Abstract
Type XII collagen is a fibril-associated collagen with multiple functional domains. The purpose of this work was to determine its role in regulating tendon matrix assembly. The temporal and spatial expression patterns of both collagen and mRNA were analysed in developing chicken metatarsal tendons using immunofluorescence microscopy, in situ hybridization and real-time quantitative PCR. Temporally, type XII collagen was present during all stages of development (day 14-hatch). However, spatially, type XII collagen expression shifted from the entire tendon at day 14, when the tendon is immature and fascicles are not well developed, to the interfacial matrix (endotendinium) associated with developing fascicles. This shift was obvious beginning at day 17, becoming prominent at day 19. Associated with this shift was a gradual decrease in type XII collagen reactivity in the tendon proper (non-sheath). By hatching, the reactivity was sequestered almost exclusively to the sheaths with some reactivity remaining at the fibroblast–matrix interface within the fascicle. In situ hybridization indicated that fibroblasts in the tendon expressed type XII collagen mRNA homogeneously at day 14. However, by hatching, when the tendon matures, type XII collagen is restricted primarily to the sheath cells. Quantitative PCR analyses, of NC3 splice variants, demonstrated highest expression levels for the short splice variant mRNA at days 14–17, followed by a significant decrease at day 19 with levels remaining constant to adult. Long variant mRNA expression was highest at day 14 then decreased and was constant from day 17 to adult. These changing patterns may be related to the spatial shift in type XII collagen expression to the sheaths. Differential temporal and spatial expression patterns indicate that type XII collagen functions to integrate the developing tendon matrices and fascicles into a functional unit.
Keywords: chicken, collagen fibril formation, development, matrix assembly, tendon, type XII collagen
Introduction
The organization and function of tissues is determined by the arrangement of cells and the extracellular matrices they produce. The avian tendon provides a model for understanding collagen fibril growth, arrangement and ultimately development of a functional tissue. The tendon is a dense connective tissue that transmits mechanical forces in a moving joint. The major components of the tendon are fibroblasts and collagen fibrils. The fibrils are highly organized and grouped together into fibres. The fibres together with the tendon fibroblasts are organized into fascicles; and the fascicles are bound together by connective tissue sheaths resulting in the mature, weight-bearing tendon (Kastelic et al. 1978; Birk & Trelstad, 1986; Birk et al. 1989a, 1997; Birk & Zycband, 1994; Birk & Mayne, 1997).
Collagen fibrils are the major elements responsible for structure stabilization and the mechanical properties of the tendon (Birk & Linsenmayer, 1994). Our general hypothesis is that fibril-associated macromolecules, including type XII collagen, maintain structural integrity in the developing tendon. The collagen fibrils are heteropolymeric structures that contain different fibrillar collagens and other macromolecules (Birk et al. 1991). Macromolecules associated with the fibril surface have been implicated as regulatory molecules mediating the progression through fibril and matrix assembly (Ezura et al. 2000).
One class of fibril-associated molecules includes the leucine-rich-repeat proteoglycans/glycoproteins. Decorin, lumican and fibromodulin are found in tendons and are known to associate with collagen fibrils. Deficiency in one or more of these proteoglycans in knock-out mice leads to the assembly of structurally abnormal tendon collagen fibrils. This phenotype is consistent with a role in regulation of fibril assembly and tendon function (Oldberg et al. 1996; Danielson et al. 1997; Ezura et al. 2000).
Fibril-associated collagens with interrupted triple helical domains (FACIT collagens) are another class of fibril-associated molecules. Two members, types XII and XIV collagen, are localized to the surface of fibrils (Keene et al. 1991; Young et al. 2000; Schuppan et al. 2001). However, little is known about their effects on fibril assembly or their roles during development. FACIT collagens are an expanding family with members that include collagen types IX, XII, XIV, XVI, XIX and XX (Prockop & Kivirikko, 1995; Ricard-Blum et al. 2000; Koch et al. 2001). Both types XII and XIV collagen are expressed in a wide variety of tissues including the developing avian tendon (Nishiyama et al. 1994; Walchi et al. 1994; Koch et al. 1995; Gordon et al. 1996; Young et al. 2000). Types XII and XIV collagen are structurally similar homotrimers, composed of two collagenous domains (COL1-COL2) and three non-collagenous domains (NC1-NC3) (Shaw & Olsen, 1991). These multidomain collagens can interact with more than one extracellular component simultaneously, allowing integration of developing matrices. For example, type XII collagen has been shown to bind to both decorin and fibromodulin (Font et al. 1996). In addition, the two carboxy terminal domains, NC1 and COL1, of type XII collagen are believed to interact with the type I collagen fibrils (Keene et al. 1991; Schuppan et al. 2001). In contrast, the large, globular, NC3 domain on the amino terminus projects into the matrix and is hypothesized to interact with other matrix components and may bridge adjacent fibrils (Shaw & Olsen, 1991).
These functional domains can be affected by alternative splicing. Collagen type XII has splice variants in its NC1 and NC3 domains (Trueb & Trueb, 1992; Kania et al. 1999). These different splice variants lead to alternative isoforms that can affect either interactions with the collagen fibril (NC1 splice variants) or interactions with other matrix components (NC3 splice variants). There are two isoforms in the NC3 domain of type XII collagen called the short form and long form. These two variants have different affinities for matrix molecules such as heparin. In addition, the long form can have an attached glycosaminoglycan while the short form cannot (Koch et al. 1995). In this paper, expression of type XII collagen during tendon development is characterized. The data indicate a role for this fibril-associated collagen in integrating different matrices during tendon development and perhaps in packing fibrils within fibres.
Materials and methods
Tissue harvest
White leghorn chicken embryos (Spafas, CT, USA) were incubated in a humidified atmosphere and staged according to Hamburger & Hamilton (1951). Metatarsal tendons were dissected from staged embryos (14–19 days) as well as hatchling and adult (2 months old) chickens.
Antibodies
Two different monoclonal antibodies were utilized in the immunofluorescence microscopy studies. One, C19, detects all forms of type XII collagen, while a second, SE14, detects only the long isoform of type XII collagen (Koch et al. 1995). Both of the antibodies were used at 5 µg mL−1 and detected by goat antimouse IgG-Alexa Fluor 488 conjugated antibody (Molecular Probes, Eugene, OR, USA).
Preparation of RNA and cDNA
Total RNA was isolated from metatarsal tendons at multiple developmental stages by homogenization in Trizol reagent (Invitrogen, Carlsbad, CA, USA) and stored at −80 °C. cDNAs were obtained by reverse transcription of 5 µg of total RNA with random primers using High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA).
Immunofluorescence microscopy
Immunofluorescence microscopy was done as previously described (Young et al. 2000). Briefly, tendons were fixed in 4% paraformaldehyde in PBS pH 7.2 for 30 min on ice, cryoprotected by incubation in 2 m sucrose and sections (6 µm) were cut and stored at −80 °C until use.
After rinsing in PBS, the sections were blocked with blocking buffer (1% Normal Goat Serum, 3% BSA in PBS). Incubation with primary antibodies was carried out at 4 °C overnight at an antibody concentration of 5 µg mL−1 in blocking buffer. After washing in PBST (0.05% Tween-20), the sections were incubated with secondary antibody, goat antimouse IgG-Alexa Fluor 488 (Molecular Probes), at 1: 400 dilution in blocking buffer for 1 h at room temperature. The sections were washed extensively in PBST followed by nuclear counterstaining with DAPI (Molecular Probes) and mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA, USA). Images were captured using an Optronics Digital camera and BioQuant Image Analysis System using set integration times and identical conditions to facilitate comparisons between samples.
In situ hybridization (ISH)
Probes were prepared as previously described (Young et al. 2002). Briefly, a PCR product within the NC3 domain of type XII collagen common to both variants was amplified using the following primers: the forward primer, 5′-GCAGAACCAAACCTCTCACT-3′ and the reverse primer, 5′-TTCTTGGTGTTCCTCTCTCC-3′. The PCR product was ligated to T7 phage RNA polymerase promoter and templates for in vitro transcription with T7 promoter at either the 5′ or the 3′ end were generated by PCR amplification. Antisense or sense RNA probes labelled with Dig-11-UTP were produced by in vitro transcription.
ISH was done as previously described (Young et al. 2002). The tendon sections were fixed in 4% paraformaldehyde in PBS pH 7.2, treated with 0.2 n HCl, and digested with pepsin. For hatchling tendons, the pepsin concentration was 0.1% in 0.2 n HCl vs. 0.15% for 14-day tissue. The 14-day tendons were incubated with 1% H2O2 in methanol at −20 °C for 30 min prior to incubation with HCl. After acetylation in 0.25% acetic anhydride, the sections were prehybridized with hybridization buffer. Hybridization was carried out at 55 °C overnight with probe at a concentration of 100 ng mL−1. The sections were washed in 2× SSC, TNE buffer (10 mm Tris pH 7.5, 500 mm NaCl, 1 mm EDTA) and then excess probe was digested with Rnase A/T1 followed by washing in 50% formamide DI and in 0.08× SSC. After blocking with 20% rabbit IgG fraction in blocking buffer, the labelled probes were detected using HRP conjugated anti-Dig antibody, the signal was amplified by consecutively incubating the sections with antibiotin-HRP, biotinyl-tyramide, and antibiotin-alkaline phosphatase. The signal was detected by incubation with Sigma Fast™ Fast Red TR/Naphthol (Sigma Chemical Co., Saint Louis, MO, USA) prepared according to the manufacturer's instructions. The sections were counterstained with haematoxylin and mounted with glycergel mounting medium.
Real-time quantitative PCR
Primers for real-time PCR were designed with Primer Expression 2.0 software (Applied Biosystems) and chosen to avoid any possible primer–dimer forming structure. The forward primer sequence for both the collagen type XII short and long variants was 5′-CCGCCGCCC TCC TCT-3′, while the reverse primer for the short variant was, 5′-AGCTTCTGC TCTGGTTCTACA TTCT-3′, and for the long variant was, 5′-GGCCTTTTCCATGACATTTGA-3′, yielding a 64-bp product for the short and 103-bp product for the long variant.
Real-time PCR was performed on the ABI PRISM 7000 Sequence Detection System using SYBR Green PCR Master Mix (Applied Biosystems) to measure the relative expression levels of the type XII collagen gene at multiple developmental stages. A serial dilution of cDNA from adult chicken RNA was used to generate relative standard curves for both the collagen XII short and long variants to which samples were compared. Identical concentrations of cDNA for each sample were used as template for PCR amplification with a primer concentration of 0.3 µm for the short variant and 0.1 µm for the long variant.
For each stage, three independent total RNA samples were isolated and, for each sample, PCR was conducted in triplicate with a 50-µL reaction volume. In each experiment, a large master mixture including SYBR green master mix, both forward and reverse primers, and PCR-grade H2O was made. PCR cycle parameters were as follows: 50 °C 2 min × 1 cycle, 95 °C 10 min × 1 cycle, 95 °C 15 s, 60 °C 1 min × 40 cycles, then raising the temperature from 60 °C to 95 °C to dissociate the PCR product and yield a dissociation curve.
The sequence detection software supplied with the ABI PRISM 7000 was used to analyse the raw data from the real time reading of the fluorescence. The threshold cycle (Ct) for each reaction, that reflects the amount of starting template, was calculated with the software. The Cts for standards were converted into a standard curve, to which each sample was compared to yield the starting relative template concentrations. For each stage, this was calculated as the mean from the three RNA preparations, each done in triplicate. The mean values at developmental stages 14, 17 and 19 days and hatching chicken were divided by the adult values to yield expression relative to the adult.
Results
The temporal and spatial expression patterns of type XII collagen isoforms in developing chicken tendon
Type XII collagen expression was studied during tendon development by immunofluorescence microscopy. The earliest time point studied, embryonic day 14, represents an immature tendon. The intermediate stages, days 17–19, are periods of rapid fibrillogenesis and assembly. The hatchling tendon represents a mature, weight-bearing tendon.
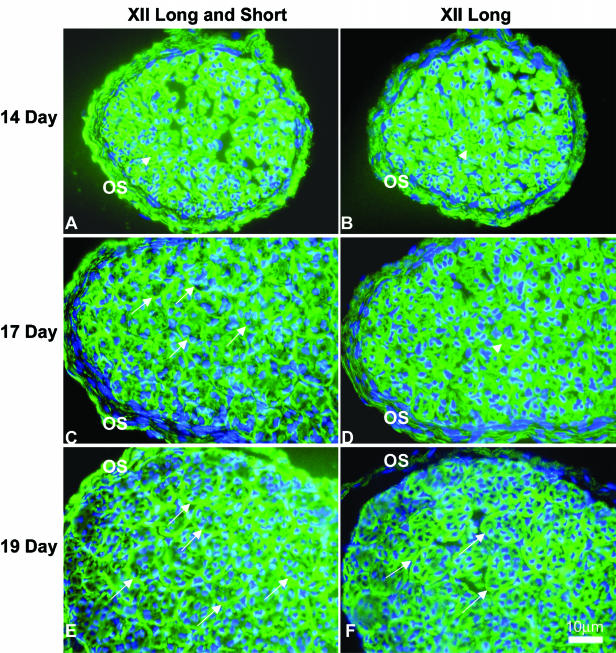
At day 14, the immature tendon fascicles were beginning to develop and the endotendinium was not yet distinguishable. Type XII collagen was detected throughout the developing tendon with a strong signal using the monoclonal antibody (C19) that reacted with both type XII collagen isoforms (Fig. 1A). At the early intermediate time point day 17, the tendon fascicles were clearly defined. Again, type XII collagen was detected throughout the developing tendon. But the fascicles showed significant reactivity in the endotendinium and the signal in tendon proper was weaker (Fig. 1C). At day 19, there was a continued decrease in reactivity in the tendon proper with an associated enrichment in the endotendinium surrounding the fascicles (Fig. 1E). After hatching, the expression of type XII collagen was predominately in the endotendinium around the fascicles. Within the tendon fascicles, reactivity appeared to be associated with processes of the tendon fibroblasts between collagen fibres while the fibres were negative (Fig. 2A). This pattern remained stable in the adult, i.e. 2 months post hatching (data not shown). Throughout development, type XII collagen was detected in the outer sheath of the tendon.
Fig. 1.
Immunofluorescence staining of type XII collagen on chicken tendon at developmental stages days 14, 17 and 19. Developing chicken tendons from day 14 to 19 were reacted with antibodies against type XII collagen either long isoform or both isoforms. Reactivity with antibodies against type XII collagen both isoforms (A, C and E) was present at all stages observed, with strong and homogenous signal at day 14. A localization of type XII collagen reactivity was observed beginning at day 17, and was prominent at day 19 in the matrix region surrounding the growing fascicles (arrows). Reactivity with antibodies against long isoform was also detected throughout the stages with homogenous distribution at day 14 and enriched endotendinium staining from day 19 (B, D and F) (arrows). Images presented demonstrated the shift in spatial distribution from the tendon proper (arrowheads) at early stages (14D) to the sheath in mature tendon (19D). In all cases, the negative controls (absence of primary antibodies) were negative (data not shown). OS = outer sheath. Bar = 10 µm.
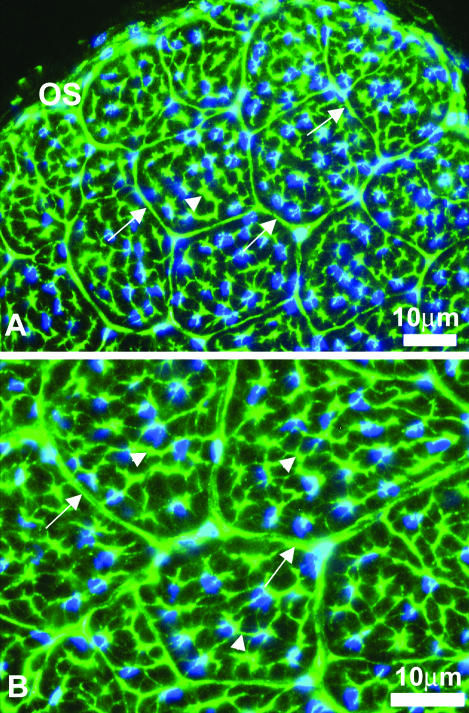
Fig. 2.
Reactivity against type XII collagen in hatchling tendon. Immunofluorescence staining of hatchling chicken tendon with antibodies against type XII collagen either for both isoforms (A) or the long isoform (B) showed the same staining pattern. The reactivity was preferentially localized and sequestered to the connective tissue sheaths between the fascicles (endotendinium) (arrows) and cytoplasmic processes that separate bundle-forming compartments (arrowheads). OS = outer sheath. Bar = 10 µm.
The long type XII isoform was localized in developing tendon using the SE14 monoclonal antibody. At the earliest time point, day 14, strong reactivity against the long isoform was found throughout the tendon proper and the outer sheath (Fig. 1B). Compared with the distribution of all type XII collagen isoforms (C19), the distribution of the long isoform showed no difference in day 14 tendon. From days 17 to 19, the long isoform was present in the tendon proper with a weaker signal than in day 14 and there was a shift in distribution of the reactivity to the developing tendon sheaths defining developing fascicles (endotendinium) (Fig. 1D,F). At hatching, as was the case for both isoforms (C19), the long XII collagen isoform was prominent in the endotendinium surrounding the fascicles and cytoplasmic processes interdigating between collagen fibres of the tendon proper (Fig. 2B). Again, the localization seen in the adult was identical to that of the hatchling tendon (data not shown).
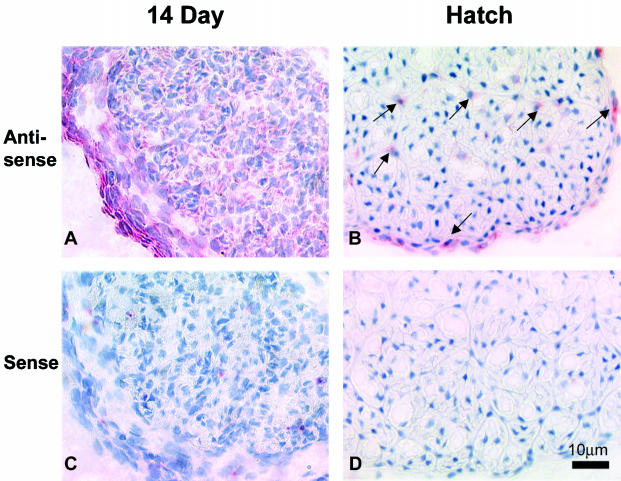
The immunolocalization data indicate differential temporal and spatial expression patterns for type XII collagen during tendon development. To determine the cellular basis for the shift of type XII collagen expression, the localization of type XII collagen mRNA in day 14 embryo and hatchling chicken tendons was characterized by in situ hybridization (Fig. 3). Using a probe that recognized mRNA common to both collagen type XII long and short variants, the type XII collagen mRNA was detected in all tendon cells including the tendon fibroblasts and cells in the outer sheath in 14-day embryo chicken tendon. However, in hatchling tendon, only fibroblasts in the endotendinium and outer sheath were positive. These data indicate that with development, the expression of type XII collagen decreases to very low levels in fibroblasts of the tendon proper with expression being increased/retained in cells of the endotendinium and outer sheath.
Fig. 3.
Type XII collagen mRNA localization on chicken tendon at developmental stages day 14 and hatching by in situ hybridization with an RNA probe common to both isoforms. At embryonic day 14, strong homogenous signal was observed throughout all the cells in tendon proper and outer sheath (A). While at hatching, only cells within the endotendinium and outer sheath (arrow) demonstrated XII collagen mRNA expression (B). Panels C and D showed sense probe on 14 day and hatching tendon. Bar = 10 µm.
The temporal expression pattern of type XII collagen variants in developing chicken tendon
The two splice variants in the NC3 domain of type XII collagen have been described in chicken (Trueb & Trueb, 1992; Koch et al. 1995). These splice variants may change the interactions between adjacent fibrils or affect the binding affinity between type XII collagen and other matrix components. To characterize the temporal expression pattern of type XII collagen variants in developing chicken tendon, real-time PCR was used to determine the relative amounts of each NC3 splice variant mRNA at multiple developmental stages. Total RNA from day 14, 17 and 19 embryos as well as hatchling and adult chicken tendons was used. For data comparisons, first strand synthesis of cDNA was performed using the same amount of total RNA for every sample, and the same amount of template cDNA was used for each PCR reaction.
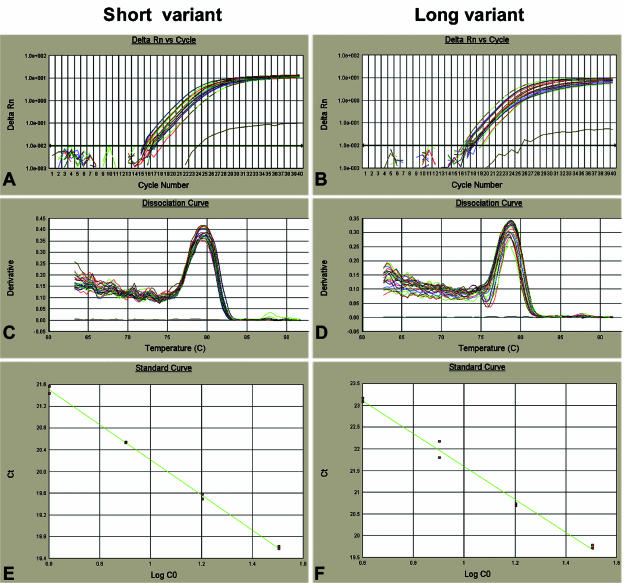
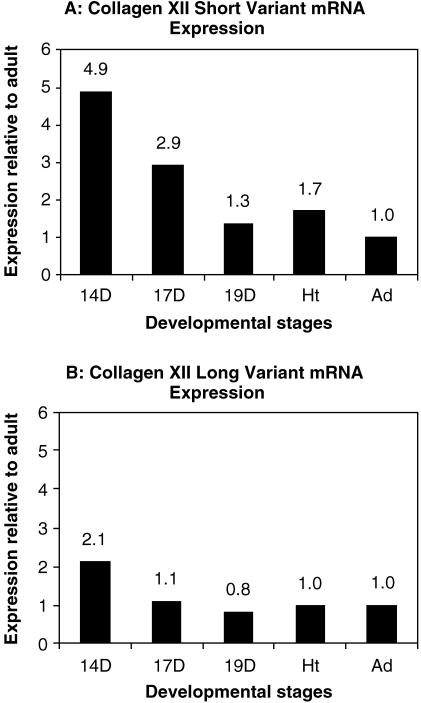
The amplification plots and dissociation curves for both long and short variants showed that the amplifications were specific with a single product in each reaction (a 64-bp product for the short variant, Tm = 79 °C; a 103-bp product for the long variant, Tm = 78 °C) (Fig. 4A–D). Standard curves for both variants were produced by amplification of a serial dilution of adult tendon cDNA. The slopes and R2-values of the standard curves indicated good amplification efficiency for each variant (theoretically slope =−3.33, R2 = 1) (Fig. 4E,F). Relative concentrations for long and short variants were determined based on extrapolation of Ct to the standard curves using the ABI software (Table 1). The results demonstrated both NC3 splice variants were expressed during the development of chicken tendon from day 14 until adult. Type XII collagen short variant mRNA expression was highest at 14 days and decreased dramatically, from day 14 to 19. From day 19 to adult, the expression was relatively stable (Fig. 5A). The long variant also had the highest expression at day 14, decreased at 17 days and was stable from 19 days to adult (Fig. 5B).
Fig. 4.
Real-time PCR amplification plot and standard curve for short and long NC3 variants. (A,B) The amplification plots of short and long variants. (C,D) The dissociation curves for the short and long variants showing that there was only one PCR product in each reaction with the right melting temperature (79 °C for the short variant, 78 °C for the long variant). (E) The standard curve for the short variant (slope =−3.336, R2 = 0.999) while (F) is for long variant (slope =−3.664, R2 = 0.995).
Table 1.
Expression of type XII collagen variants
| Stage | Day 14 | Day 17 | Day 19 | Hatch | Adult |
|---|---|---|---|---|---|
| A. Short variant | |||||
| Ct | 18.1 ± 0.03 | 18.8 ± 0.14 | 19.9 ± 0.10 | 19.6 ± 0.26 | 20.3 ± 0.04 |
| Concentration | 46.2 ± 1.00 | 27.8 ± 2.80 | 12.5 ± 0.89 | 15.8 ± 2.73 | 9.5 ± 0.30 |
| Expression level* | 4.9 | 2.9 | 1.3 | 1.7 | 1 |
| B. Long variant | |||||
| Ct | 20.5 ± 0.12 | 21.7 ± 0.38 | 22.1 ± 0.25 | 21.8 ± 0.31 | 21.8 ± 0.06 |
| Concentration | 19.1 ± 1.41 | 9.8 ± 2.10 | 7.3 ± 1.15 | 9.0 ± 1.60 | 9.0 ± 0.33 |
| Expression level* | 2.1 | 1.1 | 0.8 | 1 | 1 |
Concentration is presented in arbitrary units based on the initial cDNA concentrations used for standard curves.
Expression level relative to adult.
Fig. 5.
mRNA expression for both variants relative to adult. From the standard curve of real-time PCR amplification, the starting template amount of each reaction was calculated, which was divided by the amount of adult to yield a fold value relative to adult. The data illustrated that, at early developmental stages days 14 and 17, mRNA expression for short variant type XII collagen was much higher than adult. There was a dramatic drop at day 19 and at later stages the expression remained relatively stable (A). Similar to that seen in the short variant, the tendon at day 14 had higher mRNA expression of the long variant than other stages. The expression decreased at day 17 and was kept at a stable level the same as with the adult (B).
The temporal expression pattern of both type XII collagen mRNA variants, derived from the real-time quantitative PCR data, were consistent with the spatial and temporal expression patterns of type XII collagen isoforms. The shift in expression to the sheaths was associated with a decrease and level expression of both variant mRNAs.
Discussion
Our data demonstrate a changing pattern of type XII collagen expression in the developing tendon. At the earliest stage examined, day 14, when the tendon fascicles are beginning to form in the immature tendon, there was a strong and homogeneous expression of type XII collagen. At this stage, all the cells in tendon proper and outer sheath homogeneously express type XII collagen mRNA. As tendon development progresses, the fibrils aggregate into fibres and eventually form fascicles defined by a sheath (endotendinium); these fascicles are further organized into the functional tendon surrounded by the outer sheath or epitendinium (Kastelic et al. 1978; Birk et al. 1989b). During this period (days 17–19), when the fascicles become more pronounced, there is a shift in type XII collagen expression from the tendon fibroblasts to the sheath (endotendinium). At this stage, there is reactivity for type XII collagen throughout the fascicle with enhanced signal in the sheaths. From day 17 of development, significant type XII collagen reactivity was observed in the endotendinium. At the mature stages, hatching to adult, the type XII collagen signal remains high in the endotendinium with positive signal only at fibroblast process–fibre interfaces within the tendon fascicle. The spatial shift in expression and high reactivity of type XII collagen in the endotendinium suggests a role in integration of adjacent matrices during development. Previous studies (Birk & Mayne, 1997) demonstrated a similar shift in type III collagen expression from the fibres of the fascicle to the sheaths. Type XII collagen may have higher affinities for the heterotypic type I/III fibrils. These interactions may be important in integrating the developing tendon matrices. The expression of these two collagens may be regulated by the same or similar mechanisms. An integration of adjacent fascicles into functional units is important for the development and maintenance of structural integrity within the growing tendons.
Collagen fibril diameters are homogeneously small at day 14 of development; at hatching a large heterogeneous population of fibrils exists with days 17–19 being a transition period (Eikenberry et al. 1984; Parry, 1988; Birk & Mayne, 1997). Changes in fibril diameter do not correlate with changes in type XII collagen expression. Our data show constant expression of type XII collagen during this entire developmental period. This suggests that type XII does not regulate events in fibrillogenesis, but we cannot exclude a role in maintaining fibril packing within the fibre.
We analysed the developing tendon with two antitype XII collagen antibodies. One recognized both long and short isoforms of type XII collagen while the other specifically recognized the long isoform. Both antibodies demonstrated a similar pattern of type XII collagen reactivity at day 14 of development. Starting from day 17 when the endotendinium begins to be formed, type XII collagen starts to show a stronger signal in this region than tendon proper. A comparison of the distribution of the long isoform and both isoforms suggests that the reactivity in endotendinium probably results from short isoform expression at this stage. The long isoform exhibited a pattern of reactivity nearly identical to that of both isoforms, i.e. enrichment in the endotendinium at day 19 and at hatching, indicating that both isoforms contributed to the reactivity in the mature tendon.
The analysis of type XII collagen short splice variants demonstrated that the mRNA expression had the highest level at early stages, day 14, five-fold greater than the adult, when the fascicles were forming. Later when the fascicles were formed, the expression by the fibroblasts of the tendon proper was very low, and the mRNA level decreased significantly. As the tendon matures, from day 19 to adult, the cells in endotendinium maintained or increased type XII collagen mRNA expression. Interestingly, the temporal expression pattern of the long variant differed from that of the short variant. Although the highest expression also occurred at day 14, it decreased quickly at day 17 and remained constant to adult. Comparing the relative amounts of both variant mRNAs from the quantitative PCR data, it seemed that both variants had similar mRNA levels in adult tendons, but at earlier stages the major variant was the short variant (short-to-long, 2.4 at day 14, 2.8 at day 17, 1.7 at day 19 and hatching). The highest ratio of short vs. long variant at day 17 indicated the earlier decrease in expression of the long variant.
The temporal expression patterns of both variants of type XII collagen derived from the real-time quantitative PCR data were consistent with the spatial and temporal expression pattern of type XII collagen isoforms. With tendon development, mRNA expression of type XII collagen decreased in the fibroblasts of the tendon proper, resulting in a dramatic decrease in mRNA from day 14 to day 19, and the shift of type XII collagen localization from tendon proper to endotendinium. The shift in expression to the sheaths was associated with decreased expression of both variant mRNAs. The shift in expression to the sheaths was associated with level expression of the variants.
These splice variants generate different NC3 isoforms of type XII collagen. The NC3 domain projects into the matrix of the tissue, possibly interacting with other matrix components including adjacent fibrils. Therefore, the presence of one splice variant vs. another may affect the fibril–fibril interactions or fibril–matrix interactions involved in processes of fibril packing into fibres or integration of adjacent matrices in developing tissues.
Our earlier report demonstrated that a change in the type XIV collagen levels correlated with the stage in tendon fibrillogenesis dominated by linear fibril growth (Young et al. 2000). Our current data suggest that types XII and XIV collagen, members of the same family, have different roles in the developing tendon. The temporal and spatial expression patterns of type XII collagen suggest a possible role in organizing fibrils into larger aggregates. Type XII collagen in chicken cornea is located in the interfacial regions, Bowman's layer and Descemet's layer, on either side of the stroma, indicating a role in structural integration of the developing matrices (Marchant et al. 2002; Young et al. 2002). However, expression patterns do not correlate with specific stages or transitions in fibril formation. Therefore, type XII collagen most likely does not have a major role in regulating steps in fibrillogenesis in the developing tendon. Type XII collagen showed a differential expression pattern with development becoming enriched in the endotendinium. We suggest type XII collagen functions in integrating the adjacent developing matrices of the growing tendon fascicles. An integration of fibres into fascicles and fascicles into functional units is an important step in development of weight-bearing tendon. This integration of adjacent matrices is a critical determinant of tendon structure and the resulting functional properties.
Acknowledgments
We would like to thank Dr Manual Koch for generously supplying the antibodies. The expert technical assistance of Jessie Feng also is gratefully acknowledged. This paper was supported by the National Institutes of Health, grant no. NIAMSD AR44745.
References
- Birk DE, Trelstad RL. Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. J. Cell Biol. 1986;103:231–240. doi: 10.1083/jcb.103.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk DE, Zycband EI, Winkelmann DA, Trelstad RL. Collagen fibrillogenesis in situ: fibril segments are intermediates in matrix assembly. Proc. Natl. Acad. Sci. USA. 1989a;86:4549–4553. doi: 10.1073/pnas.86.12.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birk DE, Southern JF, Zycband EI, Fallon JT, Trelstad RL. Collagen fibril bundles: a branching assembly unit in tendon morphogenesis. Development. 1989b;107:437–443. doi: 10.1242/dev.107.3.437. [DOI] [PubMed] [Google Scholar]
- Birk DE, Silver FH, Trelstad RL. Matrix assembly. In: Hay ED, editor. Cell Biology of the Extracellular Matrix. New York: Plenum Press; 1991. pp. 221–253. [Google Scholar]
- Birk DE, Linsenmayer TF. Collagen fibril assembly, deposition and organization into tissue-specific matrices. In: Yurchenco PD, Birk DE, Mecham RP, editors. Extracellular Matrix Assembly and Structure. New York: Academic Press; 1994. pp. 91–128. [Google Scholar]
- Birk DE, Zycband EI. Assembly of the tendon extracellular matrix during development. J. Anat. 1994;184:457–463. [PMC free article] [PubMed] [Google Scholar]
- Birk DE, Mayne R. Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur. J. Cell Biol. 1997;72:352–361. [PubMed] [Google Scholar]
- Birk DE, Zycband EI, Woodruff S, Winkelmann DA, Trelstad RL. Collagen fibrillogenesis in situ: fibril segments become long fibrils as the developing tendon matures. Dev. Dyn. 1997;208:291–298. doi: 10.1002/(SICI)1097-0177(199703)208:3<291::AID-AJA1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikenberry EF, Childs B, Sheren SB, Parry DA, Craig AS, Brodsky B. Crystalline fibril structure of type II collagen I lamprey notochord sheath. J. Mol. Biol. 1984;176:261–277. doi: 10.1016/0022-2836(84)90424-8. [DOI] [PubMed] [Google Scholar]
- Ezura Y, Chakravarti S, Oldberg A, Chervoneva I, Birk DE. Differential expression of lumican and fibromodulin regulate collagen fibrillogenesis in developing mouse tendons. J. Cell Biol. 2000;151:779–788. doi: 10.1083/jcb.151.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Font B, Eichenberger D, Rosenberg LM, van der Rest M. Characterization of the interactions of type XII collagen with two small proteoglycans from fetal bovine tendon, decorin and fibromodulin. Matrix Biol. 1996;15:341–348. doi: 10.1016/s0945-053x(96)90137-7. [DOI] [PubMed] [Google Scholar]
- Gordon MK, Foley JW, Linsenmayer TF, Fitch JM. Temporal expression of types XII and XIV collagen mRNA and protein during avain corneal development. Dev. Dyn. 1996;206:49–58. doi: 10.1002/(SICI)1097-0177(199605)206:1<49::AID-AJA5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Hamberger V, Hamilton HL. A series of normal stages in development of the chick embryo. J. Molphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Kania AM, Reichenberger E, Baur ST, Karimbux NY, Taylor RW, Olsen BR, et al. Structural variation of type XII collagen at its carboxyl-terminal NC1 domain generated by tissue-specific alternative splicing. J. Biol. Chem. 1999;274:22053–22059. doi: 10.1074/jbc.274.31.22053. [DOI] [PubMed] [Google Scholar]
- Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect. Tissue Res. 1978;6:11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- Keene DR, Lunstrum GP, Morris NP, Stoddard DW, Burgeson RE. Two type XII-like collagens localize to the surface of banded collagen fibrils. J. Cell Biol. 1991;113:971–978. doi: 10.1083/jcb.113.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Bohrmann B, Matthison M, Hagios C, Trueb B, Chiquet M. Large and small splice variants of collagen XII: Differential expression and ligand binding. J. Cell Biol. 1995;130:1–10. doi: 10.1083/jcb.130.4.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Foley JE, Hahn R, Zhou P, Burgeson RE, Gerecke DR, et al. alpha 1 (Xx) collagen, a new member of the collagen subfamily, fibril- associated collagens with interrupted triple helices. J. Biol. Chem. 2001;276:23120–23126. doi: 10.1074/jbc.M009912200. [DOI] [PubMed] [Google Scholar]
- Marchant JK, Zhang G, Birk DE. Association of type XII collagen with regions of increased stability and keratocyte density in the cornea. Exp. Eye Res. 2002;75:683–694. doi: 10.1006/exer.2002.2058. [DOI] [PubMed] [Google Scholar]
- Nishiyama T, McDonough AM, Bruns RR, Burgeson RE. Type XII and XIV collagens mediate interactions between banded collagen fibers in vitro and may modulate matrix deformity. J. Biol. Chem. 1994;269:28193–28199. [PubMed] [Google Scholar]
- Oldberg A, Antonsson P, Moses J, Fransson LA. Amino-terminal deletions in the decorin core protein leads to the biosynthesis of proteoglycans with shorter glycosaminoglycan chains. FEBS Lett. 1996;386:29–32. doi: 10.1016/0014-5793(96)00407-3. [DOI] [PubMed] [Google Scholar]
- Parry DA. The molecular and fibrillar structure of collagen and its relationship to the mechanical properties of connective tissue. Biophys. Chem. 1988;29:195–209. doi: 10.1016/0301-4622(88)87039-x. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu. Rev. Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- Ricard-Blum S, Dublet B, van der Rest M. Unconventional Collagens Types VI, VII, VIII, IX, X, XIV, XVI and XIX. Oxford: Oxford University Press; 2000. [Google Scholar]
- Schuppan D, Cantaluppi MC, Becker J, Veit A, Bunte T, Troyer D, et al. Undulin, an extracellular matrix glycoprotein associated with collagen fibrils. J. Biol. Chem. 2001;265:8823–8832. [PubMed] [Google Scholar]
- Shaw LM, Olsen BR. FACIT collagens: diverse molecular bridges in extracellular matrices. Trends Biochem. Sci. 1991;16:191–194. doi: 10.1016/0968-0004(91)90074-6. [DOI] [PubMed] [Google Scholar]
- Trueb J, Trueb B. The two splice variants of collagen XII share a common 5′ end. Biochim. Biophys. Acta. 1992;1171:97–98. doi: 10.1016/0167-4781(92)90145-p. [DOI] [PubMed] [Google Scholar]
- Walchi C, Koch M, Chiquet M, Odermatt BF, Trueb B. Tissue-specific expression of the fibril-associated collagens XII and XIV. J. Cell Sci. 1994;107:669–681. doi: 10.1242/jcs.107.2.669. [DOI] [PubMed] [Google Scholar]
- Young BB, Gordon MK, Birk DE. Expression of type XIV collagen in developing chicken tendons: association with assembly and growth of collagen fibrils. Dev. Dyn. 2000;217:430–439. doi: 10.1002/(SICI)1097-0177(200004)217:4<430::AID-DVDY10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Young BB, Zhang G, Birk DE. The roles of type XII and XIV collagen in fibrillogenesis and matrix assembly in the developing cornea. J. Cell. Biochem. 2002;87:208–220. doi: 10.1002/jcb.10290. [DOI] [PubMed] [Google Scholar]