Abstract
Horses spend much of their life standing, and they are believed to be able to keep their limbs straight without muscular effort. We tested the hypothesis that the stifle (knee) and hock (tarsal) joints could be stabilized merely with the help of a passive lock mechanism whereby the patella is secured behind a hook, formed by the medial femoral trochlea. In anaesthetized animals and isolated limbs the stifle and hock flex readily under compression. In isolated limbs this collapse was prevented by a small force applied to the patella, mimicking the action of the vastus medialis muscle. In vivo, when the limb was planted loosely on the ground none of the muscles with a connection to the patella was active. However, during weight-bearing the vastus medialis (but no other muscle) was active, providing the necessary traction to stabilize the stifle. The required tension was estimated to be less than 2% of the force that would be needed in absence of a lock mechanism. Diagnosis and treatment of patellar fixation should include the possibility of overactive vastus medialis muscle as a possible cause of the disorder.
Keywords: electromyography, horse, patellar lock mechanism, stifle, vastus medialis
Introduction
In standing mammals the hind limbs are compressed between the weight of the body, acting on the hip, and the ground reaction force (GRF), acting on the hoof. Folding of the limb is prevented by the extensor muscles. If animals grow larger, muscle force – determined by cross-sectional area – would increase with body weight to the power 2/3, putting large animals at a disadvantage in maintaining body posture (Schmidt-Nielsen, 1984). To compensate, muscle cross-sectional area shows slightly positive allometric, i.e. disproportionally strong, growth (Alexander, 1985). Furthermore, larger animals shift from a crouched to a more upright limb posture, increasing the mechanical effectiveness of their limb extensors (Biewener, 1989).
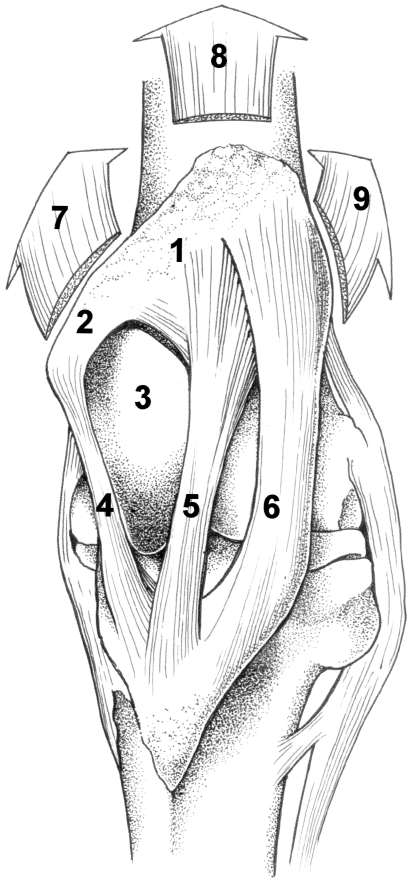
Horses, large animals adapted to fast running, spend about 80% of their time standing, even during light sleep (Dallaire, 1986; Boyd et al. 1988). At ease, they often support themselves with front limbs plus one hind limb, the latter carrying a large proportion of their body weight. It has long been believed that the so-called passive stay apparatus ensures limb stabilization with no or minimal involvement of energy consuming, fatiguing muscles (Dyce et al. 1996; Nickel et al. 1986). The key structure of the stay apparatus in the hind limb is the stifle joint. It can be prevented from flexing by fixation of the patella behind a ‘hook’, formed by the medial portion of the femoral trochlea (Fig. 1; see Shuttleworth, 1943; Sack, 1989). With a stabilized stifle, the hock joint is also protected from flexion by the taut ligamentous superficial digital flexor muscle, forming part of the reciprocal mechanism that couples the movements of both joints. The more distal joints are stabilized passively by tendons and ligaments (see above-mentioned references).
Fig. 1.
The anatomy of the left stifle joint (cranial view). When locked, the patella (1) with parapatellar fibrocartilage (2) is shifted behind the top of the prominent medial ridge of the femoral trochlea (3). Bending of the stifle joint is now prevented by the medial (4), intermediate (5) and lateral (6) patellar ligaments. The quadriceps femoris muscle, inserting to the patella, serves to extend the stifle joint. It may assist in keeping the patella in locked position. The direction of pull of its three main portions, the vastus medialis (7), rectus femoris (8) and vastus lateralis (9) is shown by arrows.
The distal portion of the vastus medialis muscle inserts to the parapatellar fibrocartilage. Other portions of the quadriceps muscle (vastus lateralis, rectus femoris) also attach directly to the patella. Four other muscles (sartorius, gracilis, tensor fasciae latae and biceps femoris) attach indirectly to the patella (Dyce et al. 1996). The possible involvement of these muscles in patellar fixation is unknown and can only be determined experimentally. So far such research has been lacking. Better insight in to this mechanism might help in improving diagnosis and treatment of upward patellar fixation, a cause of equine lameness.
In this paper the hypothesis is tested that patellar fixation is impossible without active muscular assistance. This was demonstrable in autopsy specimen and anaesthetized horses. In autopsy specimen patellar fixation could be maintained by a small force, applied to the (medial) parapatellar fibrocartilage. EMG recordings in weight-bearing and non-weight-bearing limbs confirm the hypothesis that also in vivo such a force is provided, by tonic, low-level activity of the vastus medialis muscle.
Materials and methods
Animals
In vitro measurements were carried out on fresh limbs of four Dutch Warmblood (KWPN) horses weighing 400–600 kg. Passive resistance against compression of the straightened limb was tested in eight anaesthetized adult (KWPN) horses (450–650 kg). EMG experiments were performed using three ponies (one mare, two stallions, 185–310 kg) and three adult KWPN horses (geldings, 460–530 kg). All protocols had been approved by the Animal Experimentation Committee (DEC) of the School of Veterinary Medicine (Utrecht).
In vitro loading
In fresh limbs, disarticulated at the hip joint, the musculature proximal to the patella was removed, leaving the stifle joint intact. The straighened limbs (one per horse) were placed in a frame with the hoof on a ground plate. The femoral head was positioned above the hoof and loaded with a vertical compressive force with the help of a hydraulic device, as previously described (Riemersma & Lammertink, 1988). Like in normal posture (our unpublished data), the action line of the compressive force passed 4–5 cm behind the rotation centre of the stifle and 9–10 cm in front of the hock.
In a first series of trials the optimal line of pull of the force necessary to prevent patellar unlocking and limb collapse was determined under a compressive load of 1700 N. This force was exerted to one hind limb, carrying approximately 35% of the 500-kg body weight of an adult horse. Strings connected to a spring scale to record the force were attached to the middle and the lateral top portions of the patella, and to the (medial) parapatellar fibrocartilage (Fig. 1), mimicking the respective tendons of the rectus femoris, vastus lateralis and vastus medialis muscles. Traction (in a vertical, parasagittal plane) applied to the medial side of the patella turned out to be most effective in keeping the patella in position. For this point of application the direction of traction was varied. Tested angles between the axis of the femur and the line of traction, as seen in medial view, were 15, 60 and 90°.
As traction with an angle of 60° (hence in a posterior and slightly upward direction) was most efficient, in a second series of experiments the minimum necessary traction at this angle, preventing limb collapse, was recorded for six levels of compressive loads (450– 1700 N). Compressive force and necessary tractional forces were expressed in N per kg body weight.
In vivo loading
Manual loading experiments of the intact limb were conducted in animals ready for (unrelated) surgery, under general anaesthesia, in lateral recumbency. A hind limb was straightened and the patalla kept in locked position manually. To test if an external, upward force could provoke unlocking, manual pressure was applied to the hoof in the direction of the hip; this was done with the limb in protracted, intermediate and retracted positions.
Electromyography
Seven pairs of silver surface plate electrodes (diameter 2 cm) spaced 1.5 cm apart were taped to the shaved and alcohol-cleaned skin. An adhesive metal plate (12 × 18 cm) attached to the similarly treated skin was used as a ground electrode.
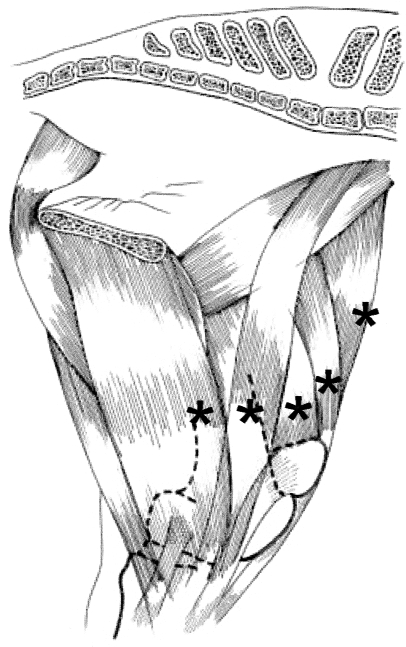
The electrodes were applied to the left muscles in the following positions in the ponies: vastus medialis, 2 cm above and medial to the tip of the parapatellar cartilage; sartorius, horizontal position as previous, on the muscle belly, as asserted by palpation; gracilis, horizontal position as previous, 1 cm cranial to saphenous vessels; tensor fasciae latae, halfway between coxal tuber and patella; rectus femoris, 3 cm above the top margin of the patella; vastus lateralis, 2 cm cranial to and halfway between the third trochanter and patella; biceps femoris, vertical position as previous muscle, 6 cm cranial to cranial margin of semitendinosus. In the KWPN horses, all aforementioned distances were about 50% larger. Electrode positions for the five medially located muscles are shown in Fig. 2.
Fig. 2.
Medial view of the left hind limb. Electrode positions for five muscles are shown by asterisks. Electrodes were directly over (from left to right): gracilis, sartorius, vastus medialis, rectus femoris and tensor fasciae latae muscles. Electrode positions for vastus lateralis and biceps femoris muscles: see text.
The signals, pre-amplified 1000× with custom-built differential amplifiers, attached to a belt fastened on the back of the horse, were sent via coaxial cable to the computer and to an oscilloscope and loudspeakers, for monitoring. The animals were brought in a quiet, dimly lit room; after a variable amount of time a state of half-sleep (immobile, drooping upper eyelid) was reached spontaneously, and recording was started. Activity of the left hind limb muscles was recorded during three support conditions, i.e. (1) on two forelimbs plus left hind limb (L), (2) on four limbs (L + R) and (3) on two forelimbs plus right hind limb (R). The non-weight-bearing limb was held slightly flexed and touched the ground with the front of the hoof. Electrodes were reapplied and all recordings repeated after 1 week. The patella of the weight-bearing limbs was always locked. Finally, the horse was walked at low speed to obtain reference EMG-signals.
Data processing
The data were AD-converted at 2500 samples per second per channel and 12-bit resolution with a PC-operated National Instruments 6030 AD converter, running with Labview (National Instruments) software. The data were run through a digital band pass filter of 15–500 Hz and a digital notch filter (49–51 Hz) to reduce noise and electrical interference. Two recordings were made of each behaviour and from each five separated samples with a total duration of 60 s were selected. After offset correction the mean rectified EMG value was calculated. To check for cross-talk between the electrodes, the correlation coefficients between the raw EMG data of the seven muscles were calculated for condition ‘L’ in two recordings of each animal.
Differences between EMG levels between the support conditions were tested with univariate anova. Of the muscles showing a significant effect, the log transformed ratios between the EMG levels during the three support conditions were tested for significant deviation from unity (Student's t-test).
Results
Passive resistance against unlocking
Small compressive loads applied manually to the hoof of a straightened, isolated fresh hind limb with the patella brought manually in a locked position always caused immediate limb flexing. To rule out the possibility that passive structures, connecting limb and trunk, assist in stifle stabilization in vivo, force was also applied to the hoof in anaesthetized animals in lateral recumbency. Gentle push to the straightened limb in the direction of the hip joint was sufficient to flex the stifle; it occurred regardless of the position of the whole limb relative to the trunk.
Prevention of unlocking by in vitro force
In the second loading experiment a force was applied to the medial parapatellar fibrocartilage, mimicking the traction of the distal portion of the vastus medialis muscle. It could keep the patella in position and prevent collapse of a loaded limb. The maximum load tested was 1700 N. Force applied to the middle of the patella in the direction of the rectus femoris muscle was about 50% less effective; force applied to the lateral side in the direction of the vastus lateralis muscle and along the tendons of the other muscles in the region failed to prevent unlocking.
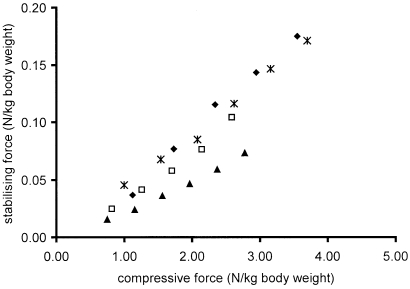
If force was applied to the parapatellar cartilage, the magnitude neccessary to prevent unlocking depended on its angle with the femur in the parasagittal plane. An angulation of 60° proved most efficient. A roughly linear relationship existed between the applied compressive force and the minimal force (at 60°) needed to keep the patella locked (Fig. 3). The latter amounted on average to only 3.5% of the applied compressive force.
Fig. 3.
Relationship between compressive force exerted on an isolated hind limb (x-axis), and the force in medio-caudal direction to the patella, necessary to prevent flexing of the stifle (y-axis). To account for the size differences between the horses both forces are expressed per kg body weight. Symbols represent data of different horses. Each data point is the mean of three measurements.
Muscle activity
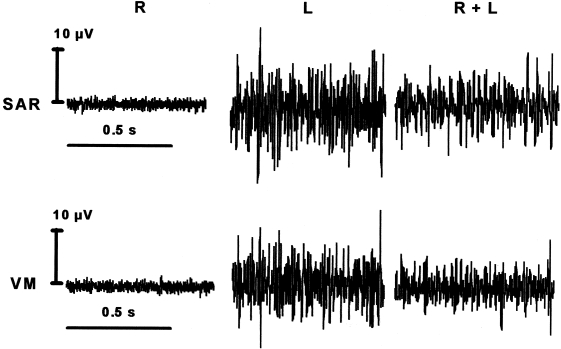
Figure 4 shows the electrical activity of the left sartorius and vastus medialis muscles under the three loading conditions, e.g. left hind limb plus forelimbs (condition L), all fours (L + R) and right hind limb plus forelimbs (R). Under condition R the signal levels are extremely low. Although the voltage is still very low, the signal level is clearly elevated at weight-bearing conditions (L + R and L), and motor unit action potentials could sometimes be monitored on oscilloscope and loudspeaker.
Fig. 4.
Example of EMG recordings. Activity of left sartorius (SAR) and vastus medialis (VM) muscles during quiet standing on the right hind limb (R), left hind limb (L) and both hind limbs (L + R). Recordings from the same horse in a single session. Note the low voltage of the signals.
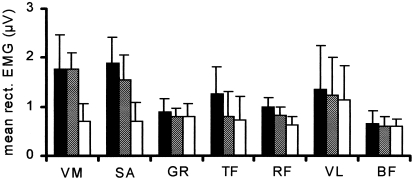
Figure 5 shows the mean rectified EMG values for all investigated muscles in three conditions. Only vastus medialis and sartorius muscles showed statistically significant differences between signal levels in the three conditions. In both muscles the ratios L/R and (L + R)/R of the signals were significantly larger than unity, showing that the loaded limb displayed a higher muscle activity than the unloaded limb. The extremely low signal levels and lack of recognizable action potentials in the other muscles in all recording conditions, and in sartorius and vastus medialis muscles during condition R, suggest that their signals represent noise. The noise levels were not the same for the different muscles, possibly because of differential pick-up of interference, due to the position of the electrodes. During a slow walk, mean rectified signal levels of sartorius and vastus medialis muscles were at least 10 times higher than during weight-bearing, suggesting that in the latter case only a small portion of the motor units were active.
Fig. 5.
Summary of EMG levels in seven muscles. Mean rectified values (plus SD) during standing on left hind limb (black columns), right hind limb (white columns) and both hind limbs (hatched columns).
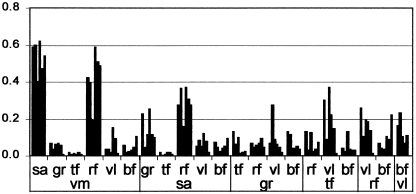
Because the sartorius muscle is a thin strap muscle overlying the bulky vastus medialis, and the recording electrodes were positioned close to each other, the possibility existed for cross-talk (Morrenhof & Abbink, 1985; De Luca, 1997). The cross-correlations between the signals of these muscles were analysed and compared with the cross-correlations between the other muscles. Figure 6 shows that the mean correlation between all possible pairs of muscles is below 0.2, with three exceptions, namely the correlations between the vastus medialis, sartorius and rectus femoris muscles. The first two of these muscles show cross-correlations between 0.4 and 0.6, and this is evidence for cross-talk. This suggests that the sartorius muscle is not active, but EMG of underlying vastus medialis muscle is picked up across its belly.
Fig. 6.
Cross-correlations (y-axis) between all possible pairs of the seven investigated muscles in six animals. Each bar represents the correlation coefficient in one animal. The figure should be read as follows: the six columns at the far left represent the correlatons (in six animals) between VM (vastus medialis) and SA (sartorius) muscles, the next group the correlations of VM with GR (gracilis), and so on.
Discussion
At variance with the widely held traditional view (e.g. Shuttleworth, 1943), our results clearly show that passive stabilization of the patella is impossible. It might be argued that in the in vitro experiments removal of the quadriceps musculature proximal to the patella leads to an artificial condition, from which it is difficult to draw conclusions. Removal was necessary because the muscle, after disappearance of rigor mortis, could, by changes in length, produce an uncontrollable effect upon the patella. However, in vivo the presence of this (passive) musculature would promote rather than obstruct patellar unlocking, by its mere weight pressing downward. The untenability of a purely passive lock mechanism was further strengthened by the experiments with anaesthetized horses.
Other authors held various muscles responsible for keeping the patella locked (e.g. Dyce et al. 1996: biceps femoris, tensor fasciae latae, sartorius, gracilis; Nickel et al. 1986: quadriceps group). Our results show that the vastus medialis is the only candidate for this action: it is active during standing, and has a suitable anatomical position.
Even if the sartorius muscle was electrically active itself, its traction, converted to the fascia of the lower limb, would be incapable of fixing the patella. The use of cross-correlation to detect cross-talk has been criticised, as false positive results might be caused by correlated motor signals to different muscles (De Luca, 1997). However, in this case cross-correlation between vastus medialis and the overlying but anatomically separate sartorius muscle was always higher than between vastus medialis/vastus lateralis, or rectus femoris/vastus lateralis, muscles joined with the vastus medialis in the quadriceps femoris muscle. Furthermore, the three highest levels of cross-correlations were found between vastus medialis, rectus femoris and sartorius muscles, and these muscles were recorded by closely adjacent electrode pairs. Therefore we suspect that the sartorius is not active, but picks up the electrical activity of the vastus medialis muscle.
The activity level of the vastus medialis was extremely low; during a slow walk the integrated signal already rose 10-fold; this suggests that activity may be only a few per cent of truly maximal activity (gallop or jump). The required muscle force (70 N) is extremely small relative to the maximal possible isometric force the relevant distal portion of the vastus medialis may develop (40 N cm−2, e.g. Maughan et al. 1983). As the more proximal parts of the muscle are inaccessible to surface electromyography, it is impossible to tell how much of the muscle is involved in the postural activity.
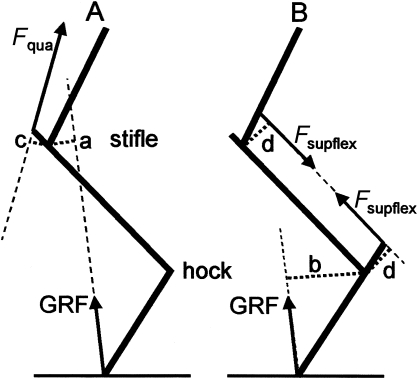
The energy cost of generating isometric force is proportional to force magnitude. To estimate the saving attained by the patellar lock mechanism, the stabilizing force in the presence and absence of this mechanism can be compared (Fig. 7). Moments are defined as force times moment arm, the latter (a–d in Fig. 7) defined as the shortest distance between the force line and the rotation centre of the joint. Without a lock mechanism, the stifle should be stabilized against the action of the (compressive) ground reaction force (GRF) by contraction of the quadriceps muscle (Fig. 7A). The GRF tends to flex the stifle with a moment MGRF(stifle). In addition (Fig. 7B), the GRF flexes the hock with a moment MGRF(hock). The hock is stabilized by the moment Msupflex(hock) provided by the superficial flexor muscle. This muscle also bridges the stifle joint. The moment arms of this muscle about hock and stifle joints (d in Fig. 7B) are equal (10 cm, our unpublished data), and consequently by its contraction the muscle produces an equal, opposite moment about the stifle −Msupflex(stifle):
Fig. 7.
Line diagram of the equine hind limb; cranial is to the left. The moment arm of the ground reaction force (GRF) about the stifle is indicated as a, about the hock as b; the moment arms of the quadriceps (Fqua), and the superficial flexor (Fsupflex) are indicated as c and d, respectively. (A) The GRF applies a flexing moment about the stifle (a*GRF), counteracted by the quadriceps muscle, applying an extensing moment c*Fquadriceps. (B) The GRF also applies a flexing moment about the hock (b*GRF) which is resisted by the force of the superficial flexor (Fsupflex). This muscle applies an extensing moment d*Fsupflex about the hock and an equal, additional flexing moment about the stifle.
| (1) |
Hence, without a lock mechanism, the quadriceps muscle must generate a moment, equal and opposite to the two flexing moments:
| (2) |
During quiet standing the GRF has a moment arm of 4 cm about the stifle and 9 cm about the hock; the moment arm of the quadriceps muscle is about 7 cm (our unpublished data). The last equation can hence be written as:
| (3) |
Consequently, without a lock mechanism Fquadriceps= 1.9*GRF. With a lock mechanism, according to our results a force of the vastus of 3.5% of the applied compressive force must be generated (0.035*GRF). This is less than 2% of the force in the absence of a lock mechanism (1.9*GRF), reducing force and energy expenditure by 98%.
Equation (3) shows that the flexing moment about the stifle is for a large part (9/13) produced via the hock by means of the reciprocal mechanism. This explains why the stifle always bends in an anaesthetized horse, whatever the direction of the limb, and, for that matter, of the upward force on the hoof. Paradoxically, the patellar lock and vastus medialis muscle thus serve primarily to stabilize the hock joint.
As normal fixation of the patella is only possible by tonic activity of the vastus medialis muscle, the muscle control mechanism is liable to disturbance. Although this has hitherto not been investigated, the possibility of spastic activity of vastus medialis should be taken into account as a possible cause of upward patellar fixation.
Acknowledgments
We are very grateful to Ton van den Bogert who read the manuscript and Rik van der Tol for his suggestions in the course of the study and help with data processing. Borden Mushonga performed some of the pilot EMG experiments. Henk van Dijk and Richard Lenters helped with the experiments and Jos Lammertink supported the electronic and computer equipment. Figures 1 and 2 were drawn by Henk Halsema.
References
- Alexander R, McNeill . Body support, scaling and allometry. In: Hildebrand M, Bramble DM, Liem KF, Wake DW, editors. Functional Vertebrate Morphology. Cambridge, MA: Harvard University Press; 1985. pp. 26–37. 10.1046/j.1469-7580.2003.00166.x. [Google Scholar]
- Biewener AA. Scaling body support in mammals: limb posture and muscle mechanics. Science. 1989;245:45–48. doi: 10.1126/science.2740914. 10.1046/j.1469-7580.2003.00166.x. [DOI] [PubMed] [Google Scholar]
- Boyd LE, Carbonaro DA, Houpt KA. The 24-hour time budget of Przewalski horses. Appl. Anim. Behav. Sci. 1988;21:5–17. 10.1046/j.1469-7580.2003.00166.x. [Google Scholar]
- Dallaire A. Sleep as behavior. Equine Prac. 1986;2:591–607. doi: 10.1016/s0749-0739(17)30708-3. 10.1046/j.1469-7580.2003.00166.x. [DOI] [PubMed] [Google Scholar]
- De Luca CJ. The use of surface electromyography in biomechanics. J. Appl. Biomechanics. 1997;13:135–163. 10.1046/j.1469-7580.2003.00166.x. [Google Scholar]
- Dyce KM, Sack WO, Wensing CJG. Textbook of Veterinary Anatomy. 2nd edn. Philadelphia: Saunders; 1996. 10.1046/j.1469-7580.2003.00166.x. [Google Scholar]
- Maughan DL, Watson JS, Weir J. Strength and cross-sectional area of human skeletal muscle. J. Physiol. 1983;338:37–49. doi: 10.1113/jphysiol.1983.sp014658. 10.1046/j.1469-7580.2003.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrenhof JW, Abbink HJ. Cross-correlation and cross-talk in surface electromyography. J. Electromyogr. Clin. Neurophysiol. 1985;25:73–79. 10.1046/j.1469-7580.2003.00166.x. [PubMed] [Google Scholar]
- Nickel R, Schummer A, Seiferle E, Wilkens H, Wille K-H, Frewein J. The Anatomy of Domestic Animals. Vol. 1. Berlin: Verlag Parey; 1986. 10.1046/j.1469-7580.2003.00166.x. [Google Scholar]
- Riemersma DJ, Lammertink JMLA. Calibration of mercury-in-silastic strain gauge in tendon load experiments. J. Biomechanics. 1988;21:469–476. doi: 10.1016/0021-9290(88)90239-4. 10.1046/j.1469-7580.2003.00166.x. [DOI] [PubMed] [Google Scholar]
- Sack WO. The stay apparatus of the horse's hind limb-explained. Equine Prac. 1989;11:31–35. 10.1046/j.1469-7580.2003.00166.x. [Google Scholar]
- Schmidt-Nielsen K. Scaling. Why Is Animal Size So Important? Cambridge, MA: Cambridge University Press; 1984. 10.1046/j.1469-7580.2003.00166.x. [Google Scholar]
- Shuttleworth ACJ. The function of the femoropatellar joint of the horse. Royal Army Vet. Corps. 1943;15:2–7. 10.1046/j.1469-7580.2003.00166.x. [Google Scholar]