Abstract
The developmental anatomy of the ventricular outlets and intrapericardial arterial trunks is a source of considerable confusion. First, major problems exist because of the multiple names and definitions used to describe this region of the heart as it develops. Second, there is no agreement on the boundaries of the described components, nor on the number of ridges or cushions to be found dividing the outflow tract, and the pattern of their fusion. Evidence is also lacking concerning the role of the fused cushions relative to that of the so-called aortopulmonary septum in separating the intrapericardial components of the great arterial trunks. In this review, we discuss the existing problems, as we see them, in the context of developmental and postnatal morphology. We concentrate, in particular, on the changes in the nature of the wall of the outflow tract, which is initially myocardial throughout its length. Key features that, thus far, do not seem to have received appropriate attention are the origin, and mode of separation, of the intrapericardial portions of the arterial trunks, and the formation of the walls of the aortic and pulmonary valvar sinuses. Also as yet undetermined is the formation of the free-standing muscular subpulmonary infundibulum, the mechanism of its separation from the aortic valvar sinuses, and its differentiation, if any, from the muscular ventricular outlet septum.
Keywords: embryo, heart, human, outflow tract, septation
Introduction
Anomalies involving the outflow channels and their valves make up a significant proportion of congenital cardiac defects, with a prevalence of at least 4 per 10 000 births (Ferencz & Neill, 1986; Edmonds & James, 1993). Recent advances in diagnosis and treatment mean that the majority of these lesions are now amenable to successful surgical correction. It goes without saying that a sound knowledge of normal development is essential for the understanding of their morphogenesis. The mechanisms involved in formation and septation of the normal outflow tracts and arterial trunks, however, continue to be controversial. There are several reasons for the lack of consensus. Some disagreements merely reflect differences in the techniques used in the various studies. Others stem from the intrinsic problems inherent in interpreting the four-dimensional events that occur during development. Still others reflect the unequivocal morphological differences that exist between humans and some of the species used in experimental studies. Underscoring all these problems is the plethora of terms used for description of the processes of development, often with the same term being used in different fashion by different investigators. There is also lack of consensus concerning the most appropriate terms for description of the definitive ventricular outflow tracts, particularly with regard to the definition and location of the arterial valvar ‘annulus’. So as to set the scene for our developmental discussions we therefore commence our review with an account of our understanding of the structure of the intrapericardial components of the ventricular outflow tracts in the postnatal heart.
The formed heart
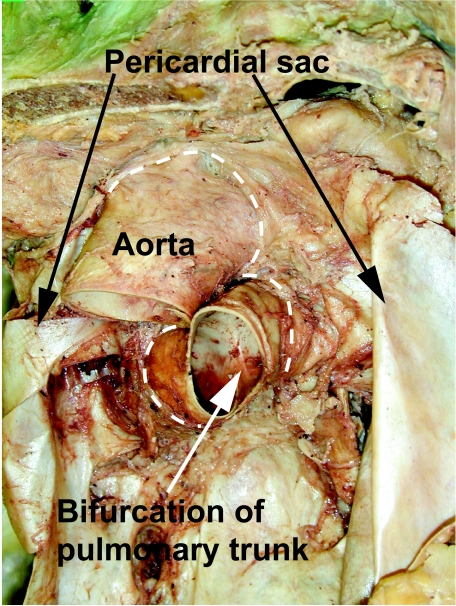
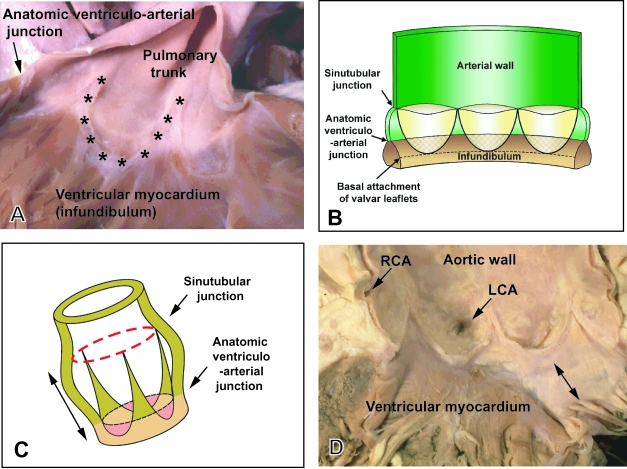
The outflow tracts in the definitive heart extend from the outflow regions of the left and right ventricles to the margins of the pericardial cavity, where they continue into the mediastinum as the ascending aorta and the right and left pulmonary arteries, respectively (Fig. 1). A key feature within each of these outflow tracts is the attachment of the leaflets of the arterial valves. These are hinged in semilunar rather than circular fashion (Fig. 2), with the semilunar attachments extending through a significant length of the two tracts (Anderson, 1990). Distally, the valvar leaflets are attached at the sinutubular junction (Merrick et al. 2000). This is a true circular boundary that marks the junctions between the arterial trunks and the more bulging parts of the arterial roots that form the walls of the valvar sinuses. Distal to the sinutubular junction, the intrapericardial components of the arterial trunks extend to the margins of the pericardial cavity. The ascending aorta is a solitary trunk within the pericardial cavity, whereas the bifurcation of the pulmonary trunk into the right and left pulmonary arteries is within the cavity. The bulging walls of the valvar sinuses, which are found proximal to the sinutubular junctions and have an arterial phenotype, also, in the case of the aortic root, give rise to the left and right coronary arteries. Proximally, the sinus walls join the ventricular myocardium at the anatomic ventriculo-arterial junctions. These junctions differ significantly in their morphology in the right and left ventricles. In the right ventricle, the anatomic junction between the infundibular musculature and the walls of the valvar sinuses is a complete circular locus (Fig. 2A). The base of each cup-shaped valvar leaflet, however, overlaps the anatomic junction, incorporating a crescent of ventricular myocardium (infundibulum) within the base of each arterial sinus (Fig. 2A,B). The distal attachments of each leaflet are at the sinutubular junction. Consequently, three triangles of arterial wall are incorporated within the right ventricular outflow tract, albeit that each triangle separates the inside of the right ventricle from extracardiac space. It is the semilunar attachment of the valvar leaflets that forms the haemodynamic junction, which straddles the anatomic ventriculo-arterial junction. When seen in the intact outflow tract therefore the circular sinutubular and anatomic ventriculoarterial junctions are distinct from the crown-like haemodynamic junction (Fig. 2C).
Fig. 1.
Heart removed from a human cadaver to show the extent of the aorta and the pulmonary trunk within the pericardial cavity. The dashed lines show the distal attachments of the fibrous pericardium.
Fig. 2.
(A) Pulmonary valve from an infant human heart. The valvar leaflets themselves have been removed to show the semilunar mode of their attachments (asterisks). Note the level of the anatomic ventriculoarterial junction between the arterial walls of the pulmonary trunk and the muscular right ventricular infundibulum. (B) Schematic drawing showing the mode of attachment of the valvar leaflets as shown in A, and their relationship to the sinutubular and ventriculoarterial junctions. Note that the bases of the valve leaflets overlap the ventricular myocardium. (C) Relationships of the valvar junctions and the valvar leaflets in three dimensions. Importantly, it demonstrates that the arterial valves have length (double headed arrows). (D) Opened aortic valve from an adult human heart subsequent to removal of the leaflets. As with the pulmonary valve, the leaflets are attached in semilunar fashion, but there is fibrous continuity between the non-coronary and left coronary leaflets of the aortic valve and the aortic leaflet of the mitral valve (double headed arrow). LCA, left coronary artery; RCA, right coronary artery.
Thus, although it possesses the same basic structure of circular sinutubular and anatomic junctions, with a crown-like haemodynamic junction, there are significant differences in the structure of the aortic as compared with the pulmonary arterial root. Because the leaflets of the pulmonary valve are supported by a complete sleeve of free-standing infundibular musculature, they are lifted away from the ventricular base (Merrick et al. 2000). In contrast, two of the leaflets of the aortic valve are in continuity posteriorly with one of the leaflets of the mitral (left atrioventricular) valve (Fig. 2D). This area of fibrous continuity then forms the roof of the left ventricle. Appreciation of the subtleties of the structure of the normal outflow tracts is crucial since, to the best of our knowledge, no account has yet been given of the processes involved in formation of the valvar leaflets, their sinusal attachments and the formation of the interleaflet triangles, nor the mechanisms of separation of the intrapericardial parts of the arterial trunks and the arterial roots. We do not claim to have solved all these problems ourselves, but their resolution will be the key to unlocking the remaining mysteries of the development of the outflow tract.
Nomenclature for the developing outflow tracts
A confusing plethora of terms has been applied to the different regions of the initially common outflow tract (Table 1, see also Arráez-Aybar et al. 2003). Numerous terms have also been employed to account for the endocardial ridges, or cushions, which divide it. Burggren (1988) highlighted the inconsistent use of the terms ‘bulbus’ and ‘conus’ to describe parts of the outlet from the heart in gill-bearing vertebrates. This problem has also existed over the years with regard to the description of mammalian cardiac development, and is further extended by inconsistent use of not only ‘conus’, but also ‘truncus’. Still further problems relate to the definition of the boundaries between the different areas of the developing outflow tract, particularly as these alter their position during the developmental period. All of these difficulties are then compounded by the same terms being used in different fashion between investigators. This is exemplified by the way different investigators have divided the outflow tract into ‘truncus’ and ‘conus’, and the way in which they have described the location of the developing valves within these components (see Laane, 1978; Pexieder, 1995). Even the popular convention of designating the area distal to the forming arterial valves as the ‘truncus’, and the area proximal to them as the ‘conus’, is fraught with difficulty, since the developing valves themselves, as they form, occupy a significant length of the developing embryonic outflow tracts. Thus, if the valvar leaflets are considered to represent the border, is the segment of outflow tract occupied by the semilunar attachments, between the sinutubular junction and the base of the leaflets (Fig. 2B), to be considered as derived from ‘truncus’ or ‘conus’?
Table 1. Comparison of the terminology used by different authors for the various regions of the outflow tract.
| Kramer (1942) | Van Mierop et al. (1963); Goor et al. (1972) | Tandler (1912); Pexider (1978) | Anderson et al. (1974a) | Shaner (1962) | Laane (1978) (early stage) | Laane (1979) (late stage) | Qayyum et al. (2001) |
|---|---|---|---|---|---|---|---|
| aortic sac | aortic sac | aortic sac | aortic sac | ventral aorta | aortic sac | truncus mesenchymalis | aortic sac |
| truncus arteriosus conus cordis | truncus conus | distal bulbus proximal bulbus | truncus distal bulbus | bulbus | distal segment middle segment Proximal segment | truncus arteriosus (myocardial segment) | distal segment proximal segment |
In our opinion, if we are to clarify the development of this important region of the heart, there is a need for a nomenclature which is explicit and unambiguous, and which is compatible both with the observed anatomy of the formed heart and with the dynamic changes seen during cardiac development. Because of the problems discussed above, we believe this mandates the use of descriptive rather than nominative terminology (Fig. 3).
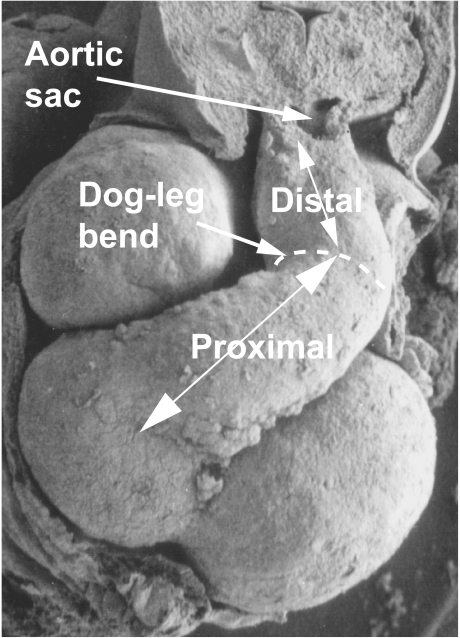
Fig. 3.
This diagram of the developing embryonic heart illustrates our suggested terminology. Note that we define proximal and distal segments of the outflow tract. In the definitive situation, as shown in Fig. 4, the boundary between these components is marked by the characteristic bend ‘dog-leg’ bend. The bend has been ignored for the purposes of this drawing. The boundary between the distal outflow segment and the arterial segment, the aortic sac, is at the level marked by the reflections of the pericardial cavity (see also Fig. 1).
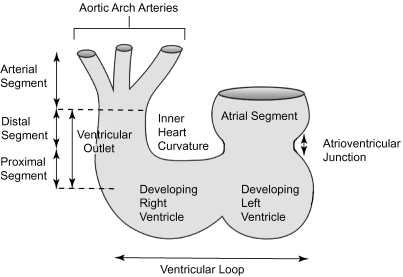
Thus we consider the developing outflow tract as commencing at the distal extent of the ventricular loop. The ventricular part of the primary heart tube itself has a desending inlet component and an ascending outlet component, from which will grow the apical components of the definitive right and left ventricles, respectively (Houweling et al. 2002). It is the component of the primary heart tube between the ventricular loop and the aortic sac that we consider to represent the outflow tract.
Initially, the common outflow tract is supported exclusively by the developing right ventricular component of the ventricular loop, and is continuous with it. It extends distally to the margins of the pericardial cavity, where it becomes continuous with the aortic sac. Eventually, it will be divided to form the outlets of both definitive ventricles, and their respective valves, along with the intrapericardial portions of the arterial trunks. When first seen, the entire wall of the undivided outflow tract, extending to the margins of the newly formed pericardial cavity, is composed of myocardium. At these early stages, the presence of a characteristic acute bend, originally termed the ‘bayonet bend’ by Orts Llorca et al. (1982), divides the outflow tract into proximal and distal parts (Fig. 4). We now describe this characteristic landmark as the ‘dog-leg’ bend. This definition of proximal and distal parts of the outflow tract follows the precedent established by Ya et al. (1998), and has recently been adopted by Yelbuz et al. (2002).
Fig. 4.
This scanning electron micrograph of a human embryo, at Carnegie stage 15 (approximately 34 days of gestation), shows a ventral view of the heart. The distal and proximal segments of the unseptated outflow tract are separated by a characteristic dog-leg bend. The proximal segment lies across the atrioventricular junction.
Early development of the primary heart tube
The heart is one of the first organs to form in amniotes. The bilateral and symmetrical cardiac primordia migrate to the midline, where they form the primary heart tube. This initially tubular heart consists of an inner endothelial layer, the endocardium, and an outer muscular layer, the myocardium. Contrary to conventional wisdom, not all regions of the developing heart are present at the initial stages of development. Lineage studies from our laboratory (N. Brown, personal communication) have shown that, in the mouse, the initial straight part of the tube becomes the left ventricular component of the definitive heart, with the other segments being recruited at a later stage. De La Cruz et al. (1991), summarizing their earlier work (De La Cruz et al. 1977), showed that, in the chick, the comparable straight part of the tube formed the apical trabecular component of the right ventricle. More recent studies have shown that the entirety of the developing outflow tract, along with the developing right ventricle, receives a contribution from a secondary, or anterior, heart field (Kelly et al. 2001; Mjaatvedt et al. 2001; Kirby, 2002). This origin from a secondary source accounts for the lengthening of the outflow tract myocardium up to Hamburger Hamilton stage 21 in the chick (Rychter, 1978; Mjaatvedt et al. 2001), to embryonic day 9.5 in the mouse (Kelly et al. 2001), and to embryonic day 11 in the rat (Ya et al. 1998). This corresponds to Carnegie stage 13 in the human. Importantly, this contribution to the outflow tract from the secondary heart field is complete by the time the outflow cushions are invaded by cells derived from the neural crest (Le Douarin, 1982; Kirby et al. 1983; Fukiishi & Morriss-Kay, 1992; Jiang et al. 2000).
The structure of the distal outflow tract
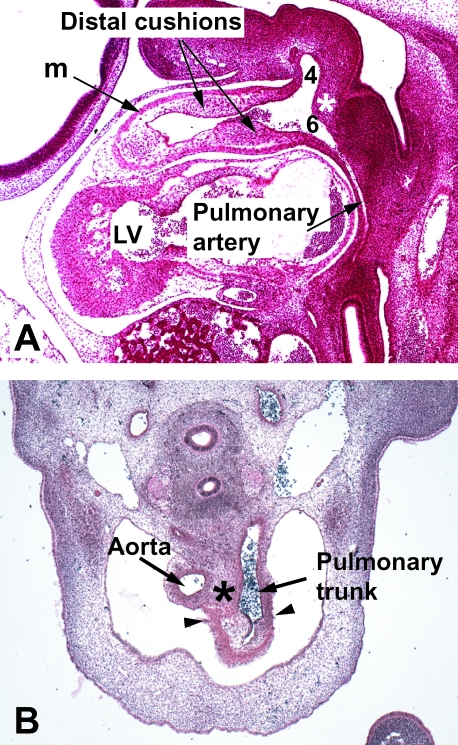
In the human, at Carnegie stage 14, the myocardial wall of the outflow tract extends to the border of the pericardial cavity (Fig. 5A), where it joins the aortic sac, the latter giving rise to the arteries that feed the pharyngeal arches. Eventually, this common outflow tract will give rise not only to the valves and sinuses of both definitive ventricular outflow tracts, but also to the intrapericardial portions of the arterial trunks (shown at Carnegie stage 16 in Fig. 5B). Our observations in the human, to be illustrated below, confirm those made by Ya et al. (1998) in the rat, showing that the definitive sinutubular junctions are formed at the level of the developing outflow tract marked initially by the dog-leg bend.
Fig. 5.
(A) Section through the outflow tract of a human embryo at Carnegie stage 14 (approximately 36 days of gestation) sectioned in the sagittal plane. It shows how the distal outflow tract with its myocardial wall (m) extends to the edge of the pericardial cavity, where it becomes continuous with the aortic sac. The aorto-pulmonary septum is seen as a wedge of tissue in the posterior wall of the sac (asterisk), separating the origins of the fourth and sixth aortic arches (4,6), the latter seen giving rise to one pulmonary artery. The outflow tract is being septated by the distal cushions. (B) Section from a human embryo at Carnegie stage 16 (approximately 37 days of gestation), which has been sectioned in the transverse plane. The distal cushions (asterisk) are shown separating into the intrapericardial parts of the aorta and the pulmonary trunk. Note the incipient arterialization of the walls of these trunks. The proximal outflow tract has retained its myocardial phenotype, proximal to the arrowheads.
Most previous studies have suggested that it is elongation and division of the aortic sac that produces the intrapericardial parts of the arterial trunks, these being distal to the dog-leg bend. The cushions initially found within the distal part of the muscular outflow tract are said to separate the developing aortic and pulmonary roots, with the proximal cushions separating the ventricular outflow tracts (Van Mierop, 1979). If correct, this process would necessitate the proximal displacement of the cushions from a position initially distal to the dog-leg bend. Our own observations do not support these interpretations. Instead, we believe that it is the distal cushions that divide the distal outflow tract into the intrapericardial parts of the aorta and pulmonary trunk, with the proximal cushions separating both the arterial roots and their ventricular outflow tracts. In the light of these differences in interpretation, it is pertinent at this stage to provide a brief review of the various previous concepts.
Previous concepts of septation of the outflow tract
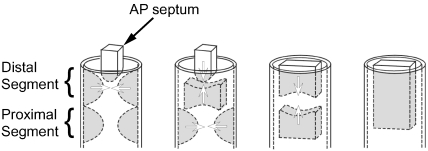
Van Mierop et al. (1963) and Van Mierop (1979) suggested that three components were needed to divide the outflow tract, namely the aortopulmonary septum, along with separate sets of distal and proximal ridges. According to this concept (Fig. 6), the ridges initially fuse distally to form a septum, which then fuses with the aortopulmonary septum, the latter growing down from between the fourth and sixth pharyngeal arches. Septation then proceeds with fusion of the proximal ridges to form a proximal conal septum, which then combines with the proximal end of the distal septum to complete the process of septation.
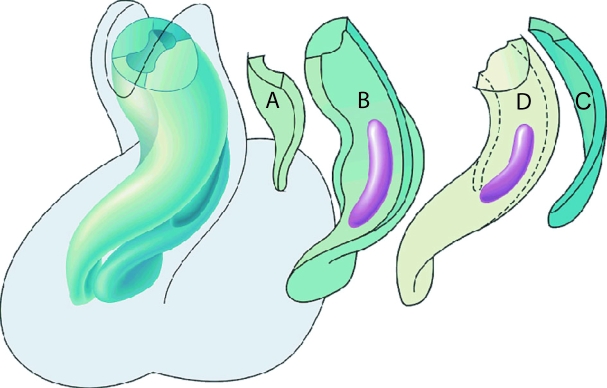
Fig. 6.
Model of development whereby three components, namely the distal cushions, the proximal cushions and the aortopulmonary septum (AP septum), contribute to the septation of the outflow tract. Only those components that give rise to the septal structures are shown, the valvar leaflets having been excluded for simplicity. The panels show the presumed steps in septation.
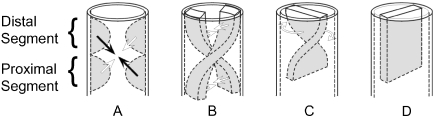
Icardo (1990), in contrast, promoted the concept of formation of a spiral septum. Like Van Mierop et al. (1963), he claimed to recognize separate proximal and distal sets of ridges, but he argued that spiralling was achieved by end-to-end fusion of each of the paired proximal ridges with an opposite partner of the paired distal ridges (Fig. 7A). This, he suggested, produced conjoined ridges, which then fused lengthwise, crossing at their midpoints.
Fig. 7.
Models for spiral septation of the outflow tract. The arrows in panel A indicate the fusion of opposite distal and proximal cushions to form spiralling longitudinal ridges, as shown in panel B. Others, however, argue that the spiralling ridges exist through the length of the outflow tract from the outset (see text for discussion). Be that as it may, having fused to form a spiralling septum, it is argued that ‘detorsion’ (panel C) is required to produce the definitive septum (panel D).
Several other groups of investigators have illustrated formation of a spiral septum but, in their view, the septum is formed by fusion of two continuous longitudinal ridges (Fig. 7B). These ridges are described as taking a spiralling course as they extend through the length of the outflow tract (Kramer, 1942; Anderson et al. 1974a; De La Cruz et al. 1977; Pexieder, 1978). Some of those who espoused this model also emphasized that it required subsequent ‘detorsion’ of the separated arterial pathways (Fig. 7C,D).
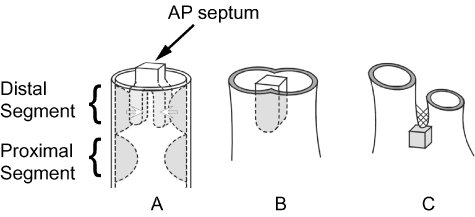
Bartelings & Gittenberger-de Groot (1989), having studied human hearts, then offered yet another hypothesis. This placed even greater emphasis on growth of a structure they call the aortopulmonary septum (Fig. 8). According to their concept, septation of the outflow tract is achieved with little or no contribution from the endocardial ridges.
Fig. 8.
Diagram illustrating the concept of separation of the outflow tracts primarily by the aortopulmonary septum. Panel A shows the distal cushions fusing with the aortopulmonary septum to form a common septal structure (panel B). By the stage shown in C, the proximal cushions remain only as the posterior wall of the subpulmonary infundibulum (grey cube), with the aortopulmonary septum having reduced to a remnant of tissue external to the heart (cross-hatched).
Over time therefore some have described separate ridges in the proximal and distal segments, while others have accounted for two elongated ridges that extend throughout the entire length of the outflow tract. But even those who have described separate sets of proximal and distal ridges have then disagreed on the number of ridges to be found distally, particularly with regard to the situation in the chick. Tonge (1869) described only two ridges proximally in the developing chick heart, but illustrated three ridges distally. Laane (1978), and Waldo et al. (1998), confirmed the existence of the three distal ridges, but Laane argued that two of the ridges fused cranially to form a common ridge, with the ridges remaining unfused caudally. Los (1978), in contrast, showed only two longitudinal ridges in his illustrations of the developing chick heart, extending throughout the length of the outflow tract. Using scanning electron microscopy, supported by reconstructions from serial sections, Qayyum et al. (2001) confirmed the initial opinion of Tonge (1869), demonstrating two ridges proximally, but three ridges distally. This avian arrangement parallels the situation in the developing reptilian heart, for which Shaner (1962) described a tripartite distal arrangement. To the best of our knowledge, this third distal ridge has never been seen in the developing mammalian heart. The three ridges seen distally in the chick are found in addition to the so-called intercalated cushions. The third distal ridge develops after the first two, but before the intercalated cushions. The intercalated cushions are seen level with the developing valves, and do not extend below them. Reinforcing the opinion first expressed by Tonge (1869), it is our belief that the intercalated cushions form one leaflet, with its supporting sinus, for each of the aortic and the pulmonary valves. Unlike most of the accounts summarized above, nonetheless, it is also our belief that the function of the cushions found in the distal outflow tract is to divide this part of the developing heart into the intrapericardial parts of the aorta and pulmonary trunk, the cushions themselves subsequently disappearing as the arterial trunks separate one from the other. At this stage therefore we will review briefly our own recent findings, supplementing them with illustrations of developing human hearts.
Formation of the intrapericardial arterial trunks
When first formed, the junction between the distal end of the outflow tract and the aortic sac is found at the distal extent of the pericardial cavity. Within the heart, this corresponds with the distal extent of the endocardial ridges or cushions (Fig. 5A). As already discussed, the walls of this distal part of the outflow tract, between the dog-leg bend and the aortic sac, possess a myocardial phenotype. It has still to be determined how this distal outflow tract, within the pericardial cavity, becomes converted to the intrapericardial components of the aortic and pulmonary trunks. Ya et al. (1998), having studied the rat heart, argued that this transformation was the consequence of transdifferentiation of the walls from a myocardial to an arterial phenotype. Arguello et al. (1978) had earlier proposed this concept, following their ultrastructural investigations of the developing outflow tract of the chick. It is possible, however, that cells from the pharyngeal mesenchyme migrate into the walls of the distal outflow tract, replacing the myocardial cells. The mechanism underscoring this crucial change therefore has still to be determined.
Clarification of the mechanism by which the intrapericardial parts of the arterial trunks are separated from one another, nonetheless, can help decide whether they are derived from the distal outflow tract or the aortic sac. According to our observations, septation of this area is achieved by fusion of the distal cushions (Fig. 5B). During this process, the cushions themselves seemingly disappear, with the aorta and pulmonary trunk developing their own walls (Fig. 9A,C). The tissue between the newly forming arterial trunks, which was initially continuous with the posterior pharyngeal mesenchyme (Fig. 5B), will eventually disappear as space is produced between the intrapericardial parts of the aorta and pulmonary trunk (Fig. 1).
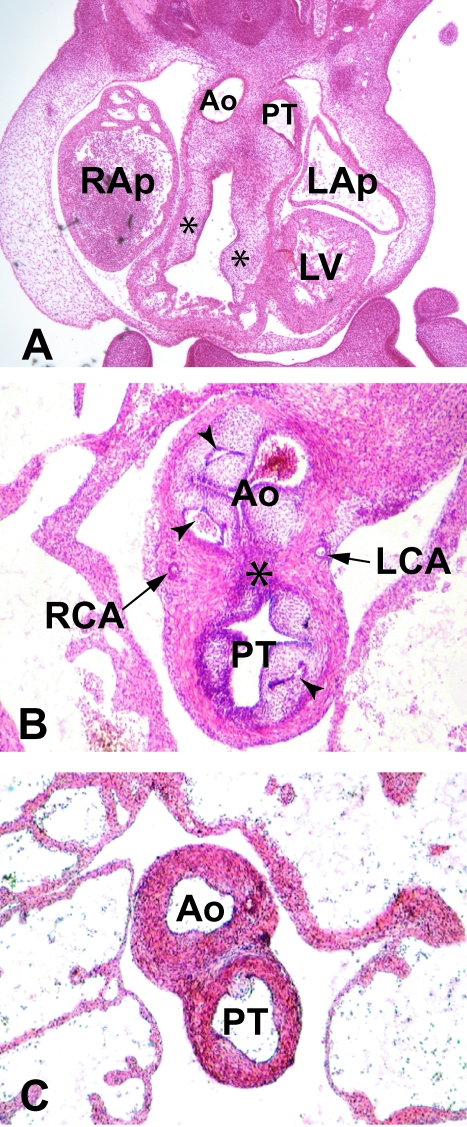
Fig. 9.
(A) Frontal section through a human embryo at Carnegie stage 14 (approximately 35 days of gestation). The dorsal outflow tract has been separated into the interpericardial parts of the aorta (Ao) and the pulmonary trunk (PT). The proximal cushions (asterisks) have yet to fuse, but the dense mesenchymal tissue that originates from the neural crest is penetrating both cushions. RAp, LAp: right and left atrial appendages; LV, left ventricle. (B) Transverse section through a human embryo at Carnegie stage 22 (approximately 54 days of gestation) shows the stage subsequent to the septation of the distal outflow tract. Now the distal parts of the proximal cushions, together with the intercalated cushions, are cavitating (arrowheads) to form the valvar leaflets of the aorta (Ao) and the pulmonary trunk (PT), along with the walls of their supporting sinuses. Note the different stages of arterialization of the different sinusal walls, and note also that the sinuses remain enclosed within the myocardial cuff, through which the left (LCA) and right (RCA) coronary arteries are penetrating to enter the valvar sinuses. The dark staining fibrous tissue (asterisk) marks the initial site of fusion of the distal parts of the proximal cushions. (C) A more cranial slide from the same embryo, which shows that, by this stage, the intrapericardial parts of the arterial trunks are separate structures, with a bar of fibro-adipose tissue now occupying the former site of the distal cushions.
The traditional concept for separation has been that the intrapericardial arterial trunks are separated by downgrowth of the so-called aortopulmonary septum, albeit that there have been various definitions for this septal complex. Our observations in the human heart show that the initial ‘aortopulmonary septum’ is no more than a relatively insignificant wedge of tissue interposed between the origins from the aortic sac of, on the one hand, the arteries supplying the third and fourth arches and, on the other hand, the arteries which feed the developing sixth arches (Fig. 5A). These observations endorse the concept of structure of the aortopulmonary septum as shown in the reconstruction of the aortic sac made by Steding & Seidl (1990). This structure at the site of bifurcation appears to be more extensive in the chick. We suggest that this reflects the topographical differences known to exist in this area of the chick as compared to mammals. As far as we are aware, none of those arguing for separation of the arterial trunks by this ‘aortopulmonary septum’ have considered how the purported ‘septal’ structure subsequently loses its septal role concomitant with the development of separate walls for the intrapericardial arterial trunks. This loss of an embryonic septal role is crucial for understanding not only the division of the distal outflow tract, but also its proximal components.
Separation of the proximal outflow tracts
Our most recent studies, conducted in mammals and birds, suggest that the basic mechanics of separation are comparable in both parts of the outflow tract, when cognisance is taken of the difference in morphology of the distal component. As explained above, most agree that the longitudinal cushions within the outflow tract can arbitrarily be divided into proximal and distal components at the dogleg bend. Initially, the opposing cushions fuse across the lumen of the outflow, with fusion starting distally and proceeding proximally. Also, as discussed above, it is our belief that the major function of the distal cushions is to separate the distal common outflow tract into the aortic and pulmonary components of the intrapericardial arterial trunks. And, as explained, it has still to be established how the walls of the intrapericardial trunks achieve their arterial phenotype, a process that occurs with remarkable rapidity. The distal cushions, nonetheless, having performed their septal function at the early stages of development, subsequently disappear by a mechanism again as yet unknown. Irrespective of the mechanisms, the aortic and pulmonary trunks are subsequently seen as separate structures, each with its own discrete walls, within the pericardial cavity (Figs 1 and 9C).
The sequence of separation seen distally (Fig. 9A) is then replicated within the proximal outflow tract. This proximal part, separated from the distal outflow tract by the dog-leg bend, itself has distal and proximal components. The distal part of this proximal outflow tract, immediately upstream of the dog-leg bend, is divided by fusion of the distal ends of the proximal cushions. These cushions, along with the intercalated cushions that have by now appeared within the outflow tract (Fig. 10), form the leaflets and supporting sinusal walls of the aortic and pulmonary valves. One of the intercalated cushions forms a leaflet and sinus of the aortic valve, while the other intercalated cushion forms the comparable components of the pulmonary valve. The other two leaflets and sinuses of each arterial valve are derived from the cushions that fused to septate this distal part of the proximal outflow tract. Each fused cushion, on its opposite face, gives rise to two valvar leaflets, one for the aortic and the other for the pulmonary valve (Fig. 9B). By a process as yet unknown, the cushions then undergo a remodelling, or cavitation, to form the definitive cup-shaped valvar leaflets along with their sinusal walls. The appearance of the cavities separates the cushions themselves into luminal and mural components. The luminal parts become the valvar leaflets, while the mural parts arterialize to form the walls of the supporting valvar sinuses. As part of this process, the distal part of the proximal cushions, like the distal cushions, must also lose their septal function, thereby separating the arterial roots.
Fig. 10.
Reconstruction made from a human heart of 5 weeks gestation (Carnegie stage 15), viewed from the ventral aspect, soon after immigration of neural crest cells has begun. The left-hand panel shows the overall arrangement, with the arrangement of the individual cushions shown to the right. (A,C) Intercalated ridges or cushions, which occupy the area of the dog-leg bend; (B) septal outflow ridge; (D) parietal outflow ridge. Localized accumulations of neural crest-derived mesenchyme form ‘prongs’, shown in purple. Note that they are located within the distal parts of the cushions of the proximal outflow tract, being positioned just proximal to the dog-leg bend.
At the start of this process of fusion, the developing arterial roots are completely encased within a myocardial cuff (Fig. 9B). Gradually, as demonstrated by Ya et al. (1998) in the rat, and endorsed by Rothenberg et al. (2002) in the chick, this cuff disappears. Concomitant with its disappearance, a plane of fibro-adipose tissue is formed at the centre of the cushions, eventually becoming continuous with the extracardiac space, and then separating the aortic from the pulmonary root (Fig. 9C).
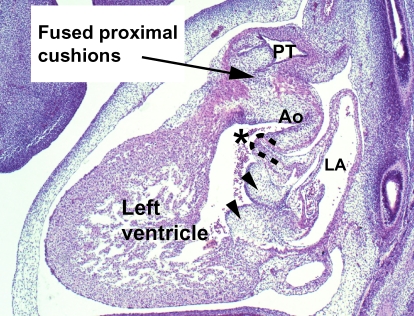
The most proximal parts of the cushions within the proximal component of the outflow tract then also fuse to form a structure that, at first, is a septum within the ventricular outflow tract (Fig. 11A). When this structure is first formed, the developing right ventricle supports the entirety of the outflow tract. This newly formed embryonic outlet septum therefore is exclusively a right ventricular structure. Its free edge overrides the cavity of the right ventricle. The endocardial cushion tissue forming this proximal embryonic outlet septum subsequently becomes muscular. This process, known as ‘myocardialization’, is the consequence of invasion of the cushions by cardiac myocytes pre-existing within the parietal walls of the outflow tract (Okamoto, 1980; McBride et al. 1981; Okamoto et al. 1981; Lamers et al. 1995; Van Den Hoff et al. 1999). As the partition, now muscularized, fuses with mesenchyme crowning the crest of the muscular ventricular septum, it walls the aorta into the left ventricle. At the same time, the muscular partition itself becomes the supraventricular crest of the right ventricle, which then separates the cavity of the right ventricle from the aortic valvar sinuses (Fig. 11B). In postnatal life therefore none of the structures that initially divided the embryonic outflow tract into aortic and pulmonary components continues to occupy a septal position.
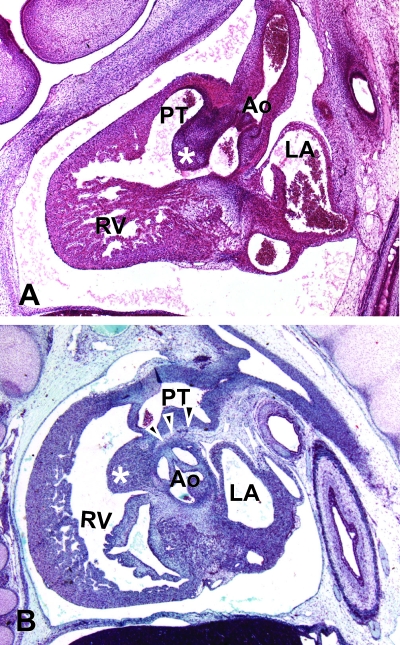
Fig. 11.
(A) Sagittal section from a human embryo at Carnegie stage 20 (approximately 50 days of gestation). The proximal cushions of the outflow tract have fused to form an embryonic outlet septum within the right ventricle (asterisk). The interventricular foramen, which links the right ventricle to the subaortic outflow, is seen as a channel positioned caudal to this outlet septum. The distal outflow segment has separated into the aortic and pulmonary trunks, each having an arterial phenotype. (B) Sagittal section from a human embryo of 11 weeks gestation. An extracardiac bar of tissue is now seen between the walls of the pulmonary trunk and the sinuses of the aorta (arrowheads). The proximal cushions have now myocardialized to form the subpulmonary infundibulum (asterisk). Abbreviations: Ao, aorta; LA, left atrium; PT, pulmonary trunk; RV, right ventricle.
Rotation of the outflow tracts
In the definitive heart, the pulmonary trunk unequivocally spirals round the aorta, from the off-setting of the arterial valves proximally to the separate arterial trunks distally (Merrick et al. 2000). In terms of development, several groups of investigators (Kramer, 1942; Patten, 1953; Van Mierop et al. 1963; de La Cruz et al. 1977; Pexieder, 1978) have previously argued that the rotation of the outflow tract, and the concomitant spiralling of the ridges, are the consequence of events occurring as part of the process of looping. Other groups (Anderson et al. 1974a; Goor et al. 1972; Los, 1978; Laane, 1979) agreed concerning rotation, and also agreed that the event would generate primary torsion. These investigators, however, argued that such primary torsion would need to be followed subsequently by anticlockwise rotation distally, thus causing ‘unwinding’ or ‘detorsion’ of the spiral pattern (Fig. 7). They stated that the ‘detorsion’ was then transferred to the arterial trunks, thus establishing the adult spiral configuration. Still others, notably De La Cruz & Da Rocha (1956) and Steding & Seidl (1980, 1981), argued that they were unable to find evidence of rotation of the ventricular outlets during normal development.
Our current findings endorse the lack of active rotation subsequent to the initial formation of the outflow tract. Indeed, we are now able to explain why there is no need to propose ‘detorsion’ as part of the mechanisms of septation. When first formed, the endocardial ridges themselves have an unequivocally spiral path within the outflow tract (Fig. 10). As the ridges fuse distally, there is concomitant arterialization of the separated aortic and pulmonary pathways (Fig. 9A). Thus, the structure that initially was a common distal outflow tract, divided by a spiralling septum, is replaced by separate arterial trunks that spiral round each other as they leave the cardiac base (Fig. 1). The retraction of the myocardial wall of the outflow tract, as it assumes a distal arterial phenotype, therefore serves simply to reveal the now separate, but still spiralling, aortic and pulmonary trunks.
Reduction of the inner heart curvature
It has often been stated that, during normal development, there is a marked shift in the position of the subaortic outlet. Initially, this proximal and posterior (dorsal) part of the outflow segment, which eventually becomes incorporated into the left ventricle, is supported exclusively by the developing embryonic right ventricle, itself formed from the distal part of the ventricular loop. Goor et al. (1972) argued that absorption of the proximal segment into the left ventricle was secondary to a process of migration, which carried the aorta over the left ventricle. Anderson et al. (1974a, b), in contrast, suggested that the process of absorption was primary, and that transfer of the aorta to the definitive left ventricle occurred concomitant with the reduction of the tissue that formed the inner heart curvature. The muscular curve itself has variously been termed the ‘conoventricular flange’ (Kramer, 1942), or the ‘bulboatrioventricular flange’ (Anderson et al. 1974a, b).
The flange forms the roof of the most direct route, in the embryonic heart, from the ventricles to the subaortic outlet, which unequivocally becomes an integral part of the left ventricle (Fig. 12). The finding of otherwise normal hearts, but with muscular tissue separating the leaflets of the aortic and mitral valves (Rosenquist et al. 1976), shows that complete reduction of the inner curve is not a prerequisite for complete transfer of the aorta to the left ventricle. The inner curvature, nonetheless, does undergo structural change during development, but this change takes place after the completion of cardiac septation, and without disturbing the topographic arrangement. Thus, examination of human embryos shows that at the time of fusion of the endocardial cushion tissue surrounding the embryonic interventricular foramen, a process which walls the aorta into the left ventricle and incorporates part of the primary ventricular foramen as the left ventricular outflow tract, a considerable portion of the myoblastic tissue of the inner curve remains interposed between the developing leaflets of the aortic and mitral valves (Fig. 12). Only much later in development does this muscular tissue become converted into the area of fibrous continuity seen between these leaflets as a characteristic feature of the normal left ventricle (Fig. 2D). The mechanism of disappearance of this musculature of the inner heart curvature has still to be established.
Fig. 12.
Section from a human embryo at Carnegie stage 17 (approximately 44 days of gestation), sectioned in the sagittal plane. It shows that, although the aortic valve is being sequestered within the left ventricle by fusion of the proximal parts of the outflow cushions to the crest of the muscular ventricular septum, the musculature of the inner heart curvature (dashed line) still separates the developing leaflets of the aortic and mitral valves. The subaortic outlet is marked by an asterisk. The arrowheads indicate the atrioventricular endocardial cushions. AO, aorta; PT, pulmonary trunk; LA, left atrium.
Formation of the subpulmonary infundibulum
Bartelings & Gittenberger-de Groot (1989) argued that invasion of the proximal outflow cushions by the limbs of the structure they call the aortopulmonary septum provided the stimulus for mobilization of myocardium to form the posterior wall of the free standing subpulmonary infundibulum. We, too, have been able to trace the ‘prongs’ of neural crest-derived condensed mesenchyme into the proximal cushions (Fig. 10). Their position marks the eventual site of septation of the proximal outlet into the aortic and pulmonary roots. We have not, however, been able to trace the rods from the true aortopulmonary septum, namely the wedge of tissue situated between the origins of the fourth and sixth arch arteries from the aortic sac. Indeed, we do not place great emphasis on this structure contributing to division of the outflow tract. In our opinion, it is more important to note that, when the most proximal parts of the cushions have fused to septate the proximal outflow tract, they subsequently lose their septal function, concomitant with the process of muscularization and formation of the free-standing subpulmonary muscular sleeve. Part of this process is the formation of space between the posterior wall of the subpulmonary infundibulum and the anterior sinuses of the aortic root (Fig. 13).
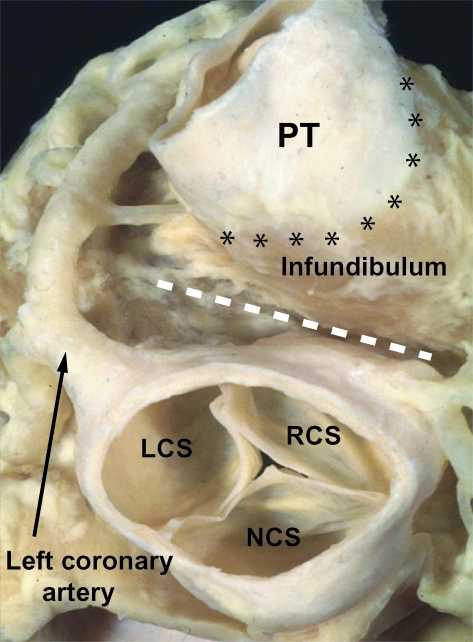
Fig. 13.
This dissection of an adult human heart shows the relationship of the definitive ventricular outflow tracts. The arterial trunks have been removed, and the base of the heart is viewed from above. The anatomic pulmonary–ventricular junction, between the arterial wall of the pulmonary trunk (PT) and the muscular right ventricular infundibulum, is shown by the line of asterisks. Note the deep tissue plane (white dashed line) that separates the free-standing subpulmonary infundibulum of the right ventricle from the wall of the right coronary sinus of the aorta. Note also the left coronary artery. LCS, RCS, NCS – left, right, and non-coronary sinuses of the aorta, respectively.
The space thus formed is continuous with the space that develops between the intrapericardial parts of the great arterial trunks (Fig. 9C). Completion of the process of separation depends on disappearance of the myocardial cuff that initially surrounded the entirety of the distal part of the proximal outflow tract (Fig. 9B). These changes explain fully the postnatal morphology of the outlet from the right ventricle, which is muscular over its entire circumference, forming a cylindrical sleeve that can be removed without encroaching the cavity of the left ventricle (Stamm et al. 1998; Merrick et al. 2000). Apoptosis is thought to be involved both in retraction of the myocardial sleeve of the proximal outflow tract (McBride et al. 1981) and in triggering the subsequent myocardialization of the proximal septum (Poelmann et al. 1998). It has also been suggested that the latter process may involve signalling by growth factors (Sanford et al. 1997). The precise mechanism of formation of the free-standing infundibulum, and its separation from the aortic root, however, currently remains unexplained.
Cardiac neural crest
Many investigators, such as Phillips et al. (1987) and Jiang et al. (2000), have now confirmed the initial studies of Kirby et al. (1983, 1985), namely that cells migrating into the heart from the cardiac neural crest are crucially important in septation of the outflow tracts. Having entered the outflow tract, the cells form two conspicuous structures, often interpreted as ‘limbs’ of the aortopulmonary septum (Laane, 1978; Los, 1978; Thompson & Fitzharris, 1979; Thompson et al. 1983, 1984; Bartelings, 1990). To the best of our knowledge, it has never been shown that the limbs are in continuity with the tissues separating the origins of the arteries supplying the fourth and sixth pharyngeal arches from the aortic sac, this tissue, as far as we can see, representing the initial aortopulmonary septum. We have confirmed that the cells from the neural crest enter the distal ridges directly, but we have found that, when the ridges fuse, a characteristic whorl of condensed mesenchyme is found at the junction between the distal and proximal parts of the outflow tract (Fig. 9A). Extensions from this whorl, the rods or prongs, run proximally within the fusing ridges (Fig. 10). These rods play a major role in dividing the distal part of the proximal outflow tract into separate aortic and pulmonary valves and their respective ventricular outflow tracts. This is confirmed by experimental ablation of the cardiac neural crest, which is known to lead to a variety of malformations involving the arterial roots, often producing a common arterial trunk (Kirby, 1983; Kirby & Bockman, 1984; Kirby et al. 1985; Nishibatake et al. 1987). It is known that the cells from the crest reach proximally as far as the arterial valvar leaflets (Takamura et al. 1990). The contribution cannot be major, however, since ablation of the neural crest has little effect on the formation of the valvar leaflets. It remains to be established with certainty whether the cells from the neural crest migrate further proximally into the heart itself (Takamura et al. 1990; Noden et al. 1995; Creazzo et al. 1998; Poelmann et al. 1998; Waldo et al. 1998). There is also uncertainty whether the cells derived from the crest persist as septal elements or are eliminated by apoptosis (Icardo, 1990; Poelmann et al. 1998; Jiang et al. 2000).
Conclusions
Many of the persisting problems concerning the development of the ventricular outflow tracts reflect the difficulties inherent in making correlations between the structure of the outflow tracts during their development and their definitive morphology. Knowledge of the fate of this developing area is pivotal to the understanding of the formation of the definitive ventriculo-arterial junctions. Crucially, these junctions shift markedly during development of the ventricular outlets. Initially, the anatomic ventriculo-arterial junction is the border between the distal end of the ventricular outflow segment and the aortic sac. This is positioned at the margins of the pericardial cavity. In the definitive heart, separate ventriculo-arterial junctions are found within the right and the left ventricles towards the bases of the arterial valves. The distal attachments of the leaflets of the arterial valves are at the sinutubular junctions. These circular landmarks are formed at the site of the dogleg bend, which initially separates the two parts of the muscular outflow tract. The valvar leaflets, along with their supporting arterial sinuses therefore are formed distally within the proximal outflow tract. The most proximal part of the outflow tract persists largely as the subpulmonary infundibulum, the aortic vestibule becoming fibrous posteriorly subsequent to disappearance of the musculature of the inner heart curve. The definitive anatomic ventriculo-arterial junctions are eventually located at markedly different levels within the right and left ventricles. It is the formation of the free-standing infundibular sleeve of the right ventricle, by muscularization of the proximal cushions, that largely accounts for the differences in these levels in the right as opposed to the left ventricles. The developmental mechanisms producing all these changes have still to be clarified.
References
- Anderson RH. The anatomy of arteial valvar stenosis. Int. J. Cardiol. 1990;26:355–359. 10.1046/j.1469-7580.2003.00168.x. [Google Scholar]
- Anderson RH, Wilkinson JL, Arnold R, Lubkiewicz K. Morphogenesis of Bulboventricular malformations I: Consideration of embryogenesis in the normal heart. Br. Heart J. 1974a;36:2–42. doi: 10.1136/hrt.36.3.242. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RH, Wilkinson JL, Arnold R, Becker AE, Lubkiewicz K. Morphogenesis of bulboventricular malformations II: observations on malformed hearts. Br. Heart J. 1974b;36:948–970. doi: 10.1136/hrt.36.10.948. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arguello C, De La Cruz MV, Sanchez C. Ultrastructural and experimental evidence of myocardial cell differentiation into connective tissue cells in embryonic chick heart. J. Mol. Cell Cardiol. 1978;10:307–315. doi: 10.1016/0022-2828(78)90380-2. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Arráez-Aybar La González-Lorrio F, Marantos-Gamarra Dg Jiménez-Collado J. Cardiac developmental onomatology: the real heart of the matter. J. Anat. 2003 doi: 10.1016/S0940-9602(03)80119-X. in press. [DOI] [PubMed] [Google Scholar]
- Bartelings MM, Gittenberger-De Groot AC. The outflow tract of the heart-embryologic and morphologic correlations. Int. J. Cardiol. 1989;22:289–300. doi: 10.1016/0167-5273(89)90270-2. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Bartelings MM. PhD Thesis. The Netherlands: University of Leiden; 1990. The outflow tract of the heart – embryologic and morphologic correlations. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Burggren WW. Cardiac design in lower vertebrates: what can phylogeny reveal about ontogeny? Experientia. 1988;44:919–930. doi: 10.1007/BF01939885. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Chen JN, Van Eden JM, Warren KS, Chin A, Nusslein-Volhard C, et al. Left-right pattern of cardiac BMP4 may drive asymmetry of the heart in zebrafish. Development. 1997;124:4373–4382. doi: 10.1242/dev.124.21.4373. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Creazzo TL, Godt RE, Leatherbury L, Conway SJ, Kirby ML. Role of cardiac neural crest cells in cardiovascular development. Annu. Rev. Physiol. 1998;60:267–286. doi: 10.1146/annurev.physiol.60.1.267. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- De La Cruz MV, Da Rocha JP. An ontogenetic theory for the explanation of congenital malformations involving the truncus and conus. Am. Heart J. 1956;51:782–805. doi: 10.1016/s0002-8703(56)80013-6. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- De La Cruz MV, Sanchez Gomez C, Arteaga MM, Arguello C. Experimental study of the development of the truncus and the conus in the chick embryo. J. Anat. 1977;123:661–686. 10.1046/j.1469-7580.2003.00168.x. [PMC free article] [PubMed] [Google Scholar]
- De La Cruz MV, Sanchez Gomez C, Cayre R. The developmental components of the ventricles: their significance in congenitalk cardiac malformations. Cardiol. Young. 1991;1:123–128. 10.1046/j.1469-7580.2003.00168.x. [Google Scholar]
- Edmonds LD, James LM. Temporal trends in the birth prevalence of selected congenital malformations. Birth Defects Monitoring Program/Commission on professional and Hospital Activities, 1979–89. Teratology. 1993;48:647–649. doi: 10.1002/tera.1420480606. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Ferencz C, Neill CA. Cardiovascular malformations: prevalence at live birth. In: Freedom RM, Benson LN, Smallhorn JF, editors. Neonatal Heart Disease. New York: Springer Verlag; 1986. pp. 19–27. 10.1046/j.1469-7580.2003.00168.x. [Google Scholar]
- Fukiishi Y, Morriss-Kay GM. Migration of cranial neural crest cells to the pharyngeal arches and heart in rat embryos. Cell Tissue Res. 1992;268:1–8. doi: 10.1007/BF00338048. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Goor DA, Dische R, Lillehei CW. The conotruncus I. Its normal inversion and conus absorption. Circulation. 1972;46:375–364. doi: 10.1161/01.cir.46.2.375. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Houweling AC, Somi S, Van Den Hoff MJ, Moorman AF, Christoffels VM. Developmental pattern of ANF gene expression reveals a strict localization of cardiac chamber formation in chicken. Anat. Rec. 2002;266:93–102. doi: 10.1002/ar.10042. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Icardo JM. Development of the outflow tract. A study in hearts with situs solitus and situs inversus. Ann. New York Acad. Sci. 1990;588:26–40. doi: 10.1111/j.1749-6632.1990.tb13194.x. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev. Cell. 2001;1:435–440. doi: 10.1016/s1534-5807(01)00040-5. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Gale TF, Stewart DE. Neural crest cells contribute to normal aortopulmonary septation. Science. 1983;220:1059–1061. doi: 10.1126/science.6844926. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Bockman DE. Neural crest and normal development: a new perspective. Anat. Record. 1984;209:1–6. doi: 10.1002/ar.1092090102. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Turnage KL, Hays BM. Characterization of conotruncal malformations following ablation of ‘cardiac’ neural crest. Anat. Record. 1985;213:87–93. doi: 10.1002/ar.1092130112. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Kirby ML. Embryogenesis of transposition of the great arteries: a lesson from the heart. Circulation Res. 2002;91:87–89. doi: 10.1161/01.res.0000028301.40791.4f. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Kramer TC. The partitioning of the truncus and conus and the formation of the membranous portion of the interventricular septum in the human heart. Anat. Record. 1942;71:343–370. 10.1046/j.1469-7580.2003.00168.x. [Google Scholar]
- Laane HM. The septation of arterial pole of the heart in the chick embryo. I. Introduction. Acta Morph. Neerlando-Scandinavica. 1978;16:17–27. 10.1046/j.1469-7580.2003.00168.x. [PubMed] [Google Scholar]
- Laane HM. The septation of the arterial pole of the heart in the chick embryo. III. Development of the truncus arterious of the heart of chick embryos from 5 to 7 days of incubation. Acta Morph. Neerlando-Scandinavica. 1979;17:1–20. 10.1046/j.1469-7580.2003.00168.x. [PubMed] [Google Scholar]
- Lamers WH, Virágh S, Wessels A, Moorman AFM, Anderson RH. Formation of the tricuspid valve in the human heart. Circulation. 1995;91:111–121. doi: 10.1161/01.cir.91.1.111. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. The Neural Crest. Cambridge, UK: Cambridge University Press; 1982. 10.1046/j.1469-7580.2003.00168.x. [Google Scholar]
- Los JA. Cardiac septation and development of the aorta, pulmonary trunk, and pulmonary veins: previous work in the light of recent observations. In: Rosenquist GC, Bergsma D, editors. Morphogenesis and Malformation of the Cardiovascular System. New York: Alan R. Liss, Inc.; 1978. pp. 109–138. 10.1046/j.1469-7580.2003.00168.x. [PubMed] [Google Scholar]
- McBride RE, Moore GW, Hutchins GM. Development of the outflow tract and closure of the interventricular septum in the normal human heart. Am. J. Anat. 1981;160:309–331. doi: 10.1002/aja.1001600308. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Merrick AF, Yacoub MH, Ho SY, Anderson RH. Anatomy of the muscular subpulmonary infundibulum with regard to the Ross procedure. Ann. Thoracic Surg. 2000;69:556–561. doi: 10.1016/s0003-4975(99)01300-4. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez RA, Norris RA, Kern MJ, Eisenberg CA, et al. The outflow of the heart is recruited from a novel heart forming field. Dev. Biol. 2001;238:97–109. doi: 10.1006/dbio.2001.0409. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Nishibatake M, Kirby ML, Van Mierop LHS. Pathogenesis of persistent truncus arteriosus and dextroposed aorta in the chick embryo after neural crest ablation. Circulation. 1987;75:255–264. doi: 10.1161/01.cir.75.1.255. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Noden DM, Poelmann RE, Gittenberger De Groot AC. Cell origins and tissue boundaries during outflow tract development. Trends Cardiovascular Med. 1995;5:69–75. doi: 10.1016/S1050-1738(99)80002-4. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Okamoto N. Congenital Anomalies of the Heart. Tokyo: Igaku-Shoin; 1980. 10.1046/j.1469-7580.2003.00168.x. [Google Scholar]
- Okamoto N, Akimoto N, Satow Y, Hidaka N, Miyabara S. In: Mechanisms of Cardiac Morphogenesis and Teratogenesis. Pexieder T, editor. New York: Raven Press; 1981. pp. 127–137. 10.1046/j.1469-7580.2003.00168.x. [Google Scholar]
- Orts Llorca F, Puerta Fonella J, Sobrado J. The formation, septation and fate of the truncus arteriosus in man. J. Anat. 1982;134:41–56. 10.1046/j.1469-7580.2003.00168.x. [PMC free article] [PubMed] [Google Scholar]
- Patten BD. Human Embryology. Michigan: The Blakiston Company, Inc; 1953. 10.1046/j.1469-7580.2003.00168.x. [Google Scholar]
- Pexieder T. Development of the outflow tract of the embryonic heart. In: Rosenquist GC, Bergsma D, editors. Morphogenesis and Malformation of the Cardiovascular System. New York: Alan R. Liss, Inc.; 1978. pp. 29–68. 10.1046/j.1469-7580.2003.00168.x. [Google Scholar]
- Pexieder T. Conotruncus and its septation at the advent of the molecular biology era. In: Clark EB, Markwald RR, Takao A, editors. Developmental Mechanisms of Heart Disease. New York: Futura Publishing; 1995. pp. 227–248. 10.1046/j.1469-7580.2003.00168.x. [Google Scholar]
- Phillips MT, Kirby MC, Forbes G. Analysis of cranial neural crest distribution in the developing heart using quail chick chimeras. Circulation Res. 1987;60:27–30. doi: 10.1161/01.res.60.1.27. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Poelmann RE, Mikawa T, Gittenberger-De Groot AC. Neural crest in outflow tract septation of the embryonic chicken heart: differentiation and apoptosis. Dev. Dynamics. 1998;212:373–384. doi: 10.1002/(SICI)1097-0177(199807)212:3<373::AID-AJA5>3.0.CO;2-E. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Qayyum SR, Webb S, Anderson RH, Verbeek FJ, Brown NA, Richardson MK. Septation and valvar formation in the outflow tract of the embryonic chick heart. Anat. Record. 2001;264:273–283. doi: 10.1002/ar.1162. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Rosenquist GC, Bharati S, McAllister HA, Lev M. Truncus arteriosus communis: truncal valve anomalies associated with small conal or truncal septal defects. Am. J. Cardiol. 1976;37:410–412. doi: 10.1016/0002-9149(76)90291-5. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Rothenberg F, Hitomi M, Fisher SA, Watanabe M. Initiation of apoptosis in the developing avian outflow tract myocardium. Dev. Dynamics. 2002;223:469–482. doi: 10.1002/dvdy.10077. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Rychter Z. Analysis of relations between aortic arches and aorticopulmonary septation. Birth Defects Orig. Artic. Series. 1978;14:443–448. 10.1046/j.1469-7580.2003.00168.x. [PubMed] [Google Scholar]
- Sanford LP, Ormsby I, Gittenberger-De Groot AC, Sariola H, Friedman R, Boivin GP, et al. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaner RF. Comparative development of the bulbus and ventricles of the vertebrate heart with special reference to Spitzer's theory of heart malformations. Anat. Record. 1962;142:519–529. doi: 10.1002/ar.1091420409. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Stamm C, Anderson RH, Ho SY. Clinical anatomy of the normal pulmonary root compared with that in isolated pulmonary valvular stenosis. J. Am. College Cardiol. 1998;31:1420–1425. doi: 10.1016/s0735-1097(98)00089-8. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Steding G, Seidl W. Contribution to the development of the heart Part I: normal development. Thoracic Cardiovascular Surgeon. 1980;28:386–409. doi: 10.1055/s-2007-1022440. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Steding G, Seidl W. Contribution to the development of the heart Part II: morphogenesis of congenital heart disease. Thoracic Cardiovascular Surgeon. 1981;29:1–16. doi: 10.1055/s-2007-1023435. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Steding G, Seidl W. Cardio-vaskulares system. In: Hinrichsen KV, editor. Humanembryologie: Lehrbuch und Atlas der Vorgeburtlichen Entwickelung Des Menschen. Berlin: Springer-Verlag; 1990. pp. 205–294. 10.1046/j.1469-7580.2003.00168.x. [Google Scholar]
- Takamura K, Okishima T, Ohdo S, Hayakawa K, Okamoto N. Sequential observation of cardiac neural cell distribution in the developing heart: effects of transplantation regions. In: Clark EB, Takao A, editors. Developmental Cardiology: Morphogenesis and Function. New York: Futura Publishing Co., Inc.; 1990. pp. 159–173. 10.1046/j.1469-7580.2003.00168.x. [Google Scholar]
- Tandler J. The development of the heart. In: Keibel F, Mall FP, editors. Manual of Human Embryology. Philadelphia: Lippincott; 1912. pp. 534–570. 10.1046/j.1469-7580.2003.00168.x. [Google Scholar]
- Thompson RP, Fitzharris TP. Morphogenesis of the truncus arteriosus of the chick embryo: tissue reorganization during septation. Am. J. Anat. 1979;156:251–264. doi: 10.1002/aja.1001560206. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Thompson RP, Wong Y-MM, Fitzharris TP. A computer graphic study of cardiac truncal septation. Anat. Record. 1983;206:207–214. 10.1046/j.1469-7580.2003.00168.x. [Google Scholar]
- Thompson RP, Wong Y-MM, Fitzharris TP. Patterns of tensile stress in the developing cardiac truncus. In: Nora JJ, Takao A, editors. Congenital Heart Disease: Causes and Processes. New York: Futura Publishing Co; 1984. pp. 387–400. 10.1046/j.1469-7580.2003.00168.x. [Google Scholar]
- Tonge M. Observations on the development of the semilunar valves of the aorta and Pulmonary artery of the heart of the chick. Phil. Trans. Royal Soc. London. 1869;159:387–412. 10.1046/j.1469-7580.2003.00168.x. [Google Scholar]
- Van Den Hoff MJB, Moorman AFM, Ruijter JM, Lamers WH, Bennington RW, Markwald RR, et al. Myocardialisation of the cardiac outflow tract. Dev. Biol. 1999;212:477–490. doi: 10.1006/dbio.1999.9366. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Van Mierop LHS, Alley RD, Kausel HW, Stranahan A. Pathogenesis of transposition complexes. I. Embryology of the ventricles and great arteries. Am. J. Cardiol. 1963;12:216–225. doi: 10.1016/0002-9149(63)90311-4. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Van Mierop LHS. Morphological development of the heart. In: Berne RM, Sperelakis N, Geiger SR, editors. The Cardiovascular System. Baltimore, MD: Waverley Press.; 1979. pp. 1–28. 10.1046/j.1469-7580.2003.00168.x. [Google Scholar]
- Waldo K, Miyagawa-Tomita S, Kumski D, Kirby M. Cardiac neural crest cells provide new insight into septation of the cardiac outflow tract: Aortic sac to ventricular septal closure. Dev. Biol. 1998;196:129–144. doi: 10.1006/dbio.1998.8860. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Ya J, Van Den Hoff MJB, De Boer PAJ, Tesink-Taekema Franco D, Moorman AFM, et al. Normal development of the outflow tract in the rat. Circulation Res. 1998;82:464–472. doi: 10.1161/01.res.82.4.464. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- Yelbuz TM, Waldo KL, Kumiski DH, Stadt HA, Wolfe RR, Leatherbury L, et al. Shortened outflow tract leads to altered cardiac looping after neural crest ablation. Circulation. 2002;106:504–510. doi: 10.1161/01.cir.0000023044.44974.8a. 10.1046/j.1469-7580.2003.00168.x. [DOI] [PubMed] [Google Scholar]