Abstract
The canine's olfactory acuity is legendary, but neither its main olfactory system nor its vomeronasal system has been described in much detail. We used immunohistochemistry on paraffin-embedded sections of male and female adult dog vomeronasal organ (VNO) to characterize the expression of proteins known to be expressed in the VNO of several other mammals. Basal cell bodies were more apparent in each section than in rodent VNO and expressed immunoreactivity to anticytokeratin and antiepidermal growth factor receptor antibodies. The thin layer of neurone cell bodies in the sensory epithelium and axon fascicles in the lamina propria expressed immunoreactivity to neurone cell adhesion molecule, neurone-specific beta tubulin and protein gene product 9.5. Some neurones expressed growth-associated protein 43 (GAP43): and a number of those also expressed neurone-specific beta tubulin-immunoreactivity. Some axon fascicles were double labelled for those two proteins. The G-protein alpha subunits Gi and Go, involved in the signal transduction pathway, showed immunoreactivity in the sensory cell layer. Our results demonstrate that the canine vomeronasal organ contains a population of cells that expresses several neuronal markers. Furthermore, GAP43 immunoreactivity suggests that the sensory epithelium is neurogenic in adult dogs.
Keywords: canine, vomeronasal, G-protein, GAP43, EGFR
Introduction
The canine vomeronasal organ (VNO) is bilaterally symmetric and lies along the ventrorostral aspect of the nasal septum (Adams & Weikamp, 1984; Salazar et al. 1984). In several vertebrate taxa, VNO sensory neurones detect chemical signals that evoke behavioural and/or physiological changes regarding prey identification, social status and reproductive state (reviewed in: Halpern, 1987; Wysocki & Meredith, 1987; Meisami & Bhatnagar, 1998; Liman, 2001; Takami, 2002; Zufall et al. 2002). These sensory neurones are the receptors of the accessory olfactory system, which is viewed as distinct from the main olfactory system because of molecular, anatomical and functional differences between the two. Although the receptor molecules expressed by VNO and main olfactory sensory neurones contain some significant differences in their amino acid sequences, there may be some overlap in the classes of compounds to which the respective sensory epithelium receptor neurones respond. Specifically, Sam et al. (2001) reported that mouse VNO neurones recognize several odourants also recognized by main olfactory sensory neurones. Those authors suggested that the odourants could act similarly to pheromones and evoke patterned behaviours (Sam et al. 2001).
The overlap in odourant recognition suggests an additional possibility, which is that responses elicited by activation in the two olfactory pathways may result in some combinatorial behaviour that is not strictly stereotypic. In that regard, the VNO may contribute to the canine's acuity relative to detection via the two olfactory pathways. We therefore undertook the study of the canine VNO and report here observations derived from an immunohistochemical analysis of the dog VNO. We used a number of antibodies to compare the expression of neuronal markers with other species and to ask if the sensory neurone population is static or if neurogenesis continues in adult dogs.
We used several markers to identify neuronal populations and to provide an estimate of those neurones’ maturation state. Neurone-specific beta tubulin (BT) (also called Class III beta tubulin) is expressed by neurones throughout the rodent nervous system (Burgoyne et al. 1988) including embryonic rodent, neonatal and adult olfactory epithelium (OE) neurones (Lee & Pixley, 1994; Roskams et al. 1998). In particular, BT is expressed in the rodent VNO (Hofer et al. 2000; Witt et al. 2002) and in the VNO of lemurs and New World monkeys (J. C. Dennis, unpublished observations). Protein gene product 9.5 (PGP9.5) is a ubiquitin hydrolase first isolated from brain (Jackson & Thompson, 1981; Wilkinson et al. 1989). It is a marker of neurones and neuroendocrine cells generally (Thompson et al. 1983) and, more particularly, is expressed in rodent main olfactory epithelium (MOE) (Iwanaga et al. 1992; Taniguchi et al. 1993), rodent VNO sensory epithelium, and rodent and canine accessory olfactory bulb (Taniguchi et al. 1993; Johnson et al. 1994; Nakajima et al. 1998a,b). The Ca2+-independent neural cell adhesion molecule (NCAM) is expressed by neurones in all vertebrates so far examined (Edelman & Chuong, 1982; reviewed in Edelman, 1984). NCAM is expressed in the postnatal rodent OE (Miragall et al. 1988) and VNO (Yoshihara et al. 1997). Growth-associated protein 43 (GAP43) is a membrane-associated protein expressed by neurones undergoing axon extension and synaptogenesis (reviewed in: Skene, 1989; Gispen et al. 1992; Oestreicher et al. 1997). In young rodents, GAP43 is highly expressed by MOE sensory neurones (Verhaagen et al. 1989, 1990) as well as in adults following bulbectomy (Schwob et al. 1992; Yamashita et al. 1998) or chemically induced lesion (Schwob et al. 1995). GAP43 is expressed in embryonic and postnatal rodent VNO (Giacobini et al. 2000; Zubair et al. 2002) as well as VNO following transplantation to brain (J. C. Dennis and E. E. Morrison, unpublished observations). To compare basal cells with the functionally homologous horizontal basal cell population in the MOE, we used antibodies directed against cytokeratin (KER) and epidermal growth factor receptor (EGFR). Finally, we probed the VNO with antibodies directed against two GTP-binding proteins: G∀i2 (Gi) and G∀o (Go). In rodents and opossums, these two proteins are expressed differentially in the VNO sensory epithelium and in the accessory olfactory bulb (AOB) (Shinohara et al. 1992; Halpern et al. 1995; Berghard & Buck, 1996; Jia & Halpern, 1996).
Materials and methods
Animals
All procedures for animal experimentation accorded with NIH animal use guidelines and were approved by the Institutional Animal Care and Use Committee of Auburn University College of Veterinary Medicine. The six animals used in this study were males and females of hound or beagle pedigree and ranged in body weight from 16.0 to 27.1 kg. They were euthanized by members of the faculty of the Small Animal Clinic at the Auburn University College of Veterinary Medicine, or by veterinarians associated therewith. Tissues were removed within an hour of death.
Tissue preparation
The nasal septum was cut in the rostrocaudal axis along the dorsal aspect of the swell body. The hard palate was cut on both sides medial to the molars to a point immediately caudal to the VNO. Transverse incisions were made every 2–3 cm and the tissue blocks were fixed by immersion for 3 days at room temperature in 100 mL 4% paraformaldehyde solution made in phosphate-buffered saline (PBS) (Sigma) and adjusted to pH 7.4. The fixative was changed on day 2. Tissue blocks were washed briefly in three changes of PBS and decalcified in 0.25 m EDTA (Fisher). Following decalcification, larger tissue blocks were cut down to 1-cm blocks, infiltrated and embedded in paraffin. Sections from the middle third along the rostrocaudal axis were cut at 7 µm thickness and mounted on glass slides.
Immunohistochemistry
Mounted tissue sections were deparaffinized in Hemo-D (Scientific Safety Products), hydrated to distilled water (dH2O) and reacted with 0.9% hydrogen peroxide (H2O2) in absolute methanol for 20 min at room temperature (23.5–25 °C) to abolish endogenous peroxidase-like activity. Some tissue sections were subjected to an antigen retrieval procedure. These sections were placed in 10 mm sodium citrate pH 6.0, heated to boiling and allowed to boil for 10 min. Tissues to be analysed by epifluoresence microscopy were transferred from dH2O directly to PBS and washed as above. All tissues were incubated for 20 min in the appropriate blocking solution which was 5% normal serum (Sigma) of the species in which the secondary antibody was made and 2.5% BSA (Sigma) in PBS, then washed twice in PBS for 3 min.
The slides were removed to humidified chambers, primary antibody (Table 1) diluted appropriately in blocking solution was applied, and the tissue sections were incubated overnight at room temperature. The following morning, sections to be analysed with bright-field optics were treated with biotinylated secondary antibodies (Vector) diluted 1 : 200. Sections to be analysed with epifluoresence microscopy received appropriate Alexa-conjugated secondaries (Molecular Probes) diluted at 1 : 500. Sections treated with biotinylated secondaries were then incubated with ABC Elite reagent (Vector) and reacted with either DAB (Vector) or True Blue (KPL), and mounted with an aqueous mountant (DAKO) or dehydrated and mounted with VectaMount (Vector). Some DAB-labelled sections were treated with the second set of immunoreagents as described above. ABC alkaline phosphatase (Vector) replaced the ABC Elite reagent and endogenous alkaline phosphatase activity was inhibited by adding Levamisole (Vector) to the Vector Blue reagent buffer. The slides were mounted with an aqueous mountant. All sections double labelled with fluorochromes received both primary and secondary antibodies as mixed cocktails. Slides prepared for epifluoresence microscopy were mounted with VectaShield (Vector) and sealed with clear nail polish.
Table 1. Antibodies used in the study.
| Antigen | Type | Supplier |
|---|---|---|
| Class III Beta-tubulin | mouse mAb | Covance |
| Cytokeratin | rabbit pAb | DAKO |
| EGFR | mouse mAb | Zymed |
| GAP43 | rabbit pAb | Novus Biologicals |
| Gi2alpha rabbit | pAb | Wako Chemicals USA |
| Goalpha | rabbit pAb | MBL |
| NCAM | goat pAb | Santa Cruz Biotech. |
| PGP9.5 | rabbit pAb | Chemicon Intl. |
All tissues were examined with a Nikon Optiphot II microscope equipped with epifluoroesence. Images were made with an RT Slider digital camera (Diagnostic Instruments) using Spot Advanced software and processed with Photoshop 5.0 (Adobe).
Results
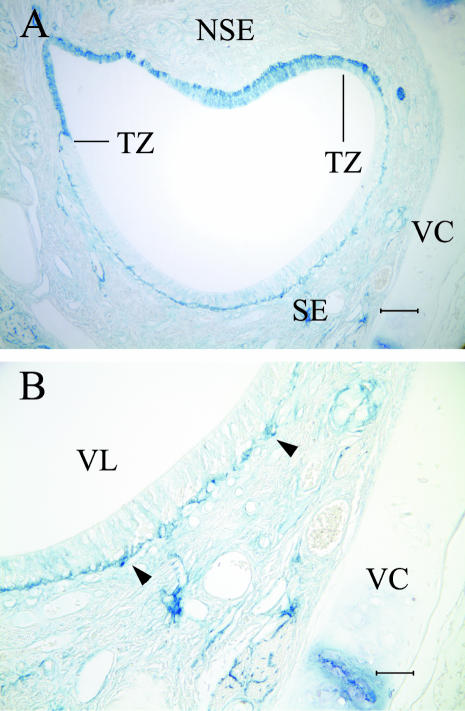
A frontal section through the VNO is shown in Fig. 1 for orientation. As previously described (Adams & Wiekamp, 1984; Salazar et al. 1984), the VNO and the lamina propria contains numerous glands and blood vessels and is largely surrounded by the vomeronasal cartilage (Fig. 1A). Fascicles of the vomeronasal nerve (VNN) occur among small blood vessels and glands in the lamina propria below a relatively thin epithelium (Fig. 1B,C). The lumen of the VNO is lined by sensory and non-sensory epithelium, the latter of which lines roughly one-quarter to one-third of the lumen throughout most of the length of the VNO (Fig. 1C). The sensory epithelium is organized into roughly two compartments: a basal compartment containing basal cells and sensory neurones and an apical compartment containing supporting cells and other apical cells (Fig. 1B,C). At the apposition of the two epithelial types, herein called the transitional zone, the sensory epithelium gives way abruptly to non-sensory epithelium (Fig. 1C).
Fig. 1.
The VNO (A) is associated with numerous blood vessels and glands that are ensheathed by the vomeronasal cartilage (scale bar = 300 µm). The SE (B) contains sensory neurones (arrowhead) in a layer 1–3 cell bodies thick. The neurones are immediately apical to basal cells (arrow) that lie on the basement membrane. Axons fasciculate (Ax) in the lamina propria (scale bar = 50 µm). In (C), the sensory epithelium (arrows) abuts the non-sensory epithelium at the transitional zone (scale bar = 50 µm).
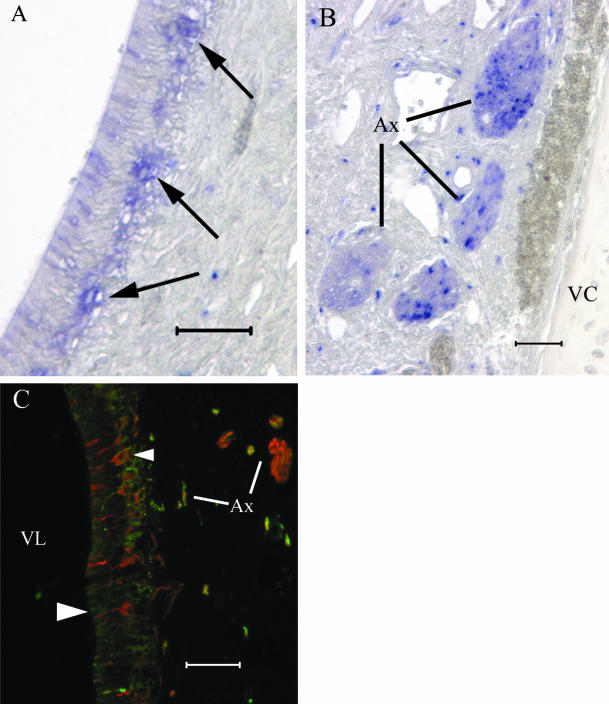
To determine if the canine VNO sensory epithelium expressed neurone-specific molecules, we tested tissue sections with antibodies to several molecules expressed by VNO sensory neurones in other mammalian species. Figure 2 shows several sections treated with anti-BT antibody. At low magnification (Fig. 2A), heavily labelled cell bodies and apical extensions demonstrate the sensory and non-sensory epithelia. At higher magnification, the antibody labels a sensory epithelium cell population that is 1–3 cell bodies thick in the basal compartment of the epithelium (Fig. 2B). In some regions, the dendritic knobs are swollen. The basal BT(+) cell population lies above cytokeratin-expressing cells one layer thick (Fig. 2B). BT signal occurs throughout the non-sensory epithelium in a dispersed punctate pattern (Fig. 2C). Axon fascicles (Ax) in the lamina propria are BT(+) but BT(−) axons are also present in some nerve profiles (Fig. 2D).
Fig. 2.
Heavily labelled BT(+) cells (A) populate the sensory epithelium and punctate BT labelling occurs in the non-sensory epithelium above the right and left transitional zones. The BT(+) sensory neurone population (B) forms a continuous layer immediately apical to KER(+) basal cells (arrowhead). In many of our preparations, the dendritic knobs exhibit a swelling artefact and give the impression of an apical BT(+) cell population (arrow). The non-sensory epithelium (C) contains BT(+) structures but no BT(+) cells were observed. In the lamina propria (D), glands and ducts are KER(+) and fascicles of the VNN contain BT(+) axon bundles that vary in signal intensity (scale bar = 50 µm in all panels).
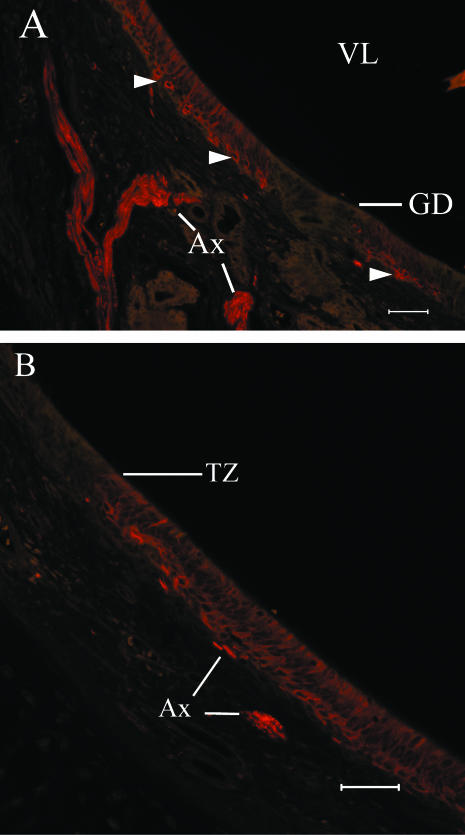
NCAM(+) cells are present in the basal compartment of the sensory epithelium and NCAM(+) axon fascicles are present in the lamina propria (Fig. 3). The antibody we used here labels some neurones intensely and other neurones just above background or not at all. Fascicles of the VNN vary in signal intensity (Fig. 3A). In some sections, the sensory epithelium is interrupted by glands that are NCAM(−) (Fig. 3A). Cellular labelling by anti-NCAM stops abruptly at the transitional zone between the sensory and non-sensory epithelial compartments (Fig. 3B). Double labelling with BT and PGP antibodies shows that at least some neurones express immunoreactivity to both markers as well as some dendrites and axon fascicles in the lamina propria (Fig. 4). In some tissue preparations, anti-PGP labels nuclei differentially: some nuclei are heavily labelled while others are labelled just above background or not at all (Fig. 4).
Fig. 3.
Anti-NCAM (A,B) labels many neurones (arrowheads) in the sensory epithelium and VNN fascicles in the underlying lamina propria. The NSE above the transitional zone (B) and glandular ducts interrupting the sensory epithelium (A) are NCAM(–) (scale bars = 50 µm).
Fig. 4.
A layer of 1–3 PGP(+) cells occupies the basal compartment of the sensory epithelium. A section double-labelled with BT in red (Alexa 594) and PGP in green (Alexa 488) reveals that the sensory neurones do not label consistently with each antibody. Cell somata that are clearly BT(+)/PGP(+) are yellow in colour (small arrowhead). Some dendrites and axon bundles contain both labels. Many nuclei are PGP(+) (small arrowhead) but some nuclei are PGP(–). At the TZ, small structures in the NSE are BT(+) and/or PGP(+) (scale bar = 50 µm).
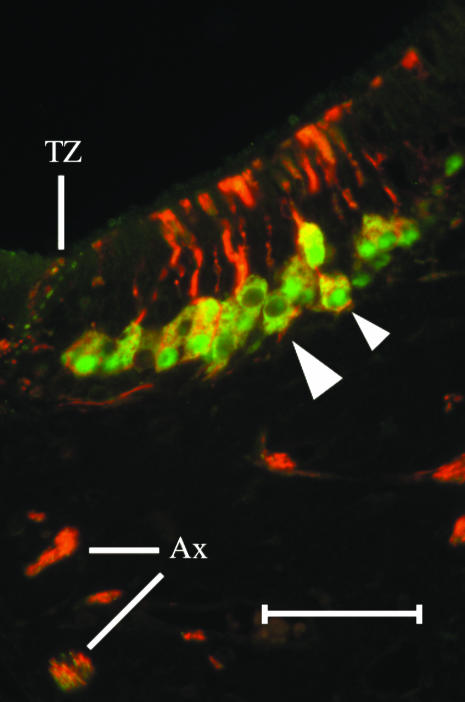
Clusters of sensory cells are GAP43(+) (Fig. 5A). A minority of axons in AOB fascicles are GAP43(+) (Fig. 5B,C). In preparations double labelled with BT and GAP43, a few cells are BT(+)/GAP43(+). Small axon bundles in the lamina propria contain a small number of double labelled axons while some nerve bundles contain only BT(+) axons (Fig. 5C).
Fig. 5.
Anti-GAP43 labels clusters (A) of cell somata in the sensory epithelium (arrowheads) and VNN fascicles in the lamina propria (B). A minority of axon fascicles in the VNN nerve bundles are GAP43(+) in (B). In a preparation (C) labelled with both BT in red (Alex 594) and GAP43 in green (Alexa 488), some neurones are BT(+)/GAP43(+) (small arrowhead) and some neurones are BT(+)/GAP43(–) (large arrowhead). Some axon bundles contain both BT(+) and GAP43(+) fibres (scale bars = 50 µm).
The VNO sensory epithelium shows immunoreactivity to antibodies directed against the G-protein alpha subunits of Gi and Go. Anti-Gi immunoreactivity is present in the basal compartment of the sensory epithelium as well as in the dendritic knobs (Fig. 6A). Small axon bundles in the lamina propria are Gi(+). The signal terminates abruptly at the transition zone (TZ) (Fig. 6B) between the sensory and non-sensory epithelial compartments although some signal above background is present in the apical-most regions of patches of non-sensory epithelium cells.
Fig. 6.
Cell somata (A) in the basal compartment and apical dendrites are Gi(+). The Gi(+) SE cell population (B) terminates abruptly at the TZ but signal above background is present in the apical extensions of some cells in the NSE (scale bars = 50 µm).
Anti-Go immunoreactivity is also expressed in the sensory epithelium (Fig. 7). Using DAB, the antibody labelled cells weakly but above background after standard tissue preparation (not shown). After an antigen retrieval procedure, the signal was identical to, although much stronger than, the labelling pattern obtained in those tissues that did not undergo the antigen retrieval step. The label is segregated to the sensory epithelial basal compartment but clearly labelled dendrites are present (Fig. 7A,B). As with the anti-Gi signal, anti-Go immunoreactivity stops abruptly at the transitional zone (Fig. 7B).
Fig. 7.
Basal compartment cells of the sensory epithelium (A) express immunoreactivity to anti-Go. The Go(+) cells lie immediately above the basal cell layer and some dendrites are clearly labelled with the antibody (arrowheads). The Go label is confined to the SE (B) and is absent in the NSE above the TZ (scale bars = 50 µm).
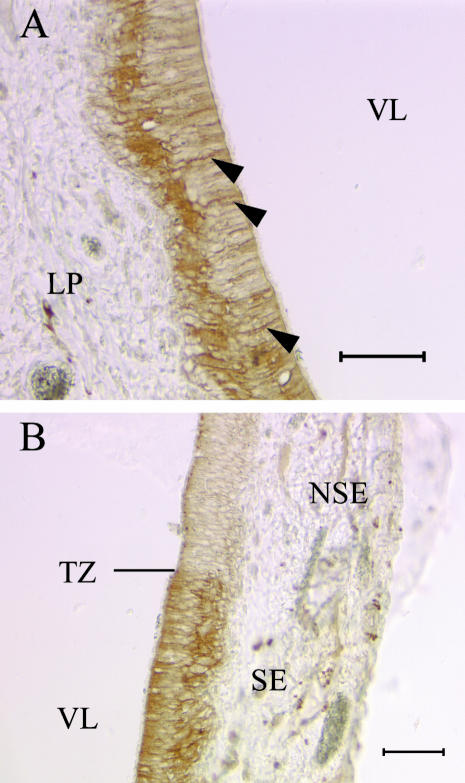
To determine if EGFR is present in dog VNO, we applied anti-EGFR antibody to some sections. Immunoreactivity to the antibody is expressed around the periphery of the VNO epithelium (Fig. 8). A strongly EGFR(+) cell layer underlies the sensory and non-sensory epithelial compartments that appears to be the cytokeratin-expressing basal cell population (Fig. 8B). In our preparations, the antibody labels the cell somata heavily but the signal is at background level throughout the apical thickness of the sensory epithelium. The non-sensory epithelium contains greater apical signal than that evident in the sensory epithelium (Fig. 8A).
Fig. 8.
Basal cells in both the SE and the NSE are EGFR(+). The EGFR(+) cells form a continuous layer (A) around the periphery of the VNO. The SE basal cell somata (B) label heavily with the antibody (arrowheads) while the signal is at background level throughout the epithelium apical to the basal cell bodies (scale bars = 50 µm).
Discussion
Our results show that the canine VNO expresses immunoreactivity to several antibodies directed against proteins expressed in the VNO of several vertebrate species. We observed immunoreactivity to four neurone-specific antibodies: BT, PGP, NCAM and GAP43. These immunohistochemical observations confirm the neuronal identity of the sensory epithelium cells previously identified as such by morphological and histological criteria. These neurones also express immunoreactivity to antibodies raised against two G protein alpha subunits, G∀o and G∀i, known to mediate signal transduction in rodents and opossums. We also show immunoreactivity of sensory and non-sensory basal cells to antibodies raised against KER and EGFR.
In any particular section, basal cell bodies are KER(+)/EGFR(+) and they are more numerous in the dog than in sections of rodent VNO. These proteins are coexpressed by horizontal basal cells in rat MOE (Holbrook et al. 1995; Rama Krishna et al. 1996) and each is expressed in rat VNO (J. C. Dennis, personal observation). That the cells express EGFR immunoreactivity does not mean that the polypeptide is present. However, three observations support the supposition that canine VNO basal cells are EGFR(+): first, the basal cells are the only EGFR(+) cell population; second, the signal pattern is consistent with a membrane-bound antigen; and, third, EGFR immunoreactivity is expressed in horse VNO (Garcia-Suarez et al. 1997). Expression of these two molecules by the basal cells indicates homology between these and the basal cells of the MOE. It further suggests similarities in growth regulation mechanisms.
It appears that most sensory neurones are BT(+)/PGP(+) although the intensity of signal varied between preparations from different individuals. We cannot assert, however, that most or all sensory neurones express both proteins. Rat VNO sections run in parallel with dog sections showed that, in our hands, not all rat sensory neurones are BT(+)/PGP(+); rather, double labelled sections showed a mosaic of cells positive for one or the other antigen but, in many cases, not both. In that regard, not all VN profiles labelled uniformly with BT suggesting that not all sensory neurones express BT or that BT is not expressed in the axons of all sensory neurones. This apparent mosaic expression pattern may mean that BT is expressed by younger sensory cells but not by mature neurones and that dog VNO sensory neurone development, with regard to BT expression, follows the MOE pattern (Lee & Pixley, 1994). Whether or not antibodies to BT and PGP are reliable markers of all dog VNO sensory neurones is yet to be determined.
Immunoreactivity to GAP43 and NCAM indicates that the canine VNO remains neurogenic because these proteins are expressed by neurones that are extending axons. The specificity of the GAP43 antibody is suggested by the signal pattern in both the epithelium and the fascicles of the VNN. In both structures, a minority of cells and axon fascicles are GAP43(+). Some but not all GAP43(+) neurones were also BT(+). Whether or not the GAP43(+)/BT(–) cells reflect a developmentally regulated difference in gene expression or whether the expression pattern is an artefact of differences in signal intensity cannot yet be determined.
The sensory epithelium showed both Go and Gi immunoreactivity in the sensory cell population. Immunoreactivity to either antibody terminated abruptly at the sensory epithelium (SE)/non-sensory epithelium (NSE) boundary. Some patchy anti-Gi signal was observed in the NSE of some sections but, in all cases, that signal was just above background. To date, our efforts at double labelling cells with these two antibodies have rendered equivocal results and therefore we cannot assert that the antibodies label the same cells or different SE cells.
We know of no published reports of G-protein immunoreactivity in the canine VNO but Takigami et al. (2000) reported that goat VNO, VN and the nerve layer of the AOB were Gi(+)/Go(–). The histology of the canine VNO and AOB is more similar to that of goats (Takigami et al. 2000) as well as sheep (Cohen-Tannoudji et al. 1989), swine (Dorries et al. 1997), and ferrets (Weiler et al. 1999) than it is to that of rodents, in that the SE is thin and the AOB is small relative to the mass of the main olfactory bulb (Adams & Wiekamp, 1984; Salazar et al. 1984, 1992, 1994; Nakajima et al. 1998a,b). One might hypothesize therefore that the VNO sensory epithelia of those species express a similar G-protein pattern and that Go would not be expressed in dogs. But one could also hypothesize that both Gi and Go are expressed in dogs given the phylogenetic distance between dogs and goats. Indeed, we used antibodies from the same commercial sources as were used by Takigami and co-workers. It should be noted, however, that the dog tissue was refractory to the anti-Go antibody in that the DAB labelling was present but faint. To obtain the DAB signal shown in Fig. 7, we boiled sections in citrate buffer before applying the primary antibody. Antigen retrieval is a routine immunohistochemical technique but the possibility remains that we amplified signal from a cross-reaction with a non-specific epitope. At this stage in our investigation of the canine VNO, we can make no meaningful comparisons of our G-protein labelling with that reported in goat VNO and AOB.
In summary, we show here that the canine VNO expresses several neuronal markers. These markers both confirm the neuronal nature of the labelled cells in the SE and suggest that neurogenesis is continuous. These observations cannot address the issue of function in the canine VNO. The VNO in ferrets (Weiler et al. 1999; Kelliher et al. 2001), swine (Dorries et al. 1997) and sheep (Cohen-Tannoudji et al. 1989) is apparently not involved in some of the functions performed by rodent VNO. We therefore need to determine whether functions inherent to the rodent VNO are, in dogs, performed by the main olfactory system as seems to be the case in the above mentioned species or whether the canine VNO, compared with the rodent VNO, has different functions, more limited functions, or any function at all. These possibilities bear on the evolution of the accessory olfactory system and its relationship to the natural history of the particular species under investigation.
Acknowledgments
This study was supported by grants FAA RG: 01-G-022 and USA RMACCN: N66001-1099–0072 to E.E.M.
References
- Adams DR, Weikamp MD. The canine vomeronasal organ. J. Anat. 1984;138:771–787. [PMC free article] [PubMed] [Google Scholar]
- Berghard A, Buck LB. Sensory transduction in vomeronasal neurons: evidence for G∀o, G∀i2, and adenylyl cyclase II as major components of a pheromone signaling cascade. J. Neurosci. 1996;16:909–918. doi: 10.1523/JNEUROSCI.16-03-00909.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD, Cambray-Deakin MA, Lewis SA, Sarkar S, Cowan NJ. Differential distribution of $-tubulin isotypes in cerebellum. EMBO J. 1988;7:2311–2319. doi: 10.1002/j.1460-2075.1988.tb03074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Tannoudji J, Lavenet C, Locatelli A, Tillet Y, Signoret JP. Non-involvement of the accessory olfactory system in the LH response of anoestrous ewes to male odour. J. Reprod. Fertil. 1989;86:135–144. doi: 10.1530/jrf.0.0860135. [DOI] [PubMed] [Google Scholar]
- Dorries KM, Adkins-Regan E, Halpern BP. Sensitivity and behavioral responses to the pheromone androstenone are not mediated by the vomeronasal organ in domestic pigs. Brain. Behav. Evol. 1997;49:53–62. doi: 10.1159/000112981. [DOI] [PubMed] [Google Scholar]
- Edelman GM. Modulation of cell adhesion during induction of histogenesis and perinatal development of the nervous system. Annu. Rev. Neurosci. 1984;7:339–377. doi: 10.1146/annurev.ne.07.030184.002011. [DOI] [PubMed] [Google Scholar]
- Edelman GM, Chuong C-M. Embryonic to adult conversion of neural cell adhesion molecules in normal and staggerer mice. Neurobiology. 1982;79:7036–7040. doi: 10.1073/pnas.79.22.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Suarez O, Germana G, Naves FJ, Ciriaco E, Represa J, Vega JA. Sensory epithelium of the vomeronasal organ express TrkA-like and epidermal growth factor receptor in adulthood. Anat. Rec. 1997;247:299–306. doi: 10.1002/(SICI)1097-0185(199703)247:3<299::AID-AR1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Giacobini P, Benedetto A, Tirindelli R, Fasolo A. Proliferation and migration of receptor neurons in the vomeronasal organ of the adult mouse. Dev. Brain Res. 2000;123:33–40. doi: 10.1016/s0165-3806(00)00080-8. [DOI] [PubMed] [Google Scholar]
- Gispen WH, Nielander HB, De Graan PNE, Oestreicher AB, Schrama LH, Schotman P. Role of the growth-associated protein B-50/GAP-43 in neuronal plasticity. Mol. Neurobiol. 1992;5:61–85. doi: 10.1007/BF02935540. [DOI] [PubMed] [Google Scholar]
- Halpern M. The organization and function of the vomeronasal system. Ann. Rev. Neurosci. 1987;10:325–362. doi: 10.1146/annurev.ne.10.030187.001545. [DOI] [PubMed] [Google Scholar]
- Halpern M, Sahpiro LS, Jia C. Differential localization of G proteins in the opossum vomeronasal system. Brain Res. 1995;677:157–161. doi: 10.1016/0006-8993(95)00159-n. [DOI] [PubMed] [Google Scholar]
- Hofer D, Shin D-W, Drenckhahn D. Identification of cytoskeletal markers for the different microvilli and cell types of the rat vomeronasal sensory epithelium. J. Neurocytol. 2000;29f:147–156. doi: 10.1023/a:1026548020851. [DOI] [PubMed] [Google Scholar]
- Holbrook EH, Szumowski KEM, Schwob JE. An immunochemical, ultrastructural, and developmental characterization of the horizontal basal cells of rat olfactory epithelium. J. Comp. Neurol. 1995;363:129–146. doi: 10.1002/cne.903630111. [DOI] [PubMed] [Google Scholar]
- Iwanaga T, Han H, Kanazawa H, Fujita T. Immunohistochemical localization of protein gene product 9.5 (PGP 9.5) in sensory paraneurons of the rat. Biomed. Res. 1992;13:225–230. [Google Scholar]
- Jackson P, Thompson RJ. The demonstration of new human brain-specific proteins by high-resolution two-dimensional polyacrylamide gel electrophoresis. J. Neurol. Sci. 1981;49:429–438. doi: 10.1016/0022-510x(81)90032-0. [DOI] [PubMed] [Google Scholar]
- Jia C, Halpern M. Subclasses of vomeronasal receptor neurons: differential expression of G proteins (Gi alpha 2 and G (o alpha) and segregated projections to the accessory olfactory bulb. Brain Res. 1996;719:117–128. doi: 10.1016/0006-8993(96)00110-2. [DOI] [PubMed] [Google Scholar]
- Johnson EW, Eller PM, Jafek BW. Protein gene product 9.5 in the developing and mature rat vomeronasal organ. Dev. Brain Res. 1994;78:259–264. doi: 10.1016/0165-3806(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Kelliher KR, Baum MJ, Meredith M. The ferret's vomeronasal organ and accessory olfactory bulb: effect of hormone manipulation in adult males and females. Anat. Rec. 2001;263:280–288. doi: 10.1002/ar.1097. [DOI] [PubMed] [Google Scholar]
- Lee VM, Pixley SK. Age and differentiation-related differences in neuron-specific tubulin immunostaining of olfactory sensory neurons. Dev. Brain Res. 1994;83:209–215. doi: 10.1016/0165-3806(94)00139-1. [DOI] [PubMed] [Google Scholar]
- Liman ER. Sex and the single neuron: pheromones excite. Trends Neurosci. 2001;24:2–3. doi: 10.1016/s0166-2236(00)01702-1. [DOI] [PubMed] [Google Scholar]
- Meisami E, Bhatnagar KP. Structure and diversity in mammalian accessory olfactory bulb. Microsc. Res. Techn. 1998;43:476–499. doi: 10.1002/(SICI)1097-0029(19981215)43:6<476::AID-JEMT2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Miragall F, Kadmon G, Husmann M, Schachner M. Expression of cell adhesion molecules in the olfactory system of the adult mouse: presence of the embryonic form of N_CAM. Dev. Biol. 1988;129:516–531. doi: 10.1016/0012-1606(88)90397-1. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Murabayashi C, Ogawa K, Taniguchi K. Immunoreactivity of protein gene product 9.5 (PGP 9.5) in the developing hamster olfactory bulb. Anat. Rec. 1998a;250:238–244. doi: 10.1002/(SICI)1097-0185(199802)250:2<238::AID-AR13>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Sakaue M, Kato M, Saito S, Ogawa K, Taniguchi K. Immunohistochemical study on the accessory olfactory bulb of the dog. Anat. Rec. 1998b;252:393–402. doi: 10.1002/(SICI)1097-0185(199811)252:3<393::AID-AR7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Oestreicher AB, De Graan PNE, Gispen WH, Verhaagen J, Schrama LH. B-50, the growth associated protein-43: modulation of cell morphology and communication in the nervous system. Prog. Neurobiol. 1997;53:627–686. doi: 10.1016/s0301-0082(97)00043-9. [DOI] [PubMed] [Google Scholar]
- Rama Krishna NS, Little SS, Getchell TV. Epidermal growth factor receptor mRNA and protein are expressed in progenitor cells of the olfactory epithelium. J. Comp. Neurol. 1996;373:297–307. doi: 10.1002/(SICI)1096-9861(19960916)373:2<297::AID-CNE11>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Roskams AJI, Cai X, Ronnett GV. Expression of neuron-specific beta-III tubulin during olfactory neurogenesis in the embryonic and adult rat. Neuroscience. 1998;83:191–200. doi: 10.1016/s0306-4522(97)00344-8. [DOI] [PubMed] [Google Scholar]
- Salazar I, Rueda A, Cifuentes JM. Anatomy of the vomeronasal organ in the dog. Folia Morph. 1984;32:331–341. [PubMed] [Google Scholar]
- Salazar I, Barbert PC, Cifuentes JM. Anatomical and immunohistological demonstration of the primary neural connections of the vomeronasal organ in the dog. Anat. Rec. 1992;233:309–313. doi: 10.1002/ar.1092330214. [DOI] [PubMed] [Google Scholar]
- Salazar I, Cifuentes JM, Quinteiro PS, Caballero TG. Structural, morphometric, and immunohistological study of the accessory olfactory bulb in the dog. Anat. Rec. 1994;240:277–285. doi: 10.1002/ar.1092400216. [DOI] [PubMed] [Google Scholar]
- Sam M, Vora S, Malnic B, Ma W, Novotny MV, Buck LB. Neuropharmacology: odorants may arouse instinctive behaviours. Nature. 2001;412:142. doi: 10.1038/35084137. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Mieleszko Szumowski KE, Stasky AA. Olfactory sensory neurons are trophically dependent on the olfactory bulb for their prolonged survival. J. Neurosci. 1992;12:3896–3919. doi: 10.1523/JNEUROSCI.12-10-03896.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob JE, Youngentob SL, Mezza RC. Reconstitution of the rat olfactory epithelium after methyl bromide-induced lesion. J. Comp. Neurol. 1995;359:15–37. doi: 10.1002/cne.903590103. [DOI] [PubMed] [Google Scholar]
- Shinohara H, Asano T, Kato K. Differential localization of G-proteins Gi and Go in the accessory olfactory bulb of the rat. J. Neurosci. 1992;12:1275–1279. doi: 10.1523/JNEUROSCI.12-04-01275.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene JHP. Axonal growth-associated proteins. Ann. Rev. Neurosci. 1989;12:127–156. doi: 10.1146/annurev.ne.12.030189.001015. [DOI] [PubMed] [Google Scholar]
- Takami S. Recent progress in the neurobiology of the vomeronasal organ. Microsc. Res. Techn. 2002;58:228–250. doi: 10.1002/jemt.10094. [DOI] [PubMed] [Google Scholar]
- Takigami S, Mori Y, Ichikawa M. Projection pattern of vomeronasal neurons to the accessory olfactory bulb in goats. Chem. Senses. 2000;25:387–393. doi: 10.1093/chemse/25.4.387. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Saito H, Okamura M, Ogawa K. Immunohistochemical demonstration of protein gene product 9.5 (PGP 9.5) in the primary olfactory system of the rat. Neurosci. Lett. 1993;156:24–26. doi: 10.1016/0304-3940(93)90430-s. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Doran JF, Jackson P, Dhillon AP, Rode J. PGP 9.5 – a new marker for vertebrate neurons and neuroendocrine cells. Brain Res. 1983;278:224–228. doi: 10.1016/0006-8993(83)90241-x. [DOI] [PubMed] [Google Scholar]
- Verhaagen J, Greer CA, Margolis FL. B-50/GAP43 gene expression in the rat olfactory system during postnatal development and aging. Eur. J. Neurosci. 1990;2:397–407. doi: 10.1111/j.1460-9568.1990.tb00432.x. [DOI] [PubMed] [Google Scholar]
- Verhaagen J, Oestreicher AB, Gispen WH, Margolis FL. The expession of the growth associated protein B-50/GAP43 in the olfactory system of neonatal and adult rats. J. Neurosci. 1989;9:683–691. doi: 10.1523/JNEUROSCI.09-02-00683.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler E, Apfelbach R, Farbman AI. The vomeronasal organ of the male ferret. Chem. Senses. 1999;24:127–136. doi: 10.1093/chemse/24.2.127. [DOI] [PubMed] [Google Scholar]
- Wilkinson KD, Lee K, Deshpande S, Duerksen-Hughes P, Boss JM, Pohl J. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989;246:670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- Witt M, Georgiewa B, Knecht M, Hummel T. On the chemosensory nature of the vomeronasal epithelium in adult humans. Histochem. Cell Biol. 2002;117:493–509. doi: 10.1007/s00418-002-0407-1. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Meredith M. The vomeronasal system. In: Finger TE, Silver WL, editors. Neurobiology of Taste and Smell. New York: John Wiley & Sons; 1987. pp. 125–150. [Google Scholar]
- Yamashita H, Kawata K, Takahashi M. Upregulation of neural growth-associated protein and neural cell adhesion molecule in mouse olfactory epithelium and axons after unilateral removal of the olfactory bulb. Eur. Arch. Otorhinolaryngol. 1998;255:441–445. doi: 10.1007/s004050050095. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y, Kawasaki M, Tamada A, Fujita H, Hayashi H, Kagamiyama H, et al. OCAM: a new member of the neural cell adhesion molecule family related to zone-to-zone projection of olfactory and vomeronasal axons. J. Neurosci. 1997;17:5830–5842. doi: 10.1523/JNEUROSCI.17-15-05830.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair M, Watanabe E, Fukada M, Noda M. Genetic labelling of specific axonal pathways in the mouse central nervous system. Eur. J. Neurosci. 2002;15:807–814. doi: 10.1046/j.1460-9568.2002.01911.x. [DOI] [PubMed] [Google Scholar]
- Zufall F, Kelliher KR, Leinders-Zufall T. Pheromone detection by mammalian vomeronasal neurons. Microsc. Res. Techn. 2002;58:251–260. doi: 10.1002/jemt.10152. [DOI] [PubMed] [Google Scholar]