Abstract
This study sought to explore the anatomical relationships between peptidergic nerves and blood vessels within human primary and permanent teeth. Extracted primary and permanent molars (n = 120) were split longitudinally, placed in Zamboni's fixative and the coronal pulps were processed for indirect immunofluorescence. Ten-micrometre-thick serial frozen pulp sections were triple-labelled using combinations of the following antisera: (1) protein gene-product 9.5 (PGP 9.5), a general neuronal marker; (2) one of the neuropeptides, calcitonin gene-related peptide (CGRP), substance P (SP), vasoactive intestinal polypeptide (VIP) or neuropeptide Y (NPY); and (iii) the lectin Ulex europeus, a label for vascular endothelium. The mid-coronal pulp region was examined, using fluorescence microscopy, to determine the proportion of blood vessels showing a positive innervation (recorded when PGP 9.5-labelled nerves appeared to intersect the vessel wall). In addition, the percentage of these vascular-related nerves expressing each of the above neuropeptides was recorded. Overall, 20% of pulpal blood vessels appeared to have a positive innervation. In the main these were thick-walled arterioles. Capillaries, venules and lymphatics were mostly devoid of an associated innervation. Ninety-two per cent of vascular-related nerves expressed CGRP, 87% expressed SP, 15% expressed VIP and 80% expressed NPY. There were no significant differences in overall innervation or peptide-related innervation between primary and permanent teeth (P < 0.05, anova, indicating that pulpal blood flow is likely to be subject to similar neurological control mechanisms in both dentitions.
Keywords: blood vessels, innervation, neuropeptides, protein gene product 9.5, tooth pulp
Introduction
Immunocytochemical investigation of human and animal tooth pulp has revealed that pulpal blood vessels are actually supplied by a variety of neuropeptide-expressing nerves. A number of studies have described close spatial relationships between blood vessels and nerve fibres immunoreactive for: substance P (SP) (Wakisaka et al. 1985); neuropeptide K (Casasco et al. 1990); neurokinin A (NKA) (Wakisaka & Akai (1989)); calcitonin gene-related peptide (CGRP) (Wakisaka et al. 1987a); vasoactive intestinal polypeptide (VIP) (Uddman et al. 1980); neuropeptide Y (NPY) (Uddman et al. 1984); somatostatin (Casasco et al. 1991); galanin (Wakisaka et al. 1996) and secretoneurin (Pertl et al. 1998). Furthermore, double-labelling techniques have shown that certain blood vessels actually receive a dual peptidergic innervation. It would appear that SP-, NKA- and CGRP-expressing fibres exhibit a similar distribution pattern around some large pulpal vessels (Wakisaka & Akai (1989)). However, SP- and VIP-immunoreactive fibres seem to display a different pattern of vascular innervation in relation to the same blood vessels (Wakisaka et al. 1987b).
More recently, immunoelectron microscopy has permitted a greater insight into pulpal neurovascular associations. Tabata et al. (1998) identified several SP-immunoreactive varicose fibres within the adventitia of large centrally positioned arterioles and observed a small number of SP-containing fibres within a few micrometres of some subodontoblastic capillaries. Zhang et al. (1998) studied the three-dimensional relationship of pulpal blood vessels and CGRP- and NPY-immunoreactive perivascular plexuses. They reported that large arterioles (40 µm or more in diameter) tended to receive a prolific peptidergic innervation whereas smaller arterioles received a much sparser innervation which tended to run spirally or longitudinally to the vessel axis. Furthermore, capillaries and venules appeared to be devoid of any innervation.
On the basis of these anatomical observations, it has been speculated that a functional relationship may exist between pulpal nerves and the microvasculature. Indeed, this hypothesis has been supported by growing evidence that the release of neuropeptides from pulpal nerves has a significant effect on vascular tone and blood flow. In brief, it has been demonstrated that the pulpal vasodilation and increased blood flow, which follows stimulation of sensory nerves, are likely to be mediated by SP, NKA and CGRP (Heyeraas et al. 1994). Following chemical, mechanical or electrical stimulation of sensory nerves there is an antidromic release of these peptides via an axon-reflex mediated response. The secreted neuropeptides subsequently regulate a number of vascular events, collectively termed ‘neurogenic inflammation’ (for review see Olgart, 1996b).
Vasoactive intestinal polypeptide has also been shown to cause vasodilation of pulpal blood vessels under experimental conditions (for review see Olgart, 1996a). However, this peptide is normally expressed by parasympathetic neurones, thus its presence within intradental nerves is considered, by some, as evidence for a parasympathetic innervation. Conversely, a sustained vasoconstriction of pulpal vessels may be induced by NPY, which is believed to coexist with noradrenaline within sympathetic nerve fibres (Edwall et al. 1985; Olgart et al. 1989).
It is therefore likely that certain neuropeptides play an important role in the regulation of pulpal vascular mechanisms both in health and disease. To date there have been no quantitative assessments of the relative contributions made by different peptidergic subpopulations to the innervation of the human pulpal microvasculature. Furthermore, even descriptive data are largely lacking for human primary teeth. A knowledge of the innervation characteristics of blood vessels within the human tooth pulp may provide a greater insight into neurovascular control mechanisms and may direct the development of novel therapeutic interventions. The aim of the present study therefore was to undertake a quantitative assessment of the innervation characteristics of pulpal blood vessels in both intact and carious human primary and permanent teeth using an immunocytochemcial approach.
Materials and methods
Experimental material
The experimental material consisted of 60 mandibular second primary molars and 60 first permanent molars obtained from children requiring dental extractions under general anaesthesia. Primary teeth were excluded from the study if there was any evidence of physiological root resorption in view of the known degenerative neural changes associated with this process (Rapp et al. 1967). Ethical approval for the study was granted by the South Sheffield Research Ethics Committee.
Immediately following simple forceps extraction, a groove was cut on the buccal aspect of each crown and teeth were split longitudinally. The mesial half of the tooth was retained and placed in fixative (4% paraformaldehyde and 0.2% picric acid in 0.1 m phosphate buffer, pH 7.4) for 24 h at 4 °C. The coronal pulp was carefully removed from the pulp chamber and placed in phosphate-buffered saline (PBS). The degree of occlusal caries in each tooth was then assessed visually under a dissection microscope at ×20 magnification. Each tooth half was categorized as intact (no colour change within dentine but with possible staining confined to enamel), moderately carious (colour changes did not extend beyond half the dentine thickness), or grossly carious (colour changes extended beyond half the dentinal thickness).
Immunocytochemistry and lectin histochemistry
The coronal pulps were left in PBS for 24 h at 4 °C before placing in 0.1 m PBS containing 30% sucrose solution for cryoprotection (5 h at 4 °C). The pulp tissue was then embedded in Tissue-Tek OCT compound (Bayer Diagnostics, Basingstoke, UK) and four sets of three 10-µm-thick longitudinal sections were cut from each tooth pulp and collected on poly D-lysine-coated glass slides. Slides were left for 60 min at room temperature to air dry prior to long-term storage at −70 °C.
Immunostaining was performed using an indirect immunofluorescence method (Coons et al. 1955). Slides were removed, as required, from storage and were left to air dry at room temperature for 60 min. Slides were then washed in PBS containing 0.2% Triton X-100 (PBST) 2 × 10 min. To reduce non-specific background staining and to increase the permeability of cell membranes to antibodies, sections were first incubated in PBST containing 10% normal goat serum (Vector Laboratories, Peterborough, UK) for 30 min at room temperature. Following this, sections were incubated with a mixture of (1) a monoclonal antibody to protein gene product 9.5 (PGP 9.5) which is a general neuronal marker (Rode et al. 1985); (2) an antibody raised against one of the neuropeptides: CGRP, SP, VIP, or NPY; and (3) biotinylated Ulex europaeus agglutinin I lectin (UEIL). This lectin, derived from the gorseplant, is specfic for α-L-fucose-containing compounds and is considered an excellent marker of human vascular endothelium (Ordóñez et al. 1987; Hoyle et al. 1996). Details regarding each of these reagents are given in Table 1. The antisera and UEIL were diluted in PBST containing 5% normal goat serum and sections were incubated for 24 h at 4 °C.
Table 1.
Details of primary reagents used in the study
| Antigen/lectin | Host species | Type | Source | Dilution |
|---|---|---|---|---|
| PGP 9.5 | mouse | Monoclonal (human) | Ultraclone, Isle of Wight, UK | 1: 1000 |
| CGRP (II) | rabbit | Polyclonal (human) | Peninsula, Merseyside, UK | 1: 800 |
| SP | rabbit | Polyclonal (human) | Genosys, Cambridge, UK | 1: 800 |
| NPY | rabbit | Polyclonal (human) | Genosys, Cambridge, UK | 1: 800 |
| VIP | rabbit | Polyclonal (human, rat, porcine) | Peninsula, Merseyside, UK | 1: 800 |
| UEIL | (gorsebush) | Lectin | Vector Laboratories, Peterborough, UK | 20 µg mL−1 |
PGP = protein gene product; CGRP = calcitonin gene-related peptide; SP = substance P; NPY = neuropeptide Y; VIP = vasoactive intestinal polypeptide; UEIL =Ulex europaeus I lectin
Slides were then washed again in PBS (2 × 10 min) before incubating, for a further 90 min at room temperature, with a mixture of goat antirabbit IgG conjugated to fluorescein isothiocyanate (FITC) (dilution 1 : 20, Vector Laboratories, Peterborough, UK), horse antimouse IgG conjugated to Texas red (TR) (dilution 1 : 100; Vector) and 7-amino-4-methyl-3-acetic acid (AMCA)-conjugated streptavidin (dilution 1: 25, Vector). The fluorescent labels were diluted in PBST containing 2% normal goat serum. Slides were finally washed again in PBS (2 × 10 min), and sections were carefully dried and mounted in Vectashield (Vector).
Immunohistochemical controls for each neuropeptide were performed by incubating sections with the primary antibody which had been pre-absorbed over a 24-h period at 4 °C with an excess (10 nmol mL−1) of the respective hapten. Controls for PGP 9.5 labelling were carried out by incubating sections with the antibody dilutent alone. The specificity of the lectin reaction was tested by inhibiting the lectin binding. This was accomplished by pre-incubing the lectin conjugate with 0.2 mα-L-fucose (Vector) dissolved in PBS containing 0.2% PBST for 60 min at room temperature prior to applying this mixture to tissue sections. No positive labelling was seen in any of the controls.
Analysis of neurovascular relationships
Sections were viewed using a Zeiss axioplan fluorescent microscope. Fluorescence images were subesquently captured and displayed on a video moniter (Sony PVM144QM) by passing light from the microscope through a low light level chilled charge-coupled device camera (Hamamatsu C5985-10) which was coupled to the secondary moniter. Descriptive and quantitative findings for each of the four different staining regimes (CGRP/SP/VIP/NPY + PGP 9.5 + UEIL) were derived from the examination of three sections taken from each tooth pulp. All analyses were performed blind.
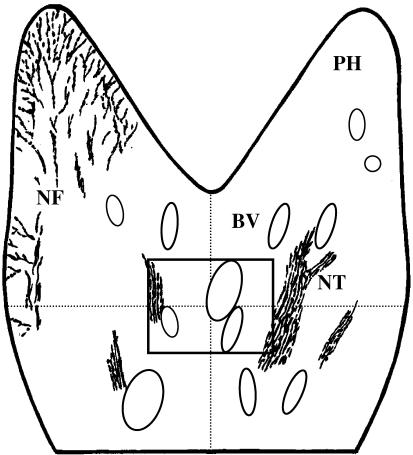
Following a qualitative examination of the labelling for PGP 9.5, UEIL and each neuropeptide throughout the entire pulp section, quantitative analysis was undertaken within the mid-coronal region only (Fig. 1). Quantitative assessment of vascular innervation was limited to this region as the mid-coronal pulp contains the greatest proportion of arterioles, which are the vessels most likely to be innervated (Okamura et al. 1994 Okamura et al. 1995). Using the ×10 objective, the cross hair of the eyepiece was positioned in the centre of the pulp section, and an image showing staining for the pulpal vasculature was captured on the secondary monitor. The tissue section was then systematically examined, using the ×40 objective and appropriate filter, to assess the spatial relationship between the wall of every blood vessel profile and any closely aligned PGP 9.5-labelled fibres. By referring to the captured image, it was ensured that every blood vessel, within the region of interest, was subject to quantitative assessment.
Fig. 1.
Schematic diagram of the coronal pulp showing the region (rectangle) in which quantitative vascular innervation data were obtained. NF = peripheral nerve fibres, NT = nerve trunk, BV = blood vessel, PH = pulp horn. Area within rectangle represents 0.98 mm2 of tissue.
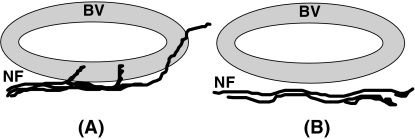
Positive innervation was only recorded when PGP 9.5-immunoreactive fibres actually appeared to intersect the vessel wall (Fig. 2A). The simple presence of closely approximated PGP 9.5-labelled fibres was not recorded as an example of positive vascular innervation if the nerve fibres did not cross the vessel wall itself (Fig. 2B). The percentage of ‘innervated’ blood vessels which also demonstrated a peptidergic innervation was then determined for each neuropeptide using the same criteria. Using previously described criteria (Ekblom & Hansson, 1984; Okamura et al. 1994; Matsumoto et al. 1997), pulpal vessels were classified as: arterioles (thick-walled vessels with a diameter of 10–50 µm); venules (vessels with thin or absent muscular layers with a diameter of 10–40 µm); capillaries (small vessels, usually with an undetectable lumen, and with a diameter of 4–10 µm), and lymphatics (irregular-shaped vessels, 20–50 µm in diameter, and displaying numerous abluminal endothelial projections).
Fig. 2.
Schematic diagram showing pulpal blood vessels classified as having a positive innervation (A) and an absent innervation (B). BV = blood vessel wall, NF = nerve fibre.
Results
Anatomical observations
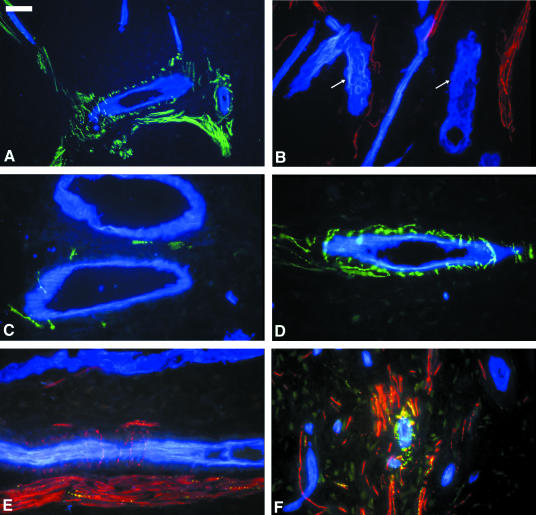
A close spatial relationship was clearly evident between PGP 9.5-immunoreactive fibres and arterioles within the mid-coronal region of both primary and permanent tooth pulps. Indeed, the majority of arterioles were intimately associated with varicose or smooth-surfaced PGP 9.5-labelled fibres (Fig. 3A). These vascular-related nerve fibres presented either as a prominent perivascular plexus or as single fibres which appeared to penetrate the vessel wall. However, no PGP 9.5-immunoreactive fibres were seen to extend as far as the endothelial layer. Within the pulp horn regions, nerve fibres were also seen to be closely associated with some small arterioles. There was no clear evidence of any direct contact between PGP 9.5-immunoreactive fibres and pulpal capillaries, although nerve fibres were often seen in close proximity to these vessels. Furthermore, venules and lymphatic vessels also appeared to be devoid of any neural contacts (Fig. 3B).
Fig. 3.
Photomicrographs showing the anatomical relationships between pulpal nerves and blood vessels in coronal tooth pulp. (A) Double-labelling for PGP 9.5 (green) and UEIL (blue, in all plates) showing the close relationship between PGP 9.5-immunoreactive fibres and arterioles. (B) Double-labelling for PGP 9.5 (red) and UEIL showing lymphatic vessels (arrows) devoid of any neural associations. (C) Double-labelling for UEIL and CGRP-expressing fibres (green) showing a minimal vascular innervation. (D) Double labelling for UEIL and SP-containing fibres (green) showing a perivascular plexus of SP-labelled fibres. (E) Triple-labelling for PGP 9.5 (red), UEIL and VIP-immunoreactive fibres (yellow) showing perivascular PGP 9.5-labelled nerve fibres but an absence of vascular-related VIP-expressing fibres. (F) Triple-labelling for PGP 9.5 (red), UEIL and NPY (yellow/green) in the pulp horn region showing some NPY-immunoreactive fibres closely associated with a small blood vessel. Scale bar = 15 µm (B–F), 30 µm (A).
The vast majority of vessels (arterioles) that were categorized as being ‘innervated’ by PGP 9.5-labelled fibres were also seen to be supplied by a peptidergic innervation. These fibres were all characteristically varicose, intensely fluorescent and made a variable contribution to the overall innervation of each vessel (Fig. 3C–E). It was also noted that there were no appreciable differences in the vascular innervation patterns demonstrated by primary and permanent samples or intact and carious samples.
The majority of those vessels that were innervated were seen to receive a subpopulation of CGRP-containing fibres. However, vessels appeared to be associated with a variable proportion of CGRP-labelled nerves, ranging from a single fibre (Fig. 3C) to a complete perivascular plexus.
Substance P-expressing fibres demonstrated a similar vascular relationship to that seen for CGRP. Essentially, the majority of thick-walled arterioles appeared to be innervated by varicose SP-immunoreactive fibres. However, the density of innervation varied, with some vessels exhibiting a dense neural network (Fig. 3D) and others showing only a single SP-immunoreactive fibre extending into the vessel wall.
The contribution of VIP-containing nerves to vascular innervation appeared to be less than that seen for the other neuropeptides. In a number of sections, no VIP-innervated vessels could be identified (Fig. 3E). However, some sections demonstrated vessels which were clearly innervated by VIP-expressing fibres.
Finally, pulpal blood vessels were commonly seen to demonstrate a prominent and varicose perivascular network of NPY-immunoreactive fibres. Dense innervation was usually associated with thick-walled arterioles; however, smaller arterioles within the pulp horn regions were also seen to be associated with NPY-labelled fibres (Fig. 3F).
Quantitative analysis of vascular innervation
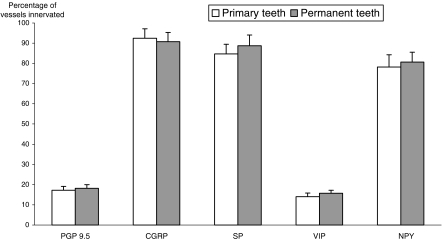
Table 2 shows the mean percentage of mid-coronal pulpal vessels that were categorized as being innervated by PGP 9.5-immunoreactive fibres. In addition, data are given for the percentage of innervated vessels that also received a peptidergic innervation. These results represent the mean data obtained from the analysis of three sections for each staining regime. However, there were no significant differences for the mean number of vessels innervated by PGP9.5 or each of the individual neuropeptides between the three sections used for each regime (P > 0.05, one-way analysis of variance). Furthermore, there were no significant differences in results according to the dentition type or the degree of caries (P > 0.05, two-way analysis of variance). Data have therefore been pooled for the three caries subgroups, but for interest have been presented separately for primary and permanent teeth. Plots of these data are also presented in Fig. 4.
Table 2.
Percentage of mid-coronal pulpal vessels innervated by protein gene product 9.5 (PGP 9.5) and the percentage of PGP 9.5-innervated vessels also supplied by a peptidergic innervation
| Innervation/dentition | Mean | SD | Range |
|---|---|---|---|
| PGP 9.5 (mean) | |||
| Primary | 17.2 | 8.57 | 14.2–20.3 |
| Permanent | 18.2 | 8.17 | 15.4–20.9 |
| CGRP | |||
| Primary | 92.5 | 20.48 | 85.9–99.2 |
| Permanent | 90.8 | 19.98 | 83.9–97.7 |
| SP | |||
| Primary | 84.7 | 21.42 | 78.8–90.5 |
| Permanent | 88.7 | 24.32 | 81.7–95.7 |
| VIP | |||
| Primary | 14.0 | 8.01 | 0.0–40.0 |
| Permanent | 15.7 | 6.59 | 4.0–27.2 |
| NPY | |||
| Primary | 78.2 | 27.40 | 17.4–27.2 |
| Permanent | 80.7 | 21.55 | 25.0–100 |
PGP = protein gene product; CGRP = calcitonin gene-related peptide; SP = substance P; NPY = neuropeptide Y; VIP = vasoactive intestinal polypeptide
Data are given for 60 primary and 60 permanent teeth.
Fig. 4.
Bar chart showing mean (± SEM) percentage of pulpal vessels innervated by protein gene product 9.5 (PGP 9.5) and percentage of PGP9.5-innervated vessels also supplied by a peptidergic innervation. CGRP = calcitonin gene-related peptide; SP = substance P; NPY = neuropeptide Y; VIP = vasoactive intestinal polypeptide. n= 20 in each series.
It can be seen that just under 20% of pulpal blood vessels within the mid-coronal region were closely associated with PGP9.5-immunoreactive fibres. Approximately 90% of these vessels were supplied by CGRP-labelled fibres, and over 80% were supplied with SP-expressing fibres. Similarly, NPY-labelled fibres were associated with approximately 80% of innervated vessels. However, there was a wide variation for NPY-innervation data between samples, with between 17.4% and 100% of vessels demonstrating a close spatial relationship with NPY-containing fibres. Only about 15% of innervated vessels appeared to be supplied by VIP-positive fibres.
Discussion
Experimental approach
One aspect of the experimental approach that warrants discussion is the assessment of vascular innervation. The criteria employed were stringent as only vessels demonstrating a definite intersection with an associated nerve fibre were classified as being innervated. Thus vessels which were closely aligned to nerve fibres, but did not demonstrate an apparent contact, were categorized as non-innervated. Neurochemicals can diffuse over some distance to exert their effects and thus it is likely that a greater proportion of pulpal vessels are under neural control than is suggested by the present investigation. This level of evaluation is acknowledged to be elementary but a more definitive assessment of vascular innervation would necessitate further ultrastructural and physiological investigation.
Comparison with other studies
The overall descriptive findings relating to the association between neuropeptide-containing pulpal nerves and the microvasculature were entirely consistent with those of previous immunocytochemical studies in human permanent teeth (Uddman et al. 1980, Uddman et al. 1984; Wakisaka et al. 1985, Wakisaka et al. 1987a). However, the relationship of peptidergic nerve fibres and pulpal blood vessels has not been previously described for the primary tooth pulp. This study therefore provides some interesting new data.
Little quantitative or comparative data exist regarding the proportion of pulpal vessels that receive a peptidergic innervation. Furthermore, previous studies have mostly been limited to the rat and cat. In their study of rat incisors, Zhang et al. (1998) reported that a similar number of vessels were associated with both CGRP- and NPY-immunoreactive fibres, although it was felt that the latter were the most prolific within the vessel wall. Wakisaka & Akai (1989) used double-labelling techniques to more fully explore the distribution of CGRP-, SP-, NPY- and VIP-labelled fibres around blood vessels in the feline dental pulp. They reported that not all blood vessels were accompanied by peptidergic fibres. They also found that the distribution of vascular-related SP- and CGRP-labelled fibres was very similar. In addition, they reported that a greater number of vessels were innervated by SP than by VIP. Within the limitations of the present study, where only single neuropeptide labelling was employed for each tissue section, it would appear there were no major differences between vascular innervation characteristics in human tooth pulp and those reported for cat and rat.
Investigation of peptidergic vascular innervation has also been performed in other human tissues including buccal mucosa (Hilliges et al. 1994), vaginal tissue (Hoyle et al. 1996) and temporal or occipital skin (Uddman et al. 1986). It is apparent from these studies that the distribution of peptidergic nerve fibres around blood vessels varies in different tissues. A notable finding was that, in vaginal tissue, the majority of vessels were associated with NPY- or VIP-immunoreactive fibres as compared to other tissue where CGRP- and SP-innervated vessels were the most well-represented.
Differences between primary and permanent teeth
Our previous studies have shown that overall innervation density and expression of CGRP, SP and VIP are greater within human permanent tooth pulp than primary tooth pulp (Rodd & Boissonade, 2001, Rodd & Boissonade, 2002). It was interesting therefore that the present study found both dentitions to have a remarkably similar prevalence of innervated blood vessels. Furthermore, the proportion of vessels that were associated with each of the four different peptidergic subpopulations was seen to be almost identical. Although this study did not attempt to quantify the density of vascular innervation, subjective observation did not reveal any marked differences in the overall density or pattern of vascular innervation between the two dentitions.
It is not easy to explain why overall pulpal innervation would appear to differ between the two dentitions but vascular innervation seems to be very similar. It is possible that neurovascular fibres, which are largely autonomic, may be subject to different developmental control mechanisms than the predominantly sensory fibres that innervate the pulpal tissue itself.
Clinical significance of findings
As the tooth pulp resides in a low compliance environment, substantial increases in pulpal blood flow and tissue pressure may result in compression of the vessels with likely pulpal ischaemia and necrosis. Close regulation of blood flow and tissue pressures are therefore critical to the survival of the pulp following dental injury and ensuing pulpal inflammation. This study has shown that pulpal arterioles appear to be well innervated by a range of peptidergic fibre subpopulations and it is therefore likely that a variety of neuropeptides may profoundly affect vascular functions.
Research in both oral and other body tissues is now being directed towards the identification and localization of peptide receptor subtypes on vascular structures (Henning et al. 1995; Fristad et al. 2000). Furthermore, experimental animal studies have succeeded in blocking antidromic-induced pulpal vasodilation with the systemic use of SP-(Rosell et al. 1981; Romerio et al. 1999) and CGRP-receptor antagonists (Olgart, 1996b). It is thus conceivable that the development of topical preparations of specific peptide receptor antagonists could have considerable therapeutic applications in the management of the inflamed and hyperaemic human dental pulp.
Acknowledgments
This investigation was supported by the Medical Research Council (UK).
References
- Casasco A, Calligaro A, Springall DR, Casasco M, Poggi P, Valentino KL, et al. Neuropeptide K-like immunoreactivity in human dental pulp. Arch. Oral Biol. 1990;35:33–36. doi: 10.1016/0003-9969(90)90111-m. [DOI] [PubMed] [Google Scholar]
- Casasco A, Calligaro A, Casasco M, Springall DR, Polak JM, Marchetti C. Immunocytochemical evidence for the presence of somatostatin-like immunoreactive nerves in human dental pulp. J. Dent. Res. 1991;70:87–89. doi: 10.1177/00220345910700021601. [DOI] [PubMed] [Google Scholar]
- Coons AH, Leduc EH, Connolly JM. Studies on antibody production. I. A method for the histochemical demonstration of specific antibody and its application to a study of the hyperimmune rabbit. J. Exp. Med. 1955;102:49–60. doi: 10.1084/jem.102.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwall B, Gazelius B, Fazekas A, Theodorsson-Norheim E, Lundberg JM. Neropeptide Y (NPY) and sympathetic control of blood flow in oral mucosa and dental pulp in the cat. Acta Physiol. Scand. 1985;125:253–264. doi: 10.1111/j.1748-1716.1985.tb07714.x. [DOI] [PubMed] [Google Scholar]
- Ekblom A, Hansson P. A thin-section and freeze-fracture study of the pulp blood vessels in feline and human teeth. Arch. Oral Biol. 1984;29:413–424. doi: 10.1016/0003-9969(84)90021-9. [DOI] [PubMed] [Google Scholar]
- Fristad I, Vandevska-Radunovic V, Wimalawansa SJ, Kvinnsland IH. NK1-, NK2-, NK3- and CGRP-receptor expression in rat dental tissues. J. Dent. Res. 2000;79:173. [Google Scholar]
- Henning IM, Laissue JA, Horisberger U, Reubi JC. Substance P receptors in human primary neoplasms: tumoral and vascular localizations. nce P and CGRP-immunoreactive nerve fibers in the low compliant cat dental pulp. Microvasc. Res. 1995;47:329–343. [Google Scholar]
- Heyeraas KJ, Kim S, Raab WM, Byers MR, Liu M. Effect of electrical tooth stimulation on blood flow, interstitial fluid pressure and substance P and CGRP-immunoreactive nerve fibers in the low compliant cat dental pulp. Microvasc. Res. 1994;47:329–343. doi: 10.1006/mvre.1994.1026. [DOI] [PubMed] [Google Scholar]
- Hilliges M, Hellman M, Ahlström U, Johansson O. Immunohistochemical studies of neurochemical markers in normal human buccal mucosa. Histochemistry. 1994;101:235–244. doi: 10.1007/BF00315910. [DOI] [PubMed] [Google Scholar]
- Hoyle CV, Stones RW, Robson T, Whiteley K, Burnstock G. Innervation of vasculature and microvasculature of the human vagina by NOS and neuropeptide-containing nerves. J. Anat. 1996;188:633–644. [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Kato S, Miura M, Yanagisawa S, Shimizu M. Fine structure and distribution of lymphatic vessels in the human dental pulp: a study using an enzyme-histochemical method. Cell Tissue Res. 1997;288:79–85. doi: 10.1007/s004410050794. [DOI] [PubMed] [Google Scholar]
- Okamura K, Kobayashi I, Matsuo K, Taniguchi K, Ishibashi Y, Izumi T, et al. Ultrastructure of the neuromuscular junction of vasomotor nerves in the microvasculature of human dental pulp. Arch. Oral Biol. 1994;39:171–176. doi: 10.1016/0003-9969(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Okamura K, Kobayashi I, Matsuo K, Taniguchi K, Ishibashi Y, Izumi T, et al. An immunohistochemical and ultrastructural study of vasomotor nerves in the microvasculature of human dental pulp. Arch. Oral Biol. 1995;40:47–53. doi: 10.1016/0003-9969(94)00147-4. [DOI] [PubMed] [Google Scholar]
- Olgart L. Neural control of blood flow. Crit. Rev. Oral Biol. Med. 1996a;7:159–171. doi: 10.1177/10454411960070020401. [DOI] [PubMed] [Google Scholar]
- Olgart L. Neurogenic components of pulp inflammation. Proceedings of the International Conference on Dentin/Pulp Complex; 1995; Quintessence Publishing Co., Ltd, Tokyo. 1996b. pp. 169–175. [Google Scholar]
- Olgart L, Edwall B, Gazelius B. Neurogenic mediators in control of pulpal blood flow. J. Endodont. 1989;15:409–412. doi: 10.1016/S0099-2399(89)80173-6. [DOI] [PubMed] [Google Scholar]
- Ordóñez NG, Brooks T, Thompson S, Batsakis JG. Use of Ulex europaeus agglutinin I in the identification of lymphatic and blood vessel invasion in previously stained microscopic slides. Am. J. Surg. Pathol. 1987;11:543–550. doi: 10.1097/00000478-198707000-00006. [DOI] [PubMed] [Google Scholar]
- Pertl C, Kaufmann W, Amann R, Heinemann A, Ebelseder K, Polansky R, et al. Secretoneurin, a novel neuropeptide in the human dental pulp. Arch. Oral Biol. 1998;43:361–365. doi: 10.1016/s0003-9969(98)00016-8. [DOI] [PubMed] [Google Scholar]
- Rapp R, Avery JK, Strachan D. The distribution of nerves in human primary teeth. Anat. Rec. 1967;159:89–104. doi: 10.1002/ar.1091590113. [DOI] [PubMed] [Google Scholar]
- Rodd HD, Boissonade FM. Innervation density of human tooth pulp: a comparative study. J. Dent Res. 2001;80:389–393. doi: 10.1177/00220345010800011601. [DOI] [PubMed] [Google Scholar]
- Rodd HD, Boissonade FM. Comparative immunohistochemical analysis of the peptidergic innervation of human primary and permanent tooth pulp. Arch. Oral Biol. 2002;47:375–385. doi: 10.1016/s0003-9969(02)00012-2. [DOI] [PubMed] [Google Scholar]
- Rode J, Dhillon AP, Doran JF, Jackson P, Thompson RJ. PGP 9.5, a new marker for human neuroendocrine tumours. Histopathology. 1985;9:147–158. doi: 10.1111/j.1365-2559.1985.tb02431.x. [DOI] [PubMed] [Google Scholar]
- Romerio SC, Linder L, Haefeli WE. Neurokinin-1 receptor antagonist R116301 inhibits substance P-induced venodilation. Clin. Pharmacol. Ther. 1999;66:522–527. doi: 10.1016/S0009-9236(99)70016-0. [DOI] [PubMed] [Google Scholar]
- Rosell S, Olgart L, Gazelius B, Panopoulos P, Folkers K, Hörig J. Inhibition of antidromic and substance P-induced vasodilation by a substance P antagonist. Acta Physiol. Scand. 1981;111:381–382. doi: 10.1111/j.1748-1716.1981.tb06752.x. [DOI] [PubMed] [Google Scholar]
- Tabata S, Ozaki HS, Nakashima M, Uemura M. Blood vessels and nerve fibers in rat incisor pulp. Immunoelectron microscopic observation with anti-substance P antibody. Eur. J. Oral Sci. 1998;106:388–391. doi: 10.1111/j.1600-0722.1998.tb02203.x. [DOI] [PubMed] [Google Scholar]
- Uddman R, Björlin G, Möller B, Sundler F. Occurrence of VIP nerves in mammalian dental pulps. Acta Odontol. Scand. 1980;38:325–328. doi: 10.3109/00016358009033600. [DOI] [PubMed] [Google Scholar]
- Uddman R, Grunditz T, Sundler F. Neuropeptide Y: occurrence and distribution in dental pulps. Acta Odontol. Scand. 1984;42:361–365. doi: 10.3109/00016358409033616. [DOI] [PubMed] [Google Scholar]
- Uddman R, Edvinsson L, Jansen I, Stiernholm P, Jensen K, Olesen J, et al. Peptide-containing nerve fibres in human extracranial tissue: a morphological basis for neuropeptide involvement in extracranial pain? Pain. 1986;27:391–399. doi: 10.1016/0304-3959(86)90162-4. [DOI] [PubMed] [Google Scholar]
- Wakisaka S, Ichikawa H, Nishikawa S, Matsuo S, Takano Y, Akai M. The distribution and origin of calcitonin gene-related peptide-containing fibres in feline dental pulp: relationship with substance P-containing nerve fibres. Histochemistry. 1987a;86:585–589. doi: 10.1007/BF00489551. [DOI] [PubMed] [Google Scholar]
- Wakisaka S, Ichikawa H, Nishikawa S, Matsuo S, Takano Y, Akai M. Immunohistochemical observation on the correlation between substance P- and vasoactive intestinal polypeptide-like immunoreactivities in the feline dental pulp. Arch. Oral Biol. 1987b;32:449–453. doi: 10.1016/0003-9969(87)90082-3. [DOI] [PubMed] [Google Scholar]
- Wakisaka S, Akai M. Immunohistochemical observation on neuropeptides round the blood vessel in feline dental pulp. J. Endodont. 1989;15:413–416. doi: 10.1016/S0099-2399(89)80174-8. [DOI] [PubMed] [Google Scholar]
- Wakisaka S, Itotagawa T, Youn SH, Kato J, Kurisu K. Distribution and possible origin of galanin-like immunoreactive nerve fibers in the mammalian dental pulp. Regul. Pept. 1996;62:137–143. doi: 10.1016/0167-0115(96)00016-x. [DOI] [PubMed] [Google Scholar]
- Wakisaka S, Nishikawa S, Ichikawa H, Matsuo S, Takano Y, Akai M. The distribution and origin of substance P-like immunoreactivity in the rat molar pulp and periodontal tissues. Arch. Oral Biol. 1985;30:813–818. doi: 10.1016/0003-9969(85)90136-0. [DOI] [PubMed] [Google Scholar]
- Zhang J, Nagata K, Iijima T. Scanning electron microscopy and immunohistochemical observations of the vascular nerve plexuses in the dental pulp of rat incisor. Anat. 1 Rec. 1998;251:214–220. doi: 10.1002/(SICI)1097-0185(199806)251:2<214::AID-AR9>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]