Abstract
Sensory neurones in the dorsal root ganglion (DRG) of the neonatal rat express the 75-kDa low-affinity neurotrophin receptor (p75NTR) and these neurones degenerate rapidly after axotomy. p75NTR belongs to the tumour necrosis factor superfamily, several members of which have a role in cell death and it is constitutively expressed within a subpopulation of DRG neurones. p75NTR has been implicated in mediating the degeneration of these neurones after axotomy. In this study, we characterize the expression of p75NTR in sensory neurones of the newborn rat DRG using immunohistochemistry. Furthermore, we investigate the change in constitutive expression pattern of p75NTR in these neurones following axotomy. In the C7 and C8 DRG of the newborn rat, p75NTR is expressed in approximately 70% of DRG neurones. Those expressing p75NTR can be classified into subpopulations with moderate or intense p75NTR expression, each present in approximately equal proportions. Whilst p75NTR expression is observed in neurones throughout the entire neuronal diameter range, a correlation exists between neuronal diameter and p75NTR expression intensity. We also found that the most vulnerable population following axotomy were those sensory neurones which constitutively express the highest levels of p75NTR, i.e. the large-diameter neurones.
Keywords: axotomy, cell death, immunocytochemistry, neonatal rat, p75NTR NGF receptor, sensory neurone
Introduction
The 75-kDa low-affinity neurotrophin receptor (p75NTR) is a membrane-bound receptor that is ubiquitously expressed in neural and non-neural tissues (Chao, 1994). The precise role of p75NTR in the survival and differentiation of neurones and its interaction with the high-affinity tyrosine kinase (trk) receptors is controversial. p75NTR acts as an accessory receptor for trk (Hempstead et al. 1991), assists in neurotrophin binding (Benedetti et al. 1993; Ip et al. 1993), facilitates retrograde transport of the neurotrophins (Bothwell, 1995) and increases trk signalling (Battleman et al. 1993; Barker et al. 1994; Hantzopoulos et al. 1994; Verdi et al. 1994). Furthermore, analysis of cultured sensory neurones from the p75NTR knockout mouse shows that expression of the p75NTR is necessary for optimal neurotrophin signalling (Davies et al. 1993; Lee et al. 1994). However, in vitro studies indicate that the trks can also achieve high-affinity receptor binding and initiate their normal signalling cascades in the absence of p75NTR (Glass et al. 1991; Klein et al. 1991; Jing et al. 1992; Verdi et al. 1994).
The p75NTR receptor is a member of the tumour necrosis factor receptor (TNFR) superfamily, and contains homologous regions to the cytoplasmic ‘death domain’ of TNFR-I and Fas (Chapman, 1995) and a juxtamembrane death domain named ‘chopper’ (Coulson et al. 2000). Reports by Rabizadeh et al. in 1993 and Barrett et al. in 1994 indicated for the first time that p75NTR was also able to induce neuronal cell death (Rabizadeh et al. 1993; Barrett & Bartlett, 1994; Barrett, 2000). Numerous studies have since indicated a direct neurotrophin-activated cytotoxic role for p75NTR, but only in the absence of the cognate trk receptor (von Bartheld et al. 1994; Casaccia-Bonnefil et al. 1996; Bamji et al. 1998; Soilu-Hanninen et al. 1999; Terrado et al. 2000). In fact, co-expression of the appropriate trk receptor has been shown to inhibit p75NTR-mediated neurotoxic signalling (Twiss et al. 1998; Yoon et al. 1998). Analysis of p75NTR knockout mice has shown reductions in the naturally occurring loss of sympathetic (Bamji et al. 1998) and basal forebrain cholinergic neurones (Van der Zee et al. 1996; Yeo et al. 1997). Furthermore, transgenic mice expressing high levels of the intracellular domain of p75NTR have reduced numbers of several neuronal types (Majdan et al. 1997). In addition, the normal extent of developmental neuronal loss in the retina is reduced by blocking nerve growth factor (NGF) binding to p75NTR (Frade et al. 1996). We have also shown that the death of axotomized sensory dorsal root ganglion (DRG) neurones is reduced after the nerve stump is treated with antisense oligonucleotides directed against p75NTR (Cheema et al. 1996).
In order to understand further the role of the p75NTR in the axotomy-induced death of sensory DRG neurones, we have analysed the p75NTR expression patterns in sensory neurones of the newborn rat, and the subsequent change in p75NTR expression following axotomy-induced cell death. Our data indicate that p75NTR expression is most intense in the large-diameter sensory neurones, and that neurones expressing the highest levels of p75NTR are preferentially lost after axotomy.
Materials and methods
Experiments were carried out on newborn (24–48 h) Wistar rat pups of either sex. All experiments were carried out in accordance with procedures approved by Monash University's Animal Ethics Experimentation Committee (Project no. 94/188). All pups were rendered unconscious via ice-induced hypothermia. One group of pups had their right median and ulnar nerves exposed and transected using a pair of iridectomy scissors. The wounds were then closed using Ethilon® 5-0 silk sutures. The pups were then warmed, and when fully conscious re-united with their respective mothers. At 1, 3 or 5 days after axotomy, the pups were again rendered unconscious via ice-induced hypothermia. The heart was exposed and the animals were perfused transcardially with phosphate-buffered saline (PBS) followed by Bouin's fixative. The second group, consisting of unoperated age-matched pups, were also perfused as described above. A laminectomy was performed to remove the cervical spinal cord with the seventh (C7) and eighth (C8) cervical DRG, followed by an overnight post-fixation in Bouin's fixative at 4 °C. The spinal cord and DRG were then dehydrated and embedded in paraffin wax. Five-micrometre-thick transverse serial sections were cut through the length of the spinal cord and mounted onto Vectabond®-coated slides. Slides that contained sections with prominent DRGs were chosen for immunohistochemistry. The first section on each slide was stained for p75NTR, and the adjacent section was immunostained for either Substance P (SP) or Calcitonin Gene Related Peptide (CGRP).
Sections were dewaxed, rinsed in PBS and placed in 3% H2O2 in methanol at 4 °C for 20 min. After a brief rinse in PBS the sections were placed in a solution of 0.4% Triton-X in PBS for 15 min at room temperature and then blocked with a solution containing 5% normal goat serum (NGS), and 5% milk powder in PBS for 1 h at room temperature. The first section on each slide was incubated in a 1 : 2000 dilution of rabbit polyclonal antibody to p75NTR (a gift from Professor Moses Chao, Ab 9650), directed against amino acids 43–161 of the extracellular domain (Huber & Chao, 1995). The adjacent section on each slide was simultaneously incubated in either a 1 : 50 dilution of rabbit polyclonal antibodies to CGRP (Cat.#RPN. 1842, Amersham, Bucks., UK) or a 1 : 1000 dilution of rabbit polyclonal antibody to SP (Cat.#8508I, AusPep, Parkville, Victoria, Australia). The antibodies against p75NTR, CGRP and SP were diluted in 5% NGS in PBS. All sections were incubated overnight at 4 °C with the primary antibody. Twenty-four hours later the sections were rinsed three times in PBS, and then exposed to a dilution of 1 : 200 biotinylated goat antirabbit antibodies (Cat.#BA-1000, Vector Laboratories, Burlingame, CA, USA), in PBS for 1 h. After a further three rinses in PBS the sections were incubated with avidin–biotin conjugated to peroxidase (Cat.#PK-4000, Vectastain® Standard Kit, Vector Laboratories) and diluted 1 : 100 in PBS for 1 h at room temperature. The peroxidase reaction product was visualized by reacting the sections with 0.05% 3,3-diaminobenzidine tetrahydrochloride solution (Cat.#S3000, DAB, Daco, Carpinteria, CA, USA), in PBS buffer containing 0.05% H2O2 until maximal (saturation) staining was obtained. The sections were carefully monitored under a light microscope until no further colour change was evident in order to standardize the immunohistochemistry. Sections were then washed in PBS, dehydrated through a graded series of alcohol and xylene, and coverslipped using DePeX. The control sections were processed in an identical manner, except that the primary antibodies were omitted. In these sections, no staining was evident (data not shown).
Approximately 1000 neurones were analysed from both C7 and C8. Two random sections of each C7 and C8 DRG were analysed from each animal. All sensory neurones within each section of DRG were analysed. Counterstaining was not required as the DAB reaction product provided low-level background staining such that all neuronal profiles were easily identified. To provide an estimate of the proportion of neurones expressing p75NTR, and to allow analysis of their diameter and coexpression with the neuropeptide markers, camera lucida drawings were made of complete DRG stained with p75NTR. All neurones were traced, and those with a clear cellular outline and a distinct nucleus were then characterized as either intensely positive for p75NTR, moderately positive for p75NTR, or negative for p75NTR. Repeated observations over periods of greater than 1 month yielded no significant differences in classification. The adjacent section, stained for either SP or CGRP, was then projected over these tracings, allowing identification of neuropeptide co-expression in p75NTR-expressing neurones. All neurones identified were then manually scanned in from the tracings via a digitizing tablet linked to a computer image analysis program (SigmaScan Pro). The computer image analysis program calculated the mean diameter of each neurone scanned. The proportion of p75NTR-negative, moderate and intense neurones from each individual section analysed was then calculated, and the mean (± SEM) obtained from these values was used to construct the histograms. Similarly, the mean neuronal diameter of the p75NTR-negative, moderate and intense neurones from each individual section analysed was calculated. The final mean neuronal diameter (± SEM) of the p75NTR-negative, moderate and intense neurones was then calculated as the average of these values. Histograms were constructed from these means. For neuronal diameter frequency analysis, all the data were pooled into negative, moderate and intense subpopulations. These data were then separated out into mean diameter groups. The raw data from within each group were then expressed as a percentage of the total number of neurones counted. Analysis of variance (anova) statistical tests were performed to determine significant differences between group means.
Results
Expression pattern of p75NTR
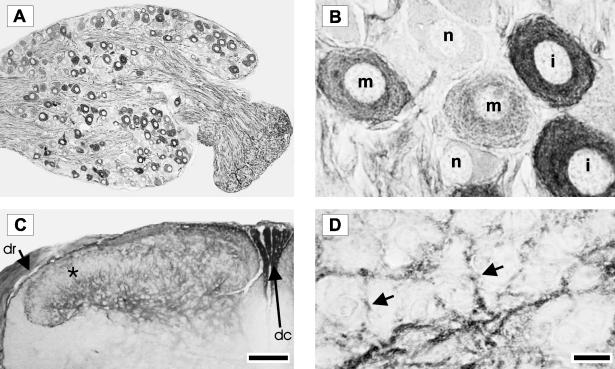
The sensory neurones that express p75NTR were analysed in the newborn C7 and C8 DRG using an antibody directed against the extracellular ligand-binding domain of the p75NTR. Approximately 70% of neurones are immunopositive for p75NTR and they are randomly distributed within the DRG. Axons entering and coursing through the DRG are also strongly p75NTR immunoreactive (Fig. 1A). Sensory neurones in the DRG were classified into three groups with respect to the p75NTR expression. The first group is intensely immunoreactive with a dense, almost black reaction product. In the second group, the neurones are moderately positive and have a lighter, more diffuse, punctate cytoplasmic reaction product. The third group consists of p75NTR-negative neurones (Fig. 1B). The dorsal horn of the spinal cord is also p75NTR immunoreactive. The staining is primarily in lamina I, and in the outer region of lamina II with little or no expression within the inner part of lamina II. The staining continues into laminae III, IV and lamina V. In addition, the dorsal roots and dorsal columns also contain p75NTR immunoreactive fibres (Fig. 1C). At higher magnification, immunoreactive sensory fibres are evident, and display fine varicosities which are presumably related to synaptic sites (Fig. 1D).
Fig. 1.
Photomicrographs of neonatal rat DRG and spinal cord immunostained for p75NTR using a polyclonal antibody directed against the extracellular domain. Low-power photomicrograph of a typical DRG is shown in Panel A. Using such p75NTR immunostained sections, sensory neurones in the DRG were classified into three groups: intense (i), moderate (m) or negative (n) for p75NTR. These differences can be seen clearly at a higher magnification (Panel B). Low-power photomicrograph of a transverse section of spinal cord dorsal horn is shown in Panel C. Note the absence of expression in the inner part lamina II (starred). In addition, the dorsal root (dr) and dorsal columns (dc) contain p75NTR immunoreactive fibres. At higher magnification (Panel D), p75NTR immunoreactive sensory fibres can be clearly seen (arrows). Scale bars in A and C represent 100 µm and in B and D represent 10 µm.
Morphometric analysis of p75NTR positive neurones
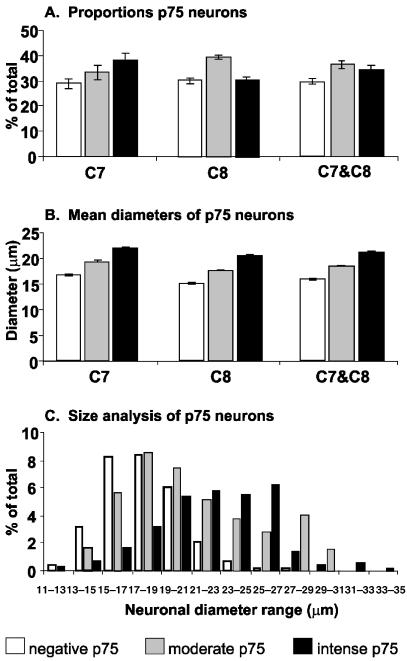
Approximately 75% of the C7 and 70% of the C8 DRG neurones are p75NTR immunopositive. The C7 DRG contains a slightly higher proportion of intense p75NTR neurones (38%) compared with moderate (33%) or negative p75NTR neurones (29%). The C8 DRG contains approximately equal proportions of intense (30%), moderate (39%) and negative (31%) p75NTR neurones. When the C7 and C8 populations are combined to form a total sample of 4668 neurones, 34% were intense, 36% were moderate and 30% were negative with respect to p75NTR expression (Fig. 2A). Further morphometric analyses showed a correlation between neuronal diameter and the intensity of p75NTR expression. Small but statistically significant differences were noted in the mean diameter of neurones in the intense, moderate and negative p75NTR groups in both the C7 and C8 DRG (Fig. 2B). The combined data of the C7 and C8 DRG revealed that neurones in the intense p75NTR group had the largest mean diameter of 21.1 µm. Neurones in the moderate p75NTR group had an intermediate mean diameter of 18.3 µm, while the negative p75NTR group consisted of the smallest neurones and had a mean diameter of 15.9 µm. anova statistics show that these differences in neuronal diameters between the three groups were all significant with P < 0.01 (Fig. 2B). Since a correlation was found between the intensity of p75NTR expression and neuronal diameter, a frequency analysis was carried out for the three groups of neurones (Fig. 2C). The neuronal diameter frequency analysis showed that there was considerable overlap between the neuronal diameter distribution of the p75NTR-moderate and p75NTR-negative groups, with a modal peak of approximately 18 µm. However, the profile for the intense p75NTR group was shifted towards the right with a modal peak of approximately 26 µm (Fig. 2C). These data confirm that there is a strong correlation between large DRG neurones and the highest intensity of p75NTR expression (Fig. 2B,C).
Fig. 2.
(A) Histograms illustrating the proportion of negative, moderate and intense p75NTR neuronal groups in the C7 and C8 DRG. In the C7 DRG, approximately 29% of neurones were negative, 33% were moderate and 38% were intense for p75NTR. In the C8 DRG, approximately 31% of neurones were negative, 39% moderate and 30% were intense for p75NTR. When the data for C7 and C8 are combined, one-third of the neurones are negative, another third moderate and the remaining third intense for p75NTR. (B) These histograms illustrate the mean diameter of the three groups of neurones within the C7 and C8 DRG. Significant differences (P < 0.01) were observed between the mean diameters of the three groups. There was a correlation between neuronal diameter and the expression of p75NTR. The negative p75NTR neurones were the smallest in diameter, while the intense p75NTR neurones were the largest. (C) Neuronal diameter frequency histogram of the three groups of neurones. While neurones in the intense group can be seen across all diameter ranges, they have a distinct bias towards the larger diameters with a modal value of approximately 26 µm. The moderate and negative p75NTR groups have similar modal values of approximately 18 µm. The means and standard errors were obtained from groups with four animals.
Co-localization of p75NTR with CGRP and SP
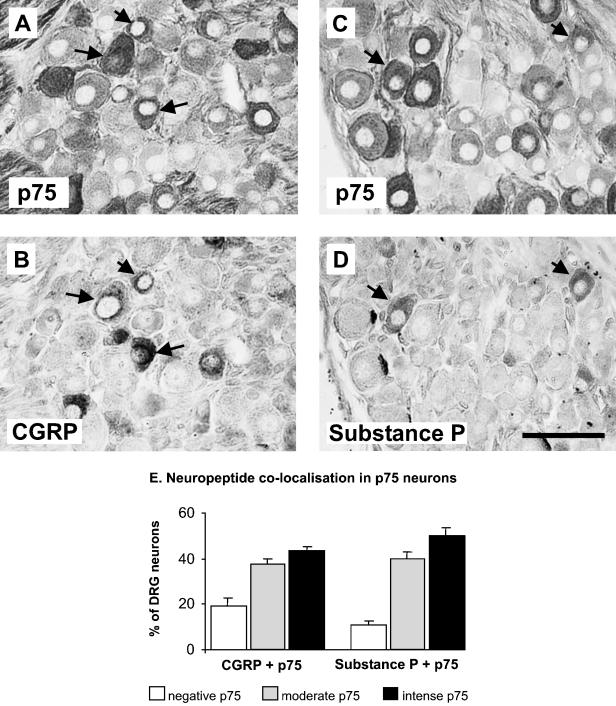
The results of the neuropeptide immunohistochemistry were, by themselves, consistent with published observations. In sections where neuropeptide immunohistochemistry was counted, 42% and 22% of neurones were positive for CGRP and Substance P (SP), respectively. The p75NTR-positive neurones were also analysed in terms of their neuropeptide expression. A proportion of neurones were observed to coexpress p75NTR and CGRP (Fig. 3A,B, arrows), or p75NTR and SP (Fig. 3C,D, arrows). Figure 3(E) is a summary of the proportions of p75NTR sensory neurones that co-localize with these neuropeptides. Approximately 43% of CGRP neurones intensely stain for p75NTR and another 38% express moderate levels of p75NTR. Approximately 19% of CGRP neurones are p75NTR negative. Of all the SP-expressing neurones, 50% intensely stain for p75NTR, 40% express moderate levels of p75NTR and 10% are p75NTR negative (Fig. 3E).
Fig. 3.
Photomicrographs of adjacent sections showing the neuronal coexpression of p75NTR with CGRP (arrows, compare A and B) or Substance P (arrows, compare C and D). The extent of such coexpression is summarized in the histograms shown in Panel E. Within the CGRP population approximately 85% (moderate and intense) coexpress p75NTR, and within the Substance P population approximately 90% (moderate and intense) coexpress p75NTR. The means and standard errors were obtained from groups with four animals. Scale bar in D represents 50 µm.
p75NTR expression following axotomy
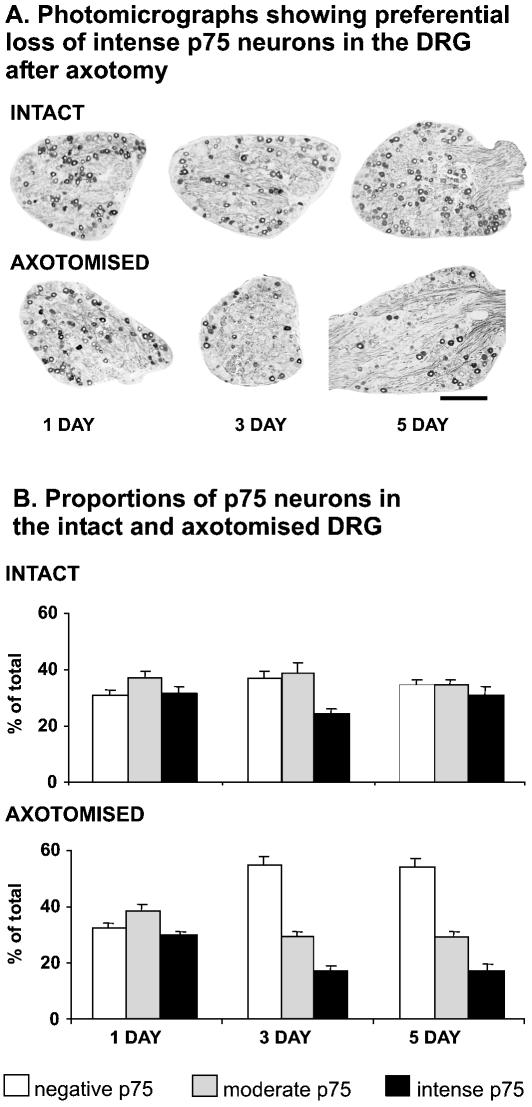
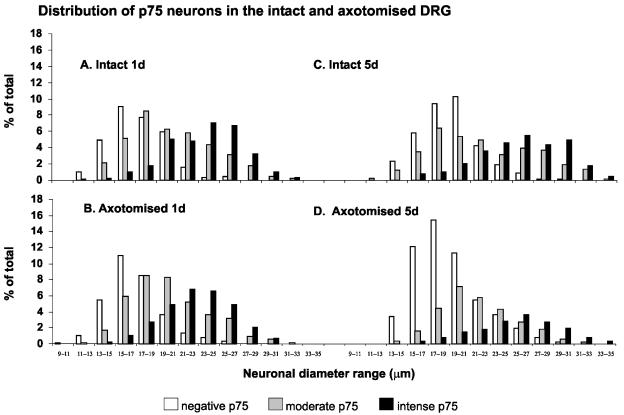
There is significant rapid and reproducible sensory neurone loss in the DRG following neonatal axotomy. In this part of the study, our aim was to test the hypothesis that this sensory neurone loss following axotomy is linked to the level of p75NTR expression. A total of 6287 sensory neurones from C7 and C8 DRG, from both axotomized and intact (contralateral) ganglia, were analysed from rat pups 1, 3 and 5 days after median and ulnar nerve transection. At 1 day after axotomy there is no change in the proportions of intense p75NTR-, moderate p75NTR- or negative p75NTR-expressing sensory neurones in either intact or axotomized DRG (Fig. 4A,B). Three days after axotomy, however, there are significant changes in the proportional distribution pattern of intense, moderate and negative p75NTR neuronal subpopulations compared to the intact side (Fig. 4A,B). Specifically, the proportion of intense p75NTR neurones has significantly reduced (from 24% to 17%) following axotomy. There is also an increase in the proportion of negative p75NTR neurones (from 37% to 55%). The changes observed at 3 days following axotomy are similar to that at 5 days after axotomy. The most prominent change is the significant reduction in the intense p75NTR neuronal population from 30% to 17% (Fig. 4A,B). The significant reduction in the proportion of p75NTR intense neurones following axotomy suggests that it is these neurones that preferentially undergo axotomy-induced death. However, the possibility remains that the p75NTR intense neurones are not dying, but are rapidly down-regulating their expression levels of p75NTR following axotomy.
Fig. 4.
Photomicrographs of p75NTR immunostained DRG sections (A), and summary data of intact and axotomized DRG at 1, 3 and 5 days after axotomy (B). At 1 day following axotomy, there is no change in the proportion of p75NTR negative, moderate and intense neurones. At 3 and 5 days after axotomy, a significant number of the intense p75NTR group are lost in the axotomized DRG. The means and standard errors were obtained from groups with four animals. Scale bar in A represents 200 µm.
In order to establish that the preferential loss of intense p75NTR neurones following axotomy was not simply the down-regulation of p75NTR expression, we carried out diameter frequency analyses of the three groups at 1 and 5 days following axotomy (Fig. 5). One day after axotomy, there was no change in the relative diameter frequency patterns of intense, moderate, and negative p75NTR neurones in either the intact or axotomized DRG (Fig. 5A,B). However, at 5 days after axotomy, the significant change is the loss of the majority of the largest neurones in the axotomized DRG (Fig. 5C,D). Importantly, the modal distribution of the intense and negative p75NTR neuronal populations does not change. The surviving intense p75NTR neurones are still the largest neurones, and they retain a modal distribution of approximately 26 µm. Similarly, the modal distribution of negative p75NTR neurones remains unchanged at approximately 18 µm (Fig. 5C,D).
Fig. 5.
Neuronal diameter frequency analysis of negative, moderate and intense p75NTR DRG neurones in intact (top) and axotomized (bottom) DRG. At 1 day after axotomy (1d), there is no change in the frequency profiles of the three groups in the intact and axotomized DRG (compare A and B). However, at 5 days after axotomy (5d), the largest neurones expressing the highest levels of p75NTR are preferentially lost in the axotomized DRG (compare C and D). The means and standard errors were obtained from groups with four animals.
Discussion
In order to understand further the role of p75NTR in the loss of axotomized sensory neurones in the cervical DRG, the present study was undertaken to characterize carefully p75NTR expression in intact and axotomized sensory neurones. Our data indicate that approximately one-third of these neurones are negative, one-third moderate and one-third intense with respect to the levels of p75NTR protein expression. The largest neurones have the highest levels of p75NTR expression while the smallest neurones are generally negative. A key finding in this study is that neurones that express the highest levels of p75NTR are especially vulnerable to axotomy-induced cell death in the newborn rat. This observation is consistent with earlier reports that p75NTR plays a role in the death of axotomized sensory DRG neurones (Cheema et al. 1996).
Here we provide evidence that following axotomy, the disappearance of p75NTR immunoreactivity in the large, intensely stained population of neurones correlates with and supports the evidence of axotomy-induced, selective loss of this population of neurones. This also accounts for the relative increase in the proportion of p75NTR-negative neurones in the axotomized DRG at 3 and 5 days following axotomy (Fig. 4A,B). The data indicate that it is the p75NTR intense neurones that are preferentially lost following axotomy, and as the p75NTR-negative neurones are relatively protected following axotomy, they exhibit a proportional increase in the declining population. It has been shown by others that the transection of the sciatic nerve in newborn rats results in the death of approximately 75% of axotomized neurones in the lumbar DRG (Himes & Tessler, 1989). Such data correlate well with the percentage of p75NTR-positive neurones in the cervical DRG reported here and suggest that p75NTR expression may be an important determinant in susceptibility or resistance to axotomy-induced cell death in the neonatal rat. However, the alternative explanation, that the large, p75NTR intense neurones may down-regulate p75NTR expression and atrophy, rather than die, cannot be excluded on the basis of our findings. It should be noted in our study that a small residual population of p75NTR intense neurones remain in the C7 and C8 DRG after transection of the median and ulnar nerve. It is important to note that not all axons belonging to sensory neurones in C7 and C8 DRG travel via the median and ulnar nerves. It is most likely that the remaining p75NTR intense neurones belong to the radial nerve, or enter into the median or ulnar nerve proximal to the site of transection.
Few studies have carefully quantified the p75NTR population in the newborn rat DRG. Our finding that approximately 70% of sensory neurones express p75NTR in the cervical DRG is higher than the 50% previously reported in the lumbar DRG of postnatal day 1–14 rats (Bennett et al. 1996). In the adult rat lumbar DRG, in situ hybridization studies have reported p75NTR-positive neuronal populations ranging from 50% to 90% (Carroll et al. 1992; Schecterson & Bothwell, 1992; Wetmore & Olson, 1995; Bergman et al. 1999). Immunohistochemical studies indicate that approximately 60% of adult rat DRG neurones are p75NTR positive (Zhou et al. 1993, 1996). Several of these investigators have also noted that p75NTR mRNA and expression varies in intensity, and is expressed throughout the complete diameter range of neurones. Our detailed analysis of p75NTR expression and neuronal diameter revealed that the neurones with intense expression of p75NTR were generally of a larger diameter. It should be noted that there was a small but distinct proportion of large neurones that were clearly negative, and a similar proportion of small neurones that were clearly intensely positive for p75NTR. These observations are consistent with the reported observations that p75NTR-positive neuronal profiles have a mean cross-sectional area somewhat larger than the average of the total cell population (Bergman et al. 1999) and that many small neurones do not contain p75NTR expression (Molliver & Snider, 1997). It was recently reported that approximately 35% of DRG neurones that are small do not express any neurotrophin receptor and these are responsive to GDNF (Bennett et al. 1998). These neurones also project to the inner region of lamina II in the spinal cord dorsal horn, and are likely to correspond to the population of neurones that we have found to be p75NTR negative.
The expression of p75NTR is closely linked to neurotrophin signalling. All p75NTR-positive neurones have been shown to coexpress at least one trk receptor, and there are no reports of p75NTR expression independent of trk (Verge et al. 1989; Wright & Snider, 1995). The profile of trk receptor expression is well correlated to the sensory modality of DRG neurones (Snider, 1994). For example, the trkA population of sensory neurones tends to be small and predominantly cutaneous, thermal or nociceptive, while the trkC neuronal population tends to be large and proprioceptive (Ernfors et al. 1993; Mu et al. 1993; McMahon et al. 1994). In contrast to the high-affinity trk receptors, p75NTR expression is not modality specific since it is found throughout the entire diameter range of sensory neurones, which represent all the sensory modalities. There is, however, a correlation between the intensity of p75NTR expression and neuronal diameter. While the significance of the differing intensities is unclear, DRG neurones that express the highest levels of p75NTR tend to belong mainly to the large-diameter group, and these neurones are most likely proprioceptive or tactile cutaneous sensory neurones. In contrast, neurones lacking p75NTR tend to be in the smaller diameter group, and are most likely to be nociceptive or thermal sensory neurones. Interestingly, analysis of the p75NTR knockout mouse indicates that there is a significant developmental loss of sensory neurones in these mice since p75NTR is necessary for survival during embryonic development (Murray et al. 1999). The extent of this loss corresponds approximately to the level of loss found in the current study. It should be noted, however, that analysis of the cell size distribution in p75NTR knockout mouse DRG shows no selective loss of neurones of particular diameters (Bergmann et al. 1997). In addition, all types of sensory neurones are equally depleted in the p75NTR knockout mouse, indicating that p75NTR plays a significant role in the development of more than one neuronal modality (Bergmann et al. 1997).
Axotomy has been used to demonstrate neuronal plasticity within the adult nervous system, but in contrast it has also been shown to cause widespread neuronal death in the neonatal nervous system (Snider et al. 1992b). The p75NTR has been implicated in the death signalling of multiple neuronal populations in vitro and this laboratory has shown that the in vivo reduction of p75NTR expression prevents the loss of axotomized sensory neurones in the dorsal root ganglion of newborn rats (Cheema et al. 1996). This provides evidence of a death-signalling role for the p75NTR in these neurones that is triggered by axotomy. While it is difficult to define a causal relationship between p75NTR expression and axotomy-induced cell death in vivo, this correlative analysis provides new insights into the consequence of p75NTR expression with respect to neuronal injury. While the parameters that control the level of p75NTR expression in sensory DRG neurones remain unknown, we have found that neurones expressing high levels of p75NTR are particularly vulnerable to axotomy-induced loss. The converse is also true. The neurones that are p75NTR negative remain relatively protected from axotomy-induced loss.
The functional role of the p75NTR receptor in DRG sensory neurones has been studied in vitro (Barrett & Bartlett, 1994). Analysis of embryonic sensory neurones indicates that the p75NTR receptor promotes the survival of these neurones in vitro; however, the same analysis of early postnatal sensory neurones indicates that the p75NTR switches to a death-signalling role in the absence of NGF (Barrett & Bartlett, 1994). Furthermore, the observation that PC 12 cells expressing the highest levels of p75NTR die most rapidly following NGF deprivation (Barrett & Georgiou, 1996) suggests there is a general relationship between p75NTR levels and susceptibility to cell death. The evidence that the constitutive p75NTR can switch to a cytotoxic molecule under certain circumstances is compelling (Bredesen et al. 1998; Casaccia-Bonnefil et al. 1998).
How p75NTR switches to a cytotoxic role in the axotomized DRG sensory neurone in vivo remains unclear. Since p75NTR is constitutively expressed, the cytotoxic capacity of p75NTR must be kept in check by the action of the coexpressed trk receptors. The ratio of trk to p75NTR expression has been shown to alter the outcome of neurotrophin signalling (Twiss et al. 1998; Yoon et al. 1998), and the trk receptors have been shown to inhibit neurotoxic p75NTR (Dobrowsky et al. 1995; Bamji et al. 1998; Davey & Davies, 1998). One hypothesis is that axotomy results in the deprivation of target-derived neurotrophins. This reduces or removes trk-mediated signalling, resulting in the removal of the inhibition to p75NTR death signalling. This notion is supported by the fact that transgenic mice engineered to express high levels of p75NTR in the intracellular domain have significantly reduced numbers of sympathetic, peripheral sensory and neocortical neurones (Majdan et al. 1997). Although there is no direct evidence, axotomized neurones with high levels of p75NTR could rapidly generate and accumulate cytotoxic signalling molecules such as ceramide, and activate caspases, which have been linked to p75NTR signalling (Dobrowsky & Carter, 1998; Gu et al. 1999; Khursigara et al. 1999). An alternative hypothesis is that axotomized neurones down-regulate their high-affinity trk receptors. Since sensory neurones also express neurotrophins in an autocrine manner (Schecterson & Bothwell, 1992; Sebert & Shooter, 1993), these neurotrophins could activate p75NTR that leads to the death of the axotomized neurone. There is evidence that axotomized spinal motor neurones in vivo can be actively killed by the application of exogenous NGF, and these data support a role for NGF-mediated p75NTR cytotoxicity (Terrado et al. 2000). The possibility also remains that novel as yet undiscovered non-neurotrophin ligands that bind to p75NTR may exist. For example, CRNF in molluscs, has been shown to bind to p75NTR and is up regulated by nerve lesion (Fainzilber et al. 1996). New insights may be gained by exploring the above possibilities with respect to the mechanisms by which constitutively expressed p75NTR could switch to a cytotoxic molecule.
Less compelling arguments, not involving p75NTR as a cytotoxic molecule, could account for the selective loss of large-diameter neurones expressing high levels of p75NTR. It may be that the trophic support from non-myelinating Schwann cells ensheathing bundles of small-diameter axons are more effective in preventing the death of these neurones. Alternatively, the large p75NTR intense neurones may not die, but rather shrink and down-regulate p75NTR in response to axotomy. Following sciatic nerve transection in the adult rat, p75NTR mRNA in situ hybridization and immunohistochemistry reveals a reduction in the number of neurones expressing p75NTR, a diminution in the intensity of labelling, and a decrease in the mean cross-sectional area of p75NTR-labelled neurones in the DRG (Verge et al. 1992; Bergman et al. 1999). However, in contrast to the newborn, axotomy in the adult does not result in rapid neuronal cell death, but rather a plastic response to injury (Verge et al. 1989, 1992). This age-dependent change in DRG neurone susceptibility to axotomy is developmentally regulated by a decrease in dependence on growth factors for survival (Snider et al. 1992a). The mechanism for this is unclear, but it may be controlled by the relative expression of members of the bcl-2 gene family (Vogelbaum et al. 1998) that are also implicated in p75NTR-mediated neuronal death (Coulson et al. 1999).
Acknowledgments
We are grateful to Professor Moses Chao for providing us with the antibody against p75NTR (9650), and for reading this manuscript and providing useful comments. We thank Dr David Finkelstein for assistance with the digitizing tablet and software, Liz Lopes for technical assistance and Ms Kim Lowry and Mr Bradley Turner for comments on the manuscript. S.S.M. was supported by a research scholarship from The Bethlehem Griffiths Foundation and a Monash University Post-Graduate Publication Award. We are also grateful for the financial support of The Motor Neurone Disease Research Institute of Australia, The Motor Neurone Disease Association of the United Kingdom and the Australian NH & MRC (Veterans Affairs #143510). We thank Alvin Quah, Rachel Scott, Michael Azari, Bradley Turner, Da Wei Zang and Alan Rembach for their comments. Mr Greg Poynter provided editorial assistance.
References
- Bamji SX, Majdan M, Pozniak CD, Belliveau DJ, Aloyz R, Kohn J, et al. The p75 neurotrophin receptor mediates neuronal apoptosis and is essential for naturally occurring sympathetic neuron death. J. Cell Biol. 1998;140:911–923. doi: 10.1083/jcb.140.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker PA, Barbee G, Misko TP, Shooter EM. The low affinity neurotrophin receptor, p75LNTR, is palmitoylated by thioester formation through cysteine 279. J. Biol. Chem. 1994;269:30645–30650. [PubMed] [Google Scholar]
- Barrett G, Bartlett P. The p75 nerve growth factor receptor mediates survival or death depending on the stage of sensory neuron development. Proc. Natl Acad. Sci. USA. 1994;91:6501–6505. doi: 10.1073/pnas.91.14.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett GL, Georgiou A. The low-affinity nerve growth factor receptor p75NGFR mediates death of PC12 cells after nerve growth factor withdrawal. J. Neurosci. Res. 1996;45:117–128. doi: 10.1002/(SICI)1097-4547(19960715)45:2<117::AID-JNR4>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Barrett GL. The p75 neurotrophin receptor and neuronal apoptosis. Prog. Neurobiol. 2000;61:205–229. doi: 10.1016/s0301-0082(99)00056-8. [DOI] [PubMed] [Google Scholar]
- von Bartheld CS, Kinoshita Y, Prevette D, Yin QW, Oppenheim RW, Bothwell M. Positive and negative effects of neurotrophins on the isthmo-optic nucleus in chick embryos. Neuron. 1994;12:639–654. doi: 10.1016/0896-6273(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Battleman DS, Geller AI, Chao MV. HSV-1 vector-mediated gene transfer of the human nerve growth factor receptor p75hNGFR defines high-affinity NGF binding. J. Neurosci. 1993;13:941–951. doi: 10.1523/JNEUROSCI.13-03-00941.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti M, Levi A, Chao MV. Differential expression of nerve growth factor receptors leads to altered binding affinity and neurotrophin responsiveness. Proc. Natl Acad. Sci. USA. 1993;90:7859–7863. doi: 10.1073/pnas.90.16.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DL, Averill S, Clary DO, Priestley JV, McMahon SB. Postnatal changes in the expression of the trkA high-affinity NGF receptor in primary sensory neurons. Eur. J. Neurosci. 1996;8:2204–2208. doi: 10.1111/j.1460-9568.1996.tb00742.x. [DOI] [PubMed] [Google Scholar]
- Bennett DL, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, et al. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J. Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann I, Priestley JV, McMahon SB, Brocker EB, Toyka KV, Koltzenburg M. Analysis of cutaneous sensory neurons in transgenic mice lacking the low affinity neurotrophin receptor P75. Eur. J. Neurosci. 1997;9:18–28. doi: 10.1111/j.1460-9568.1997.tb01349.x. [DOI] [PubMed] [Google Scholar]
- Bergman E, Fundin BT, Ulfhake B. Effects of aging and axotomy on the expression of neurotrophin receptors in primary sensory neurons [In Process Citation] J. Comp. Neurol. 1999;410:368–386. doi: 10.1002/(sici)1096-9861(19990802)410:3<368::aid-cne2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Bothwell M. Functional interactions of neurotrophins and neurotrophin receptors. Annu. Rev. Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- Bredesen D, Ye X, Tasinato A, Sperandio S, Wang J, Assa-Munt N, et al. p75NTR and the concept of cellular dependence: seeing how the other half die. Cell Death Differentiation. 1998;5:365–371. doi: 10.1038/sj.cdd.4400378. [DOI] [PubMed] [Google Scholar]
- Carroll SL, Silos-Santiago I, Frese SE, Ruit KG, Milbrandt J, Snider WD. Dorsal root ganglion neurons expressing trk are selectively sensitive to NGF deprivation in utero. Neuron. 1992;9:779–788. doi: 10.1016/0896-6273(92)90040-k. [DOI] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P, Carter BD, Dobrowsky RT, Chao MV. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- Casaccia-Bonnefil P, Kong H, Chao M. Neurotrophins: the biological paradox of survival factors eliciting apoptosis. Cell Death Differentiation. 1998;5:357–364. doi: 10.1038/sj.cdd.4400377. [DOI] [PubMed] [Google Scholar]
- Chao MV. The p75 neurotrophin receptor. J. Neurobiol. 1994;25:1373–1385. doi: 10.1002/neu.480251106. [DOI] [PubMed] [Google Scholar]
- Chapman BS. A region of the 75 kDa neurotrophin receptor homologous to the death domains of TNFR-I and Fas. FEBS Lett. 1995;374:216–220. doi: 10.1016/0014-5793(95)01113-s. [DOI] [PubMed] [Google Scholar]
- Cheema SS, Barrett GL, Bartlett PF. Reducing p75 nerve growth factor receptor levels using antisense oligonucleotides prevents the loss of axotomized sensory neurons in the dorsal root ganglia of newborn rats. J. Neurosci. Res. 1996;46:239–245. doi: 10.1002/(SICI)1097-4547(19961015)46:2<239::AID-JNR12>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Coulson EJ, Reid K, Barrett GL, Bartlett PF. p75 Neurotrophin Receptor-mediated Neuronal Death Is Promoted by Bcl-2 and Prevented by Bcl-xL. J. Biol. Chem. 1999;274:16387–16391. doi: 10.1074/jbc.274.23.16387. [DOI] [PubMed] [Google Scholar]
- Coulson EJ, Reid K, Baca M, Shipham KA, Hulett SM, Kilpatrick TJ, et al. Chopper, a new death domain of the p75 neurotrophin receptor that mediates rapid neuronal cell death. J. Biol. Chem. 2000;275:30537–30545. doi: 10.1074/jbc.M005214200. [DOI] [PubMed] [Google Scholar]
- Davey F, Davies AM. TrkB signalling inhibits p75-mediated apoptosis induced by nerve growth factor in embryonic proprioceptive neurons. Curr. Biol. 1998;8:915–918. doi: 10.1016/s0960-9822(07)00371-5. [DOI] [PubMed] [Google Scholar]
- Davies AM, Lee KF, Jaenisch R. p75-deficient trigeminal sensory neurons have an altered response to NGF but not to other neurotrophins. Neuron. 1993;11:565–574. doi: 10.1016/0896-6273(93)90069-4. [DOI] [PubMed] [Google Scholar]
- Dobrowsky RT, Jenkins GM, Hannun YA. Neurotrophins induce sphingomyelin hydrolysis. Modulation by co-expression of p75NTR with Trk receptors. J. Biol. Chem. 1995;270:22135–22142. doi: 10.1074/jbc.270.38.22135. [DOI] [PubMed] [Google Scholar]
- Dobrowsky RT, Carter BD. Coupling of the p75 neurotrophin receptor to sphingolipid signaling. Ann. NY Acad. Sci. 1998;845:32–45. doi: 10.1111/j.1749-6632.1998.tb09660.x. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Rosario CM, Merlio JP, Grant G, Aldskogius H, Persson H. Expression of mRNAs for neurotrophin receptors in the dorsal root ganglion and spinal cord during development and following peripheral or central axotomy. Brain Res. Mol. Brain Res. 1993;17:217–226. doi: 10.1016/0169-328x(93)90005-a. [DOI] [PubMed] [Google Scholar]
- Fainzilber M, Smit AB, Syed NI, Wildering WC, Hermann van der Schors RC, Jimenez C, et al. CRNF, a molluscan neurotrophic factor that interacts with the p75 neurotrophin receptor. Science. 1996;274:1540–1543. doi: 10.1126/science.274.5292.1540. [DOI] [PubMed] [Google Scholar]
- Frade JM, Rodriguez-Tebar A, Barde YA. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996;383:166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- Glass DJ, Nye SH, Hantzopoulos P, Macchi MJ, Squinto SP, Goldfarb M, et al. TrkB mediates BDNF/NT-3-dependent survival and proliferation in fibroblasts lacking the low affinity NGF receptor. Cell. 1991;66:405–413. doi: 10.1016/0092-8674(91)90629-d. [DOI] [PubMed] [Google Scholar]
- Gu C, Casaccia-Bonnefil P, Srinivasan A, Chao MV. Oligodendrocyte apoptosis mediated by caspase activation. J. Neurosci. 1999;19:3043–3049. doi: 10.1523/JNEUROSCI.19-08-03043.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantzopoulos PA, Suri C, Glass DJ, Goldfarb MP, Yancopoulos GD. The low affinity NGF receptor, p75, can collaborate with each of the Trks to potentiate functional responses to the neurotrophins. Neuron. 1994;13:187–201. doi: 10.1016/0896-6273(94)90469-3. [DOI] [PubMed] [Google Scholar]
- Hempstead BL, Martin-Zanca D, Kaplan DR, Parada LF, Chao MV. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor [see comments] Nature. 1991;350:678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Himes BT, Tessler A. Death of some dorsal root ganglion neurons and plasticity of others following sciatic nerve section in adult and neonatal rats. J. Comparative Neurol. 1989;284:215–230. doi: 10.1002/cne.902840206. [DOI] [PubMed] [Google Scholar]
- Huber LJ, Chao MV. A potential interaction of p75 and trkA NGF receptors revealed by affinity crosslinking and immunoprecipitation. J. Neurosci. Res. 1995;40:557–563. doi: 10.1002/jnr.490400415. [DOI] [PubMed] [Google Scholar]
- Ip NY, Stitt TN, Tapley P, Klein R, Glass DJ, Fandl J, et al. Similarities and differences in the way neurotrophins interact with the Trk receptors in neuronal and nonneuronal cells. Neuron. 1993;10:137–149. doi: 10.1016/0896-6273(93)90306-c. [DOI] [PubMed] [Google Scholar]
- Jing S, Tapley P, Barbacid M. Nerve growth factor mediates signal transduction through trk homodimer receptors. Neuron. 1992;9:1067–1079. doi: 10.1016/0896-6273(92)90066-m. [DOI] [PubMed] [Google Scholar]
- Khursigara G, Orlinick JR, Chao MV. Association of the p75 Neurotrophin Receptor with TRAF6. J. Biol. Chem. 1999;274:2597–2600. doi: 10.1074/jbc.274.5.2597. [DOI] [PubMed] [Google Scholar]
- Klein CM, Guillamondegui O, Krenek CD, La Forte RA, Coggeshall RE. Do neuropeptides in the dorsal horn change if the dorsal root ganglion cell death that normally accompanies peripheral nerve transection is prevented? Brain Res. 1991;552:273–282. doi: 10.1016/0006-8993(91)90092-a. [DOI] [PubMed] [Google Scholar]
- Lee KF, Davies AM, Jaenisch R. p75-deficient embryonic dorsal root sensory and neonatal sympathetic neurons display a decreased sensitivity to NGF. Development. 1994;120:1027–1033. doi: 10.1242/dev.120.4.1027. [DOI] [PubMed] [Google Scholar]
- Majdan M, Lachance C, Gloster A, Aloyz R, Zeindler C, Bamji S, et al. Transgenic mice expressing the intracellular domain of the p75 neurotrophin receptor undergo neuronal apoptosis. J. Neurosci. 1997;17:6988–6998. doi: 10.1523/JNEUROSCI.17-18-06988.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Snider WD. Nerve growth factor receptor TrkA is down-regulated during postnatal development by a subset of dorsal root ganglion neurons. J. Comparative Neurol. 1997;381:428–438. doi: 10.1002/(sici)1096-9861(19970519)381:4<428::aid-cne3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Mu X, Silos-Santiago I, Carroll SL, Snider WD. Neurotrophin receptor genes are expressed in distinct patterns in developing dorsal root ganglia. J. Neurosci. 1993;13:4029–4041. doi: 10.1523/JNEUROSCI.13-09-04029.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray S, Bartlett P, Cheema S. Differential loss of spinal sensory but not motor neurons in the p75NTR knock out mouse. Neurosci. Lett. 1999;266:1–4. doi: 10.1016/s0304-3940(99)00330-4. [DOI] [PubMed] [Google Scholar]
- Rabizadeh S, Oh J, Zhong LT, Yang J, Bitler CM, Butcher LL, et al. Induction of apoptosis by the low-affinity NGF receptor. Science. 1993;261:345–348. doi: 10.1126/science.8332899. [DOI] [PubMed] [Google Scholar]
- Schecterson LC, Bothwell M. Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron. 1992;9:449–463. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- Sebert ME, Shooter EM. Expression of mRNA for neurotrophic factors and their receptors in the rat dorsal root ganglion and sciatic nerve following nerve injury. J. Neurosci. Res. 1993;36:357–367. doi: 10.1002/jnr.490360402. [DOI] [PubMed] [Google Scholar]
- Snider WD, Elliott JL, Yan Q. Axotomy-induced neuronal death during development. J. Neurobiol. 1992a;23:1231–1246. doi: 10.1002/neu.480230913. [DOI] [PubMed] [Google Scholar]
- Snider WD, Zhang L, Yusoof S, Gorukanti N, Tsering C. Interactions between dorsal root axons and their target motor neurons in developing mammalian spinal cord. J. Neurosci. 1992b;12:3494–3508. doi: 10.1523/JNEUROSCI.12-09-03494.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Soilu-Hanninen M, Ekert P, Bucci T, Syroid D, Bartlett PF, Kilpatrick TJ. Nerve growth factor signaling through p75 induces apoptosis in Schwann cells via a Bcl-2-independent pathway. J. Neurosci. 1999;19:4828–4838. doi: 10.1523/JNEUROSCI.19-12-04828.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrado J, Monnier D, Perrelet D, Sagot Y, Mattenberger L, King B, Kato AC. NGF-induced motoneuron cell death depends on the genetic background and motoneuron sub-type. Neuroreport. 2000;11:1473–1477. [PubMed] [Google Scholar]
- Twiss JL, Wada HG, Fok KS, Chan SD, Verity AN, Baxter GT, Shooter EM, Sussman HH. Duration and magnitude of nerve growth factor signaling depend on the ratio of p75LNTR to TrkA. J. Neurosci. Res. 1998;51:442–453. doi: 10.1002/(SICI)1097-4547(19980215)51:4<442::AID-JNR4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Van der Zee CE, Ross GM, Riopelle RJ, Hagg T. Survival of cholinergic forebrain neurons in developing p75NGFR-deficient mice. Science. 1996;274:1729–1732. doi: 10.1126/science.274.5293.1729. [DOI] [PubMed] [Google Scholar]
- Verdi JM, Birren SJ, Ibanez CF, Persson H, Kaplan DR, Benedetti M, et al. p75LNGFR regulates Trk signal transduction and NGF-induced neuronal differentiation in MAH cells. Neuron. 1994;12:733–745. doi: 10.1016/0896-6273(94)90327-1. [DOI] [PubMed] [Google Scholar]
- Verge VM, Riopelle RJ, Richardson PM. Nerve growth factor receptors on normal and injured sensory neurons. J. Neurosci. 1989;9:914–922. doi: 10.1523/JNEUROSCI.09-03-00914.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verge VM, Merlio JP, Grondin J, Ernfors P, Persson H, Riopelle RJ, et al. Colocalization of NGF binding sites, trk mRNA, and low-affinity NGF receptor mRNA in primary sensory neurons: responses to injury and infusion of NGF. J. Neurosci. 1992;12:4011–4022. doi: 10.1523/JNEUROSCI.12-10-04011.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelbaum MA, Tong JX, Rich KM. Developmental regulation of apoptosis in dorsal root ganglion neurons. J. Neurosci. 1998;18:8928–8935. doi: 10.1523/JNEUROSCI.18-21-08928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmore C, Olson L. Neuronal and nonneuronal expression of neurotrophins and their receptors in sensory and sympathetic ganglia suggest new intercellular trophic interactions. J. Comp. Neurol. 1995;353:143–159. doi: 10.1002/cne.903530113. [DOI] [PubMed] [Google Scholar]
- Wright DE, Snider WD. Neurotrophin receptor mRNA expression defines distinct populations of neurons in rat dorsal root ganglia. J. Comp. Neurol. 1995;351:329–338. doi: 10.1002/cne.903510302. [DOI] [PubMed] [Google Scholar]
- Yeo TT, Chua-Couzens J, Butcher LL, Bredesen DE, Cooper JD, Valletta JS, et al. Absence of p75NTR causes increased basal forebrain cholinergic neuron size, choline acetyltransferase activity, and target innervation. J. Neurosci. 1997;17:7594–7605. doi: 10.1523/JNEUROSCI.17-20-07594.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SO, Casaccia-Bonnefil P, Carter B, Chao MV. Competitive signaling between TrkA and p75 nerve growth factor receptors determines cell survival. J. Neurosci. 1998;18:3273–3281. doi: 10.1523/JNEUROSCI.18-09-03273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XF, Gai WP, Rush RA. CGRP immunoreactive neurons in rat dorsal root ganglia do not all contain low-affinity NGF receptor immunoreactivity. Brain Res. 1993;612:322–325. doi: 10.1016/0006-8993(93)91679-m. [DOI] [PubMed] [Google Scholar]
- Zhou XF, Rush RA, McLachlan EM. Differential expression of the p75 nerve growth factor receptor in glia and neurons of the rat dorsal root ganglia after peripheral nerve transection. J. Neurosci. 1996;16:2901–2911. doi: 10.1523/JNEUROSCI.16-09-02901.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]