; Lance-Jones, 1988). The somatopleure is likely to be the primary source of positional cues, because when it is displaced anteriorly, prior to axon outgrowth and limb bud formation, motoneurones show parallel shifts in trajectory (Landmesser & Dias, 1991). Many possible candidates to provide the basis of these cues, for example netrins, semaphorins/collapsins, hepatocyte growth factor, neuropilins, Eph/ephrins as well as components of the extracellular matrix, are differentially expressed in the developing chick embryo at the time when axons are travelling towards the developing limb (Oakley & Tosney, 1991; reviewed in Varela-Echavarria & Guthrie, 1997; Stoeckli & Landmesser, 1998). For example, the ephrin receptor, EphA7, is expressed by cells flanking the specific sites where axons penetrate the developing cartilaginous limb girdles to enter the limbs (Araujo et al. 1998). Furthermore, expression of EphA7 is integrated with DV patterning of the limb bud itself since removal of the dorsal ectoderm results in rapid loss of EphA7 expression (Araujo et al. 1998). This manipulation is accompanied by the failure of the limb plexus to converge properly at the base of the bud, thus implicating correct EphA7 expression in normal plexus organization. Once within the limb bud itself it is known that motor axons are sensitive to anterio-posterior positional information (Lance-Jones & Landmesser, 1981b; Stirling & Summerbell, 1985, 1988) and so may be using cues related to, or regulated by, positional signals that pattern the limb itself.
A further possibility is that nerve guidance in the limb bud is a non-specific passive process, with motoneurones following pathways of least mechanical resistance (Horder, 1978), which may create the stereotyped anatomical innervation pattern. These routes seem related to limb bud identity, since the pattern of nerves within a limb bud is characteristic of the limb type (Stirling & Summerbell, 1983), and even foreign motoneurones can follow them (Swanson & Lewis, 1982). Experiments by Straznicky (1963) showed that when the limbs are left in situ, and sections of the spinal cord are grafted from non-limb levels into brachial or lumbar sites (limb bud levels), the nerves still grow into the limb along the characteristic pathways, and form an apparent normal innervation pattern. Similarly, when limb buds are transplanted to ectopic sites on the body they show patterns of innervation that resemble normal limbs (Swanson & Lewis, 1982; Stirling & Summerbell, 1983).
Not only can limbs be innervated in characteristic patterns by ‘foreign’ neurones but there is also evidence that neurones do not actively seek specific target muscles in the periphery. When the DV axis of the chick wing bud is reversed (by transplanting the right wing on to the left side thus maintaining the anterio-posterior orientation), the incoming nerves innervate any muscle they encounter (Stirling & Summerbell, 1985). Further experimental support for the passive guidance model comes from chick experiments in which the limbs were modified so that they had a double complement of dorsal thigh musculature. Neurones that normally innervated the ventral muscles (from the LMCM) innervated the dorsal muscles that were now in place on the ventral half of the limb bud (Lance-Jones, 1988). Together, these experimental data suggest that the characteristic and stereotyped pattern of innervation in the limb itself is independent of the type of neurone innervating the limb and that neurones can innervate non-target muscles. To date, the exact nature of these non-specific pathways remain uncharacterized, although recently interest has focused on possible associations between the extending nerve axons and developing blood vessels, since in the adult the innervation and vascular patterns show intriguing correspondence (Mukouyama et al. 2002).
It seems likely that elements of all these possibilities are deployed along the axon's route towards and within the limb. The challenge remains to establish the relative roles of each and to characterize how one set of guidance mechanisms interacts with the others. For example, the molecular identity of a motoneurone may affect how repulsive or attractive cues are perceived and responded to (Kania et al. 2000). We have begun to address this question by investigating the relationship between the presence of a limb bud and the outgrowth of motoneurones to innervate it. We have done this by implanting fibroblast growth factor (FGF)-soaked beads into the presumptive flank of stage 13/14 chick embryos. This treatment induces an additional limb (Cohn et al. 1995), which contains muscles (Ohuchi & Noji, 1999). Thus, this approach enables the role of differential gene expression by the motoneurones in the spinal cord to be distinguished from guidance cues in the limb bud. We have analysed whether these extra limbs are innervated, and used retrograde tracing techniques to establish the location of the motoneurone cell bodies in the spinal cord. Finally we have also established whether the anterio-posterior orientation of the innervation pattern is in register with that of the induced limb bud.
Materials and methods
Embryo manipulation
Fertile Hen's eggs were obtained from Winter Farm (Royston, Herts., UK) and were incubated in a humidified incubator at 38 °C until they reached stage 13/14 (embryonic day 2; Hamburger & Hamilton, 1951). Heparin beads (Sigma H5263) were soaked for 1 h with 1 mg mL−1 FGF-4 (R & D systems), then implanted into the chick embryos by cutting a small slit in the lateral plate mesoderm adjacent to somites 21–25 (future flank), using techniques as described by Cohn et al. (1995). The manipulated embryos were then returned to the incubator and incubated for either a further 48, 72 or 120 h.
Immunohistochemistry
Nerve axons were revealed by whole-mount immunohistochemistry with the 3A10 antibody (Developmental Studies Hybridoma Bank, Iowa, USA; DSHB), which detects a neurofilament-associated protein. Selected embryos were fixed in 4% paraformaldehyde for 3 h, then treated with 3% H2O2 in PBS and dehydrated in 100% methanol, all at room temperature. After this the embryos were rehydrated in PBS containing 0.1% Triton X-100 (PBTx), then preblocked in 10% goat serum in PBTx, before incubating with a 1 : 25 dilution of the 3A10 antibody for at least 24 h at 4 °C. Embryos were washed extensively in PBTx prior to preblocking as before, then incubated with a peroxidase-conjugated goat anti-mouse antibody (Jackson Laboratories) overnight at 4 °C at a 1 : 100 dilution. Following repeated washes in PBTx at room temperature the embryos were placed in 0.1 m Tris pH 7.5 prior to reacting with DAB in the presence of 0.001% H2O2 to visualize the pattern of antibody staining. After the reaction had completed the embryos were cleared in first 50% then finally 80% glycerol in PBS.
Embryos analysed with the LIM1/2 antibody (DSHB) were fixed in 4% PFA, then rinsed in PBS and placed in 40% sucrose overnight. They were then embedded in 1.5% agar (Gibco) containing 5% sucrose, frozen on dry ice and then cryosectioned. The sections were placed in PBS at 37 °C to remove the agar, then rinsed in PBTx before preblocking with 10% goat serum in PBTx. Sections were incubated in a 1 : 50 dilution of the LIM1/2 antibody. Antibody labelling was detected with an FITC-conjugated secondary antibody (Jackson Laboratories). Prior to mounting in Citifluor, sections were briefly counterstained by rinsing in PBS containing 0.001% DAPI. Sections were viewed by epifluorescence.
Retrograde tracing
Embryos chosen for axonal tracing experiments were labelled in ovo with the retrograde tracer horseradish peroxidase (HRP; Sigma type VI). This was done by making a small cut through the chorionic and amniotic membranes so that the additional limb became accessible. A small quantity of 40% HRP in H2O was injected into the dorsal side of the limb through a drawn glass microcapillary (Clarke Instruments). The egg was resealed and placed back in the incubator for 6 h to allow time for the HRP to be transported back along the axons to the motorneuron cell body in the spinal cord. Labelled embryos were then fixed overnight in 2% glutaraldehyde, then infiltrated overnight with 20% sucrose in PBS prior to frozen sectioning. Sections were processed in DAB and H2O2 to detect the HRP. Those sections containing HRP were mounted in DPX, and analysed by light microscopy.
Embryos used for whole-mount in situ hybridization were fixed overnight in 4% paraformaldehyde, and then hybridized with the EphA7 probe (Araujo et al. 1998; kindly provided by A. Nieto and M. Ros), using methods as described by Wilkinson (1992).
Results
Normal innervation pattern of the chick wing bud
The motor axons that innervate the chick wing bud emerge from the spinal cord and extend towards the wing in spinal nerves (XII to XVII; Roncali, 1970). These begin to grow towards the wing base from stage 20 (Hamburger & Hamilton, 1951; embryonic day 3; Fig. 2A), and once there congregate to form the plexus (Fig. 2B,C). The axons then wait for approximately 24 h before finally entering the wing bud at stage 25/6 (4.5 days incubation), branching to follow either a dorsal or a ventral trajectory (Fig. 2D). Over the next 5 days the definitive nerve trunks are established as the axons make a further series of reproducible branches across the three axes of the growing limb, AP, DV and proximo-distal (Fig. 2E–G, Roncali, 1970). The definitive adult innervation pattern is established by stage 36 (10 days of incubation, Fig. 2H) as are other elements of the limb bud, for example the cartilaginous skeleton (Fig. 2I).
FGF-induced additional limbs are innervated
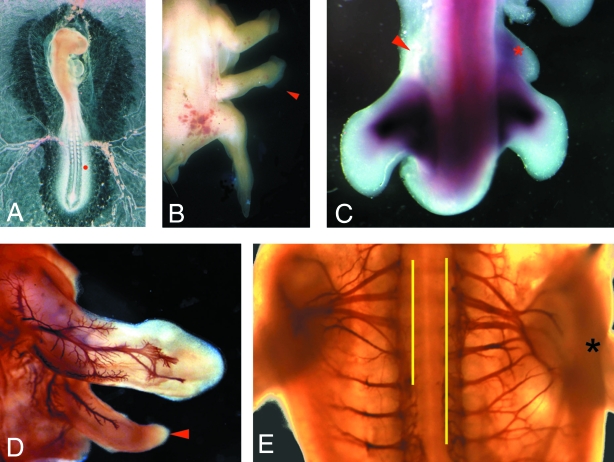
FGF4-soaked beads were placed in a small slit cut in the presumptive flank (adjacent to somites 21–25) of stage 13/14 chick embryos (Fig. 3A). This treatment led to the formation of an additional limb structure (Cohn et al. 1995; Fig. 3B). Depending upon the exact site of the implanted bead either discrete additional wings or legs grew (19/47), or less complete limbs formed which in some cases fused with either the normal wing or the leg (28/47; Cohn et al. 1997). By 72 h after bead implantation, expression of the EphA7 tyrosine kinase receptor was up-regulated in the additional limb bud, compared to the contralateral flank (n = 5; Fig. 3C). In order to assay whether these additional limbs were innervated, manipulated embryos were incubated for 5 days after bead implantation, then whole-mount immunohistochemistry performed with the 3A10 antibody (n = 47). We found that even in cases in which only a small additional structure formed as a result of FGF treatment, axons could be seen growing into the extra outgrowth (for example, Fig. 3D). In addition, these smaller outgrowths led to alterations in the overall appearance of the brachial plexus on the manipulated side of the embryo (Fig. 3E), such that spinal nerves that would normally supply the flank were instead drawn towards the extra bud.
Fig. 3.
FGF-induced additional limb buds are innervated and the plexus on the manipulated side of the embryo is altered. (A) FGF-4-soaked beads (depicted by red dot) were implanted into a stage 13/14 (embryonic day 2) chick embryo. This treatment led to the formation of an additional limb structure (arrowed in B). (C) Dorsal view of a whole-mount in situ with the EphA7 riboprobe, showing up-regulation of EphA7 expression in the additional bud (red asterix), compared to the contralateral, non-manipulated, side (red arrow). (D) Whole-mount immunohistochemistry with 3A10 shows that additional outgrowths are innervated (arrowed) and that the plexus on the manipulated side of the embryo (black asterix; right-hand side) is altered compared to the contralateral side (left; E). Ventral view. Extent of plexus marked by yellow lines.
Motoneurones that innervate the flank are diverted from their normal trajectories and enter the additional limb
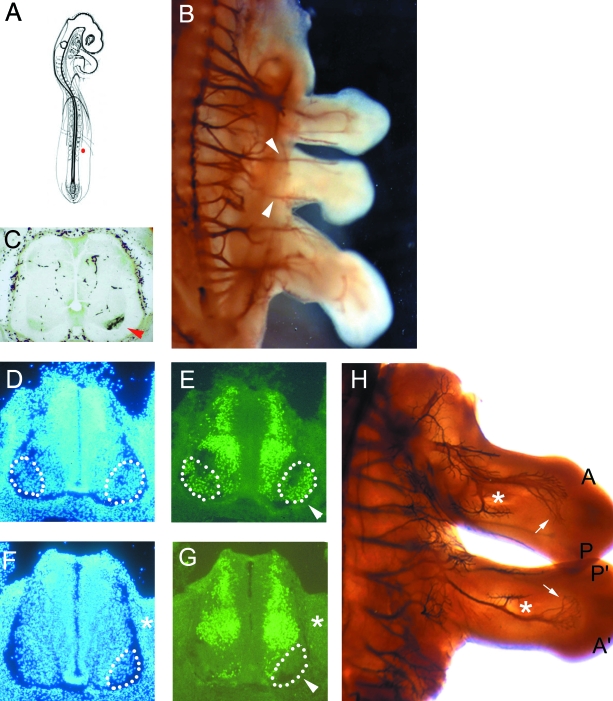
Discrete additional limbs formed after FGF bead implantation were also innervated (Fig. 4A,B; n = 19). In these cases, whole-mount immunohistochemistry suggested that these axons originated from motoneurones that would normally innervate the flank (Fig. 4B). To confirm this, five ectopic limbs were injected with the retrograde axonal tracer, HRP. These were then processed to reveal the location of the HRP-labelled motoneurone cell bodies in the spinal cord. HRP-labelled motoneurones were not found within the LMC, but instead were present at flank levels of the spinal cord within the MMC (Fig. 4C).
Fig. 4.
FGF-induced limbs are innervated by flank level motoneurones, which are responsive to its anterio-posterior orientation. (A) Schematic diagram of stage 13/14 chick embryo showing location of the implanted FGF-4 bead in the presumptive flank (red dot). (B) Whole-mount immunohistochemistry with 3A10 showing axons from flank levels of the spinal cord enter the additional limb (arrowheads). (C) Transverse section of the spinal cord showing HRP-labelled motoneurones on the side of the FGF-induced additional limb at flank levels (arrowhead). (D,E) Transverse section of a stage 26 chick embryonic spinal cord at wing bud level counterstained with DAPI (D) and reacted with the LIM1/2 antibody (E). The LMCL which expresses LIM1 is outlined by dots. Additional staining outside the LMCL is due to LIM2-positive neurons. (F,G) Transverse section of a stage 26 chick embryonic spinal cord at the level of the additional limb counterstained with DAPI (F) and reacted with the LIM1/2 antibody (G). LIM1 is not expressed by the MMC motoneurones (arrowed). (H) Whole-mount antibody staining with 3A10 showing reversed anterio-posterior orientation of the major nerve bundles in the additional bud (asterix and arrows). A and A′= anterior; P and P′= posterior.
Motoneurones that enter the additional limb from flank levels of the spinal cord do not express LIM-1
Tsuchida et al. (1994) showed that the motor columns within the spinal cord differentially express LIM homeodomain proteins. In particular, motoneurones lying in the LMCL, which innervate dorsal muscle limb targets, can be distinguished since they express LIM-1 (Fig. 4D,E). Therefore, to investigate whether the altered behaviour of the MMC motoneurones at the level of the additional limb reflected a change in molecular identity we looked for up-regulation of LIM-1 in these neurons, but no such change was detected (n = 3; Fig. 4F,G).
Flank level motoneurones are responsive to the anterio-posterior orientation of the additional limb bud
Cohn et al. (1995) showed that the orientation of ectopic limbs induced by FGF are reversed anterior-posteriorly compared to normal limbs (Figs 3B and 4H). As the innervation pattern in the normal wing bud is polarized across this axis (Fig. 2E–H) we were able to test whether the axons in extra limb buds responded to its anterio-posterior orientation. We show that, in well-formed ectopic wings, in keeping with their reversed orientation the anterio-posterior pattern of nerve branches is inverted too (Fig. 4H). This reversed innervation pattern is not obvious in all of the additional limbs (e.g. Figure 4B), and it is likely in these instances that the identity of the extra bud is less well formed or a wing/leg mosaic (see Ohuchi & Noji, 1999).
Discussion
We have addressed the problem of how the vertebrate limb bud is innervated in such a highly reproducible and accurate fashion. We have investigated this problem by providing an additional limb target for flank level motoneurones, which would not normally innervate a limb. Using this approach we have been able to test whether such motoneurones can respond to the presence of the extra limb, and if so whether they send out axons which then trace out an innervation pattern that resembles that of the normal limb. Further, this method allows us to start to investigate the relative roles of the interacting sets of guidance mechanisms that appear to work together to guide the axons accurately to their muscle targets. We chose to create the additional limb by applying an FGF-soaked bead to the flank of the embryo at a stage prior to normal limb initiation. This approach has the advantage that the intervention is at the time when motoneurones are first starting to emerge from the spinal cord. Further, it is likely to be less disruptive than the ‘cut and paste’ experiments employed previously (Straznicky, 1963; Hollyday 1981), which may affect the axon's ability to grow out.
We found that in all cases where an extra limb structure formed, it was innervated. Even if the additional structure had fused with the existing limbs and was not fully formed, nerve fibres grew into it, showing that the presence of the additional bud exerted a powerful attractive force for axons. This is in keeping with in vitro experiments in which explants of ventral spinal cord cultured with pieces of limb bud mesenchyme send out axons that grow towards the limb tissue (Ebens et al. 1996). HGF/SF is expressed by the early limb mesenchyme and was identified by the authors as being the source of the limb-derived chemoattractive ability. We also showed that expression of the tyrosine kinase receptor EphA7 was expressed by the ectopic buds. This is in keeping with its proposed role in channelling axons into the limb bud (Araujo et al. 1998).
Our retrograde labelling experiments showed that the spinal nerves that grew into the additional limbs arose from flank levels of the spinal cord rather than being diverted from the existing LMC motoneurones. Therefore, the presence of the additional limb was able to divert them from their normal trajectories. According to their lack of LIM-1 expression, these motoneurones maintained a different identity to motoneurones in the LMCL which normally innervate dorsal muscles in the limb. Therefore motoneurones that have a different identity from LMCL motoneurones can still grow into the limb, be responsive to the anterio-posterior orientation of the bud and respond to the cues that result in an innervation pattern which certainly resembles that of the normal limb supplied by LMC neurones. Our results support retrograde tracing experiments with supernumerary limbs by Hollyday (1981), who showed that although foreign neurones were innervating these limbs they did so in an orderly fashion across the anterio-posterior (AP) axis. She found that the AP position of a motor pool in the spinal cord that supplied the supernumerary limb depended on the AP position and orientation of that limb. Therefore limb tissue plays an important role in shaping the branching pattern of the peripheral nerves that supply it. Our data do not address whether dorsal/ventral targets in the additional limb are selectively innervated. This is still an area of doubt since Hollyday's data suggest that there is selective innervation of dorsal/ventral muscle targets whereas Stirling & Summerbell's (1985) data do not (see Introduction).
We have not tested whether the innervation pattern in the FGF-induced additional limbs is fully functional. Supernumerary limbs do move but only do so in a limited and uncoordinated fashion (Straznicky, 1963). This suggests that although the gross anatomical pattern of the extra limb approximates that of the normal it is not completely accurate or that LMC motoneurones, with their particular molecular identity and central connections, are specifically required for normal and co-ordinated motor function.
Studying the mechanisms of guidance of motor axons in the developing limb bud is complicated by the combination of genetic and epigenetic guidance cues, making it difficult to tease out their relative contributions to the acquisition of the stereotyped accurate anatomical innervation pattern. The process of limb innervation clearly involves interactions between motoneurone axons with intrinsic differences or identities, with the properties of the local environment of the limb into which they grow. The creation of an innervated additional limb, in which foreign axons from non-limb axial levels and non-limb motor columns can still produce grossly normal innervation pathways within the limb bud mesenchyme, may be a good means of separating out the different mechanisms required to accurately innervate a limb bud. In the future, this experimental approach may enable the contributions of motoneurone identity, positional cues and passive deployment to be placed in an ordered hierarchy of importance for achieving accurate innervation of the vertebrate limb bud.
Acknowledgments
We wish to thank Tim Horder, Gillian Morriss-Kay and Susanna Blackshaw for helpful comments on this manuscript. This research was funded by the Wellcome Trust. J.M.B. is a Lloyd's Tercentenary Fellow. A.M.R. is a Daphne Jackson Fellow.
References
- Araujo M, Piedra ME, Herrera MT, Ros MA, Nieto MA. The expression and regulation of chick EphA7 suggests roles in limb patterning and innervation. Development. 1998;125:4195–4204. doi: 10.1242/dev.125.21.4195. [DOI] [PubMed] [Google Scholar]
- Bell E, Wingate RJ, Lumsden A. Homeotic transformation of rhombomere identity after local Hoxb1 misexpression. Science. 1999;284:2168–2171. doi: 10.1126/science.284.5423.2168. [DOI] [PubMed] [Google Scholar]
- Cohn MJ, Izpisua-Belmonte J-C, Abud H, Heath JK, Tickle C. Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell. 1995;80:739–746. doi: 10.1016/0092-8674(95)90352-6. [DOI] [PubMed] [Google Scholar]
- Cohn MJ, Patel K, Krumlauf R, Wilkinson DG, Clarke JD, Tickle C. Hox9 genes and vertebrate limb specification. Nature. 1997;387:97–101. doi: 10.1038/387097a0. [DOI] [PubMed] [Google Scholar]
- Ebens A, Brose K, Leonardo ED, Hanson MG, Bladt F, Birchmeier C, et al. Hepatocyte growth factor/scatter factor is an axonal chemoattractant and a neurotrophic factor for spinal motor neurons. Neuron. 1996;17:1157–1172. doi: 10.1016/s0896-6273(00)80247-0. [DOI] [PubMed] [Google Scholar]
- Ensini M, Tsuchida TN, Belting HG, Jessell TM. The control of rostrocaudal pattern in the developing spinal cord: specification of motor neuron subtype identity is initiated by signals from paraxial mesoderm. Development. 1998;125:969–982. doi: 10.1242/dev.125.6.969. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morph. 1951;88:49–92. [PubMed] [Google Scholar]
- Hollyday M. Organisation of motor pools in the chick lumbar lateral motor column. J. Comp. Neurol. 1980;194:143–170. doi: 10.1002/cne.901940108. [DOI] [PubMed] [Google Scholar]
- Hollyday M. Rules of motor innervation in chick embryos with supernumerary limbs. J. Comp. Neurol. 1981;202:439–465. doi: 10.1002/cne.902020312. [DOI] [PubMed] [Google Scholar]
- Horder TJ. Functional adaptability and morphogenetic opportunism the only rules for limb development? Zoon. 1978;6:447–466. [Google Scholar]
- Kania A, Johnson RL, Jessell TM. Coordinate roles for LIM homeobox genes in directing the dorsoventral trajectory of motor axons in the vertebrate limb. Cell. 2000;102:161–173. doi: 10.1016/s0092-8674(00)00022-2. [DOI] [PubMed] [Google Scholar]
- Keynes RJ, Stern CD. Segmentation in the nervous system. Nature. 1984;310:786–789. doi: 10.1038/310786a0. [DOI] [PubMed] [Google Scholar]
- Lance-Jones C, Landmesser LT. Pathway selection by embryonic chick motoneurones in an experimentally altered environment. Proc. R. Soc. London. 1981a;214:19–52. doi: 10.1098/rspb.1981.0080. [DOI] [PubMed] [Google Scholar]
- Lance-Jones C, Landmesser LT. Pathway selection by chick lumbosacral motoneurones during normal development. Proc. Royal Soc. London. 1981b;214:1–18. doi: 10.1098/rspb.1981.0079. [DOI] [PubMed] [Google Scholar]
- Lance-Jones C. The effect of somite manipulation on the development of motoneurone projection patterns in the embryonic chick hindlimb. Dev. Biol. 1988;126:408–419. doi: 10.1016/0012-1606(88)90150-9. [DOI] [PubMed] [Google Scholar]
- Landmesser LT. The distribution of motoneurones supplying chick hind limb muscles. J. Physiol. 1978;284:371–389. doi: 10.1113/jphysiol.1978.sp012545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser C, Dias M. The influence of presumptive limb connective tissue on motoneurone axon guidance. Dev. Biol. 1991;143:93–110. doi: 10.1016/0012-1606(91)90057-a. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The origin and development of the visceral system in the spinal cord of the chick embryo. J. Morph. 1950;86:253–283. [Google Scholar]
- Lewis J, Chevallier A, Kieny M, Wolpert L. Muscle nerve branches do not develop in chick wings devoid of muscle. J. Embryol. Exp. Morph. 1981;64:211–232. [PubMed] [Google Scholar]
- Lin JH, Saito T, Anderson DJ, Lance-Jones C, Jessell TM, Arber S. Functionally related motor neuron pool and muscle sensory afferent subtypes defined by coordinate ETS gene expression. Cell. 1998;95:393–407. doi: 10.1016/s0092-8674(00)81770-5. [DOI] [PubMed] [Google Scholar]
- Liu JP, Laufer E, Jessell TM. Assigning the positional identity of spinal motor neurons. Rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and retinoids. Neuron. 2001;32:997–1012. doi: 10.1016/s0896-6273(01)00544-x. [DOI] [PubMed] [Google Scholar]
- Matise MP, Lance-Jones C. A critical period for the specification of motor pools in the chick lumbosacral spinal cord. Development. 1996;122:659–669. doi: 10.1242/dev.122.2.659. [DOI] [PubMed] [Google Scholar]
- Mukouyama Y-S, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Oakley RA, Tosney KW. Peanut agglutinin and chondroitin-6-sulphate are molecular markers for tissues that act as barriers to axon advance in the avian embryo. Dev. Biol. 1991;147:187–206. doi: 10.1016/s0012-1606(05)80017-x. [DOI] [PubMed] [Google Scholar]
- Ohuchi H, Noji S. Fibroblast-growth-factor-induced limbs in the study of limb formation, limb identity, myogenesis, and innervation. Cell Tissue Res. 1999;296:45–56. doi: 10.1007/s004410051265. [DOI] [PubMed] [Google Scholar]
- Price SR, De Marco Garcia NV, Ranscht B, Jessell TM. Regulation of motor neuron pool sorting by differential expression of type II cadherins. Cell. 2002;109:205–216. doi: 10.1016/s0092-8674(02)00695-5. [DOI] [PubMed] [Google Scholar]
- Roncali L. The brachial plexus and the wing nerve pattern during early developmental phases in chicken embryos. Monitore Zool. Ital. 1970;4:81–98. [Google Scholar]
- Stoeckli E, Landmesser LT. Axon guidance at choice points. Curr. Op. Neurobiol. 1998;8:73–79. doi: 10.1016/s0959-4388(98)80010-x. [DOI] [PubMed] [Google Scholar]
- Stirling RV, Summerbell D. Familiarity breeds contempt: the behaviour of axons in foreign and familiar environments. Prog. Clin. Med. Res. 1983;110A:217–226. [PubMed] [Google Scholar]
- Stirling RV, Summerbell D. The behaviour of growing axons invading developing chick wing buds with dorsoventral or anteroposterior axis reversed. J. Embryol. Exp. Morph. 1985;85:251–269. [PubMed] [Google Scholar]
- Stirling RV, Summerbell D. Specific guidance of motor axons to duplicated muscles in the developing amniote limb. Development. 1988;103:97–110. doi: 10.1242/dev.103.1.97. [DOI] [PubMed] [Google Scholar]
- Straznicky K. Function of heterotopic spinal cord segments investigated in the chick. Acta Biol. Hung. 1963;14:145–155. [PubMed] [Google Scholar]
- Swanson GJ, Lewis J. The timetable of innervation and its control in the chick wing bud. J. Embryol. Exp. Morph. 1982;71:121–137. [PubMed] [Google Scholar]
- Tiret L, Le Mouellic MM, Brulet P. Increased apoptosis of motorneurones and altered somatotopic maps in the brachial spinal cord of Hoxc8-deficient mice. Development. 1998;125:279–291. doi: 10.1242/dev.125.2.279. [DOI] [PubMed] [Google Scholar]
- Tosney KW, Landmesser LT. Development of the major pathways for neurite outgrowth in the chick hindlimb. Dev. Biol. 1985;109:193–214. doi: 10.1016/0012-1606(85)90360-4. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographical organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Varela-Echavarria A, Guthrie S. Molecules making waves in axon guidance. Genes Dev. 1997;11:545–557. doi: 10.1101/gad.11.5.545. [DOI] [PubMed] [Google Scholar]
- Wang G, Scott SA. The ‘waiting period’ of sensory and motor axons in early chick hindlimb. its role in axon pathfinding and neuronal maturation. J. Neurosci. 2000;20:5358–5366. doi: 10.1523/JNEUROSCI.20-14-05358.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG. Situ Hybridisation. Oxford: IRL Press; 1992. pp. 257–276. [Google Scholar]