Abstract
We studied the early stages of the degeneration of skeletal muscles using the venom of Notechis scutatus as the myotoxic agent. The venom was used at a dose equivalent to the LD50 in the mouse. There was no mortality amongst the rats. Electron microscopy was used to show the progressive hypercontraction of sarcomeres and the loss of alignment of myofibrils in individual muscle fibres. Between areas of hypercontraction sarcomeres were torn, shedding loosened myofilaments into the cytosol. Western blotting and Coomassie staining were used to compare the respective rates of loss of desmin, titin, actin, myosin and dystrophin. We showed that desmin and titin were the first proteins to be degraded with a time to 50% loss of approximately 1 h and 3 h, respectively. The loss of major contractile proteins, myosin and actin, was rather slower. The loss of dystrophin was also slower than the loss of desmin and titin. Early damage to the plasma membrane of the muscle fibre caused the cells to depolarize, probably promoting the hypercontraction of the sarcomeres, but actual loss of membrane was incomplete even at 24 h. We suggest that the early degradation of desmin and titin was responsible for the disaggregation of the sarcomeres; the liberated contractile proteins myosin and actin were shed into the cytosol, where they were degraded. Phagocytic cells that had invaded the degenerating muscle fibres were primarily involved in the clearance of damaged mitochondria.
Keywords: desmin, Notechis scutatus, sarcomere, soleus, titin
Introduction
The large bulk of many skeletal muscles and the unique characteristics of the skeletal muscle fibre – its syncitial nature, its length compared to its diameter and the highly regulated organization of its myofibrils and sarcomeres – have a major impact on the response of the tissue to injury. A muscle fibre may respond to an injurious assault with either a highly localized lesion, or with the development of a segment of damage bounded by otherwise normal tissue, or with the degeneration of the entire muscle fibre. Within an entire muscle, individual muscle fibres may be affected, and the damaged muscle fibres may be dispersed throughout otherwise normal muscle. These observations create major problems for both the clinical pathologist trying to interpret a muscle biopsy and the experimental pathologist trying to understand the molecular and cellular basis of muscle degeneration, because the muscle may contain a mixed population of destroyed, partially damaged and undamaged muscle fibres (Cullen & Mastaglia, 1982).
Muscle damage is a major component of the clinical features that follow an effective envenoming bite by a number of dangerous snakes. Notechis scutatus (Peters), the common tiger snake, is indigenous to south-eastern regions of Australia. It is a very dangerous elapid snake, not to be confused with the non-venomous common tiger snake Telescopus semiannulatus of Southern Africa. The venom of Notechis scutatus contains a high proportion of myotoxic agents, notably notexin and notechis II-5. These toxic phospholipases constitute 10–15% of the total protein content of the venom and are generally considered responsible for the severe rhabdomyolysis that is a feature of envenoming bites by the animal in both man and non-human animals (Hood & Johnson, 1975; Frost, 1980, 1981; Lewis, 1994). The degeneration of muscle induced by the inoculation of either venom or pure myotoxins is rapid and is followed by the vigorous regeneration of the affected tissue, initiated by the activation of the satellite cells of the damaged muscle fibres (Klein-Ogus & Harris, 1983). The reproducibility of this cycle of degeneration and regeneration has led to the adoption of the myotoxic venoms and purified toxins as tools for the study of numerous aspects of muscle biology, including the control of myosin gene expression, myoblast allotransplantation and the expression of vimentin and desmin in regeneration (Whalen et al. 1990; Skuk et al. 1999). In view of the widespread use of venoms and toxins as the primary myotoxic agent, it is perhaps surprising that so little attention has been paid to the sequence of events. Dixon & Harris (1996) have shown that the sarcolemma is the binding site for myotoxic phospholipases A2, and have suggested that the hydrolytic activity of the toxin causes the appearance of small lesions in the sarcolemma, the loss of ion gradients and hypercontraction. In the work described in this paper we have used a combination of electrophysiology, morphology, morphometrics and quantitative Western blotting to identify the earliest stages of degeneration and the relative rates of loss of a number of cytoskeletal, structural and contractile proteins associated with the myofibrils.
Materials and methods
Animals
Female Wistar rats, 90–120 g (n = 36), were obtained from an accredited breeder and maintained in accordance with the requirements of the Animals (Scientific Procedures) Act of 1986 under the day to day supervision of a veterinarian. The animals were anaesthetized (halothane/N2O/O2) and a single subcutaneous injection of 15 µg of whole venom (0.2 mL of 75 µg mL−1 in 0.9% w/v NaCl) from Notechis scutatus (Peters), the common tiger snake of Australia, was made into one hind limb at the line of demarcation between lateral gastrocnemius and tibialis anterior (Harris & Johnson, 1978). The injection was made so that the venom was introduced into the vicinity of the underlying soleus rather than directly into the muscle. The inoculation of venom was followed immediately by the injection of a non-steroidal analgesic (buprenorphine 0.05 mg kg−1 s.c.).
Muscles
At 1, 3, 6 or 24 h post inoculation nine randomly selected animals were killed by stunning and exsanguination and the inoculated and contralateral soleus muscles were removed. Three pairs of muscles were used for electron microscopy, three pairs for polyacrylamide gel electrophoresis and Western blotting, and three pairs for electrophysiology. In each case the contralateral muscles were used as controls.
Electron microscopy
Pairs of soleus muscles were pinned side by side to dental wax under slight longitudinal tension. They were fixed by immersion for 1 h in 5% w/v glutaraldehyde in 0.1 m phosphate buffer (composition 0.02 m NaH2PO42H2O; 0.08 m Na2HPO4) at pH 7.35 and room temperature. The muscles were then trimmed and mid-belly segments were cut into small blocks (1.0 mm × 0.5 mm × 0.5 mm). The blocks were fixed for a further 1 h in 5% w/v glutaraldehyde, post-fixed in 1% OsO4 in 0.1 m phosphate buffer, dehydrated in graded alcohols and embedded in Araldite™. Semi-thin transverse sections, 0.5 µm thick, were cut using a Reichert OMU2 ultramicrotome, collected onto glass slides and stained with hot 1% w/v toluidine blue containing borax, 1% w/v for 10 s. The sections were washed, mounted in Araldite resin and used to monitor the general pathology of the tissue. The blocks were then remounted and longitudinal sections 60–80 nm thick and a characteristic silver/gold colour were cut on a Reichert OMU5 ultramicrotome, collected onto grids, stained with 30% w/v uranyl acetate in methanol and 1% w/v aqueous lead citrate and viewed in a Philips CM100 Compustage transmission electron microscope. The sections were used for morphometry only if the A-bands within a given myofibril could be measured from end to end in at least three successive sarcomeres. This criterion allowed us to be confident that we were not including obliquely cut myofibrils in our measurements. Uncorrected measurements of A-band length, sarcomere length and Z-line slippage were made directly from the displayed images generated by the microscope using the software ‘Analysis 2.0’ (Soft Imaging, Münster, Germany) then corrected using the length of the A-band in control muscles as a standard. A-band length was considered not to vary between control muscles and to be 1.55 µm (Cullen et al. 1984). To measure the congruence of muscle fibre basal lamina and plasma membrane images of randomly selected lengths of muscle fibre sarcolemma were prepared at a magnification of c. 30 000×. The lengths of basal lamina and associated plasma membrane were measured and the length of the latter expressed as a function of the former. Each image covered c. 2.5 µm of membrane and 10 images were analysed at each time point. For the examination of infiltrating cells, randomly encountered cells were examined provided the nucleus was visible and the entire profile of the cell could be viewed within the grid bars. All electron microscopy was performed by a colleague who had no detailed knowledge of the specific details of the investigation.
Western blotting
Small (20–25 mg) blocks of freshly removed muscle tissue were homogenized in 20 volumes of SDS buffer. Aliquots of 30 µL were used for polyacrylamide gel electrophoresis and Western blotting (Nicholson et al. 1989). Blots to be probed for desmin were resolved on non-gradient 10% gels; those for titin were resolved on 2–12% gradient gels; those for dystrophin were resolved on 4–7% gradient gels. In all cases blots were blocked for 1 h at room temperature using a buffer containing 10 mm Tris, 0.15 m NaCl, 0.05% w/v Tween 20 and 5% w/v dried milk. Blots were incubated with an appropriate 1° Ab and defined using an HRP-conjugated 2° Ab. The blots were scanned at 400 dpi using an Epson GT 800 flat bed scanner and the scanned images, stored as TIFF files, were quantified using Bioscan Optima software. Control densitometric data were generated by preparing blots of serial dilutions of homogenates of normal tissue. In each case plots of optical density vs. load were linear, correlation coefficients ranging from r = 0.99 to r = 0.995. Experimental data were expressed in terms of relative optical density. Because the blotting of myosin and actin was unreliable, unblotted gels were stained with Coomassie blue and the myosin and actin bands were scanned and analysed as described above.
Resting membrane potential
Pairs of soleus muscles were pinned onto dental wax under slight longitudinal tension and mounted in a constant flow bath (1 mL min−1) containing a bathing fluid (composition: K+ 5.0 mm; Na+ 150 mm; Ca2+ 2.0 mm; Mg2+ 1.0 mm; Cl− 148 mm; H2PO4− 1.0 mM; HCO3− 12.0 mM; glucose 11.0 mM) at pH 7.2 and room temperature and equilibrated with 5% CO2 in O2. Glass microelectrodes, filled with 3 M KCl and with a resistance of 5–15 MΩ were used to measure intracellular resting membrane potentials. Three pairs of muscles were used at each time point and data obtained at any given time point were aggregated.
Antibodies and venoms
Venom from captive born specimens of Notechis scutatus was obtained from Venom Supplies Pty Ltd, Tanunda, S. Australia. Monoclonal antibody Dy4/6D3 (Novocastra) was used to identify dystrophin. Desmin was identified using the monoclonal antibody D33 (Dako, code M670) and titin was recognized using the monoclonal antibody AB5 (from Trinnick and Wardale, formerly of ARC Meat Research Institute, Bristol, UK). HRP-conjugated anti-mouse IgG (DAKO, code P260) was used routinely as the secondary antibody.
Results
Behaviour of animals
The animals recovered rapidly from the anaesthetic and all survived the procedures. They tended to favour the inoculated limb but continued to eat and drink normally, there was no ruffling of the fur and they did not object to handling or the palpation of the inoculated limb. The inoculated limb was paralysed within 1 h and remained paralysed for the entire period under investigation. There was no bleeding from the site of inoculation and no evidence of systemic poisoning.
General pathology
Muscles removed 1 h after the inoculation of venom were pale and oedematous. The wet weight of the inoculated muscles was 40–45% higher than that of contralateral muscles. Muscles removed 6 h after inoculation were also pale and oedematous and wet weight was 60–65% higher than that of contralateral muscles. There was no evidence of haemorrhage into the muscle. Focal hypercontraction was seen in a minority of fibres at 1 h but by 6 h almost all muscle fibres showed evidence of hypercontraction, and myofibrillar degradation was common. Extensive perivascular invasion by phagocytic cells was evident at 3–6 h but very few muscle fibre profiles contained intracellular phagocytic cells. By 24 h the internal architecture of the muscle fibres was lost and the damaged muscle fibres consisted of little more than occasional clumps of cellular debris, damaged mitochondria and phagocytic cells within the otherwise empty fibre. The general pathology observed in these studies was indistinguishable from that described in detail on a number of occasions following the inoculation of venom myotoxins (Harris & Johnson, 1978; Cullen & Mastaglia, 1982) and so are not illustrated here.
Electron microscopy
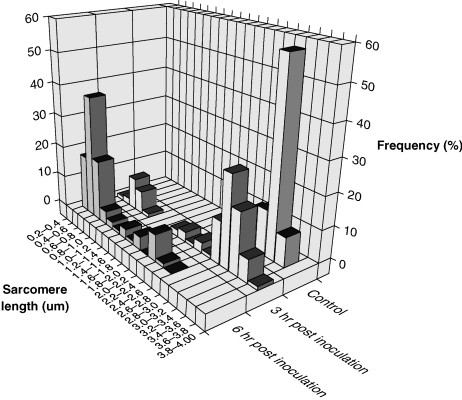
Detailed studies of the ultrastructure of inoculated and contralateral muscles were made at 3 h, 6 h and 24 h post inoculation. Control muscle fibres exhibited the regular repeat of sarcomeres, with sarcomeres of adjacent myofibrils in close lateral alignment (Fig. 1). Sarcomere length was normally distributed about a mean of 3.4 µm (SEM 0.02; range 2.7–3.7 µm; n = 100). The data are illustrated in Fig. 2.
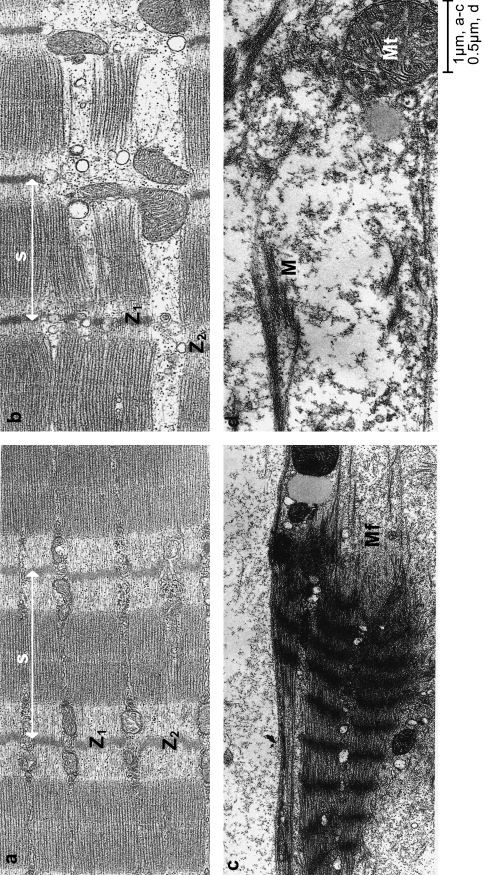
Fig. 1.
Longitudinal sections of soleus muscles of the rat at various times after the inoculation of tiger snake venom. (a) Control muscle fibre showing regularly organized sarcomeres(s) with very little displacement between discs of adjacent myofibrils (Z1 and Z2). (b) Three hours after the inoculation of tiger snake venom. Note the shortening of the sarcomere(s) and the slippage between Z discs of adjacent myofibrils (Z1 and Z2). The arrow points to a region where the shortened myofibril has buckled, taking it out of the plane of section. (c) At 6 h after the inoculation of venom, sarcomeres were greatly shortened and the myofibrils were torn (Mf). (d) By 24 h, muscle fibres were devoid of structural organization, and characteristic features were an amorphous cytoplasm containing clumps of contractile myofilaments (M), damaged mitochondria (Mt) and damaged plasma membrane punctuated by lesions.
Fig. 2.
Sarcomere lengths plotted as a frequency histogram in control soleus muscles and in muscles 3, 6 and 24 h after the inoculation of tiger snake venom. n = 100 in each case.
The slippage between adjacent myofibrils, calculated by measuring the longitudinal distance between opposing Z-lines in 100 pairs of myofibrils, was 0.14±0.01 µm (mean ±SEM). Both plasma membrane and basal lamina were intact. No phagocytic cells were seen in the muscle tissue.
The most significant change in muscle fibres examined 3 h after the inoculation of venom was the contraction of some sarcomeres (Figs 1 and 2). Mean sarcomere length fell to 2.7±0.1 µm (mean ±SEM; n = 100) but the range of lengths was much greater than in control muscles (0.83–3.9 µm). Alignment between adjacent myofibrils was within the normal range in more than 30% of 100 pairs of adjacent Z-lines studied, but overall there were clear signs of misalignment and mean slippage had increased from 0.14±0.01 µm to 0.39±0.04 µm. Twenty-one full profiles of phagocytic cells were identified in tissue sections, only one of which was unequivocally intracellular. The basal lamina of the muscle fibres was always intact, but plasma membranes exhibited numerous lesions up to 500 nm in length. Overall, 19% of basal lamina was devoid of contiguous plasma membrane.
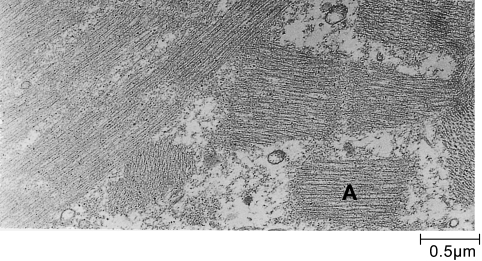
By 6 h hypercontraction resulted in the appearance of clumps of sarcomeres with a mean length of 1.43±0.06 µm (n = 100). These clumps were separated by tears, at the site of which myofilaments were frayed and loose myofilaments could be identified in the cytosol (Figs 1 and 2). It was of interest that there were very few ‘stretched-but-intact’ sarcomeres. In a small number of myofibrils the Z-lines of hypercontracted myofibrils had degenerated. In these myofibrils intact A-bands and sometimes small clumps of A-bands were released (Fig. 3). The basal lamina remained undamaged, but lesions in the plasma membrane were more frequent, and only 60% of the basal lamina was associated with plasma membrane. Phagocytic cells were common in the perivascular space but only two of the 23 phagocytic cells we examined had invaded the damaged muscle fibres. Neither of the two internalized phagocytic cells contained identifiable muscle mitochondria or contractile material.
Fig. 3.
Loss of Z discs and the release of entire A-band (A) was seen occasionally at 6 h.
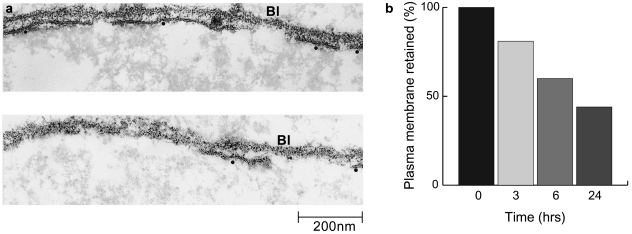
By 24 h clumps of myofibrillar proteins were still common but there was no discernible sarcomeric organization. Less than 50% of basal lamina was associated with plasma membrane (Fig. 4). Profiles of phagocytic cells were commonly found in the cytoplasm of the damaged muscle fibres. Fifty-six profiles of internalized phagocytic cells were examined. Thirty-five contained or were in the process of engulfing damaged muscle mitochondria, and six appeared to be engulfing ill-defined amorphous debris as well as mitochondria. No phagocytic cells were observed to contain, or be in the act of engulfing, either intact A-bands or definable clumps of myofilaments.
Fig. 4.
Longitudinal sections of soleus muscle fibres 6 h after the inoculation of tiger snake venom. The muscle fibre plasma membrane was punctuated by lesions of varying size (a). The survival of plasma membrane was calculated by measuring basal lamina (Bl) and plasma membrane (•) contiguity at 3, 6 and 24 h. The loss was time dependent (b).
Resting membrane potential
The mean resting membrane potential of muscle fibres of control soleus muscles was −78±0.5 mV (mean ±SEM; n = 90). One hour after the inoculation of venom, the mean resting potential had fallen to −28±3.9 mV (n = 50). Distribution histograms showed that there were two broad categories of muscle fibre: those in which the resting membrane potential had fallen to less than −10 mV and those in which resting membrane potential was preserved. By 6 h, resting membrane potentials were uniformly low (mean −5 ±±0.38 mV, n = 93), indicating the effective loss of all ion gradients across the plasma membrane.
Loss of major muscle protein
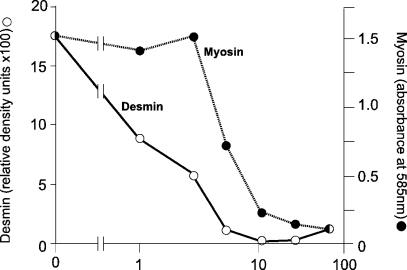
The ultrastructural study of the muscles inoculated with the venom of Notechis scutatus suggested that the disruption of both inter- and intramyofibrillar organization preceded the loss of the major contractile proteins. Previous qualitative studies on myotoxic venoms and toxins suggested that desmin, α-actinin and titin were lost before there was widespread degradation of myofibrillar components (Helliwell, 1988; Gutiérrez et al. 1990; Vater et al. 1992a, 1992b). This would be consistent with the early disruption of sarcomeric organization but no quantitative study of the loss of relevant proteins has previously been made and so it has not been possible to make formal comparisons of the relative rates of loss of the respective classes of contractile, structural and cytoskeletal proteins. We took advantage of the stored, digitized images of the gels and blots prepared by Vater et al. (Vater et al. 1992a, 1992b) to reanalyse the data using quantitative scanning densitometry. Our data showed that desmin was lost particularly rapidly from the muscles with a time to 50% loss (t50) of approximately 1 h. This compared with a t50 of approximately 6 h for actin and myosin and a similarly slow loss of dystrophin (see Table 1 and Fig. 5).
Table 1. Estimated rate of loss of major muscle proteins following exposure to the venom of Notechis scutatus.
Estimates were based on the analysis of Western blots or (*) Coomassie blue staining.
Fig. 5.
The relative rates of loss of desmin and myosin from soleus muscles at various times after the inoculation of tiger snake venom were calculated from Western blots for desmin- and Coomassie-stained gels for myosin. The data were collected by densitometry for desmin and absorbance for myosin. The original descriptive data were published by Vater et al. (1992b). See Materials and methods for details.
It was more difficult to make such precise measurements from Western blots of titin, because all antibodies available to us recognized a range of high-molecular-weight proteins, but a clearly discernible low-molecular-weight band first appeared 1 h after the inoculation of venom and grew steadily more intense over 6 h (not shown). We considered this band to be a major breakdown product of native titin. If we are correct, it would indicate that the breakdown of titin was also an extremely early event in venom-induced muscle damage.
Discussion
This investigation was made in an attempt to understand better the cellular processes involved in the experimentally induced degeneration of skeletal muscle. The crude venom of Notechis scutatus was used as the myotoxic agent and the soleus muscle of the rat as the substrate. We first address these choices.
Snake venoms are complex farragoes of toxic and non-toxic components. They are secreted from modified salivary glands and have two principal functions – the subjugation of prey items and the initiation of exodigestion. The venom of Notechis scutatus contains, inter alia, toxins that block the nAChR at the neuromuscular junction (Halpert et al. 1979) procoagulant toxins mimicking factor Xa (Hutton & Warrell, 1993), toxic phospholipases that promote haemorrhage and hypotension (Francis et al. 1998), and phospholipases that destroy skeletal muscle and motor nerve terminals (Harris et al. 1973, 1975, 2000; Dixon & Harris, 1996). The complexity of crude venoms has persuaded many investigators that working with venoms as biological reagents results in a loss of precision and that myotoxicity, for example, is best studied when a single, purified toxin with myotoxic activity is used. For this reason, the crude venom of Notechis scutatus is used in such work much less frequently than the purified myotoxic phospholipase A2, notexin, which is the major toxic fraction of the venom of this snake. The use of purified toxins allows a precise definition of their particular characteristic activities but they are of limited value to the clinician trying to interpret physiological or structural pathology in victims of an envenoming snake bite. Since rhabdomyolysis and consequential acute renal failure is a major feature of envenoming bites by Notechis scutatus in both humans and non-human animals (Hood & Johnson, 1975; Frost, 1980, 1981; Lewis, 1994) we chose to work with whole venom. We chose to use the rat soleus muscle because this muscle is composed entirely of oxidative and oxidative/glycolytic muscle fibres. Muscle fibres with high oxidative capacity are particularly susceptible to myotoxic phospholipases A2 and it is possible to initiate the destruction of the entire muscle following a single injection of either crude venom or pure toxin (Harris et al. 1975; Harris & Johnson, 1978). The dose of venom used (15 µg in 0.2 mL solution) was calculated as follows. The myotoxic phospholipases A2 notexin and notechis 11–5 are the most toxic fractions of the venom, and 2.0 µg of either toxin will destroy the soleus muscle (weighing 60–100 mg) of a rat weighing approximately 100 g (Harris & Johnson, 1978). The toxic phospholipases A2 constitute 10–15%, by mass, of the dried venom of Notechis scutatus (Karlsson et al. 1972). Thus, 15 µg of crude venom should contain approximately 2.0 µg of toxic phospholipases A2. This calculation is clearly inexact because venom composition varies with age, season and feeding habits, but with this dose of venom we have consistently achieved total degeneration of the soleus muscle with zero mortality and no long-term morbidity. It is of incidental interest that the dose is equivalent to the inoculation of 0.15 mg venom kg−1. This is comparable to the LD50 of the venom in the mouse (0.12 mg kg−1 s.c.; Broad et al. 1979; 0.18 mg kg−1 s.c., 0.04 mg kg−1 i.p., Minton & Minton, 1971) and suggests that the rat is more resistant to the venom than the mouse. Unfortunately, no figures for comparative LD50s are available to allow a formal distinction between species.
The inoculation of venom resulted in muscle degeneration in the underlying soleus muscle. There was no apparent damage to the contralateral soleus muscle. Animal behaviour was similar to that in normal laboratory rats. There was neither bleeding from the site of inoculation nor haemorrhage within or adjacent to the damaged muscle. We did not check coagulation times but on all other available evidence we can confidently assert that the damage to the soleus muscle was not associated with any signs of systemic poisoning. The animals favoured the inoculated limb and this may indicate venom-induced hyperalgesia or allodynia. For this reason all inoculated animals received analgesia at veterinary therapeutic levels. Since animals allowed palpation of the inoculated limb we believe any pain associated with the inoculation was minimized.
The earliest physiological change seen in muscles exposed to venom was the depolarization of the muscle fibres. By 6 h muscle fibre depolarization was complete, reflecting we suggest the extensive loss of plasma membrane at 6 h and the consequential loss of all ion gradients. Since the ion with the largest concentration gradient across the plasma membrane is Ca2+ ([Ca2+]o = 10−3 m; [Ca2+]i = 10−7 m), the hypercontraction may reasonably be considered the result of the entry of Ca2+ into the muscle fibre cytosol. The very early loss of desmin and titin was also probably related to the rapid elevation of [Ca2+]i. Desmin is the major intermediate filament protein in smooth, cardiac and skeletal muscle. In mature skeletal muscle desmin forms a reticulated collar around the Z-discs of myofibrils, interlinking both sequential Z-discs within a myofibril and the Z-discs of adjacent myofibrils, and also linking peripheral myofibrils to the plasma membrane. It is enriched at the myotendinous junction and thus provides both mechanical stability and tensile strength to the myofibril. Desmin is also associated with other subcellular organelles such as mitochondria and nuclei (Lazarides, 1980; Tokuyasu et al. 1983; Traub, 1985). The role of desmin in the development and regeneration of the muscle fibre is unclear. It is expressed early during myogenesis but does not become associated with the Z-disc until myofibrils are laid down and aligned (Tokuyasu et al. 1984; 1985; Schultheiss et al. 1991). Myogenesis and primary muscle formation is not affected in desmin knockout mice (i.e. Des−1−) and the animals develop normally. The muscles, however, are weak and fatique easily (Li et al. 1997). The load-bearing muscles, such as soleus, are particularly susceptible to fatique and degeneration. The regeneration of skeletal muscle in Des−1− mice is delayed and the regenerated muscles exhibit a number of abnormalities including persistent, small muscle fibres, Z-line streaming and the lack of alignment of myofibrils (Milner et al. 1996; Agbulut et al. 2002). It would appear therefore that desmin plays a limited role in normal muscle formation and development but does have a major role in the stabilization of the sarcomere, in force transduction and in the coherent alignment of adjacent myofibrils. Titin is a large sarcomeric protein that spans the sarcomere from Z-line to M-line and is generally considered to regulate the length, and structural integrity of the sarcomere during cycles of contraction and relaxation (Minajeva et al. 2001). Both titin and desmin are rapidly hydrolysed by Ca2+-activated proteases. Titin is so rapidly hydrolysed that it presents significant problems during purification from skeletal muscle (Wang, 1982). The selective hydrolysis of titin does not result in any change in the maximum rate of shortening but there is a reduction in both resting and active tension, and the dislocation of A- and I-bands (Higuchi, 1992). Stretched sarcomeres were particularly susceptible to damage (Higuchi, 1992; Kellermayer et al. 2001). Desmin is rapidly hydrolysed in situ by a highly specific proteinase activated by Ca2+ at a concentration of 10 µm (Nelson &Traub, 1982, 1983). The rapid loss of desmin and titin is an early feature of stress- and disease-related muscle degeneration (Cullen et al. 1992; Lieber et al. 1996; Kellermayer et al. 2001).
These observations on the hydrolysis of desmin and titin and the morphological consequences of their loss suggest that the hydrolysis of the phospholipids of the plasma membrane by myotoxic phospholipases in the venom of Notechis scutatus results in the localized loss of ion homeostasis and the entry of Ca2+. The initiation of hypercontraction by elevated [Ca2+]i will coincide with the activation of Ca2+-dependent proteolytic activity. The consequential loss of desmin would then lead to the loss of register between adjacent sarcomeres, and the stretching of some sarcomeres between segments of hypercontraction (Dixon & Harris, 1996) would expose titin to hydrolytic attack, leading to the disaggregation of A- and I-bands and the shedding of loose myosin and actin filaments into the cytosol. The hydrolysis of stretched titin filaments in the extensible, but easily fatigued I-band region (Kellermayer et al. 2001) explains why we never observed highly stretched but intact sarcomeres.
Compared to the rapid loss of desmin and titin, the hydrolysis of myosin and actin was relatively slow. We expected to identify clumps of myofibrils and intact A-bands in invading phagocytic cells. There has been much debate on the importance of this process (myophagia) in the clearance of contractile filaments (Cullen & Mastaglia, 1982). On the basis of our data, we suggest that myophagia is principally a process for clearing dead and dying mitochondria and that the clearance of contractile material is primarily cytosolic.
Dystrophin is a subsarcolemmal cytoskeletal protein linked to the sarcolemma via a dystrophin–glycoprotein complex (DGC) and to the basal lamina via protein α-dystroglycan (Anderson, 2002). The absence of dystrophin from the skeletal muscle fibre leads to the progressive degeneration of skeletal muscle that characterizes Duchenne-type muscular dystrophy in man and the closely related x-linked disease in the dog (Kakulas, 1996). We speculated, privately, that the rapid degeneration of skeletal muscle exposed to phospholipase-rich venoms might be related to the rapid loss of dystrophin exposed by the hydrolysis of the sarcolemma. Our observation that dystrophin was lost very much more slowly than desmin and titin shows our speculation to be unsupported but does not change the general conclusion that the primary cause of degeneration is the selective hydrolysis of desmin and titin induced by Ca2+ entry via the damaged plasma membrane.
Our data have clear implications for those managing victims of bites by snakes with myotoxic venoms. The onset of muscle damage is very rapid and is unlikely to be controlled by the use of appropriate antivenom because the delay between bite and admission to a competent clinic, even in developed countries, may be several hours. Treatment should be conservative and designed to maintain respiration and adequate renal function. It is generally recognized that, for elapid bites in particular, the application of a firm crepe bandage to the bitten limb, combined with immobilization is a beneficial first-aid measure (Sutherland et al. 1979). The rationale for this treatment is that venom movement can be delayed for long periods, thus reducing the danger of systemic poisoning. It may have an additional benefit since immobilization may well reduce the mechanical demands on damaged muscle and limit the rate of degradation and degeneration. Muscle regeneration begins at 3 days (Harris & Johnson, 1978) and the victim should then recover rapidly.
Acknowledgments
We thank the Wellcome Trust and Muscular Dystrophy Campaign for financial support, and John Walsh, Carol Young and Keith Davison for their technical expertise.
References
- Agbulut O, Li ZL, Perie S, Ludosky MA, Paulin D, Cartaud J, et al. Lack of desmin results in abortive muscle regeneration and modifications in synaptic structure. Cell Motil. Cytoskel. 2002;49:51–66. doi: 10.1002/cm.1020. [DOI] [PubMed] [Google Scholar]
- Anderson LVB. Dystrophinopathies. In: Karpati G, editor. Structural and Molecular Basis of Skeletal Muscle Diseases. Basel: ISN Neuropath Press; 2002. pp. 6–23. [Google Scholar]
- Broad AJ, Sutherland SK, Coulter AR. The lethality in mice of dangerous Australian and other snake venoms. Toxicon. 1979;17:664–667. doi: 10.1016/0041-0101(79)90245-9. [DOI] [PubMed] [Google Scholar]
- Cullen MJ, Mastaglia FL. Pathological reactions of skeletal muscle. In: Mastaglia FL, Walton JN, editors. Skeletal Muscle Pathology. Edinburgh: Churchill Livingstone; 1982. pp. 88–139. [Google Scholar]
- Cullen MJ, Hollingworth S, Marshall MW. A comparative study of the transverse tubular system of the rat extensor digitorum longus and soleus muscle. J. Anat. 1984;138:297–308. [PMC free article] [PubMed] [Google Scholar]
- Cullen MJ, Fulthorpe JJ, Harris JB. The distribution of desmin and titin in normal and dystrophic human muscle. Acta Neuropathol. 1992;83:153–169. doi: 10.1007/BF00308475. [DOI] [PubMed] [Google Scholar]
- Dixon RW, Harris JB. Myotoxic activity of the toxic phospholipase, notexin, from the venom of the Australian tiger snake. J. Neuropath. Exp. Neurol. 1996;55:1230–1237. doi: 10.1097/00005072-199612000-00006. [DOI] [PubMed] [Google Scholar]
- Francis BR, Da Silva NJ, Kaiser I. A new type of toxic snake venom phospholipase A2 which promotes hypotension and haemorrhage in mice. In: Bailey GS, editor. Enzymes from Snake Venom. Colorado: Alaken Inc; 1998. pp. 481–502. [Google Scholar]
- Frost J. Tiger snake envenomation. Med. J. Aust. 1980;1:440. doi: 10.5694/j.1326-5377.1981.tb112999.x. [DOI] [PubMed] [Google Scholar]
- Frost J. Tiger snake envenomation and muscle spasm. Med. J. Aust. 1981;2:579. doi: 10.5694/j.1326-5377.1981.tb112999.x. [DOI] [PubMed] [Google Scholar]
- Gutiérrez JM, Arce V, Brenes F, Chaves F. Changes in myofibrillar components after skeletal muscle necrosis induced by a myotoxin isolated from the venom of the snake Bothrops asper. Exp. Mol. Pathol. 1990;52:25–36. doi: 10.1016/0014-4800(90)90055-i. [DOI] [PubMed] [Google Scholar]
- Halpert J, Fohlman J, Eaker D. Amino acid sequence of a postsynaptic neurotoxin from the veonom of the Australian tiger snake Notechis scutatus scutatus. Biochemie. 1979;61:719–723. doi: 10.1016/s0300-9084(79)80172-8. [DOI] [PubMed] [Google Scholar]
- Harris JB, Karlsson E, Thesleff S. Effects of an isolated toxin from Australian tiger snake (Notechis scutatus scutatus) venom at the mammalian neuromuscular junction. Br. J. Pharmacol. 1973;47:141–146. doi: 10.1111/j.1476-5381.1973.tb08168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JB, Johnson MA, Karlsson E. Pathological responses of rat skeletal muscle to a single subcutaneous injection of a toxin isolated from the venom of the Australian tiger snake, Notechis scutatus scutatus. Clin. Exp. Pharmacol. Physiol. 1975;2:383–404. [Google Scholar]
- Harris JB, Johnson MA. Further observations on the pathological responses of rat skeletal muscle to toxins isolated from the venoms of the Australian tiger snake Notechis scutatus scutatus. Clin. Exp. Pharmacol. Physiol. 1978;5:587–600. doi: 10.1111/j.1440-1681.1978.tb00714.x. [DOI] [PubMed] [Google Scholar]
- Harris JB, Grubb BD, Maltin CA, Dixon R. The neurotoxicity of the venom phospholipaes A2, notexin and taipoxin. Exp. Neurol. 2000;161:517–526. doi: 10.1006/exnr.1999.7275. [DOI] [PubMed] [Google Scholar]
- Helliwell TR. Lectin binding and desmin expression in bupivicaine-induced necrosis and regeneration of rat skeletal muscle. J. Pathol. 1988;155:317–326. doi: 10.1002/path.1711550407. [DOI] [PubMed] [Google Scholar]
- Higuchi H. Changes in contractile properties with selective digestion of connectin (titin) in skinned fibres of frog skeletal muscle. J. Biochem. (Tokyo) 1992;111:291–295. doi: 10.1093/oxfordjournals.jbchem.a123752. [DOI] [PubMed] [Google Scholar]
- Hood VL, Johnson JR. Acute renal failure with myoglobinuria following tiger snake bite. Med. J. Aust. 1975;2:638–641. [PubMed] [Google Scholar]
- Hutton RA, Warrell DA. Action of snake venom components on the hemostatis system. Blood Rev. 1993;7:176–189. doi: 10.1016/0268-960x(93)90004-n. [DOI] [PubMed] [Google Scholar]
- Kakulas BA. The spectrum of dystrophinopathies. In: Lane RJM, editor. Handbook of Muscle Disease. New York: Marcel Dekker Inc; 1996. pp. 235–244. [Google Scholar]
- Karlsson E, Eaker D, Rydén L. Purification of a presynaptic neurotoxin from the venom of the Australian tiger snake, Notechis scutatus scutatus. Toxicon. 1972;10:405–413. doi: 10.1016/0041-0101(72)90066-9. [DOI] [PubMed] [Google Scholar]
- Kellermayer MJ, Smith SB, Bustamante C, Granzier HL. Mechanical fatigue in repetitively stretched single molecules of titin. Biophys. J. 2001;870:852–860. doi: 10.1016/S0006-3495(01)76064-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Ogus C, Harris JB. Preliminary observations of satellite cells in undamaged fibres of the rat soleus muscle assaulted by a snake-venom toxin. Cell Tiss. Res. 1983;230:671–676. doi: 10.1007/BF00216210. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980;283:249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lewis PP. Myotoxicity and nephrotoxicity of common tiger snake (Notechis scutatus) venom in the dog. Aust. Vet. J. 1994;71:136–139. doi: 10.1111/j.1751-0813.1994.tb03366.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Mericskay M, Butler-Browne G, Carlsson L, Thornell L-E, Babinet C, et al. Desmin is essential for the tensile strength an integrity of myofibrils but not for myogenic commitment, differentiation and fusion of skeletal muscle. J. Cell Biol. 1997;139:129–144. doi: 10.1083/jcb.139.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber RL, Thornell LE, Friden J. Muscle cytoskeleton disruption occurs within the first 15 min of cyclic eccentric contraction. J. App. Physiol. 1996;80:278–284. doi: 10.1152/jappl.1996.80.1.278. [DOI] [PubMed] [Google Scholar]
- Milner DJ, Weitzer B, Trau D, Bradley A, Capetanaki Y. Disruption of muscle architecture and myocardial degeneration in mice lacking desmin. J. Cell Biol. 1996;134:1255–1270. doi: 10.1083/jcb.134.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minajeva A, Kulke M, Fernandez JM, Linke WA. Unfolding of titin domains explains the visoeleastic behaviour of skeletal muscle. Biophys. J. 2001;80:1442–1451. doi: 10.1016/S0006-3495(01)76116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton SA, Minton MR. Venomous Reptiles. London: George Allen & Unwin; 1971. [Google Scholar]
- Nelson WJ, Traub P. Purification and further characterization of the Ca2+-activated proteinase specific for the intermediate filament proteins vimentim and desmin. J. Biol. Chem. 1982;257:5544–5553. [PubMed] [Google Scholar]
- Nelson WJ, Traub P. Proteolysis of vimentin and desmin by the Ca2+-activated protease specific for these intermediate filament proteins. Mol. Cell. Biol. 1983;3:1146–1156. doi: 10.1128/mcb.3.6.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson LVB, Davison K, Falkous G, Harwood C, O'Donnel E, Slater CR, et al. Dystrophin in skeletal muscle. 1. Western blot analysis using a monoclonal antibody. J. Neurol. Sci. 1989;94:125–136. doi: 10.1016/0022-510x(89)90223-2. [DOI] [PubMed] [Google Scholar]
- Schultheiss T, Lin Z, Ishikawa H, Zamir I, Stoeckert CJ, Holzer H. Desmin/vimentin intermediate filaments are dispensable for many aspects of myogenesis. J. Cell Biol. 1991;114:953–966. doi: 10.1083/jcb.114.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuk D, Roy B, Goulat M, Tremblay JP. Successful myoblast transplantation in primates depends on appropriate cell delivery and induction of regeneration in the host muscle. Exp. Neurol. 1999;155:22–30. doi: 10.1006/exnr.1998.6973. [DOI] [PubMed] [Google Scholar]
- Sutherland SK, Coulter AR, Harris AD. Rationalisation of first aid measures for elapid snake bite. Lancet. 1979;1:183–186. doi: 10.1016/s0140-6736(79)90580-4. [DOI] [PubMed] [Google Scholar]
- Tokuyasu KT, Dutton AH, Singer SJ. Immunoelectron microscopic studies of desmin (skeletin) localization and intermediate filament organization in chicken skeletal muscle. J. Cell. Biol. 1983;96:1727–1735. doi: 10.1083/jcb.96.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu KT, Maher PA, Singer SJ. Distributions of vimentin and desmin in developing chick myotubes in vivo. I. Immunofluorescence study. J. Cell. Biol. 1984;98:1961–1972. doi: 10.1083/jcb.98.6.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu KT, Maher PA, Singer SJ. Distributions of vimentin and desmin in developing chick myotubes in vivo. II. Immunoelectron microscopic study. J. Cell. Biol. 1985;100:1157–1166. doi: 10.1083/jcb.100.4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub P. Intermediate filaments. A Review. Berlin: Springer-Verlag; 1985. [Google Scholar]
- Vater R, Cullen MJ, Harris JB. The fate of dystrophin during the degeneration and regeneration of the soleus muscle of the rat. Acta Neuropathol. 1992a;83:140–148. doi: 10.1007/BF00308473. [DOI] [PubMed] [Google Scholar]
- Vater R, Cullen MJ, Harris JB. The fate of desmin and titin during the degeneration and regeneration of the soleus muscle of the rat. Acta Neuropathol. 1992b;84:278–283. doi: 10.1007/BF00227821. [DOI] [PubMed] [Google Scholar]
- Wang K. Purification of titin and nebulin. Meth. Enzymol. 1982;85:264–274. doi: 10.1016/0076-6879(82)85025-8. [DOI] [PubMed] [Google Scholar]
- Whalen RG, Harris JB, Butler-Browne GS, Sesodia S. Expression of myosin isoforms during notexin-induced regeneration of rat soleus muscles. Dev. Biol. 1990;141:24–40. doi: 10.1016/0012-1606(90)90099-5. [DOI] [PubMed] [Google Scholar]