Abstract
Embryonic tissues, in common with other tissues, including tumours, tend to develop a substantial vasculature when transplanted onto the chorioallantoic membrane (CAM). Studies conducted to date have not examined in any detail the identity of vessels that supply these grafts, although it is known that the survival of transplanted tissues depends on their ability to connect with CAM vessels supplying oxygen and nutrients. We grafted the mesonephros, a challenging model for studies in vascular development, when it was fully developed (HH35). We used reciprocal chick-quail transplantations in order to study the arterial and venous connections and to analyse the cell invasion from the CAM to the organ, whose degeneration in normal conditions is rapid. The revascularization of the grafted mesonephros was produced by the formation of peripheral anastomoses between the graft and previous host vasculatures. The assembly of graft and CAM blood vessels occurred between relatively large arteries or veins, resulting in chimeric vessels of varying morphology depending on their arterial or venous status. Grafts showed an increased angiogenesis from their original vasculature, suggesting that the normal vascular degeneration of the mesonephros was partially inhibited. Three types of isolated host haemangioblast were identified in the mesonephros: migrating angioblast-like cells, indicating vasculogenesis, undifferentiated haematopoietic cells and macrophages, which might have been involved in the angiogenesis. Tomato lectin was found to bind activated macrophages in avian embryos.
Keywords: angiogenesis, avian embryo, embryonic grafts, mesonephros, vasculogenesis
Introduction
The literature on organ and tumour transplantation, and on the importance of angiogenesis as a survival factor in the revascularization of these transplanted tissues, is extensive. However, these studies do not examine the identity of the vessels that supply the grafts. Given that the survival of transplanted tissues depends on their ability to connect with vessels that transport oxygen and nutrients, the specification of arterial and venous identity, and the study of arterial and venous connections between host and graft vasculatures, is fundamental to our understanding of how transplanted tissues survive.
The literature on embryonic kidneys grafts on the chorioallantoic membrane (CAM) is somewhat scant. Only a few studies have been reported, mainly using mouse metanephros, consisting of undifferentiated and avascular kidneys (Ekblom et al. 1982; Sariola et al. 1983, 1984a,b; Sariola, 1984). Therefore, little is known about what would happen when a mesonephros with a fully developed vascular system is transplanted onto the CAM.
The CAM is a suitable site for transplanting tissues, including the embryonic kidneys (Preminger et al. 1981), due to its great microvascular bed and, indeed, it has been widely used as a model (Ribatti et al. 2001). Transplanted tissues can survive and develop in the CAM by peripheral anastomoses between graft and original CAM vasculatures or by new angiogenic vessels grown from the CAM that invade the graft. Previous studies have demonstrated that the formation of peripheral anastomoses between host and pre-existing donor vessels is the main, and the most common, mechanism involved in the revascularization of embryonic grafts, whereas the growth of CAM-derived vessels into the graft is only stimulated in tumour grafts (Ausprunk et al. 1975; Ausprunk & Folkman, 1976).
Avian kidney development proceeds in three stages. Both the pronephros and the mesonephros degenerate. Only the third embryonic excretory organ, the metanephros, persists as the definitive kidney. Here, we chose the avian mesonephros since its transient nature offers the possibility of studying a fully developed vascular organ in a short period of time. Moreover, we have previously studied the normal development of its vascular system in detail (Carretero et al. 1993, 1995, 1997, 2001; Arcalís et al. 2002). The mesonephros is a source of multiple stem cells, vascular endothelial cells and haematopoietic stem cells. Thus it constitutes a ‘challenging’ model for studies on stem cell differentiation (see review by Sainio & Raatikainen-Ahokas, 1999).
The aim of this study was to analyse the vessel interactions in the revascularization of a fully developed vascular organ, in this case the avian mesonephros, using the CAM model. The study focused on the identity and assembly of blood vessels between host and donor vasculatures. A second aim involved analysing the vascular remodelling of the mesonephric vessels when this organ had been taken out of its normal biological placement.
We grafted mesonephros at stage HH35, when its vascular system was fully developed. This was then removed at stage HH40, when the normal degeneration of the mesonephros had become severe (Lillie, 1952). Reciprocal transplantations (quail/chick and chick/quail) were performed, in order to analyse the constitution of connecting vessels and the identity of isolated cells, by means of quail cellular markers such as QH1 and QCPN. To analyse vascular corrosion casts of the grafts, we used scanning electron microscopy (SEM), a method that offers many facilities for studying the specimen, including quasi three-dimensional images of the angioarchitecture. We were thus able to make the first vascular corrosion casts of grafts onto the CAM, modifying our injection technique described elsewhere for embryonic specimens (Carretero et al. 1993).
Materials and methods
General procedures
Fertilized eggs of White Leghorn chicks (Gallus domesticus) and Japanese quails (Coturnix coturnix japonica) were incubated at 38.5 °C and 60% relative humidity. For reciprocal transplantations between chick and quail, donor embryos were incubated until stage HH35 (embryonic days 7 and 9 in quail and chick, respectively). At this stage, when the mesonephros is fully developed, the urogenital organs were dissected out from the embryo. Then the gonads and the mesonephric ducts were carefully removed and each mesonephros was grafted separately onto the CAM of the host, which was at the same stage of development (HH35). The host eggs were previously windowed at stage HH18 (day 3 of development approximately). The grafted eggs were resealed with cellophane tape and returned to the incubator for 4 or 5 days until stage HH40 (embryonic days 11 and 14 in quail and chick, respectively), when the degeneration of the mesonephros has become severe in normal conditions. The chick and quail embryos were staged according to Hamburger & Hamilton's (1951) and Zacchei's (1961) criteria, respectively.
The eggs were examined daily. Dead hosts were not included in the study. We were able to evaluate 28 chick grafts and 33 quail grafts that had been successfully revascularized.
Scanning electron microscopy study
Approximately half of the chick grafts were injected with prepolymerized Mercox (Mercox-Jap. Vilene Co. supplied by Ladd Research Ind., Inc. Williston, VT, USA). To obtain a Mercox viscosity similar to that of blood, the resin was diluted with 25% methyl-methacrylate (Miyoshi et al. 1995). A CAM vessel of approximately 50 µm, which vascularized the graft, was chosen as the injection site. To improve graft vascular casting, retrograde CAM vessels were clamped with microclips, devised from a transmission electron microscopic carrier grid (Hogers et al. 1997) to avoid resin wastage. A Pasteur's pipette was used as a cannula. The pipette was fire polished to a gauge similar to that of the vessel that was to be injected. Then the pipette was inserted into the vessel and sealed with a chemical ligature (Cyanocrylate, Loctite) (see Carretero et al. 1993 for details of the technique). After polymerization, the injected grafts were corroded in 2% KOH and washed in distilled water; afterwards they were mounted on stubs, sputtered with gold (5 min, 14–17 mA, 0.07 mbar), and observed under a Hitachi S-570 scanning electron microscope at an accelerating voltage of 10–15 kV.
Immunohistochemistry on paraffin sections
The grafts were removed, together with a part of the adhering host CAM, fixed in 2% acetic acid in absolute ethanol and embedded in paraffin. The transplanted mesonephroi were serially sectioned at 5 µm and analysed using both histology (Haematoxylin-eosin) and immunohistochemistry (quail-specific monoclonal antibodies QCPN and QH1). The QCPN antibody recognizes an antigenic determinant in the perinuclear membrane common to all quail cells, except for the blood cells (Carlson and Carlson, unpublished). The QH1 antibody was used in order to stain endothelial and angioblast quail cells. This antibody also recognizes the haematopoietic quail cells and its epitope is expressed on antigens found both within the cytoplasm and on the cell surface (Pardanaud et al. 1987).
Some of the grafts, in which connections between chick-quail vessels had previously been observed, were also stained with the muscle-actin-specific monoclonal antibody, HHF35 (Dako) (Tsukada et al. 1987), in order to stain the smooth muscle cells of the vessel walls.
The deparafinized and rehydrated sections were immersed in phosphate-buffered saline (PBS), pH 7.3, to which 0.3% H2O2 was added in order to inhibit endogenous peroxidase activity. After rinsing twice in PBS for 5 min and once in PBS with 0.05% Tween-20, overnight incubation in a humidity chamber (at 4 °C) took place with the first monoclonal antibodies (1 : 500 QH1, 1 : 1 QCPN and 1 : 500 HHF35) all diluted with 1% ovalbumin (Sigma) in PBS-Tween-20. After rinsing twice in PBS for 5 min and once in PBS with 0.05% Tween-20, the second incubation was performed for 2 h with the rabbit antimouse peroxidase-conjugated antibody (Dako P260), diluted 1 : 200. Subsequently, all sections were rinsed twice in PBS for 5 min. The staining reaction was performed by exposing the slides for 8 min to diaminobenzidine (DAB, Sigma) diluted in TRIS-maleate buffer, pH 7.6, to which 0.006% hydrogen peroxide was added for the location of peroxidase activity, followed by washing in distilled water. The stained sections were briefly counterstained with Mayer's Haematoxylin and dehydrated in ethanol, after which they were mounted in Entellan (Merck) and studied and photographed under a light microscope (Nikon Eclipse E800).
A selection of the slides were incubated with QH1 diluted 1 : 500 in wash buffer and normal goat serum (10%) for 2 h, then rinsed in washing buffer and incubated with the second antibody biotinylated antimouse IgG (Vector) diluted in washing buffer 1 : 250 for 1 h. Subsequently, the stained sections were revealed with avidin-rhodamine diluted 1 : 100 in PBS for 1 h, then rinsed in washing buffer and PBS and mounted with aqueous medium designed for the preservation of fluorescence. The slides were examined under a Leica TCS-4D confocal laser-scanning microscope.
To study the isolated QH1-positive cell populations invading the chick grafts, various markers were used. The LEP100 IgG is an avian-specific monoclonal antibody that stains a lysosomal membrane glycoprotein identified from chicken (Lippincott-Schwartz & Fambrough, 1986). The protocol was similar (1 : 1 first antibody dilution) to those described above for the other monoclonal antibodies. We also performed acidic phosphatase (AcPase) histochemistry to differentiate the macrophages from other cell types. After the removal of paraffin in chloroform, and hydration, sections were incubated overnight at 37 °C in a solution containing 0.01% naphtol AS BI phosphate, 1% dimethylformamide and 0.02% fast red violet in Walpole acetate – acetic acid buffer (pH 5.2). Sodium tartrate, an inhibitor of some types of AcPase activity, was added. These slides were counterstained with nuclear fast green. Finally, we used a biotin-conjugated lectin from Lycopersicon esculentum (tomato) (Sigma). Lycopersicon esculentum agglutinin (LEA) is specific for N-acetyl glucosamine residues and it is a known marker of cells with macrophagic activity in mammals. Sections were treated with 0.1% trypsin buffer supplemented with cations (0.1 mm CaCl2, MgCl2 and MnCl2) at 37 °C for 10 min. Preparations were incubated overnight at 4 °C with LEA (working solution 20 µg mL−1) implemented with PBS containing cations and 0.1% triton X-100.
Immunohistochemistry on whole mounts
Twelve grafts were studied using the QH1 antibody on whole mount preparations. Fixed grafts in 10% neutral buffered formalin (NBF) were permeabilized overnight at 4 °C and their endogenous peroxidase activity was inhibited with 0.1% triton X-100, methanol and 3% hydrogen peroxide. The procedure then followed was similar to that conducted for the paraffin sections. The specimens were stored and cleared in a sodium azide glycerine solution, and photographed under a dissection microscope (Olympus SZH) fitted with a camera (Olympus C-35 AD-4). Whole mounts were then sectioned (100 µm) using a vibratome (Series 1000) and studied under a light microscope.
Results
Microvascular connections between host and graft
Most of the grafts were well revascularized by large vessels from the CAM (Fig. 1). The vascular casts showed large vessels coming from outside that connected with the graft vasculature at its periphery (Fig. 2A). On the cast surface of these main vessels, we observed round endothelial imprints with no consistent orientation, corresponding with the classical endothelial nuclear pattern in a vein. These vessels ramified markedly providing several main branches towards the graft. In some of the connections between the host and graft vessels, it was possible to observe clear constrictions, probably at the anastomosing sites (Fig. 2B). This observation was consistent with the presence of subendothelial small cushions in the histological sections, which pushed the endothelium towards the lumen at the anastomosing sites (Fig. 4A).
Fig. 1.
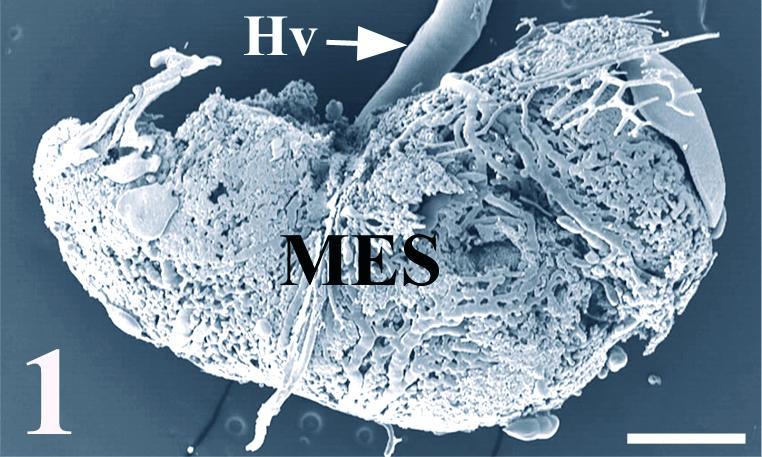
SEM micrograph showing the general appearance of a vascular cast of the chick mesonephros grafted onto quail CAM. Note the great vessel from the CAM (Hv) supplying the graft (MES). Scale bar = 0.6 mm.
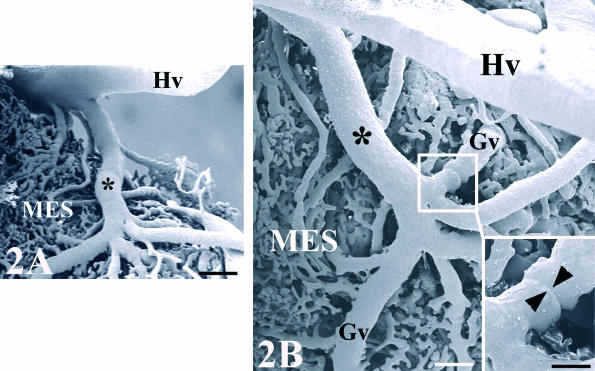
Fig. 2.
Cast of the chick mesonephros vasculature grafted onto quail CAM. A venous branch (*) coming from the host vasculature (Hv) supplies the graft (MES) (A). General appearance. Scale bar = 0.2 mm (B). Detail of Fig. 2(A). The venous branch connects with the original vasculature of the graft (Gv). Scale bar = 0.1 mm. The inset shows a vascular connection which presents circular constrictions (arrowheads). Scale bar = 60 µm.
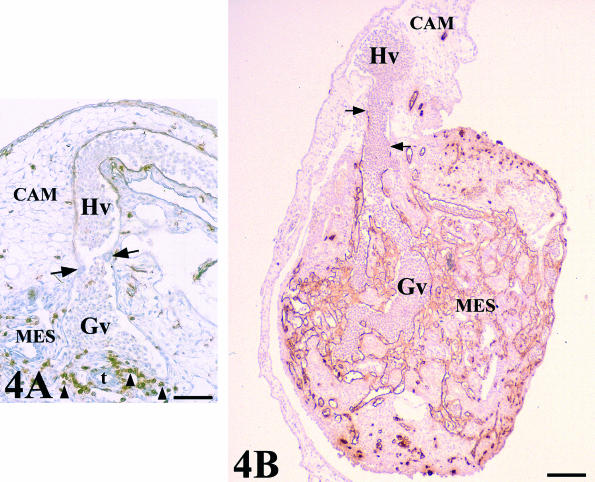
Fig. 4.
QH1-stained sections showing two venular assemblies between CAM and graft-mesonephros (MES) vasculatures. (A) Chick mesonephros grafted onto quail CAM (QH1 positive). The arrows indicate a constriction at the level of the endothelial junction between host (Hv) and graft (Gv) veins. There is no invasion of host vessels into the graft. Peripheral regions presented a great invasion of QH1 quail-positive cells (arrowheads). t, tubule. Scale bar = 55 µm. (B) Quail mesonephros (QH1 positive) grafted onto chick CAM. The arrows indicate the endothelial junction between host (Hv) and graft (Gv) veins. Note the adequate vascularization of the grafted mesonephros. Scale bar = 250 µm.
The analysis with quail-specific monoclonal antibodies failed to rule out either an invasion of host-derived vessels into the graft or a penetration of graft-mesonephric vessels on the CAM (Figs 3 and 4). Moreover, clear anastomoses between the CAM and graft vessels were observed. These anastomoses produced connecting vessels of chimeric structure (Fig. 4).
Fig. 3.
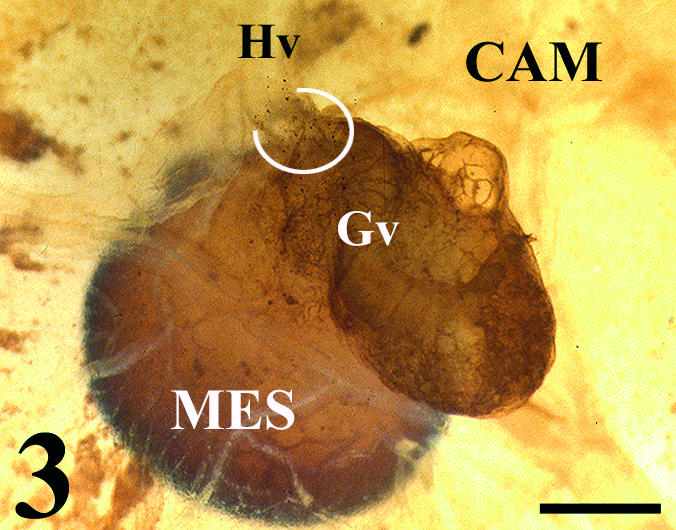
Whole mount of a quail mesonephros (MES) grafted onto chick CAM. Peripheral vascular connection (circle) between a host vessel (Hv) (QH1 negative) and a graft vessel (Gv). Scale bar = 0.3 mm.
Graft vascular casts showed increased angiogenesis at their periphery, presenting enlarged vessels with angiogenic holes and vascular sprouts (Fig. 5). The QH1 signal in whole mounts revealed the fine structure of these vascular sprouts, showing tufts of filopodia emerging from the endothelial cells to the vessel periphery. Whole mounts also confirmed that angiogenic sprouts were of mesonephric origin (Fig. 6).
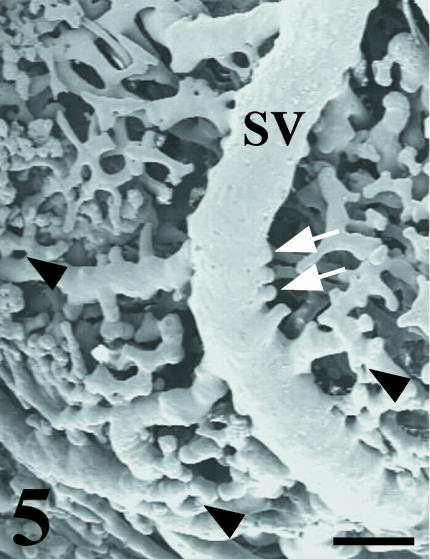
Fig. 5.
SEM micrograph of a grafted chick mesonephros vascular cast showing the appearance of the superficial subcardinal veins (SV) presenting classical angiogenic structures such as vascular sprouts (arrows) and angiogenic holes (arrowheads). Scale bar = 100 µm.
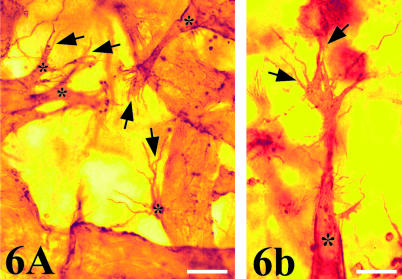
Fig. 6.
(A,B) Superficial QH1-positive vessels in whole mount of grafted quail mesonephros showing vascular sprouts (*) that present large stuff of filopodia (arrows). (A) Scale bar = 14 µm; (B) scale bar = 15 µm.
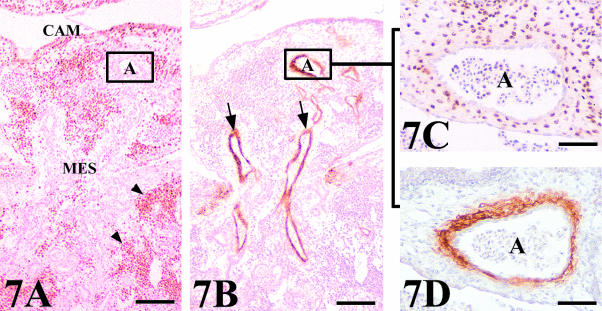
Connecting vessels were identified as being either arteries or veins using the HHF35 antibody, which marks smooth muscle cells (Fig. 7). Only arteries presented smooth muscle cells surrounding their endothelium (Fig. 7B). It is important to note that we only found connections between host and donor arteries or veins and that we observed no artery–vein junction.
Fig. 7.
Arterial assembly in a chick mesonephros (MES) grafted onto quail CAM. (A,B) General view of QCPN and HHF35 stained serial sections showing the positive binding of the HHF35 to the arteries (arrows) which penetrate to supply the graft. Peripheral regions presented a great invasion of QCPN quail-positive cells (arrowheads). Scale bar = 100 µm. (C) Detail of a consecutive section stained with QCPN showing the connection between quail and chick arteries. The arterial assembly shows as the endothelial cells of the graft are surrounded by host smooth muscle cells. (D) View of a serial section stained with HHF35 demonstrating the nature of the smooth muscle cells around the artery. A, artery. Scale bar = 48 µm.
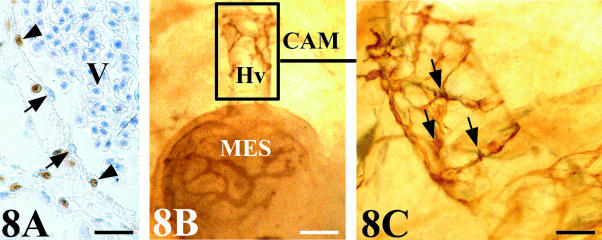
Whereas the anastomoses in arteries were produced by the invasion of host-quail smooth muscle cells surrounding the endothelial cells of the graft (Fig. 7C,D), the anastomoses in veins showed a chimeric endothelial composition of mosaic-type (Fig. 8A). Whole mounts revealed QH1-positive cells, with the morphological features of migrating angioblast, invading the connecting veins (Fig. 8B,C). Figure 9 shows a schematic structural representation of both artery–artery and vein–vein connections found between mesonephros and CAM vasculatures.
Fig. 8.
Venous assembly. (A) Chick mesonephros grafted onto quail CAM. QCPN-stained section showing a chimeric endothelial composition in a vein anastomosis. Arrow: chick endothelial cell. Arrowhead: quail endothelial cell. V, vein lumen with numerous erythrocytes. Scale bar = 25 µm. (B) Whole mount quail mesonephros (MES) grafted onto chick CAM stained with QH1. A host vein (Hv) that connects with the graft vasculature are surrounded by QH1-positive cells of graft origin. Scale bar = 120 µm. (C) Detail of Fig. 8(B) showing the aspect of these cells of graft origin (arrows) that present large filopodia and the morphology as migrating angioblast-like cells. Scale bar = 46 µm.
Fig. 9.
chematic structural representation of both artery–artery and vein–vein connections found between mesonephros and CAM vessels.
Host cell invasion into the graft
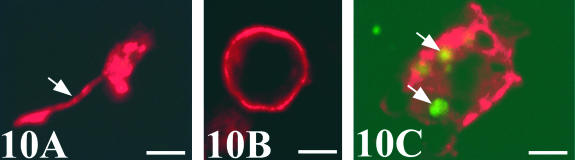
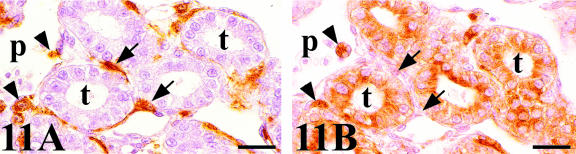
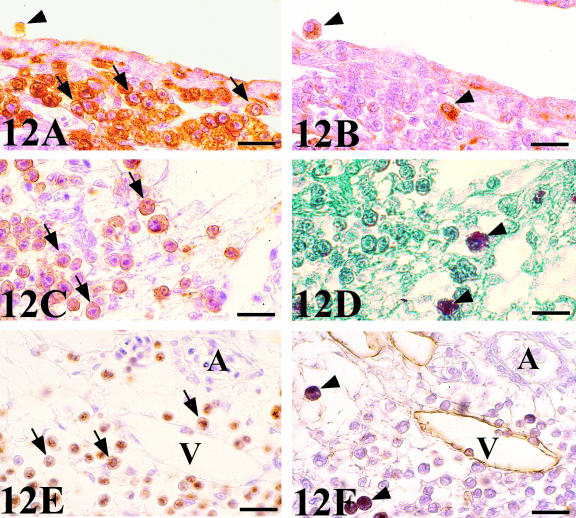
Chick-mesonephros were grafted onto quail-CAM, since the quail-specific antibodies allowed us to observe the quail cells inside the graft. The peripheral areas of the grafts contained a great invasion of QH1- and QCPN-positive cells (Figs 4A and 7A). Observation of the QH1-positive cells using confocal microscopy allowed us to classify into three categories depending on their morphological aspect: angioblast-like cells with filopodial processes (Fig. 10A), rounded haematopoietic-like cells (Fig. 10B) and swollen activated macrophage-like cells containing numerous cell fragments in their cytoplasm (Fig. 10C). We therefore applied other immunohistochemical and histochemical techniques, based on the detection of lysosomes, which are typical of active phagocyte cells, to confirm these morphological results. No immunoreactivity to the LEP100 IgG antibody or AcPase or LEA activity was found in angioblast-like cells (Fig. 11B) or rounded haematopoietic cells (Fig. 12B,D,F). In contrast, macrophages gave positive readings for these techniques (Figs 11B and 12B,D,F). Some angioblast-like cells were elongated and situated around the tubules corresponding to the location of the peritubular mesonephric capillary plexus, and, therefore, they have already been differentiated into endothelial cells (Fig. 11A). The rounded haematopoietic cells were the most numerous and were found in all the peripheral areas of the graft (Fig. 12A,C,E). Activated macrophages might have invaded the graft from the blood vessels by traversing the endothelial wall, since they were evident in the intraluminal blood flow (Fig. 11B). Tomato lectin bound only to the vessel endothelium of vein type (Fig. 12F), and was therefore a good marker for differentiating veins from arteries, even in the case of the smallest vessels which could not be easily distinguished with the HHF35 antibody.
Fig. 10.
Confocal fluorescence micrographs of different QH1-positive quail cells inside the chick graft. (A) Angioblast-like cell with a filopodium (arrow). (B) Undifferentiated haematopoietic cell. (C) Activated macrophage. Phagosomes appear as green autofluorescent fragments in the cytoplasm (arrows). Scale bar = 3.75 µm.
Fig. 11.
(A) QH1-stained section of a chick mesonephros grafted onto quail CAM showing a great invasion of QH1-positive cells of host origin: endothelial cells (arrows) that are located around the tubules forming part of the peritubular capillary plexus (p), and macrophages (arrowheads). (B) LEP 100 IgG-stained consecutive section. The endothelial cell (arrows) do not present the LEP 100 epitope and only macrophages (arrowheads) are positive to this antibody. Note the intense reaction of the proximal tubules (t) cells. Scale bar = 18.5 µm.
Fig. 12.
QH1-(A,C) and QCPN-(E) stained sections of chick mesonephros grafted onto quail CAM showing a great invasion of QH1- and QCPN-positive cells of host origin in the periphery of the graft. Mostly rounded and undifferentiated haematopoietic cells (arrows). (B,D,F) Consecutive sections to the previous figures stained with LEP 100 IgG, AcPase and LEA, respectively. Only macrophages (arrowheads) are positive to these techniques. Note the spotted signal in the cytoplasm using LEP 100 IgG and AcPase which marks lysosomes. However, LEA binds to citoplasmatic membrane. Note in (F) that LEA bound only to endothelium of venous type. V, vein; A, artery. Scale bar (A,B,E,F) = 19 µm; (C,D) = 16 µm.
Discussion
Previous studies in which tissues have been grafted onto CAM suggest that the formation of peripheral anastomoses between host and graft vessels is the most common mechanism involved in the revascularization of grafted embryonic tissues, whereas sprouting of CAM-derived vessels into the transplant only occurs in grafts of tumour tissue (Ausprunk et al. 1975; Ausprunk & Folkman, 1976). In agreement with these authors, the QH1 and QCPN antibodies used here showed that blood reperfusion of the chick mesonephros implanted onto quail CAM takes place by means of the establishment of peripheral anastomoses between host and graft blood vessels. No new growth of vessels from the host microvasculature into the graft, or vice versa, was noticed.
In all cases the connection between the host and donor vasculatures gave rise to chimeric vessels. Histological results revealed that all host–graft connections were formed between relatively large vessels and no connections were found at the capillary level. Although here we have referred to the vessels as arteries and veins, it would be more accurate to describe them as arterioles and venules, in line with descriptions elsewhere of the CAM microvasculature (Ausprunk et al. 1974; Shumko et al. 1988; Ribatti et al. 2001). In fact, the diameters of the connecting vessels recorded in our study and the negative stain of the venous vessels with HHF35 are in accordance with this vessel classification.
Studies of the early development of the vascular system have shown that, in general, the actin-negative vessels are involved in vascular remodelling, which implies the formation of new blood vessels, while muscular actin spreads throughout the complete vascular system when vessels mature (DeRuiter et al. 1990). Staining of smooth muscle cells actin with HHF35 could confirm, at least in arteries, that the arterial connections observed between host and graft vasculatures when the grafts were removed are produced in mature vessels.
The vascular connections observed were always artery–artery or vein–vein. The connections in veins were of mosaic-type, intermingled quail and chick endothelial cells, whereas host smooth muscle cells surrounding the donor endothelial cells formed the union between arteries. In fact, this is partially in agreement with the explanation offered by Carmeliet (2000) where it is described how smooth muscle cells ‘muscularize’ the nascent vasculature migrating alongside pre-existing vessels.
Previously, arterial–venous differentiation was thought to occur as a consequence of haemodynamic forces (reviewed by Yancopoulos et al. 1998). However, several molecules have recently been shown in mouse, zebrafish and chick embryo to be expressed selectively by developing arterial or venous endothelial cells, thereby providing evidence that the differences between arteries and veins are, at least in part, genetically determined. Arterial-specific markers include some Notch genes (Shutter et al. 2000; Lawson et al. 2001), the transcription factor gridlock (Zhong et al. 2000), the transmembrane growth factor ephrin B2 (Wang et al. 1998; Carretero et al. 2001), and a transmembrane receptor neuropilin-1 (Moyon et al. 2001). Venous endothelial cells express the receptor for ephrin B2, EphB4 (Wang et al. 1998; Adams et al. 1999) and a transmembrane receptor TIE2 (Moyon et al. 2001).
However, Moyon et al. (2001) showed that already differentiated arterial endothelial cells, in the absence of the vascular wall, were able to colonize again both host arteries and veins. These results seem to confirm that the endothelium remains plastic independently of its arterial or venous differentiation. At the moment of grafting, 9 days of development, the vessels of the chick CAM are just leaving the proliferation stage (4–8 days), and are just entering the differentiation stage (9–13 days). When the grafts are removed at 14 days of incubation, the CAM vessels have started the maturation stage (14–18 days) (Shumko et al. 1988). Therefore, we grafted fully developed mesonephros onto the CAM when the immaturity of the endothelial cells helps this membrane to support grafted tissues. An interesting hypothesis emerging from these results is that non-mature CAM vessels could differentiate into arterial or venous phenotypes in response to a kind of signal produced by the graft in order to assemble with its mature arteries or veins, respectively.
This distinctive behaviour for arterial and venous endothelial cells at the time of connection could be due to structural differences during their development. For instance, Shumko et al. (1988) described that the CAM chick arterioles at stage 9–13 days of incubation display extensive interdigitating cell junctions with multiple membrane contact points. In contrast, venular endothelial cell appositions remain as simple contact points, even in the latter stages of development. This might explain why venous endothelial cells seemed more able to separate themselves and also to incorporate new endothelial cells producing the mosaic-type connection observed in our experiment.
Lectins specifically stain a particular type of endothelial cell, discriminating arteries from veins (Mills & Haworth, 1986). In our experiments, the tomato lectin bound to chick and quail endothelium. However, we did not find the LEA signal in arteriolar endothelial cells, as observed by Nanka et al. (2001). This difference could be due to spatial and temporal changes in lectin binding to endothelial luminal glycoconjugates during microvascular development. For instance, Charmaine & DeFouw (1995) observed significant variations in LEA binding to N-acetyl glucosamine of precapillary and postcapillary vessels during chick CAM development.
Histological and vascular cast observations showed constrictions in the connections between CAM and graft veins. Constrictions of this type have been described as sphincters by numerous authors and may correspond to smooth muscle cells accumulated into subendothelial small cushions (Aharinejad & Böck, 1992). Normally, they have been described in precapillary arterioles (Rhodin, 1967; Miyoshi et al. 1995) and they probably modify the blood flow and pressure in the capillary network. However, we observed constrictions in connecting venules, which did not contain smooth muscle cells.
Corrosion casts studied by SEM are widely used for three-dimensional studies of blood vessels. This method offers many advantages such as being able to analyse the origin, course and branching of individual vessels and to distinguish between arteries and veins by the difference in endothelial imprints on the cast surfaces (Ditrich & Splechtna, 1987). However, the use of this technique to study the vascularization of grafts is much less common (Miyoshi et al. 1995) and this is the first time that corrosion casts of tissues grafted onto the CAM have been obtained, enabling us to delineate whole graft microvasculature.
The graft casts showed a more developed vascular system than that of a degenerating mesonephros on the same day of development (HH40). The appearance of these graft vascular casts showed a vascular aspect more similar to that of a developing mesonephros (Carretero et al. 1995). The angiogenic structures found in whole mount mesonephros and in the cast surface have been previously described by numerous authors as true angiogenic structures: sprouting of capillaries from pre-existing vessels (Coffin & Poole, 1988; Arcalís et al. 2002), and holes characteristic of intussusceptive angiogenesis (Caduff et al. 1986; Pérez-Aparicio et al. 1996). The quail-specific antibodies showed that this growth of new blood vessels was produced from the original graft vasculature and not from the CAM host vessels. This result demonstrates that the normal vascular degeneration of the mesonephros at stage HH40 was inhibited when it was taken out of its normal biological placement. This probably means that the degeneration of the mesonephros is regulated, at least in part, by extrinsic factors. The irremediable hypoxia produced in grafting techniques has long been recognized as a stimulus for angiogenesis, since in response to lowered oxygen concentration an up-regulation of VEGF mRNA and its receptors is produced (reviewed in Ferrara & Gerber, 1999). In agreement with this, we found enhanced angiogenesis in the mesonephros when the embryos were previously treated with exogenous HCl (Navarro et al. 1996), a potent inducer of vascular endothelial growth factor (VEGF).
The quail haemangioblast-specific QH1 antibody was used to detect cells of donor origin inside the graft. The term haemangioblast refers to a common ancestor of endothelial and haematopoietic cells (Pardanaud &Dieterlen-Lièvre, 1995, 1999; Choi et al. 1998). Although no invasion of host vessels into the graft was noticed, we did observe the presence of different QH1-positive cell populations in peripheral areas. One of these cell populations had the morphological and immunohistochemical features of angioblast, the endothelial cell precursor. Cells with similar features have been described by other authors as migrating angiogenic cells (Wilms et al. 1991; Arcalís et al. 2002). In our study, endothelial cells were located at the level of the peritubular capillary plexus but we do not know if they would have formed new blood vessels by vasculogenesis. Studies suggest that metanephric blood vessels are also formed by vasculogenesis (Hyink et al. 1996; Robert et al. 1996; Abrahamson et al. 1998).
The other cell populations of host origin could be distinguished in the graft peripheral areas and were classified as: undifferentiated haematopoietic cells and macrophages. The negative cells for LEP100, AcPase and LEA were often found in clusters around and inside the vessels. These round-shaped cells have been described as haematopoietic cells by other authors (Cuadros et al. 1992; Pardanaud &Dieterlen-Lièvre, 1995, 1999; Jaffredo et al. 1998; Takakura et al. 2000), and could play an important role in angiogenesis, since they produce angiopoietin-1 which directly promotes the migration of endothelial cells (Takakura et al. 2000). The positive cells to LEP100, AcPase and LEA have been described as macrophages, containing numerous vacuoles filled with phagocytic inclusions.
An intense positive reaction to LEP100 IgG was observed in mesonephric tubules. This is a normal reaction indicating that they were proximal tubules, whose cells contain abundant secretory granules, LEP100 positive, in their apical pole. The AcPase activity, a typical macrophagic enzymatic activity in paraffin-embedded sections, was also a useful cytochemical technique to distinguish macrophages from other haematopoietic cells (Cuadros et al. 1992). Moreover, we used another technique, the LEA binding, to confirm the macrophage cell phenotype. This lectin has been defined as a macrophage marker in species such as humans and mice (Sato & Hughes, 1994; Andjelkovic et al. 1998) but it is the first time that avian macrophages have been demonstrated by LEA binding.
We were unable to determine the origin of the QH1-positive cells found inside the graft, though it seems they might have originated from the splachnopleural mesoderm of the CAM. While paraxial mesoderm produces pure angioblast unassociated with haemopoiesis, the splachnopleural mesoderm gives rise to progenitor cells with a dual angioblastic and haematopoietic potential (Pardanaud et al. 1996; Pardanaud & Dieterlen-Lièvre, 1999).
Evidence for circulating endothelial cells or their precursors has been definitively provided (Shi et al. 1998) and the incorporation of these cells into the neovasculature associated with wound healing, tumorigenesis or myocardial ischaemia has also been demonstrated (Asahara et al. 1999). Unlike angiogenesis, vasculogenesis is not induced by hypoxia (Maltepe et al. 1997; Semenza, 1998), and thus the invasion of host cells observed in our grafts must have been induced by factors other than hypoxia.
In this study, we have presented evidence of the involvement of both vascular morphogenetic mechanisms – angiogenesis and vasculogenesis – in the revascularization of grafted mesonephros. The mesonephros, an exclusively mesodermal organ, was an example of angiogenic vascularization; however, our results confirm those reported in our previous study using younger mesonephros in which the involvement of vasculogenic processes was also identified in their vascularization (Arcalís et al. 2002).
Acknowledgments
The QH1 and QCPN monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank (DSHB, Department of Biological Sciences, University of Iowa), maintained by the Department of Anatomy and Embryology at the University of Leiden. The LEP100 IgG antibody was obtained directly from the DSHB. Special thanks to M. M. T. Mentink and L. J. Wisse of the Department of Anatomy and Embryology, Leiden University, for their excellent technical assistance; and to Dr J. Navascués, from the Departamento de Biología Celular de la Facultad de Ciencias, University of Granada, Spain, for advice in the use of LEP100 antibody. We thank Granja Urgel Ganadera S.A. for providing the quail eggs. This work was performed with the support of a CICYT (AGF98-1036-C02-01) grant from the Spanish Government.
References
- Abrahamson DR, Robert B, Hyink DP, St John PL, Daniel TO. Origins and formation of microvasculature in the developing kidney. Kidney Int. 1998;54:S7–S11. doi: 10.1046/j.1523-1755.1998.06702.x. [DOI] [PubMed] [Google Scholar]
- Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, et al. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharinejad S, Böck P. Appearance of venous sphincters in the pulmonary microvascular bed of normotensive and spontaneously hypertensive rats. Scan. Microsc. 1992;6:865–875. [PubMed] [Google Scholar]
- Andjelkovic AV, Nikolic B, Pachter JS, Zecevic N. Macrophages/microglial cells in human central nervous system during development: an immunohistochemical study. Brain Res. 1998;814:13–25. doi: 10.1016/s0006-8993(98)00830-0. [DOI] [PubMed] [Google Scholar]
- Arcalís T, Carretero A, Navarro M, Ayuso E, Ruberte J. Vasculogenesis and angiogenesis in the subcardinal venous plexus of quail mesonephros: spatial and temporal morphological analysis. Anat. Embryol. 2002;205:19–28. doi: 10.1007/s00429-001-0225-6. [DOI] [PubMed] [Google Scholar]
- Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ausprunk DH, Knighton DR, Folkman J. Differentiation of vascular endothelium in the chick chorioallantois: a structural and autoradiographic study. Dev. Biol. 1974;38:237–249. doi: 10.1016/0012-1606(74)90004-9. [DOI] [PubMed] [Google Scholar]
- Ausprunk DH, Knighton DR, Folkman J. Vascularization of normal and neoplasic tissues grafted to the chick chorioallantois. Am. J. Pathol. 1975;79:597–618. [PMC free article] [PubMed] [Google Scholar]
- Ausprunk DH, Folkman J. Vascular injury in transplanted tissues. Fine structural changes in tumour, adult, and embryonic blood vessels. Wirchows Arch. B. Cell. Pathol. 1976;1:31–44. [PubMed] [Google Scholar]
- Caduff JH, Fischer LC, Burri PH. Scanning electron microscopic study of the developing microvasculature in the postnatal rat lung. Anat. Rec. 1986;216:154–164. doi: 10.1002/ar.1092160207. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nature Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Carretero A, Ditrich H, Navarro M, Splechtna H, Ruberte J. Technical improvements in corrosion casting of small specimens: a study on mesonephric tubules and vessels of chicken embryos. Scanning Microsc. 1993;7:1333–1338. [PubMed] [Google Scholar]
- Carretero A, Ditrich H, Pérez-Aparicio FJ, Splechtna H, Ruberte J. Development and degeneration of the arterial system in the mesonephros and metanephros of chick embryos. Anat. Rec. 1995;243:120–128. doi: 10.1002/ar.1092430114. [DOI] [PubMed] [Google Scholar]
- Carretero A, Ditrich H, Navarro M, Ruberte J. Afferent portal venous system in the mesonephros of chick embryos: development and degeneration. Anat. Rec. 1997;247:63–70. doi: 10.1002/(SICI)1097-0185(199701)247:1<63::AID-AR9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Carretero A, Blanco MJ, Navarro M, Armengol C, Nieto MA, Ruberte J. Ephrin B2 mRNA expresión during chick mesonephros development and degeneration. Int. J. Dev. Biol. 2001;45(Suppl. 1):S184. [Google Scholar]
- Charmaine BSH, DeFouw DO. Differential lectin binding to microvascular endothelial glycoconjugates during normal angiogenesis in the chick chorioallantoic membrane. Microvasc. Res. 1995;49:201–211. doi: 10.1006/mvre.1995.1016. [DOI] [PubMed] [Google Scholar]
- Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- Coffin JD, Poole TJ. Embryonic vascular development: immunohistochemical identification of the origin and subsequent morphogenesis of the major vessel primordial. Development. 1988;102:735–748. doi: 10.1242/dev.102.4.735. [DOI] [PubMed] [Google Scholar]
- Cuadros MA, Coltey P, Nieto MC, Martín C. Demonstration of a phagocytic cell system belonging to the hematopoietic lineage and originating from the yolk sac in the early avian embryo. Development. 1992;115:157–168. doi: 10.1242/dev.115.1.157. [DOI] [PubMed] [Google Scholar]
- DeRuiter MC, Poelmann RE, Van Iperen L, Gittenberger-de Groot AC. The early development of the tunica media in the vascular system of the rat embryos. Anat. Embryol. 1990;181:341–349. doi: 10.1007/BF00186906. [DOI] [PubMed] [Google Scholar]
- Ditrich H, Splechtna H. Scanning electron microscopy of vascular corrosion casts in comparative studies on renal vascular structure. Scanning Microsc. 1987;191:145–149. [PubMed] [Google Scholar]
- Ekblom P, Sariola H, Karkinen-Jääskeläinen M, Saxén L. The origin of the glomerular endothelium. Cell Differ. 1982;11:35–39. doi: 10.1016/0045-6039(82)90014-8. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP. The vascular endothelial growth factor family. In: Ware JA, Simons M, editors. Angiogenesis and Cardiovascular Disease. New York: Oxford University Press; 1999. pp. 101–127. [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J. Morph. 1951;88:49–92. [PubMed] [Google Scholar]
- Hogers B, DeRuiter MC, Gittenberger-de Groot A, Poelman RE. Unilateral vitelline vein ligation alters intracardiac blood flow patterns and morphogenesis in the chick embryo. Circulation Res. 1997;80:473–481. doi: 10.1161/01.res.80.4.473. [DOI] [PubMed] [Google Scholar]
- Hyink DP, Tucker DC, St. John PL, Leardkamolkarn V, Accavitti MA, Abrass CK, et al. Endogenous origin of glomerular endothelial and mesangial cells in grafts of embryonic kidneys. Am. J. Physiol. 1996;270:F886–F899. doi: 10.1152/ajprenal.1996.270.5.F886. [DOI] [PubMed] [Google Scholar]
- Jaffredo T, Gautier R, Eichmann A, Dieterlen-Lièvre F. Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development. 1998;125:4575–4583. doi: 10.1242/dev.125.22.4575. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- Lillie FR. Development of the Chick. An Introduction to Embryology. New York: Holt, Rinehart and Wilson.; 1952. [Google Scholar]
- Lippincott-Schwartz J, Fambrough DM. Lysosomal membrane dynamics: structure and interorganellar movement of a major lysosomal membrane glycoprotein. J. Cell. Biol. 1986;102:1593–1605. doi: 10.1083/jcb.102.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon CM. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature. 1997;386:403–407. doi: 10.1038/386403a0. [DOI] [PubMed] [Google Scholar]
- Mills AN, Haworth SG. Changes in lectin binding patterns in the developing pulmonary vasculature of the pig lung. J. Pathol. 1986;149:191–199. doi: 10.1002/path.1711490305. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y, Date I, Ohmoto T. Neovascularization of fetal neocortical grafts transplanted into a previously prepared cavity in the cerebral cortex: a three-dimensional morphological study using the scanning electron microscope. Brain Res. 1995;681:131–140. doi: 10.1016/0006-8993(95)00304-9. [DOI] [PubMed] [Google Scholar]
- Moyon D, Pardanaud L, Yuan L, Bréant C, Eichmann A. Plasticity of endothelial cells during arterial-venous differentiation in the avian embryo. Development. 2001;128:3359–3370. doi: 10.1242/dev.128.17.3359. [DOI] [PubMed] [Google Scholar]
- Nanka O, Peumans WJ, Van Damme EJM, Pfüller U, Valášek P, Halata Z, et al. Lectin histochemistry of microvascular endothelium in chick and quail musculature. Anat. Embryol. 2001;204:407–411. doi: 10.1007/s004290100212. [DOI] [PubMed] [Google Scholar]
- Navarro M, Carretero A, Pérez-Aparicio FJ, Ruberte J. Effect of the acidosis on the degenerating mesonephric vascular system of the chick embryos. Int. J. Dev. Biol. Suppl. 1996;1:287S–288S. [PubMed] [Google Scholar]
- Pardanaud L, Altmann C, Kitos P, Dieterlen-Lièvre F, Buck CA. Vasculogenesis in the early quail blastodisc as studied with a monoclonal antibody recognizing endothelial cells. Development. 1987;100:339–349. doi: 10.1242/dev.100.2.339. [DOI] [PubMed] [Google Scholar]
- Pardanaud L, Dieterlen-Lièvre F. Does the paraxial mesoderm of the avian embryo have hemangioblastic capacity? Anat. Embryol. 1995;192:301–308. doi: 10.1007/BF00710099. [DOI] [PubMed] [Google Scholar]
- Pardanaud L, Luton D, Prigent M, Bourcheix LM, Catala M, Dieterlen-Lièvre F. Two distinct endothelial lineages in ontogeny, one of them related to hemopoiesis. Development. 1996;122:1363–1371. doi: 10.1242/dev.122.5.1363. [DOI] [PubMed] [Google Scholar]
- Pardanaud L, Dieterlen-Lièvre F. Manipulation of the angiopoietic/hemangiopoietic commitment in the avian embryo. Development. 1999;126:617–627. doi: 10.1242/dev.126.4.617. [DOI] [PubMed] [Google Scholar]
- Pérez-Aparicio FJ, Carretero A, Navarro M, Ruberte J. Angiogenesis in the gonadal capillary network of the chick embryo. Scanning Microsc. 1996;10:859–874. [PubMed] [Google Scholar]
- Preminger GM, Koch WF, Fried FA, Mandell J. Chorioallantoic membrane grafting of the embryonic murine kidney. Invest. Urol. 1981;18:377–381. [PubMed] [Google Scholar]
- Rhodin JAG. The ultrastructure of mammalian arterioles and precapillary sphincters. J. Ultrastruct. Res. 1967;18:181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Nico B, Vacca A, Roncali L, Burri PH, Djonov V. Chorioallantoic membrane capillary bed: a useful target for studying angiogenesis and anti-angiogenesis in vivo. Anat. Rec. 2001;264:317–324. doi: 10.1002/ar.10021. [DOI] [PubMed] [Google Scholar]
- Robert B, St. John PL, Hyink DP, Abrahamson DR. Evidence that embryonic kidney cells expressing flk-1 are intrinsic, vascular angioblast. Am. J. Physiol. 1996;271:F744–F753. doi: 10.1152/ajprenal.1996.271.3.F744. [DOI] [PubMed] [Google Scholar]
- Sainio K, Raatikainen-Ahokas A. Mesonephric kidney-a stem cell factory? Int. J. Dev. Biol. 1999;43:435–439. [PubMed] [Google Scholar]
- Sariola H, Ekblom P, Lehtonen E, Saxén L. Differentiation and vascularization of the metanephric kidney grafted on the chorioallantoic membrane. Dev. Biol. 1983;96:427–435. doi: 10.1016/0012-1606(83)90180-x. [DOI] [PubMed] [Google Scholar]
- Sariola H. Incomplete fusion of the epithelial and endothelial basement membranes in interspecies hybrid glomeruli. Cell Differ. 1984;14:189–195. doi: 10.1016/0045-6039(84)90045-9. [DOI] [PubMed] [Google Scholar]
- Sariola H, Timpl R, Von Der Marck K, Mayne R, Fitch JM, Linsenmayer F, et al. Dual origin of glomerular basement membrane. Dev. Biol. 1984a;101:86–96. doi: 10.1016/0012-1606(84)90119-2. [DOI] [PubMed] [Google Scholar]
- Sariola H, Peault B, LeDouarin N, Buck C, Dieterlen-Lièvre F, Saxén L. Extracellular matrix and capillary ingrowth in interspecies chimeric kidneys. Cell Differ. 1984b;15:43–51. doi: 10.1016/0045-6039(84)90028-9. [DOI] [PubMed] [Google Scholar]
- Sato S, Hughes RC. Regulation of secretion and surface expression of Mac-2, a galactoside-binding protein of macrophages. J. Biol. Chem. 1994;269:4424–4430. [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr. Opin. Genet. Dev. 1998;8:588–594. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, et al. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- Shumko JZ, DeFouw DO, Feinberg N. Vascular histodifferentiation in the chick chorioallantoic membrane: a morphometric study. Anat. Rec. 1988;220:179–189. doi: 10.1002/ar.1092200209. [DOI] [PubMed] [Google Scholar]
- Shutter JR, Scully S, Fan W, Richards WG, Kitajewski J, Deblandre GA, et al. Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Dev. 2000;14:1313–1318. [PMC free article] [PubMed] [Google Scholar]
- Takakura N, Watanabe T, Suenobu S, Yamada Y, Noda T, Ito Y, et al. A role for hematopoietic stem cells in promoting angiogenesis. Cell. 2000;102:199–209. doi: 10.1016/s0092-8674(00)00025-8. [DOI] [PubMed] [Google Scholar]
- Tsukada T, Tippens D, Gordon D, Ross R, Gown AM. HHF35, a muscle-actin-specific monoclonal antibody. I. Immunocytochemical and biomedical characterization. Am. J. Path. 1987;126:51–60. [PMC free article] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Wilms P, Christ B, Wilting J, Wachtler F. Distribution and migration of angiogenic cells from grafted avascular intraembryonic mesoderm. Anat. Embryol. 1991;183:371–377. doi: 10.1007/BF00196838. [DOI] [PubMed] [Google Scholar]
- Yancopoulos GD, Klagsbrun M, Folkman J. Vasculogenesis, angiogenesis and growth factors: ephrins enter the fray at the border. Cell. 1998;93:661–664. doi: 10.1016/s0092-8674(00)81426-9. [DOI] [PubMed] [Google Scholar]
- Zacchei AM. The embryonic development of the Japanese quail Coturnix coturnix japonica. Arch. Ital. Anat. Embriol. 1961;66:36–62. [PubMed] [Google Scholar]
- Zhong TP, Rosenberg M, Mohideen M-APK, Weinstein B, Fishman MC. Gridlock, an HLH gene required for assembly of the aorta in zebrafish. Science. 2000;287:1820–1824. doi: 10.1126/science.287.5459.1820. [DOI] [PubMed] [Google Scholar]