Abstract
The conus (bulbo-ventricular) valves of teleosts perform a key function in the control of blood backflow during ventricular diastole. However, the structural characteristics of these valves are almost unknown. This paper presents a systematic anatomical, histological and structural study of the conus valves of the adult gilthead seabream (Sparus auratus). S. auratus shows two major left and right valves consisting of the leaflet and the supporting sinus. Each valvar leaflet can be divided into a stout proximal body and a flap-like distal region. The proximal body is structured into three layers: a luminal fibrosa, a dense cellular core and a parietal fibrosa. The luminal fibrosa is a collagenous structure extending the entire length of the leaflet, while the parietal fibrosa is restricted to the most proximal area. The dense cellular core consists of fibroblastic cells and a matrix rich in glycoconjugates, collagen and elastin. The histochemical and structural data suggest that the luminal fibrosa bears most of the force associated with valvar closure, while the cellular core acts as a cushion dampening vibrations and absorbing the elastic recoil. The sinus wall is a fibrous layer which shows proximal–distal differences in thickness. It also shows compositional differences that can be related to mechanical function. We describe the presence of a fibrous cylinder formed by the sinus wall, the fibrous interleaflet triangles and the fibrous layer that covers the inner surface of the conus myocardium. This fibrous cylinder constitutes the structural nexus between the ventricle, the conus and the bulbus arteriosus, provides support for the conus valves and separates the valvar complex from the surrounding tissues. The structure of the conus valves in S. auratus is different from that found in other vertebrates. Anatomical similarities between the conus valves and the mammalian arterial valves are emphasized. Each phyletic group appears to have developed specific structures in order to perform similar functions.
Keywords: conus valves, gilthead seabream, heart, lectins, teleost
Introduction
The cardia outflow tract in teleostean fishes is furnished with a single transverse row (rarely two rows) of valves, the number and shape of which vary between species and, to a lesser extent, between members of the same species (Boas, 1880a, b ; Senior, 1907a, b; Smith, 1918; Parsons, 1930; Bertin, 1958). Classically, only primitive teleost species have been considered to display a more or less developed muscular conus arteriosus interposed between the ventricle and the bulbus cordis. In these species, the valvar leaflets are anchored to this muscular segment and are termed conus valves (Smith, 1918). In evolutionary advanced teleost species, which have presumably lost the conus arteriosus, the valvar leaflets are described as being attached to the cephalic end of the ventricle or to the wall of the bulbus arteriosus (Smith, 1918; Bertin, 1958). As a result, the cardiac outflow tract valves of teleosts have usually been termed bulbo-ventricular valves (Munshi & Mishra, 1974; Priede, 1976; Thomas, 1976; Greer-Walker et al. 1985; Santer, 1985; Raso, 1993; Raso et al. 1994). However, we have recently demonstrated that in the adult gilthead seabream (Sparus auratus), and probably in most adult teleosts, the conus is a conserved, distinct muscular segment which supports the outflow tract valves (Schib et al. 2002). Therefore, these valves are more appropriately termed conus valves. As a direct continuation of the previous report on the structure of the conus arteriosus (Schib et al. 2002), we present here a structural study of the conus valves.
The conus valves open and close during the heart contractile cycle, preventing the reflux of blood into the ventricle. Despite their obvious importance, little information is available on the structural characteristics of the conus valves in teleosts. Brief accounts of their histological characteristics have been published for Macrourids (Greer-Walker et al. 1985) and for the adult white bass (Morone chrysops) (Raso, 1993). However, systematic analyses have not been carried out on the structure of the conus valves. This type of study would be of great value and would lead to a better understanding of the relationship between structure and function in fish valves. We have undertaken a study of the anatomical, histological and immunohistochemical characteristics of the conus valves in the adult gilthead seabream. This species represents a wide group of perciforms and is commonly used for cardiovascular research in our laboratory. In addition, the morphological basis of cardiovascular performance in this fish species is most interesting, since the gilthead seabream is intensively farmed and has increasing commercial value. Our findings may provide a suitable basis to assess the relationships between the structural design of the valves and their mechanical function, as well as to gain further insight into the biomechanical principles governing the performance of the valvular system of the cardiac outflow tract in teleosts.
Materials and methods
Twenty-one adult gilthead seabream (19 male, aged 1–2 years; and two female, older than 3 years) were either purchased at the local fishmarket or obtained alive at the fish farms Cupimar and Pemares, Cádiz, Spain. Live specimens were killed with an overdose of MS-222 (tricaine methane sulfonate, Sigma Chemical Co., UK). Then, the hearts were removed from the pericardial cavity, transferred to an ice cold phosphate-buffered saline (PBS) (pH 7.4), and lightly perfused with the same buffer.
Semithin sections and transmission electron microscopy (TEM)
Two hearts were fixed in 2% glutaraldehyde and 1% paraformaldehyde in PBS for 3 h. Then, fragments of the bulbus containing valvular structures were post-fixed in 1% osmium tetroxide, dehydrated in graded acetone and propylene oxide, and embedded in Araldite (Fluka). Semithin sections were cut with a LKB III ultratome, stained with 1% toluidine blue, and observed with a Zeiss III photomicroscope. Ultrathin sections were cut with a Leica Ultracut UCT, stained with uranyl acetate and lead citrate, and examined with a Zeiss ME 10C microscope.
Scanning electron microscopy (SEM)
Four hearts were dissected longitudinally under a stereomicroscope to expose the conus valves, pinned to a paraffin bed, and immersion-fixed in 3% glutaraldehyde in PBS for 3 h. The specimens were then dehydrated in graded acetone, dried by the critical point method, and gold-sputter-coated. Observations were made using a Philips SEM 501 scanning microscope.
Conventional histology and histochemistry
Twelve hearts were fixed in Bouin's or in MAW fixative (methanol–acetone–water, 2 : 2 : 1), embedded in paraffin and serially cut at 10 µm. Four specimens were sectioned longitudinally, three sagittally and five transversely. The sections were stained with either haematoxylin and eosin for a general assessment of tissue structure, Mallory's trichrome for connective tissue, resorcin-fuschin stain or orcein for the detection of elastic fibres, 1% alcian blue (pH 2.5) for a general localization of glycosaminoglycans, and the periodic acid-Schiff (PAS) reaction to detect mucosubstances.
Immunohistochemistry
Immunolabeling of sections taken from MAW-fixed specimens was performed by using a monoclonal antibody to myosin heavy chain (MF20) (Developmental Studies Hibridoma Bank, University of Iowa, USA), and a polyclonal antibody to laminin (LN) (L9393, Sigma Chemical Co.).
Sections assigned to LN immunostaining were mildly digested with 0.5% papain in 0.02 m phosphate buffer (pH 4.7) at 37 °C for 10 min. Endogenous peroxidase activity was quenched by incubation for 30 min with 6% hydrogen peroxide in TRIS-phosphate-buffered saline (TPBS). Then, the slides were washed twice in TPBS and non-specific binding sites were saturated for 1 h with 3% bovine serum albumin (BSA) in TPBS. Endogenous biotin was blocked with the avidin-biotin blocking kit (Vector, Burlingame, CA, USA).
The primary antibodies were allowed to bind overnight at 4 °C in PBS concentrations of 1 : 100 for MF20 or 1 : 1000 for LN. Control slides were incubated in BSA only. After washing, the slides were incubated for 1 h at room temperature in biotin-conjugated anti-mouse goat IgG (Sigma) diluted 1 : 100 in PBS (for MF20), or in biotin-conjugated anti-rabbit goat IgG (Sigma) diluted 1 : 100 in PBS (for LN). The slides were washed again and incubated for 1 h in avidin–peroxidase complex (Sigma) diluted 1 : 150 in TPBS. Peroxidase activity was developed with Sigma Fast® 3,3′-diaminobenzidine tablets according to the supplier's instructions. The slides were counterstained with Delafield's haematoxylin.
Lectin-binding studies
Three hearts were immersed in cold 96% ethanol–glacial acetic acid (99 : 1) (Saint-Marie, 1962) for 2 h, dehydrated in cold 100% ethanol, cleared in cold xylene, embedded in Paraplast, and serially cut at 8 µm. Dewaxed sections were stained for 2 h with fluorescein isothiocyanate (FITC)-conjugated lectins, 50 U mL−1 in PBS, of different nominal sugar specificities (Table 1), purchased from Vector or Sigma. In addition, some sections were treated for 10 min with Texas red-conjugated propidium iodide, which binds to ribonucleic acids and labels the cell nuclei. The slides were then washed three times (10 min each) in PBS and mounted with Vectashield (Vector).
Table 1. Lectins used in the study.
| Lectin source | Abbreviation | Nominal saccharide specificity |
|---|---|---|
| Canavalia ensiformis | ConA | α-d-mannose; α-d-glucose |
| Maclura pomifera | MPA | α-acetylgalactosamine, > α-galactosamine |
| Arachy hypogaea | PNA | d-galactose(1–3)-N-acetyl-d-galactosamine |
| Triticum vulgaris | WGA | N-acetyl-d-glucosamine |
| N-acetyl neuraminic acid | ||
| Triticum vulgaris | WGAs* | N-acetyl-d-glucosamine |
| Ricinus communis | RCA-I | β-d-galactose |
Succinylated
Selected tissue sections were treated with neuraminidase prior to lectin staining. Neuraminidase from Clostridium perfringens (Type V, Sigma) was diluted (1 Ul mL−1) in 0.05 m acetate buffer, pH 5.3, and applied to the sections for 4 h at 37 °C. This procedure exposes the penultimate carbohydrate residues blocked by sialic acid (Uehara et al. 1985). The sections were then washed in acetate buffer and stained. Lectin-binding specificity was tested by pre-incubation of the lectin conjugates with 0.2 m solutions of the nominal specific sugars (Sigma) (Sarkar et al. 1981; Goldstein & Poretz, 1986; Damjanov, 1987). All the controls were routinely negative. Labelling was assessed by means of a laser confocal Bio-Rad MRC-1024.
Results
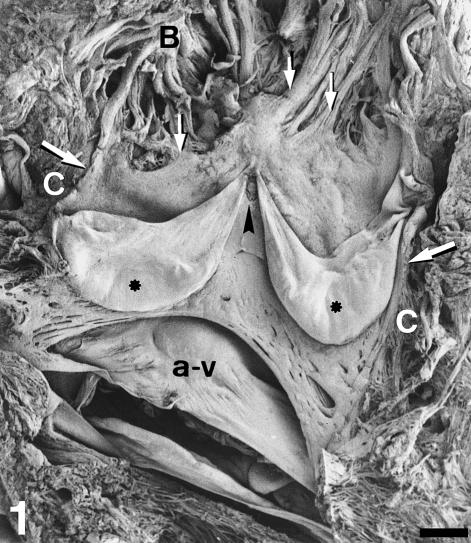
The conus valves of the adult gilthead seabream are anchored to the myocardial conus arteriosus, extending from the conus to the bulbus arteriosus (Fig. 1). All the hearts examined showed two major valves, left and right. In addition, we found accessory valves in 12 specimens. In nine there was a dorsal accessory valve, while in the other three there were two accessory valves, one dorsal and one ventral. The size of the accessory valves ranged between about one-quarter of the normal valvar size and simple rudiments (Fig. 1). Each valve had two components, i.e. the leaflet and the supporting sinus. The leaflet is the mobile, pocket-like component of the valve. Each leaflet presents an adherent border attached to the supporting sinus, a free border lying distally, a luminal side, and a parietal side facing the sinus. The attachment border of the leaflet crosses the conus arteriosus and extends to the bulbus arteriosus distally. As a result of the semilunar design, the valvar leaflets approach each other distally and meet (Fig. 1). The space between adjacent valvar leaflets is triangular. In a few cases the leaflets do not join distally. Then, the space between adjacent leaflets adopts a trapezoidal shape. The accessory valves always appear in this trapezoidal space. The sinus valve can be defined as the more or less hollow portion of the cardiac outflow tract which supports the leaflet. The wall of the sinus constitutes the boundary between the valve and the surrounding tissues.
Fig. 1.
Open-cut view of the conus valves. SEM. The conus arteriosus has been opened along the ventral midline. The left and right semilunar conus valves are exposed. A posterior valvar rudiment (arrowhead) is also present. Each valvar leaflet consists of a stout proximal body (asterisk) and a distal flap-like area. The smooth appearance of the inner heart surface at the valvar level is due to the presence of the fibrous cylinder. Its distal rim (small arrows) is irregular. Note the continuity with the bulbus (B) ridges. Large arrows indicate the section of the cylinder. a-v, atrioventricular valve. C, conus myocardium. Scale bar = 2 mm.
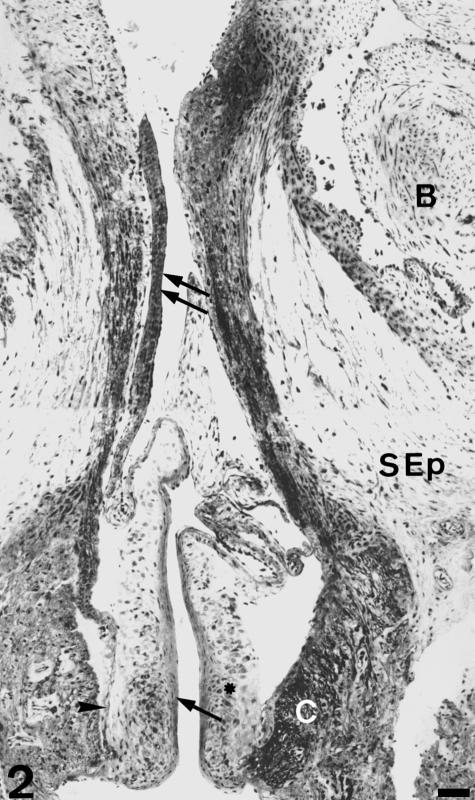
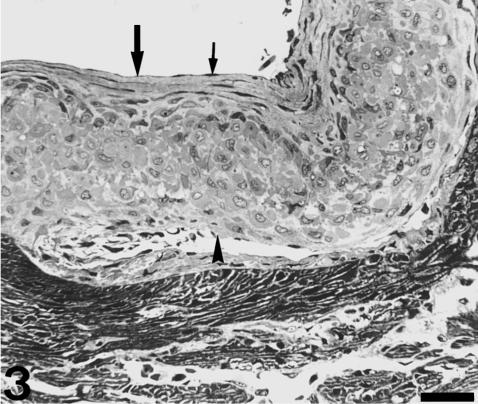
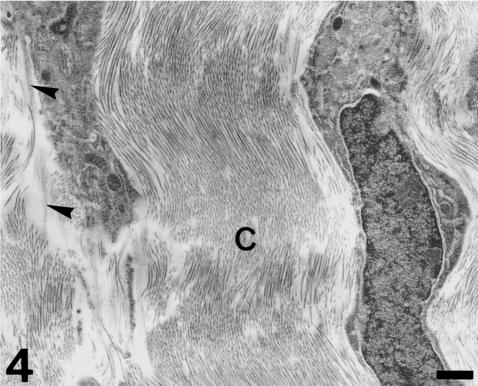
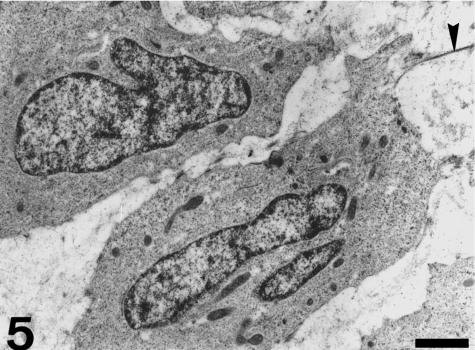
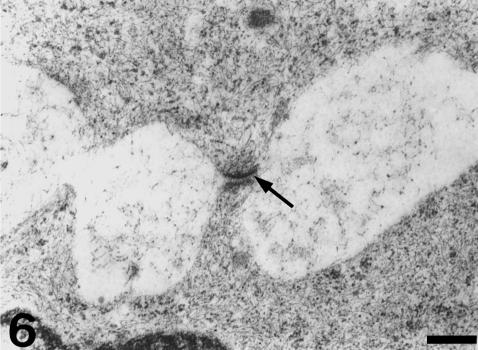
Each valvar leaflet consists of two main portions (Figs 1 and 2). There is a stout proximal body, wit a crescentic shape, and a much thinner, flap-like distal region. The boundary between these two portions follows the general contour of the leaflet, although the lateral parts of the proximal body do not extend distally. At the level of the proximal body, the valvar leaflet is formed by three distinct tissue layers (Figs 2 and 3). There is a fibrous layer on the luminal side, a cellular core, and a fibrous layer on the parietal side. The luminal fibrosa is a thick collagenous layer containing elongated cells which are mostly orientated in the circumferential direction (Fig. 3). TEM revealed that this fibrosa is made up of wavy collagen bundles and elongated fibroblasts which appear aligned in parallel to each other (Fig. 4). Fibroblasts show elongated nuclei, small mitochondria, free ribosomes and profiles of rough endoplasmic reticulum. The surface of these cells is in contact with collagen fibres and bundles and with discrete elastin fibres. Solitary cilia may also be present. The core of the proximal body is a compact tissue consisting of densely grouped rounded or polygonal cells, embedded in an extracellular matrix (Fig. 3). The cell density is higher on the proximal than on the distal part of the proximal body. These cells show a euchromatic, often lobed nucleus, and a clear cytoplasm containing a ground filamentous meshwork, small mitochondria and some free ribosomes and cisternae of endoplasmic reticulum (Fig. 5). A solitary cilium may also be present. The cells are joined to each other by numerous tight junctions (Fig. 6). The extracellular space is occupied by fibrillar and granular material, collagen fibrils and elastin material. The parietal fibrosa shows the same structural components as the luminal fibrosa, but its organization is looser. It is also restricted to the most proximal part of the leaflet, becoming irregular and disappearing at about the middle height of the proximal body. The endocardium covering the leaflets is formed by unconspicuous flattened cells (Fig. 3) which may present short cell prolongations extending into the subjacent matrix. The endocardial basement membrane is well developed on the luminal side but appears patchy and irregular on the parietal side.
Fig. 2.
Semithin longitudinal section of the conus valves. Each valvar leaflet shows a luminal (single arrow) and a parietal (arrowhead) fibrosa. The former continues distally (double arrow) in the flap-like valvar portion. The stout proximal body (asterisk) consists of a dense cellular tissue. The sinus wall is composed of a dense fibrous tissue which is bounded, from proximal to distal, by the conus myocardium (C), the loose subepicardial tissue (SEp) and the bulbus arteriosus (B). The sinus wall is much thicker when it is bounded by the subepicardial tissue. Scale bar = 1 mm.
Fig. 3.
Transverse section of the valve. The stout proximal body consists of a dense cellular tissue bounded by a thick luminal fibrosa (large arrow). The parietal fibrosa (arrowhead) is barely discernible at this section level. Small arrow indicates flattened endocardial cells. Scale bar = 50 µm.
Fig. 4.
Transverse section of the luminal fibrosa. This fibrosa consists of wavy collagen (C) bundles, typical fibroblasts and discrete elastin fibres (arrowheads). Scale bar = 0.5 µm.
Fig. 5.
Cells in the proximal body display euchromatic nucleus and a clear cytoplasm containing numerous small mitochondria and a ground matrix. Arrowhead, collagen fibre. Scale bar = 1 µm.
Fig. 6.
Detail of two proximal body cells. Note the presence of numerous ribosomes and polyribosomes, and a ground filamentous meshwork. A tight junction is indicated (arrow). The extracellular matrix contains a network of filaments and small granules, and some amorphous material. Scale bar = 300 nm.
The flap-like, distal region of the leaflet is composed of the luminal fibrosa and the endocardial covering, although the fibrosa is less organized in this area. Thus, the luminal fibrosa (together with the endocardium) is the only layer common to the entire leaflet (Fig. 2).
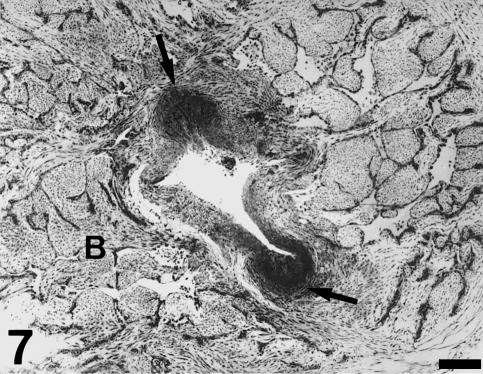
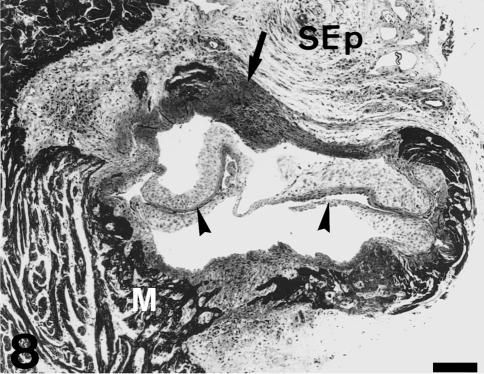
The sinus wall is formed by a fibrous layer covered by the endocardial endothelium on its inner side. The fibrous sinus wall is attached to the surrounding tissues on its external side (Fig. 2). Its distal part is attached to the inner surface of the bulbus arteriosus (Fig. 7). We could not detect interposed structures or specific junctional features, except for a higher density of cells and extracellular fibres (Fig. 2). Its middle portion is delimited by the loose subepicardial tissue (Fig. 2). At the proximal body level, it is attached to the conus myocardium (Fig. 8). On the other hand, the fibrous sinus wall is continuous with the fibrous tissue present in the interleaflet triangles. Thus, a fibrous cylinder is formed, which constitutes the structural nexus between the single ventricle, the conus and the bulbus arteriosus. Furthermore, it provides support for the valvar leaflets. This fibrous cylinder can be seen not only in transverse sections (Figs 7 and 8), but also in open-cut views of the heart (Fig. 1). Its upper limit is irregular and covers the proximal part of the bulbar ridges. The thickness of this cylinder is irregular (Figs 7 and 8). It is very thick at the bulbus arteriosus level, but is reduced to a thin layer when it is surrounded by the conus myocardium (the inner fibrous layer of the conus; see Schib et al. 2002). Downstream, the fibrous cylinder is continuous with a thick collagenous layer present in the subendocardium of the main ventricular lumen (Fig. 2).
Fig. 7.
Transverse section of the heart through the proximal part of the bulbus arteriosus (B). The fibrous cylinder attaches to the bulbar ridges. The anterior and posterior areas (arrows) appear denser and correspond to the points of valvar insertion. Scale bar = 250 µm.
Fig. 8.
Transverse section of the heart through the upper part of the proximal body. Same specimen as in Fig. 7. Owing to the semilunar shape, the middle portion of the leaflets is only formed by the inner fibrosa (arrowhead). The fibrous cylinder is very thin when bounded by the conus myocardium, but appears thick (arrow) when bounded by the loose subepicardial (SEp) tissue. Scale bar = 250 µm.
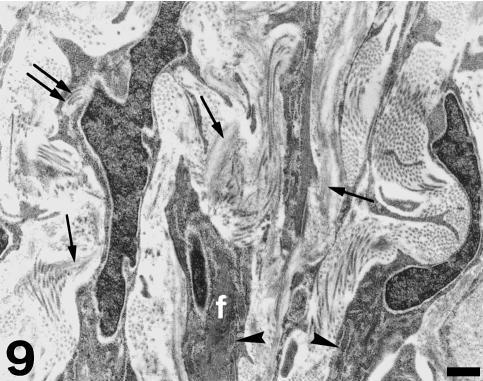
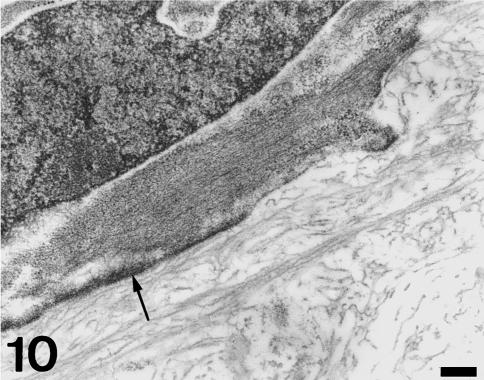
The thick part of the fibrous cylinder consists of cells showing microfilament bundles, dilated cisternae of rough endoplasmic reticulum, mitochondria, secretory vacuoles and discrete subplasmalemmal condensations (Figs 9 and 10). The extracellular matrix is mostly composed of collagen bundles orientated in several directions and abundant elastin fibres. The thin part of the fibrous cylinder, in contact with the conus myocardium, corresponds to the inner fibrous conus layer. This area contains typical fibroblasts, large amounts of collagen and discrete amounts of elastin. It has been described previously by Schib et al. (2002).
Fig. 9.
TEM view of the fibrous cylinder at the sinus valvar level. Cells show elongated, irregular shapes, filament bundles (f) and cisternae of the rough endoplasmic reticulum (arrowheads). The extracellular matrix contains collagen bundles and numerous elastin fibres (single arrows). Double arrow indicates a cytoplasmic invagination containing collagen fibrils. Scale bar = 0.5 µm.
Fig. 10.
Detail of an interstitial cell showing a bundle of microfilaments surrounded by polyribosomes. Arrow indicates a long subplasmalemmal condensation. The extracellular matrix contains elastin material. Scale bar = 300 nm.
The histochemical and immunohistochemical studies help to elucidate the composition and architecture of the valvar tissue. Mallory's trichrome (Fig. 11) illustrates the collagenous composition of the valvar fibrous layers. Furthermore, these studies revealed that the luminal fibrosa of the valvar leaflets is continuous with the fibrous cylinder at the leaflet adherent border in such a way that a kind of fibrous joint is formed along that border. This coupling was more apparent in the central part of the valve. Resorcin–fuschin and orcein established further compositional differences within the fibrous cylinder (Figs 12 and 13). Thus, the part of the fibrous cylinder corresponding to the valvar sinus is very rich in elastin. However, the interleaflet triangles and the areas corresponding to the leaflet attachment are almost exclusively formed by collagenous material. Within the leaflets, delicate elastin strands traverse the proximal body from the luminal to the parietal side (Fig. 14). Furthermore, elastin fibres run longitudinally along the parietal side of the leaflets. The matrix within the valvar leaflets appears to be very rich in acidic glycoconjugates since it stained intensely with Alcian blue (Fig. 15). This matrix was also PAS-positive (Fig. 16). On the other hand, the cells forming the dense core of the stout proximal body were negative for both Alcian blue and PAS (Figs 15 and 16). Laminin was detected on the luminal surface of the valvar leaflets, but not on the parietal side (Fig. 17). The positive reaction of the supporting muscular conus to the MF20 antibody has been described previously (Schib et al. 2002).
Fig. 11. Histochemistry and immunohistochemistry of the conus valves. B, bulbus; C, conus; S, sinus wall; SEp, subepicardial tissue.
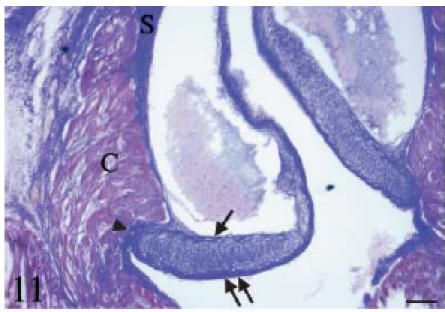
Mallory's trichrome. Longitudinal section. The leaflet parietal (arrow) and luminal (double arrow) fibrosa are intensely positive for collagen (blue colour). The differences in thickness and extension between the two fibrous layers are evident. The cellular core of the proximal body also stains for collagen. The valvar system is bounded by the conus myocardium (C). Note the fibrous continuity (arrowhead) between the valvar fibrosa, the collagenous sinus wall and the subendocardial collagen. Scale bar = 50 µm.
Fig. 13. Histochemistry and immunohistochemistry of the conus valves. B, bulbus; C, conus; S, sinus wall; SEp, subepicardial tissue.
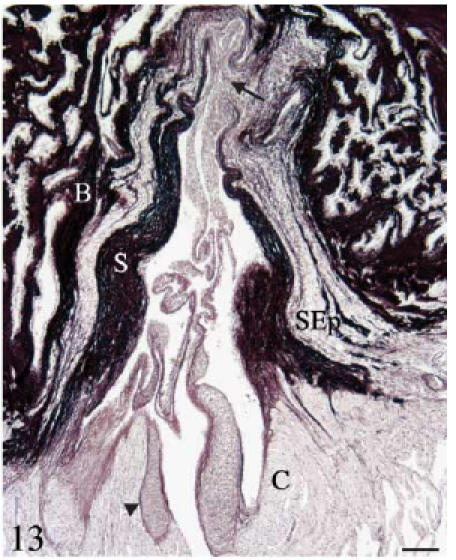
Orcein. Longitudinal section. The fibrous cylinder shows two different parts. The area corresponding to the sinus wall is very rich in elastin. The areas corresponding to the valvar attachments (arrow) are poor in elastin but rich in collagen. The subepicardial tissue also shows elastin fibres. Scale bar = 100 µm.
Fig. 16. Histochemistry and immunohistochemistry of the conus valves. B, bulbus; C, conus; S, sinus wall; SEp, subepicardial tissue.
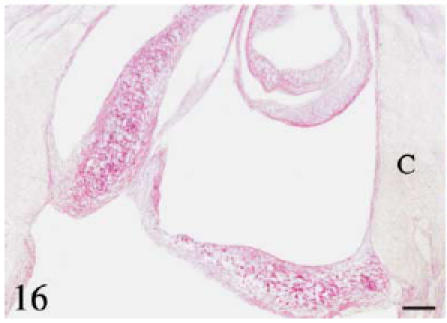
Longitudinal section through the conus valves. PAS. Positive staining is observed in the proximal body and along the subendocardial line. Scale bar = 50 µm.
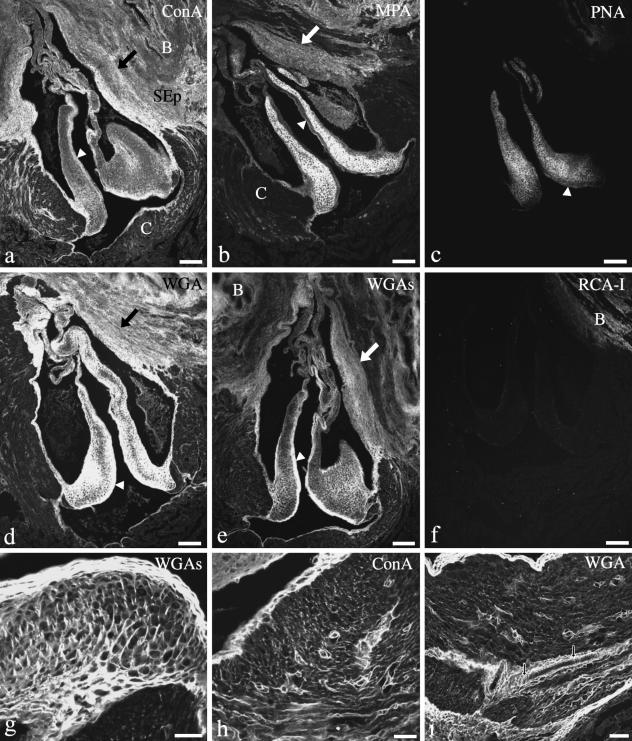
The presence of sugar residues was detected by lectin immunohistochemistry. Table 2 summarizes the lectin distribution and the levels of staining. The luminal and parietal fibrosa were highly positive to ConA, WGA and WGAs, but appeared negative to MPA, PNA and RCA-I (Fig. 18). The dense cellular core was highly positive to MPA and WGA. However, the intensity of staining decreased greatly when the succinylated form of the WGA was used. This decrease also occurred in the flap-like part of the valve, the fibrous cylinder and the subepicardial tissue, but not in the fibrous layers of the proximal body or in the conus myocardium (Fig. 18d,e). Lectin-binding in the cellular core always corresponded to the extracellular matrix (Fig. 18g). In addition, the lectin-binding studies helped to establish further compositional differences in the conus valves. ConA, MPA, PNA and WGAs did not bind to the flap-like portion of the valve while they did bind to one or more valvar components at the level of the proximal body. In the muscular conus arteriosus, lectin staining was mostly found in association with the collagen accompanying the coronary vessels (Fig. 18h–j). Of all the lectins tested, only RCA-I was negative to all the valvar components. No cryptic PNA-binding sites were observed after the neuraminidase treatment.
Table 2. Lectin distribution in the conus valves.
| Valvar leaflet | ||||||
|---|---|---|---|---|---|---|
| Stout Proximal Body | ||||||
| Lectin | Lumen. fibr. | Pariet. fibr. | Cell. core | Flap-like Part | Fibr. Cylin. | Conus |
| ConA | + | + | + | – | + + + | + + + |
| MPA | – | – | + + + | – | + | + (–) |
| PNA | – | – | + + | – | – | – |
| NPNA | – | – | + + | – | – | – |
| WGA | + + + | + + + | + + + | + + | + + (+) | + |
| WGAs | + + + | + + + | + | – | + | + |
| RCA | – | – | – | – | – | – |
Fibr. Cylin., fibrous cylinder; Lumen. Fibr., luminal fibrosa; Pariet. Fibr., parietal fibrosa. For lectin abbreviations, see Table 1.
Fig. 18.
Lectin-binding pattern of the conus valves. B, bulbus; C, conus; SEp, subepicardial tissue. The thick part of the fibrous sinus wall (arrows) and the luminal fibrosa (arrowheads) are indicated. At the level of the proximal body the fibrosa are highly positive to ConA (a), WGA (d) and WGAs (e), faintly positive to PNA (c) and negative to MPA (b). In the flap-like part of the valve, the fibrosa is negative to all lectins except to WGA (d). The dense cellular core is highly positive to MPA (b) and WGA (d), moderately positive to ConA (a), PNA (c) and WGAs (e), and negative to RCA-I (f). Lectin-binding localizes outside the cells as shown in a detailed view of the cellular core (g). Lectin-binding in the conus myocardium is mostly associated with the collagenous component of blood vessels and connective septa (h–i). Black and white arrows in (i) indicate fine collagenous fibres wrapping a vessel. Scale bars = a–f, 100 µm; g–h, 25 µm.
Discussion
The gilthead seabream possesses a single row of conus valves formed by two major, left and right, valves. This coincides with the anatomical arrangement in most teleost species (Senior, 1907a, b; Parsons, 1930; Bertin, 1958). Only a few teleostean genera belonging to the primitive order of the Elopiformes have two rows of valves (Senior, 1907a, b, c). The presence of accessory valves located in a dorsal and/or ventral position is also a common feature in teleosts. The small number of conus valves contrasts with the situation in most elasmobranches (Parsons, 1930; White, 1936; Bertin, 1958; Santer, 1985) and in the primitive actynopterigians (Boas, 1880a, b; Parsons, 1930; Bertin, 1958; Icardo et al. 2002), in which the number of valvar rows and of conus valves is very high. The decrease in the number of valves in teleosts is a consequence of the reduction in the size of the conus arteriosus that has occurred in this fish group.
Each conus valve consists of two components, the leaflet and the sinus. Each valvar leaflet is structured into three layers: the luminal fibrosa, the cellular core and the parietal fibrosa. The collagenous luminal fibrosa appears to be the strongest portion of the valve mechanically. It should create a stiff border providing the valvar leaflets with enough strength to sustain their apposition during valvar closure. The parietal fibrosa is less organized and only appears in the most proximal part of the leaflet. The distribution of the two fibrous layers is similar in the white bass Morone chrysops (Raso, 1993; Raso et al. 1994) and in other members of the Sparid and Serranid families (Icardo, unpublished observations). However, there are some differences with other fish groups. In elasmobranches (Sans-Coma et al. 1995) there is a luminal fibrosa and a parietal fibrosa (termed outer and inner, respectively), the latter extending the entire length of the leaflet while, in the sturgeon, there is only a luminal fibrosa (Icardo et al. 2002). Thus, despite differences between species, a feature common to all these fish groups is the presence of a thick luminal fibrosa, which appears to be the principal structure bearing the stress generated by the backflow of blood (Iwamizu & Itazawa, 1989; Raso, 1993; Raso et al. 1994; Sans-Coma et al. 1995; Icardo et al. 2002).
This situation contrasts with that observed in mammals, where the fibrosa is located on the parietal (aortic) side of the cardiac semilunar leaflets (Gross & Kugel, 1931; Thubrikar, 1990). The luminal side is lined by the so-called ventricularis, a thin layer – sometimes absent – consisting of loosely arranged elastic and collagen fibres (Gross & Kugel, 1931; Thubrikar, 1990; Icardo & Colvee, 1995). The reasons for this phylogenetic shift remain unclear.
In the gilthead seabream, the middle layer of the leaflets is a dense cellular tissue consisting of fibroblastic cells rich in microfilaments, and an extracellular matrix rich in glycoconjugates and some collagen and elastin fibres. Glycoconjugates are able to absorb water and to control tissue hydration (Mathews, 1967), thereby regulating the viscoelastic properties of the leaflet and providing a compressible internal milieu capable of absorbing the tissue deformations during opening and closure of the valves. They may also be involved in the maintenance of the tissue architecture by controlling cell interactions (Toole, 1981) within the proximal body. Thus, the cellular core may have an important role in leaflet mechanics, probably acting as a cushion that absorbs the recoil initiated from the front of the valve (Raso et al. 1994). The distinct organization of the elastin fibres in the leaflet suggests that elastin also performs an important function in the maintenance of the valvar structure and in the elastic recovery of the leaflet. On the other hand, the mechanical requirements of the proximal body and of the flap-like portion of the valve are obviously different.
The existence of distinct lectin-binding patterns between the two valvar portions further indicates that the conus valves are composite structures. The cellular core was positive to all the lectins tested (except to RCA), while the flap-like part was positive to WGA only. The use of the sequence WGA/WGAs revealed that the affinity of the luminal fibrosa and of the parietal fibrosa to WGA was exclusively due to the presence of N-acetyl-d-glucosamine. On the contrary, the positivity of the flap-like part of the valve was exclusively due to N-acetyl neuraminic acid (sialic acid). This indicates that the flap-like part of the valve has a sialoprotein that provides this part of the valve with numerous anionic sites. The anionic sites control the electrostatic properties and the degree of hydration of the tissues (Kerjaschki et al. 1984). The fact that we have not found differences in the staining with PNA after neuraminidase treatment indicates that the valves lack PNA cryptic sites. However, it must be underscored that the exact significance of the lectin-binding patterns can only be analysed in a comparative context. Lectin-binding studies on the cardiac outflow tract valves of vertebrates are lacking. However, our unpublished observations suggest that the gilthead seabream represents a model for teleosts belonging to the Sparid family. Whether the gilthead seabream represents a model for other teleost species remains unknown.
In an earlier study of the conus valves of a different teleost species, the freshwater white bass Morone chrysops, collagen, elastin and proteoglycans were reported to be distributed into layers (Raso, 1993). Whilst the distribution of collagen and laminin agrees with the present findings, elastin and mucopolysaccharides were found to accumulate on the parietal and luminal sides of the valvar leaflets, respectively. The reasons for these differences are uncertain, and we can only speculate that the existence of specific haemodynamic parameters results in different types of stress on the valvar tissue.
On a comparative basis, the middle portion of the semilunar leaflets consists, in elasmobranches (Sans-Coma et al. 1995), chondrosteans (Icardo et al. 2002) and mammals (Gross & Kugel, 1931), of a loose connective tissue termed the spongiosa. In general, the spongiosa contains a small number of cells and large amounts of glycoconjugates whose main functions are to dampen the vibrations of the fibrosa associated with valvar closure (Sauren et al. 1980). It also ties the inner and outer leaflet surfaces into a coherent biomechanical whole (Broom, 1988). As discussed above, our structural findings indicate that the dense cellular core performs a similar function in the gilthead seabream. Thus, different species and group species appear to have developed different structures to perform similar functional roles. While phyletic diversification is accompanied by structural adaptations of the cardiovascular system (Burggren et al. 1997), the exact role of phylogeny, adaptation of or other factors in morphological diversification is presently unknown.
The presence of a fibrous cylinder surrounding the conus valvular complex and establishing the structural nexus between the ventricle, the conus and the bulbus arteriosus is a surprising finding. However, the ventricle–bulbus region described in Macrourids resembles our fibrous cylinder (Greer-Walker et al. 1985). Macrourids are benthic teleost species that have been reported to contain a gelatinous tube inside the heart extending from the valves to the proximal half of the bulbus. This tube is complemented by another layer of gelatinous tissue arising from the proximal rim of the bulbus and invaginating upward to fuse with the inner tube. The drawing reconstructions (Greer-Walker et al. 1985) appear to depict, albeit with the limitations of the techniques used, both the fibrous cylinder (this paper) and the outer fibrous layer of the conus (Schib et al. 2002). The conus is not recognized as a distinct segment, but can clearly be observed in a panoramic view of the heart (Plate I, Greer-Walker et al. 1985). The present results, the observations in Macrourids, and our own unpublished observations in other species of the Sparid and Serranid families, suggest that the presence of a fibrous cylinder is common to many teleost species.
The distribution pattern of collagen and elastin within the fibrous cylinder is also surprising, but it can be explained in mechanical terms. The areas of valvar attachment are considered to bear the greatest force and therefore need to be very stiff. Indeed, resistance is increased in some teleosts by the presence of hyaline cartilage at the sites of valvar attachment (Blanco et al. 2001). In the gilthead seabream, most of the fibrous cylinder is elastin-rich, suggesting that it is very deformable and can be adapted to cyclical deformation. Furthermore, it is mostly bounded by the bulbus arteriosus, an elastic chamber (Priede, 1976; Santer, 1985), and by the loose subepicardial tissue. If the fibrous cylinder were a stiff structure, it would create an internal rigidity that could interfere with heart mechanics and create unnecessary tissue stress. The elasticity of the fibrous cylinder should decrease tissue stress in the conus region, favour valvar closure and support deformation during the dilation and subsequent elastic recovery of the bulbus arteriosus. The richness in elastin of the sinus fibrous wall also correlates with cell morphology. The cells in these areas show structural characteristics (filament bundles, subplasmalemmal condensations, micropinocytotic-like vesicles, etc.) of myofibroblasts (see Powell et al. 1999), and appear to be involved in the synthesis of elastin. In contrast, the leaflet fibrosa is mainly collagenous and the cells in this region are typical fibroblasts. The distribution of laminin under the luminal endocardial surface of the leaflets indicates a better development of the basement membrane on this side of the valve, and agrees with previous findings in other teleosts (Raso et al. 1994). The significance of the distribution of laminin in the conus myocardium has been discussed in the previous paper (Schib et al. 2002).
Are the gilthead seabream conus valves and the mammalian arterial valves comparable structures?
We have discussed above the presence of species differences in the fine structure of the conus valves. On a broader sense, it may be pertinent to emphasize the presence of gross anatomical similarities (and differences) with the arterial valves of the higher vertebrates. In mammals and birds, the valvar structures located at the base of the aorta and of the pulmonary trunk are designated as aortic valve and pulmonary valve, respectively. Each arterial valve is considered to be a single entity composed of the leaflets, the sinuses and the interleaflet triangles. In this sense, the gilthead seabream (and in fact, most teleosts) shows a comparable anatomical arrangement. Hence, the simpler term of conus valve could have been properly used in this study. However, fish constitute a very diverse group of vertebrates. Primitive teleosts (Senior, 1907a, b, c), and elasmobranches, polipteriformes, chondrosteans and holosteans (Bertin, 1958; Santer, 1985; Sans-Coma et al. 1995; Icardo et al. 2002), present several rows of outflow tract valves entirely attached to a muscular conus. In all these cases, the anatomical arrangement is not comparable (except for the presence of leaflets and sinuses) to that observed in the gilthead seabream or in mammals. The terminology used here (Sans-Coma et al. 1995) describes the valvar anatomy in fish adequately, and can be applied to all fish groups.
In mammals, several characteristics of the arterial valves have been emphasized because of their anatomical, embryological or surgical importance. For instance, the aortic annulus has been described as a ring-like annulus supporting the valvar leaflets. However, the presence of an aortic annulus has been discredited (Anderson et al. 1991, 1996) owing to the semilunar attachment of the leaflets. This situation is similar in the gilthead seabream. The ventriculoarterial junction has been described in humans as the circular connection between the arterial wall and the underlying ventricle (Stamm et al. 1998). This is clearly different in fish. Although the distal rim of the conus arteriosus is roughly circular, at least in the gilthead seabream (Schib et al. 2002), the teleost ventral aorta is separated from the conus by the interposed bulbus arteriosus. Even so, the semilunar attachment of the gilthead seabream leaflets crosses the distal rim of the conus linking the conus myocardium, the fibrous wall of the sinuses, the interleaflet triangles, and the distal bulbus arteriosus. The fibrous cylinder described here is very specific. It is the result of the structural continuity between the fibrous layer covering the inner face of the myocardial conus arteriousus, the fibrous wall of the sinuses and the fibrous interleaflet triangles. The fact that the fibrous cylinder extends in the gilthead seabream distally, covering the proximal inner side of the bulbus arteriosus, creates a true sinutubular junction that corresponds to the distal, irregular rim of the fibrous cylinder (see Fig. 1). As in humans, the sinutubular junction marks the distal limit of the sinus wall and corresponds to the zones of apposition of the leaflets. Furthermore, the interleaflet triangles are related both to the pericardial cavity (the middle part in direct contact with the subepicardial tissue) and to the supporting musculature (for description in humans, see Anderson et al. 1991; Sutton et al. 1995; Stamm et al. 1998). Only the relations of the distal part of the interleaflet triangles are different: they correspond to the arterial wall in humans, and to the elastic bulbus arteriosus in teleosts.
Finally, a key component of the outflow tract in the mammalian heart is constituted by the supporting ventricular structures: the pulmonary infundibulum on the right side, and the aortic leaflet of the mitral valve and the ventricular septum on the left side (Anderson et al. 1996; Stamm et al. 1998). The fish heart possesses a single ventricle and a single outflow tract. Nevertheless, it shows a distinct muscular segment, the conus arteriosus, which carries the proximal attachment of the outflow valves. Contrary to what occurs in higher vertebrates, the conus arteriosus has not been divided and has not been incorporated into the ventricular mass. It stands free on top of the ventricular musculature. The species differences emphasized above should be of no surprise. The gilthead seabream is an evolutionary advanced teleost species, having evolved phyletically as such. It appears that there is a general developmental pattern for the vertebrate heart, which is modified through evolution to satisfy the physical and physiological demands of a given species or group of species. We assume that any specific pattern matches the needs of the beholder in a close to perfect manner. The number of teleost species sharing the valvar pattern described here is unknown as yet. In this respect, the lack of appropriate studies makes drawing clades of evolutionary significance impossible at this time.
Fig. 12. Histochemistry and immunohistochemistry of the conus valves. B, bulbus; C, conus; S, sinus wall; SEp, subepicardial tissue.
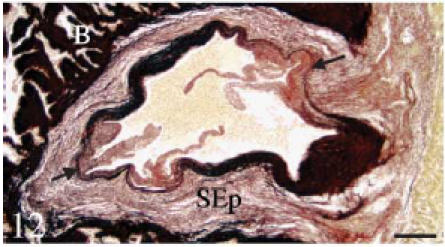
Resorcin-fuschin. Transverse section. The fibrous sinus wall contains large amounts of elastin (black colour). The areas of valvar attachment (arrows) are rich in collagen but contain very little elastin. The bulbus (B) is highly positive. Scale bar = 100 µm.
Fig. 14. Histochemistry and immunohistochemistry of the conus valves. B, bulbus; C, conus; S, sinus wall; SEp, subepicardial tissue.
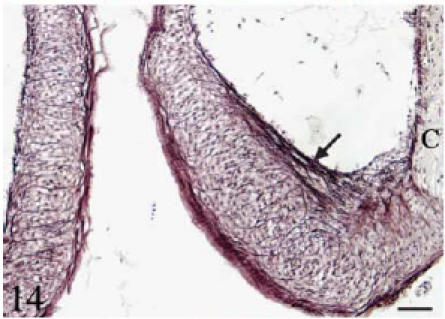
Orcein. Longitudinal section. Individual elastin fibres run longitudinally along the luminal side of the leaflets (arrow). Elastin fibres also course transversally from the parietal to the luminal side of the leaflet. Scale bar = 25 µm.
Fig. 15. Histochemistry and immunohistochemistry of the conus valves. B, bulbus; C, conus; S, sinus wall; SEp, subepicardial tissue.
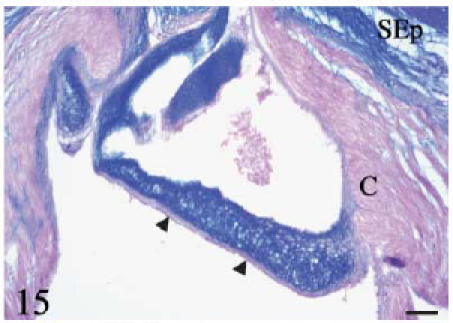
Alcian blue–eosin. Longitudinal section. The valvar leaflet and the subepicardial space are highly positive. Cells in the dense cellular core are negative. The conus myocardium, the valvar fibrosa (arrowheads) and the fibrous cylinder are mostly negative. Scale bar = 25 µm.
Fig. 17. Histochemistry and immunohistochemistry of the conus valves. B, bulbus; C, conus; S, sinus wall; SEp, subepicardial tissue.
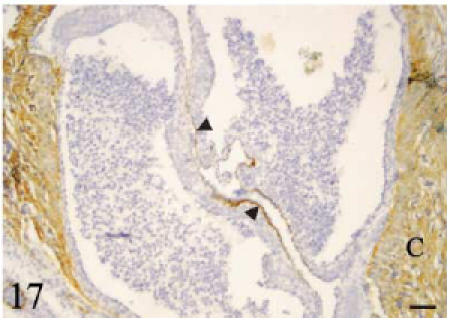
Anti-laminin antibody. Transverse section. The conus myocardium and the luminal side of the leaflets (arrowheads) are positive. No signal is detected on the parietal side of the leaflets. Scale bar = 50 µm.
Acknowledgements
We thank Dr M. T. Berciano for useful insights and R. Garcia-Ceballos, M. Mier and L. González for expert technical assistance. The study was supported by grants PB98-1418-C02-01, PB98-1418-C02-02 and BMC2000-0118-CO2-01 from the Ministerio de Ciencia y Tecnología.
References
- Anderson RH, Devine WA, Ho SY, Smith A, McKay R. The myth of the aortic annulus: the anatomy of the subaortic outflow tract. Ann. Thoracic Surg. 1991;52:640–646. doi: 10.1016/0003-4975(91)90966-t. [DOI] [PubMed] [Google Scholar]
- Anderson RH, Lal M, Ho SY. Anatomy of the aortic root with particular emphasis on options for its surgical arrangement. J. Heart Valve Dis. 1996;5(Suppl. 3):S249–S257. [PubMed] [Google Scholar]
- Bertin L. Appareil circulatoire. In: Grassé PP, editor. Traité de Zoologie. Vol. XIII. Paris: Masson; 1958. pp. 1399–1458. [Google Scholar]
- Blanco C, Lopez D, De Andres AV, Schib JL, Gallego A, Duran AC, et al. Cartilage in the bulbus arteriosus of teleostean fishes. Neth. J. Zool. 2001;51:361–370. [Google Scholar]
- Boas JEV. Über Hertz und Arterienbogen bei Ceratodus und Protopterus. Morph. Jahrbücher. 1880a;6:321–354. [Google Scholar]
- Boas JEV. Über den Conus arteriosus bei Butirinus und bei anderen Knochenfischen. Morph. Jahrbücher. 1880b;6:527–534. [Google Scholar]
- Broom ND. Connective tissue function and malfunction: a biomechanical perspective. Pathology. 1988;20:93–104. doi: 10.3109/00313028809066618. [DOI] [PubMed] [Google Scholar]
- Burggren WW, Farrell A, Lillywhite H. Vertebrate cardiovascular systems. In: Dantzler WH, editor. Handbook of Physiology, Sect13, Comparative Physiology. Vol. 1. New York: Oxford University Press; 1997. pp. 215–308. [Google Scholar]
- Damjanov I. Biology of disease. Lectin cytochemistry and histochemistry. Lab. Invest. 1987;57:5–20. [PubMed] [Google Scholar]
- Goldstein IJ, Poretz RD. Isolation, physiochemical characterization and carbohydrate-binding specificity of lectins. In: Liner RD, Sharon N, Goldstein IJ, editors. The Lectins, Properties, Functions and Applications in Biology and Medicine. New York: Academic Press; 1986. pp. 33–247. [Google Scholar]
- Greer-Walker M, Santer M, Benjamin M, Norman D. Heart structure of some deepsea fish (Teleostei: Macrouridae) J.Zool., London (a) 1985;205:75–89. [Google Scholar]
- Gross L, Kugel MA. Topographic anatomy and histology of the valves in the human heart. Am. J. Pathol. 1931;7:445–474. [PMC free article] [PubMed] [Google Scholar]
- Icardo JM, Colvee E. Atrioventricular valves of the mouse. III. Collagenous skeleton and myotendinous junction. Anat. Record. 1995;243:367–375. doi: 10.1002/ar.1092430311. [DOI] [PubMed] [Google Scholar]
- Icardo JM, Colvee E, Cerra MC, Tota B. The structure of the conus arteriosus of the sturgeon (Acipenser naccarii) heart. I. The conus valves and the subendocardium. Anat. Record. 2002;267:17–27. doi: 10.1002/ar.10080. [DOI] [PubMed] [Google Scholar]
- Iwamizu N, Itazawa Y. Gross anatomy of the vascular system and lateral musculature of the yellow tail Seriola quingueradiata (Carangidae) Jap. J. Ichthyol. 1989;36:232–251. [Google Scholar]
- Kerjaschki D, Sharkey DF, Farquhar MG. Identification and characterization of podocalyxin the major sialoprotein of renal glomerular epithelial cells. J. Cell Biol. 1984;98:1591–1596. doi: 10.1083/jcb.98.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews MB. Biophysical aspects of acid mucopolysaccharides relevant to connective tissue. Structure and function. In: Wagner BM, Smith DE, editors. The Connective Tissue. Baltimore: Williams & Wilkins; 1967. pp. 304–329. [Google Scholar]
- Munshi JSD, Mishra N. Structure of the heart of Amphipnous cuchia (Ham.) (Amphipnoidae; Pisces) Zool. Anzeiger. 1974;193:228–239. [Google Scholar]
- Parsons CW. The conus arteriosus in fishes. Q. J. Microscop. Sci. 1930;73:145–176. [Google Scholar]
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am. J. Physiol. Cellular Physiol. 1999;277:C1–C19. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- Priede IG. Functional morphology of the bulbus arteriosus of rainbow trout (Salmo gairdneri Richardson) J. Fish Biol. 1976;9:209–216. [Google Scholar]
- Raso DS. Functional morphology of laminin, collagen type IV, collagen bundles, elastin, and proteoglycans in the bulbus arteriosus of the white bass, Morone chrysops (Rafinesque) Can. J. Zool. 1993;71:947–952. [Google Scholar]
- Raso DS, Terracio L, Borg TK. Extracellular matrix components in the heart of the white bass, Morone chrysops (Rafinesque) Can. J. Zool. 1994;72:449–454. [Google Scholar]
- Saint-Marie G. A paraffin embedding technique for studies employing immunofluorescence. J. Histochem. 1962;10:250–256. [Google Scholar]
- Sans-Coma V, Gallego A, Muñoz-Chápuli R, De Andrés AV, Durán AC, Fernández B. Anatomy and histology of the cardiac conal valves of the adult dogfish (Scyliorhinus canicula) Anat. Record. 1995;241:496–504. doi: 10.1002/ar.1092410407. [DOI] [PubMed] [Google Scholar]
- Santer RM. Morphology and innervation of the fish heart. Adv. Anat. Embryol. 1985;89:1–102. doi: 10.1007/978-3-642-70135-1. [DOI] [PubMed] [Google Scholar]
- Sarkar M, Wu AM, Kabat EA. Immunocytochemical studies on the carbohydrate specifity of Maclura pomifera lectin. Arch. Biochem. Biophys. 1981;209:204–220. doi: 10.1016/0003-9861(81)90273-3. [DOI] [PubMed] [Google Scholar]
- Sauren AAHS, Kuijpers W, van Streenhoven AA, Veldpaus FE. Aortiv valve histology and its relation to mechanics: Preliminary report. J. Biomechanics. 1980;13:97–104. doi: 10.1016/0021-9290(80)90183-9. [DOI] [PubMed] [Google Scholar]
- Schib JL, Icardo JM, Durán AC, Guerrero A, López D, Colvee E, et al. The conus arteriosus of the adult gilthead sea bream (Sparus auratus) J. Anat. 2002;201:395–404. doi: 10.1046/j.0021-8782.2002.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior HD. The conus arteriosus in Tarpon atlanticus (Cuvier & Valenciennes) Biol. Bull. 1907a;12:146–151. [Google Scholar]
- Senior HD. Teleosts with a conus having more than one row of valves. Anat. Record. 1907b;4:83–84. [Google Scholar]
- Senior HD. Note on the conus of Megalops cyprinoides (Broussonet) Biol. Bull. 1907c;12:378–379. [Google Scholar]
- Smith WC. On the process of disappearance of the conus arteriosus in teleosts. Anat. Record. 1918;15:65–71. [Google Scholar]
- Stamm C, Anderson RH, Ho SY. Clinical anatomy of the normal pulmonary root compared with that in isolated pulmonary valve stenosis. J. Am. College Cardiol. 1998;31:420–425. doi: 10.1016/s0735-1097(98)00089-8. [DOI] [PubMed] [Google Scholar]
- Sutton JP, Ho SY, Anderson RH. The forgotten interleaflet triangles: a review of the surgical anatomy of the aortic valve. Ann. Thoracic Surg. 1995;59:419–427. doi: 10.1016/0003-4975(94)00893-c. [DOI] [PubMed] [Google Scholar]
- Thomas EI. The minute anatomy of the heart of Anabas testudineus (Cuvier) Zool. Anzeiger. 1976;196:397–404. [Google Scholar]
- Thubrikar M. The Aortic Valve. Boca Raton: CRC Press; 1990. [Google Scholar]
- Toole BP. Glycosaminoglycans in morphogenesis. In: Hay ED, editor. Cell Biology of Extracellular Matrix. New York: Plenum Press; 1981. pp. 259–294. [Google Scholar]
- Uehara F, Muramatsu T, Sameshima M, Kawano K, Koide H, Ohaba N. Effects of neuraminidase on lectin binding sites in photoreceptor cells of monkey retina. Jpn. J. Ophthalmol. 1985;29:54–62. [PubMed] [Google Scholar]
- White E. The heart valves of the Elasmobranch fishes. Am. Museum Novitates. 1936;838:1–21. [Google Scholar]