Abstract
Recent research has demonstrated that not only haemodynamic factors but also genetic programmes control arterial–venous cell fate and blood vessel identity. The identification of arteries and veins was previously based solely on morphological criteria and is now greatly facilitated by specific molecular markers. Moreover, signalling pathways controlling the arterial–venous decision during embryonic development have been outlined for the first time. This review gives an up-to-date overview of differentially expressed genes and the regulatory processes leading to the differentiation of arteries and veins.
Keywords: angioblast, angiogenesis, arterial–venous, artery, Eph, ephrin, Gridlock, Notch, vasculogenesis, vein
Introduction
Arteries and veins have evolved as anatomically distinct but closely interconnected blood vessel types in the vertebrate circulatory system. The aorta, the biggest artery, receives the blood from the heart and distributes it through a branching network of large- and small-calibre arteries and finally arterioles to extensive capillary beds in organs and tissues (see Fig. 1). Blood pressure and flow speed are highest in the aorta and decrease progressively towards capillary beds, reflecting the steady expansion of vessel numbers and the enlargement of their combined luminal diameter along this axis. Their circular cross-sections together with thick layers of smooth muscle cells (SMCs) and extracellular matrix (ECM) provides arteries with mechanical strength and elasticity. Conversely, the network of venules and veins, which collects the blood from the periphery and directs it back to the heart, is adapted to lower pressure and thus its vessels have much thinner walls, more irregular outlines and contain valves to prevent the backflow of blood.
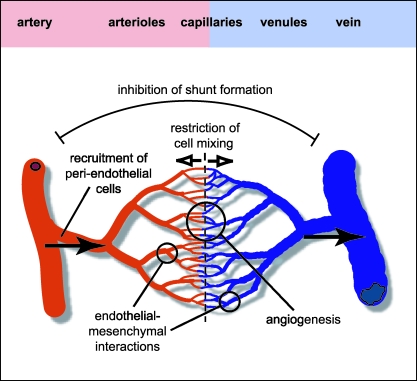
Fig. 1.
Organization of the arterial–venous network. Distinct molecular AV identity could play various roles during early vascular morphogenesis. Repulsive cues (open arrows) provided by Eph/ephrin signalling and other pathways might restrict cell movement across an AV boundary and inhibit the formation of shunts between large-calibre blood vessels. Formation of capillary beds by angiogenesis might be stimulated at the AV interface. Distinct molecular properties of arteries and veins could contribute to the recruitment of pericytes and vascular smooth muscle cells, and perhaps mediate specific interactions between endothelial cells and the adjacent mesenchyme. Haemodynamic factors are also likely to contribute to AV differentiation (arrows indicate the direction of blood flow).
Flow dynamics and distinct physiological requirements have been long considered the main or even sole driving forces for arterial–venous (AV) specialization and, as the SMC layer thickness and morphology was used to distinguish arteries from veins, it was not clear if separate populations of endothelial cells (ECs) existed. Recent work has not only addressed this question by the identification of molecular markers specific for the arterial or venous endothelium but has also provided us with a first glimpse of the molecular machinery controlling formation of these vessel types in the developing embryo. Remarkably, much of this differentiation programme is already in place before blood circulation is established, indicating that at least some steps of genetic control precede regulation by haemodynamic factors. It is appealing to assume that both processes are closely integrated and interdependent. Moreover, one might expect that the molecular pathways controlling AV morphogenesis in the early embryo will be also of relevance for human arteriovenous malformations in which shunts permit the direct entry of arterial blood into the venous system without passing through a capillary network.
This review summarizes the current knowledge about artery- and vein-specific gene expression, its regulation and role during blood vessel morphogenesis in the embryo.
The role of Eph receptors and ephrins
Much of the recent interest in the molecular specification of arteries and veins has been sparked by work done in the field of Eph receptor tyrosine kinases (RTKs) and ephrins. Eph receptors, which constitute the largest tyrosine kinase subfamily in vertebrates, trigger signal transduction in response to extracellular stimuli, i.e. the interaction with ephrin ligands. Sequence homology and ligand binding preference have been the criteria for dividing the Eph receptors into two subclasses. The eight so-called EphA receptors (EphA1–A8) bind to six ephrin-A proteins (ephrin-A1–A6), which are presented on the cell surface due to a glycosylphosphatidylinositol (GPI) anchor modification. The second class contains six EphB receptors (EphB1–B6) and three ephrin-B transmembrane proteins (ephrin-B1–B3). Binding within each subclass is highly promiscuous, resulting in a large number of potential Eph–ephrin interactions. Previous work has also demonstrated that ephrins are capable of receptor-like active signalling, resulting in bi-directional signal transduction (recently reviewed in Kullander & Klein, 2002).
Eph/ephrin molecules are versatile regulators with roles in a wide range of morphogenetic processes (Boyd & Lackmann, 2001; Wilkinson, 2001; Adams, 2002; Kullander & Klein, 2002). In the cardiovascular system, the observation that ephrin-B2 is expressed by arterial endothelial cells but not on the venous endothelium was a first key step towards understanding molecular AV identity (Wang et al. 1998; Adams et al. 1999; Gerety et al. 1999; Gale et al. 2001; Shin et al. 2001). Wang et al. (1998) generated mutant mice carrying an insertion of the bacterial lacZ gene in the ephrin-B2 locus. β-galactosidase staining revealed ephrin-B2 expression in various regions of early embryos, but in the vasculature it was restricted to arteries as judged by anatomical criteria. It also turned out that expression of the EphB4 receptor, an interaction partner of ephrin-B2, was largely but not completely confined to veins (Wang et al. 1998; Adams et al. 1999; Gerety et al. 1999). Surprisingly, AV-specific expression of the two molecules preceded the formation of morphologically distinct arteries and veins in the early and very primitive vascular network, which is formed de novo by the fusion of blood islands in a process termed vasculogenesis (Risau & Flamme, 1995; Wang et al. 1998).
Further studies confirmed AV-specific expression of the ephrin and its receptor during later embryonic development and in adult mice, although arterial SMCs as well as podocyte progenitors and glomerular ECs in the kidney were also positive for ephrin-B2 (Gale et al. 2001; Shin et al. 2001; Takahashi et al. 2001). Ephrin-B2 was also found on the arterial endothelium in chick and zebrafish, making it a consistent and reliable marker in a wide range of species (Lawson et al. 2001, 2002; Moyon et al. 2001a; Othman-Hassan et al. 2001; Zhong et al. 2001).
Despite these important findings, it is still not clear if ephrin-B2 and EphB4 play an active role during the specification of arteries and veins. Knockout mice lacking ephrin-B2 either uniformly or specifically in the endothelium and mutants lacking the ephrin-B2 cytoplasmic domain showed very similar severe defects in the whole vasculature (Wang et al. 1998; Adams et al. 1999, 2001; Gerety et al. 1999; Gerety, 2002). Primitive blood vessels were formed but the remodelling of the early and uniform vascular network into a hierarchical system of small and large blood vessels (angiogenesis) failed. Consequently, this defect also disrupted the differentiation of blood vessels into morphologically distinguishable arteries and veins. However, the biggest artery, the dorsal aorta, did form in ephrin-B2 mutant embryos whereas the biggest vein, the cardinal vein, was rendered into a non-functional loose network of endothelial cells (Adams et al. 1999). These two major vessels are formed by direct assembly of angioblasts (endothelial precursor cells), i.e. vasculogenesis. A very similar phenotype was described for EphB4-deficient mice, indicating strong interdependence of ephrin and receptor activity during blood vessel development (Gerety et al. 1999).
Despite strong evidence suggesting that ephrin-B2 and EphB4 are not involved in the earliest steps of the AV decision (see below), the two molecules seem to mediate critical communication between the arterial and venous endothelium (see Fig. 1). Knockout phenotypes and in vitro sprouting assays indicate that the ephrin-B2–EphB4 interaction promotes angiogenesis, a property that could contribute to the formation of extensive capillary beds at the arterial–venous interface. Since it is known from the work of many groups that Eph/ephrin molecules can restrain cell movements and create tissue boundaries (recently reviewed in Wilkinson, 2001), ephrin-B2 and EphB4 might establish some sort of AV boundary across which the migration of ECs is restricted. The fate of individual endothelial cells can be studied by grafting tissue from quail donors into chick host embryos. Transplanted endothelial cells, which can be identified by species-specific surface markers, respect the arterial or venous character of their host blood vessels, in other words arterial cells tend to integrate into arteries and venous cells into veins (Moyon et al. 2001a; Othman-Hassan et al. 2001). In some cases, grafted cells retained plasticity, permitting them to colonize both arteries and veins but these cells changed the expression of markers such as ephrin-B2 to match AV properties of their host vessels (Moyon et al. 2001a). Although it is currently not clear which and how many different molecules restrict the mixing of arterial and venous cells (ephrin-B2 and EphB4 certainly appear to be promising candidates), the work described above has now firmly established that two distinct EC populations exist.
It is noteworthy that additional Eph/ephrin molecules are expressed in endothelial cells and the adjacent mesenchyme and several experimental findings suggest that they contribute to the patterning of blood vessels (Adams et al. 1999; Helbling et al. 2000; Oike et al. 2002; reviewed in Adams, 2002). Future work will have to address whether arteries and veins respond differently to signals from the surrounding tissue.
More markers – additional regulatory pathways?
Over the last few years, more genes with selective expression on arteries or veins have been identified but in most cases specific roles in the process of AV differentiation remain elusive (see Table 1). For example, the cytoplasmic tyrosine kinase Bmx was found to be expressed in the endothelium of large arteries and in the endocardium of embryonic and adult mice, but Bmx-deficient mutants were viable and lack obvious developmental defects (Ekman et al. 1997; Rajantie et al. 2001). Bmx tyrosine phosphorylation and activity can be triggered by the RTK Tie2, a receptor for a family of soluble ligands called angiopoietins (reviewed in Jones et al. 2001; Loughna & Sato, 2001).
Table 1.
Known molecular markers of the arterial and venous endothelium
| Arterial endothelial cells | Venous endothelial cells | |
|---|---|---|
| Mouse | ephrin-B21,2,3, neuropilin-14, connexin-404, Bmx5,6 Notch17, 37 and 47, Delta-like47,8, Jagged17 and 27 | EphB41,2,3 |
| Chick | ephrin-B29,10, neuropilin-19,11, | Neuropilin-211, Tie29,12,* |
| Zebrafish | Notch513,14, gridlock14,15, ephrin-B2a13,14,15 | Flt413,14,15, EphB415 |
| Xenopus | EphB416 |
Tie2 is also expressed in aorta (see text)
In the developing chick embryo, expression of tie2 was confined to the venous endothelium and the aorta whereas the other arteries showed no staining (Moyon et al. 2001a, 2001b). Experimental evidence suggests that tie2 expression might be not equally restricted to veins in mouse, indicating that species-specific differences might exist (Schlaeger et al. 1997). Mesenchymal cells surrounding blood vessels are known to express angiopoietin ligands. Remarkably, in situ hybridization signal for angiopoietin-1 was restricted to cells around veins in chick embryos, whereas angiopoietin-2 was transcribed in the tissue enclosing arteries that express little or no tie2 (Moyon et al. 2001b). Whereas both angiopoietins can bind the Tie2 receptor, they seem to trigger different responses in vivo and in vitro (Jones et al. 2001; Loughna & Sato, 2001). Tie2 and angiopoietin-1 knockout mice have been generated and displayed severe angiogenesis defects in the whole vascular system similarly to ephrin-B2- and EphB4-deficient mutants (Sato et al. 1995; Suri et al. 1996).
Neuropilins are cell surface receptors for soluble (class-3) semaphorins, members of a large gene family controlling axon guidance in the nervous system and other processes (reviewed in Miao & Klagsbrun, 2000; Neufeld et al. 2002). It has been recently demonstrated that neuropilins can also bind to specific isoforms of vascular endothelial growth factor (VEGF) and act as accessory receptors facilitating signalling by VEGF receptors (Soker et al. 1998; Gluzman-Poltorak et al. 2000, 2001). Neuropilin expression in the chick vascular system was detected at the earliest stages of vascular development: Transcripts for the two related molecules neuropilin-1 (np1) and neuropilin-2 (np2) were located to blood islands and blood vessels. As soon as arteries and veins became distinguishable, expression of np1 was confined to the arterial endothelium and np2 to veins (Herzog et al. 2001; Moyon et al. 2001a).
Notch proteins are cell-surface receptors for membrane-bound ligands, termed Delta-like and Jagged in higher vertebrates, and are involved in cell fate decisions and patterning during embryonic development (reviewed in Artavanis-Tsakonas et al. 1999). Gene targeting studies in mouse and the identification of mutations in human patients have shown that several of the molecules in this pathway play important roles in the cardiovascular system (Xue et al. 1999; Krebs et al. 2000; McCright et al. 2001; Uyttendaele et al. 2001). It was recently reported that the receptors notch1, notch3 and notch4 and the ligands dLl4, jagged1 and jagged2 are expressed in arteries but not in veins of mouse embryos (Shutter et al. 2000; Villa et al. 2001). Localization of Jagged1 protein to arterial SMCs was also observed (Villa et al. 2001).
It is important to keep in mind that most of the findings described above are based on in situ hybridization results or the expression of reporter genes. Significant differences could exist on the levels of protein expression and activity.
Genetic experiments continue to provide significant functional insight into the processes leading to AV differentiation. Mice with a targeted inactivation of the activin receptor-like kinase-1 gene develop large shunts between arteries and veins reminiscent of arteriovenous malformations in humans, lose arterial ephrin-B2 expression and die at midgestation (Urness et al. 2000).
The AV decision – lessons from zebrafish development
Much of the recent progress in the area of arterial–venous differentiation was obtained from studies in zebrafish, a powerful model organism accessible to genetic approaches and other forms of experimental manipulation. Zebrafish mutants lacking the arterial protein gridlock (grl), a basic helix–loop–helix (bHLH) protein with homology to transcriptional repressors, were isolated in a large genetic screen because they showed disrupted assembly of the aorta in the posterior part of the body (see Fig. 2) (Zhong et al. 2000, 2001). Reduced levels of gridlock expression were directly proportional to the extent of the defect in the artery. Conversely, gridlock overexpression caused a similar disruption of the vein without affecting the artery (Zhong et al. 2001).
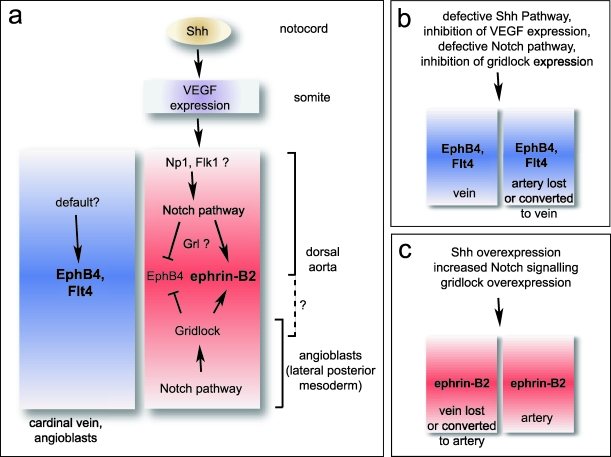
Fig. 2.
Arterial–venous cell fate decision in zebrafish embryos. Signalling pathways in the lateral posterior mesoderm and in the large blood vessels are shown (a). Sonic Hedgehog provided by the notochord has been recently shown to induce VEGF expression in somites and regulate the arterial identity of endothelial cells in the aorta. Neuropilin-1, which is expressed by arterial ECs in mouse, could perhaps mediate artery-specific VEGF signalling. Differences between pathways in the lateral posterior mesoderm and the main blood vessels might exist. Venous cell fate might be driven by unknown pathways or could be the default outcome in the absence of arterial signals. Blood vessels are disrupted or change their molecular AV identity in certain zebrafish mutants or in response to experimental manipulation (b,c). Note that arrows do not necessarily indicate direct interaction or regulation.
Gridlock expression starts very early during development in the lateral posterior mesoderm (LPM), a region that contains the angioblast precursors that will eventually migrate to the midline of the embryo and assemble into dorsal aorta and cardinal vein. Strikingly, angioblasts already appear to have some AV identity, since dye-labelled cells contributed either to the arterial or venous endothelium but never to both blood vessels (Zhong et al. 2001). Substantial evidence suggests that gridlock might be triggering the arterial differentiation programme in a subset of angioblasts whereas the remaining (gridlock-negative) cells acquire a venous fate by default. As the number of endothelial precursors is probably limited, manipulating gridlock expression could perhaps alter the normal numbers of cells with an arterial or venous commitment and thus interfere with the assembly of arteries or veins, respectively. Consistent with a key role in the early AV decision, diminishing grl levels did indeed result in decreased ephrin-B2 expression whereas EphB4 was elevated at the same time (Zhong et al. 2001). But is gridlock the master regulator controlling arterial–venous identity? Probably not. Gridlock belongs to a family of so-called hairy-related transcription factors, which are often involved in cell fate decisions made by the Notch pathway (reviewed in Fisher & Caudy, 1998; Davis & Turner, 2001). Zhong et al. (2001) observed that Notch signalling positively regulated gridlock expression in the lateral posterior mesoderm. Moreover, disruption of the Notch pathway reduced mesodermal gridlock and interfered with the normal development of the aorta (see Fig. 2). A similar approach was employed by Lawson et al. (2001, 2002) and, in their hands, loss of Notch signalling eliminated the arterial markers ephrin-B2 and notch5 but did not affect gridlock expression in the aorta. It is currently not clear if the findings by the two laboratories reflect regional differences between angioblasts in the LPM and endothelial cells in the major blood vessels or simply different extents of Notch inhibition.
In early embryos, the aorta is located in close proximity to the notochord, an important signalling centre that controls development of multiple adjacent tissues such as the neural tube, somites and blood vessels (Lassar & Munsterberg, 1996; Fouquet et al. 1997; Litingtung & Chiang, 2000). It is known that a molecule named Sonic Hedgehog (Shh) mediates many of these regulatory processes. Zebrafish mutants with defects in the Shh pathway lacked arterial ephrin-B2 expression and retained a single big blood vessel, which was positive for venous markers. Shh overexpression had the opposite effect and led to an expansion of the ephrin-B2-expressing endothelium (Lawson et al. 2002). This regulation seemed to depend critically on VEGF expression in the somites, which are placed to both sides of the notochord and the main blood vessels. Loss of Shh signal diminished somitic VEGF whereas Shh overexpression increased VEGF levels. Further experiments showed that the VEGF signal is upstream of Notch5 expression and Notch signalling, which in turn controls ephrin-B2 expression and arterial cell fate (Lawson et al. 2002). Remarkably, VEGF secreted by the peripheral nerves in mouse embryos was recently shown to control arterial differentiation and patterning in the skin (Mukouyama et al. 2002), indicating that the mechanism underlying the AV decision might be conserved among vertebrate organisms.
Open questions and perspectives
Despite the tremendous recent increase in our understanding of vascular morphogenesis and the arterial–venous cell fate decision, many questions remain open. For the first time, pathways mediating the AV decision have been outlined in zebrafish but closer examination is needed in order to find more molecules involved in these signalling cascades. A few candidate molecules have already been identified on the basis of their expression patterns but functional assays are required to assess their roles. Furthermore, we can expect that one or two pathways are probably not sufficient to control the AV decision and a more complex regulatory network might emerge in the future. It will also be important to find out whether the same molecular mechanisms apply to the AV decision in the endothelium of smaller blood vessels and in different vertebrate organisms. Although the work summarized in this review has suddenly opened the door to an exciting new research area, at least one fundamental problem has already become evident. The roles of gene products like Notch receptors, Eph RTKs, ephrins and, in particular, VEGF are not solely confined to cell fate decisions and the same pathways seem to mediate multiple other processes in the developing and adult vascular system. Many of these genes have been inactivated in mouse but quite often the severity of the resulting vascular defects together with embryonic lethality have permitted only rather limited analysis. Spatially and temporally controlled genetic manipulations could prove more useful in these cases.
The contribution of haemodynamic factors in the morphogenesis of arteries and veins has been largely ignored in this review but the important role of physical parameters such as blood pressure and shear stress is far from obsolete despite newly discovered genetic pathways. One interesting possibility might be that control of AV identity by physical and genetic factors is fully integrated and mutually regulated.
As saphenous and femoral veins are commonly used for vascular and coronary artery by-pass surgery, one can assume that a certain degree of plasticity permits reprogramming of endothelial cells. We are still far away from molecular therapies for human arteriovenous malformations, which are currently treated by surgical removal, radiosurgery or related methods. However, a key step will be to understand if and how molecular factors contribute to AV malformations and the knowledge gained from developmental processes could prove invaluable for this purpose.
Acknowledgments
I would like to thank Amelia Compagni, Vassiliki Kostourou, Milenko Cicmil and Shane Foo for critically reading the manuscript.
References
- Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RH, Diella F, Hennig S, Helmbacher F, Deutsch U, Klein R. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104:57–69. doi: 10.1016/s0092-8674(01)00191-x. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Adams RH. Vascular patterning by Eph receptor tyrosine kinases and ephrins. Semin. Cell Dev. Biol. 2002;13:55–60. doi: 10.1006/scdb.2001.0289. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Boyd AW, Lackmann M. Signals from Eph and ephrin proteins: a developmental tool kit. Sci. STKE. 2001;2001:RE20. doi: 10.1126/stke.2001.112.re20. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Davis RL, Turner DL. Vertebrate hairy and Enhancer of split related proteins: transcriptional repressors regulating cellular differentiation and embryonic patterning. Oncogene. 2001;20:8342–8357. doi: 10.1038/sj.onc.1205094. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Ekman N, Lymboussaki A, Vastrik I, Sarvas K, Kaipainen A, Alitalo K. Bmx tyrosine kinase is specifically expressed in the endocardium and the endothelium of large arteries. Circulation. 1997;96:1729–1732. doi: 10.1161/01.cir.96.6.1729. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Fisher A, Caudy M. The function of hairy-related bHLH repressor proteins in cell fate decisions. Bioessays. 1998;20:298–306. doi: 10.1002/(SICI)1521-1878(199804)20:4<298::AID-BIES6>3.0.CO;2-M. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Fouquet B, Weinstein BM, Serluca FC, Fishman MC. Vessel patterning in the embryo of the zebrafish: guidance by notochord. Dev. Biol. 1997;183:37–48. doi: 10.1006/dbio.1996.8495. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Gale NW, Baluk P, Pan L, Kwan M, Holash J, DeChiara TM, et al. Ephrin-B2 selectively marks arterial vessels and neovascularization sites in the adult, with expression in both endothelial and smooth-muscle cells. Dev. Biol. 2001;230:151–160. doi: 10.1006/dbio.2000.0112. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol. Cell. 1999;4:403–414. doi: 10.1016/s1097-2765(00)80342-1. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Gerety SS, Anderson DJ. Cardiovascular ephrin B2 function is essential for embryonic angiogenesis. Development. 2002;129:1397–1410. doi: 10.1242/dev.129.6.1397. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G. Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165. J. Biol. Chem. 2000;275:29922. doi: 10.1074/jbc.M909259199. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Gluzman-Poltorak Z, Cohen T, Shibuya M, Neufeld G. Vascular endothelial growth factor receptor-1 and neuropilin-2 form complexes. J. Biol. Chem. 2001;276:18688–18694. doi: 10.1074/jbc.M006909200. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Helbling PM, Saulnier DM, Brandli AW. The receptor tyrosine kinase EphB4 and ephrin-B ligands restrict angiogenic growth of embryonic veins in Xenopus laevis. Development. 2000;127:269–278. doi: 10.1242/dev.127.2.269. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Herzog Y, Kalcheim C, Kahane N, Reshef R, Neufeld G. Differential expression of neuropilin-1 and neuropilin-2 in arteries and veins. Mech. Dev. 2001;109:115–119. doi: 10.1016/s0925-4773(01)00518-4. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Jones N, Iljin K, Dumont DJ, Alitalo K. Tie receptors: new modulators of angiogenic and lymphangiogenic responses. Nat. Rev. Mol. Cell Biol. 2001;2:257–267. doi: 10.1038/35067005. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, et al. Notch signaling is essential for vascular morpho-genesis in mice. Genes Dev. 2000;14:1343–1352. 10.1046/j.1469-7580.2003.00137.x. [PMC free article] [PubMed] [Google Scholar]
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat. Rev. Mol. Cell Biol. 2002;3:475–486. doi: 10.1038/nrm856. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Lassar AB, Munsterberg AE. The role of positive and negative signals in somite patterning. Curr. Opin. Neurobiol. 1996;6:57–63. doi: 10.1016/s0959-4388(96)80009-2. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, et al. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev. Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Litingtung Y, Chiang C. Control of Shh activity and signaling in the neural tube. Dev. Dyn. 2000;219:143–154. doi: 10.1002/1097-0177(2000)9999:9999<::aid-dvdy1050>3.3.co;2-h. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Loughna S, Sato TN. Angiopoietin and Tie signaling pathways in vascular development. Matrix Biol. 2001;20:319–325. doi: 10.1016/s0945-053x(01)00149-4. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- McCright B, Gao X, Shen L, Lozier J, Lan Y, Maguire M, et al. Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development. 2001;128:491–502. doi: 10.1242/dev.128.4.491. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Miao HQ, Klagsbrun M. Neuropilin is a mediator of angiogenesis. Cancer Metastasis Rev. 2000;19:29–37. doi: 10.1023/a:1026579711033. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Moyon D, Pardanaud L, Yuan L, Breant C, Eichmann A. Plasticity of endothelial cells during arterial-venous differentiation in the avian embryo. Development. 2001a;128:3359–3370. doi: 10.1242/dev.128.17.3359. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Moyon D, Pardanaud L, Yuan L, Breant C, Eichmann A. Selective expression of angiopoietin 1 and 2 in mesenchymal cells surrounding veins and arteries of the avian embryo. Mech. Dev. 2001b;106:133–136. doi: 10.1016/s0925-4773(01)00425-7. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial diff-erentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Shraga N, Lange T, Kessler O, Herzog Y. The neuropilins: multifunctional semaphorin and VEGF receptors that modulate axon guidance and angiogenesis. Trends Cardiovasc. Med. 2002;12:13–19. doi: 10.1016/s1050-1738(01)00140-2. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Oike Y, Ito Y, Hamada K, Zhang X-Q, Miyata K, Arai F, et al. Regulation of vasculogenesis and angiogenesis by EphB/ephrin-B2 signaling between endothelial cells and surrounding mesenchymal cells. Blood. 2002;1000:1326–1333. 10.1046/j.1469-7580.2003.00137.x. [PubMed] [Google Scholar]
- Othman-Hassan K, Patel K, Papoutsi M, Rodriguez-Niedenfuhr M, Christ B, Wilting J. Arterial identity of endothelial cells is controlled by local cues. Dev. Biol. 2001;237:398–409. doi: 10.1006/dbio.2001.0383. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Rajantie I, Ekman N, Iljin K, Arighi E, Gunji Y, Kaukonen J, et al. Bmx tyrosine kinase has a redundant function downstream of angiopoietin and vascular endothelial growth factor receptors in arterial endothelium. Mol. Cell Biol. 2001;21:4647–4655. doi: 10.1128/MCB.21.14.4647-4655.2001. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W, Flamme I. Vasculogenesis. Annu. Rev. Cell Dev. Biol. 1995;11:73–91. doi: 10.1146/annurev.cb.11.110195.000445. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Schlaeger TM, Bartunkova S, Lawitts JA, Teichmann G, Risau W, Deutsch U, et al. Uniform vascular-endothelial-cell-specific gene expression in both embryonic and adult transgenic mice. Proc. Natl Acad. Sci. USA. 1997;94:3058–3063. doi: 10.1073/pnas.94.7.3058. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D, Garcia-Cardena G, Hayashi S, Gerety S, Asahara T, Stavrakis G, et al. Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev. Biol. 2001;230:139–150. doi: 10.1006/dbio.2000.9957. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Shutter JR, Scully S, Fan W, Richards WG, Kitajewski J, Deblandre GA, et al. Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Dev. 2000;14:1313–1318. 10.1046/j.1469-7580.2003.00137.x. [PMC free article] [PubMed] [Google Scholar]
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Takahashi K, Gerety S, Wang H, Anderson DJ, Daniel TO. Temporally compartmentalized expression of ephrin-B2 during renal glomerular development. J. Am. Soc. Nephrol. 2001;12:2673–2682. doi: 10.1681/ASN.V12122673. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Urness LD, Sorensen LK, Li DY. Arteriovenous malformations in mice lacking activin receptor-like kinase-1. Nat. Genet. 2000;26:328–331. doi: 10.1038/81634. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Uyttendaele H, Ho J, Rossant J, Kitajewski J. Vascular patterning defects associated with expression of activated Notch4 in embryonic endothelium. Proc. Natl Acad. Sci. USA. 2001;98:5643–5648. doi: 10.1073/pnas.091584598. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa N, Walker L, Lindsell CE, Gasson J, Iruela-Arispe ML, Weinmaster G. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech. Dev. 2001;108:161–164. doi: 10.1016/s0925-4773(01)00469-5. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. Multiple roles of EPH receptors and ephrins in neural development. Nat. Rev. Neurosci. 2001;2:155–164. doi: 10.1038/35058515. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Xue Y, Gao X, Lindsell CE, Norton CR, Chang B, Hicks C, et al. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum. Mol. Genet. 1999;8:723–730. doi: 10.1093/hmg/8.5.723. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Zhong TP, Rosenberg M, Mohideen MA, Weinstein B, Fishman MC. gridlock, an HLH gene required for assembly of the aorta in zebrafish. Science. 2000;287:1820–1824. doi: 10.1126/science.287.5459.1820. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]
- Zhong TP, Childs S, Leu JP, Fishman MC. Gridlock sig-nalling pathway fashions the first embryonic artery. Nature. 2001;414:216–220. doi: 10.1038/35102599. 10.1046/j.1469-7580.2003.00137.x. [DOI] [PubMed] [Google Scholar]