Abstract
To test the hypothesis that chronic immune stimulation of a peripheral lymph node induces the formation of additional mature adipocytes in adjacent adipose tissue, one popliteal lymph node of large male rats was stimulated by local injection of 10 µg or 20 µg lipopolysaccharide three times a week for 6 weeks. Adipocyte volumes in sites defined by their anatomical relations to the stimulated and homologous unstimulated popliteal lymph nodes were measured, plus adipocyte complement of the popliteal depot, and the lipid and protein content of adipocytes and adipose stroma. The higher dose of lipopolysaccharide doubled the mass of the locally stimulated lymph node and the surrounding adipose tissue enlarged by the appearance of additional mature adipocytes. Similar but smaller changes were observed in the popliteal adipose depot of the unstimulated leg and in a nodeless depot. The lipid content of the adipocytes decreased and that of the stroma increased dose-dependently in all samples measured but the changes were consistently greater in the depot surrounding the stimulated lymph node. The protein content of both adipocytes and stroma increased in samples surrounding the stimulated node. We conclude that chronic immune stimulation of lymphoid tissues induces the formation of more adipocytes in the adjacent adipose tissue. These findings suggest a mechanism for the selective hypertrophy of lymphoid-containing adipose depots in the HIV-associated adipose redistribution syndrome.
Keywords: adipocyte volume, HIV-related adipose redistribution syndrome (HARS), lipodystrophy, perinodal adipose tissue, site-specific properties
Introduction
Adipocytes in depots that enclose lymph nodes or other dense masses of lymphoid tissue have many site-specific physiological properties (Pond, 1999, 2003b). Changes in the manifestation of receptors for pro-inflammatory cytokines can be detected on perinodal adipocytes situated within 2 mm of a lymph node less than an hour after mild local stimulation of lymphoid cells (MacQueen & Pond, 1998). Such local interactions modulate lipolysis, sometimes inducing very high rates in perinodal tissues (Pond & Mattacks, 1998, 2002 ; Mattacks & Pond, 1999; Mattacks et al. 2003). The fatty acids, and possibly other nutrients, that support the enlargement of lymph nodes in response to immune stimulation are derived mainly from the triacylglycerols in the perinodal adipocytes (Pond & Mattacks, 2003).
HIV-related adipose redistribution syndrome (HARS) is an increasingly prevalent complication of otherwise successful treatment of HIV infection: over months or years, patients report distressing changes in body conformation as certain adipose depots hypertrophy, while others atrophy. HARS is more common and worse in those treated with either or both of the major classes of antiretroviral drugs, nucleoside reverse transcriptase inhibitors (NRTIs), and protease inhibitors (PIs), although it has also been described in patients in whom the progression from HIV infection to AIDS is delayed naturally (Madge et al. 1999). The syndrome may accompany constant, increasing or decreasing body mass, with or without changes in average energy intake, and, once established, reverses very slowly during interruptions to antiviral therapy, in many cases not at all (Hatano et al. 2000). In these respects, the metabolism and anatomical changes of adipose tissue in HARS contrast with those of natural and pathological obesity.
HARS has been attributed to interactions between antiviral drugs and proteins involved in lipid metabolism whose structures resemble that of the target viral proteins (Carr, 2000; John et al. 2001; Shevitz et al. 2001). However, none of these hypotheses can account for the almost universal finding that the changes in adipose tissue are selective, with certain depots undergoing hypertrophy, while others atrophy. Pond (2003a) have proposed that the key distinction between depots that hypertrophy and those that atrophy is the presence in the former of embedded lymphoid tissue.
Chronic infection with HIV raises the rate of turnover of lymph node lymphoid cells (Orenstein et al. 1999; Kaur et al. 2000). With continuous replacement of lymphoid cells, the paracrine signals (MacQueen & Pond, 1998; Pond & Mattacks, 1998) that elicit the release from perinodal adipocytes of nutrients that support their growth and metabolism would persist for much longer than normal. The best characterized such response of perinodal adipocytes is cytokine-controlled lipolysis (Pond & Mattacks, 1995, 2002 ; Mattacks et al. 2002); as well as making fatty acids and glycerol available to adjacent lymphoid cells, prolonged lipolysis raises the extracellular concentration of these products around the adipocytes, conditions that promote the expansion of adipose tissue and hence obesity (Amri et al. 1994; Schling & Löffler, 2002). Similar mechanisms operating locally would lead to proliferation of adipocytes that are associated anatomically and metabolically with activated lymphoid tissues, and thus to permanent hypertrophy of those adipose depots (Pond, 2003a). This paper tests this hypothesis: we study the long-term effects of chronic local inflammation of a peripheral lymph node on the anatomical structure and chemical composition of the adipose tissue associated with it. The popliteal lymph nodes are chosen for study because they are quiescent in healthy animals but can be stimulated locally by minimally invasive procedures (Smith & Morris, 1970). The small adipose depots enclosing the popliteal nodes are very consistent in size and structure, thus facilitating the identification of exactly homologous samples from the left and right hind legs, and are composed of adipocytes that interact strongly with lymphoid cells (Pond & Mattacks, 1995).
Materials and methods
Animals
Three similar groups of 18 adult male Sprague–Dawley rats fed ad libitum on RM3 chow (Special Diet Services, Witham, UK) were aged about 6 months when this experiment started. Chronic, local, low-level immune disturbance was generated by injecting 10 µg (about 5 µg kg−1 body mass) for the low-dose group, or 20 µg (about 10 µg kg−1 body mass) for the high-dose group of lipopolysaccharide (LPS) dissolved in buffered saline, pH 7.4 (injected volume about 0.1 mL), with a similar volume of sterile saline administered to a third group as a control. The LPS or saline was injected subcutaneously into the lateral skin of the left lower leg (the ‘catchment’ area of the left popliteal lymph nodes), three times a week for the final 6 weeks. From weaning, the rats were caged in permanent groups of three, and all cage-mates were injected and killed at the same time.
After being killed by carbon dioxide anaesthesia, each rat was weighed and the epididymal, perirenal/retroperitoneal, mesenteric, omental, inguinal, interscapular and popliteal adipose depots dissected out, washed in Hank's Balanced Salt Solution (HBSS) and weighed. These depots together comprise almost all visible adipose tissue and can be used to calculate fatness (fresh weight of adipose tissue as percentage live body mass). The stimulated and unstimulated popliteal lymph nodes, and some conspicuous mesenteric lymph nodes, were also dissected out and weighed.
The perinodal adipose tissue is defined as within 2 mm the popliteal lymph nodes, ‘middle’ as about 5 mm from the nodes and ‘remote’ as more than 10 mm from the lymph nodes (Pond & Mattacks, 2002). Samples were chosen entirely on the basis of their anatomical relations to the nodes; apart from a small difference in adipocyte volume, the microscopic appearance of fresh perinodal adipose tissue is similar to that of the rest of the depot, though these specialized adipocytes can be distinguished in situ by differences in the manifestation of surface proteins such as cytokine receptors (MacQueen & Pond, 1998). Perinodal, middle and remote samples were dissected from both popliteal depots together with samples of the nodeless perirenal depots. The average yield of perinodal adipose tissue from each popliteal depot was only 27 mg wet weight, so all methods were adapted for use on such small samples. Collagenase isolation of adipocytes and cell counting and sizing were performed within 4 h of dissection.
Adipocyte isolation
The adipose tissue samples were cut up with scissors into approximately 2-mm pieces, washed in HBSS and incubated in 10 mL Krebs-Hensliet buffer with 2 mg collagenase, at 37 °C for 30 min in a shaking waterbath (approx. 100 cycles min−1). The released adipocytes floated to the surface and were removed using a siliconized pasteur pipette, washed in fresh HBSS twice and then resuspended in 0.1 mL HBSS. The remaining stroma was spun at 1500 r.p.m. in MSE Coolspin centrifuge, washed twice with HBSS and resuspended into 0.1 mL HBSS.
Size and number of adipocytes
The numbers of adipocytes in each of three 10-µL samples of isolated adipocytes from both popliteal and the nodeless perirenal depots were counted using a haemocytometer on a light microscope. The adipocyte complement of each of these depots was calculated from these values and its gross mass. At the same time (and using the same samples), the diameters of about 50 adipocytes were measured with a low-power objective and an eye-piece graticule by an experienced operator (CAM). The mean adipocyte volume of each such sample was calculated from the mean and standard deviation of measurements of adipocyte diameter using Goldrick's equations (Goldrick, 1967). In pilot experiments, these adipocyte volume measurements were compared with those obtained from whole mounts of fresh tissue, using our previous procedure (Pond et al. 1984). No consistent differences were found, so we adopted the haemocytometer method because the smaller quantities of tissue it required enabled us to compare exactly homologous samples in each rat.
Lipid and protein assays
The remaining material from each sample was used to assay total lipid (i.e. neutral triacylglycerols, phospholipids and non-esterified fatty acids) and total protein. The adipocyte and stroma suspensions were homogenized in HBSS and centrifuged at 1500 r.p.m. to precipitate the protein. The lipid was extracted from the supernatant with 1 mL of a mixture of 2 g Tween 80/L acetone and stored overnight at 4 °C. Four × 200 µL of 2 m sodium acetate was added to the 1 mL acetone/Tween mixture, and vortexed with each addition. Then the turbidity of duplicate 100-µL samples was measured with a Titertek Multiskan plate reader with 440-nm filter, using corn oil as the standards. The results were calculated as percentage lipid in fresh weight of adipose tissue.
Total protein was measured by the Bradford method (Bradford, 1976) against standards of 1 mg mL−1 BSA stock solution. The precipitated protein pellet was resuspended in 1 mL of 1 m sodium hydroxide, vortexed and placed in a water bath for 1 h at 60 °C, resuspended in 100 µL of 10 mm 1 m sodium hydroxide and vortexed. Duplicate 10-µL samples from each preparation were mixed with 30 µL sodium hydroxide and 250 µL Bradford reagent in a 96-well plate and allowed to stand at room temperature for 10 min. The readings were taken using a Titertek Multiskan plate reader with 595-nm filter and the results were calculated from standard curve as mg g−1 fresh weight of adipose tissue.
The data were analysed with Microsoft Excel. The differences between means were assessed using Student's t-test.
Results
Gross anatomy
Table 1 lists data on the gross anatomy at death of the three groups of rats. There was no evidence that the experimental regimes impaired appetite, induced fever or caused emaciation. The rats given the high dose of LPS were in fact slightly heavier and fatter than the controls. The lower dose of LPS induced a small increase in mass of the popliteal lymph node in the stimulated (left) leg only. The higher dose of LPS induced significant enlargement of both popliteal lymph nodes, with that of the stimulated side swelling to twice the size of the saline-injected controls. The mass of the popliteal adipose tissue around this lymph node was also 29% larger than that of the saline-injected controls, although these rats were not significantly bigger or fatter. However, as the standard deviations show, the magnitude of the responses differed between specimens.
Table 1. Gross anatomy of the rats. N = 18 mature male rats in each group. Values are mean ± SD.
| Saline-injected controls | Low-dose LPS (10 µg) | High-dose LPS (20 µg) | |
|---|---|---|---|
| Body mass (g) | 494 ± 56 | 463 ± 43 | 511 ± 27 |
| Fatness (total adipose tissue %BM) | 3.50 ± 0.75 | 3.45 ± 0.71 | 3.68 ± 0.68 |
| Mass of unstimulated popliteal lymph node (mg) | 11.4 ± 1.34 | 11.0 ± 1.25 | 15.4 ± 2.47†† |
| Mass of stimulated popliteal lymph node (mg) | 11.4 ± 1.33 | 11.9 ± 1.17* | 23.9 ± 2.93††** |
| Mass of popliteal adipose tissue around unstimulated node (mg) | 249 ± 87 | 243 ± 79 | 284 ± 91 |
| Mass of popliteal adipose tissue around stimulated node (mg) | 253 ± 65 | 255 ± 89 | 327 ± 105† |
Significantly different from saline-injected controls at:
P < 0.05;
P < 0.001.
Difference between locally stimulated and homologous unstimulated structures significant at:
P < 0.05;
P < 0.001.
Adipocyte size and number
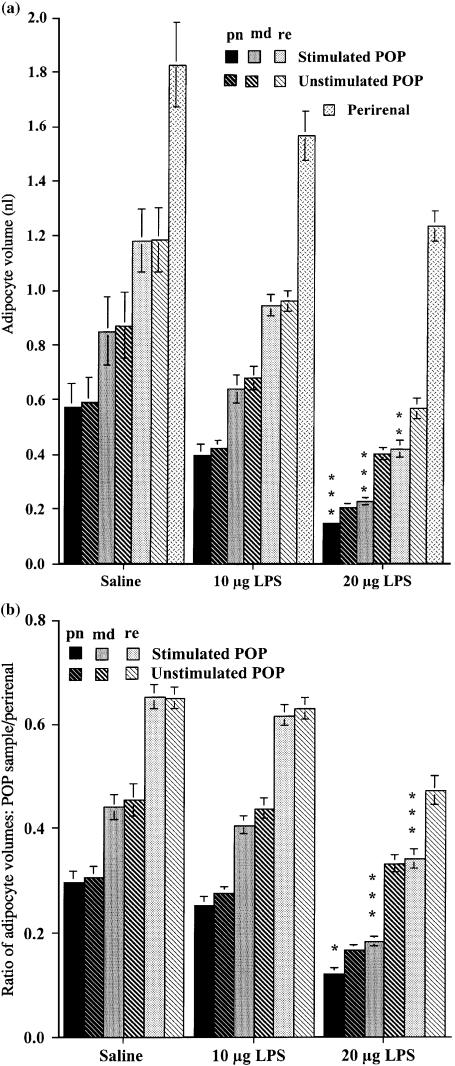
Figure 1A shows the mean volume of about 50 adipocytes from three sample sites, perinodal, 5 (middle) and 10 mm (remote) from the lymph node of both popliteal depots and from the nodeless perirenal depots. The large standard errors (SEs) of the means reflect the substantial variation between individual rats in the cellular composition of all adipose depots studied (i.e. the adipose tissue of some specimens consists of many small adipocytes, while others of similar overall body composition have fewer, larger adipocytes). However, the very high concordance between the measurements from homologous samples from the left and right legs of the saline-injected controls demonstrates the reproducibility that we can achieve using this technique. As expected from previous measurements from wild and laboratory animals (Pond et al. 1986; Pond, 1992), the perirenal adipocytes were consistently about 50% larger than those in the popliteal depots. There were clear and consistent gradients in adipocyte size within the popliteal depots, with the perinodal samples containing adipocytes that averaged around half the volume of those in the remote samples. Differences in size between homologous samples of adipocytes surrounding the stimulated and unstimulated lymph nodes were significant only in the high-dose group, with trends towards similar site-specific differences recorded from the low-dose group.
Fig. 1.
Volume of perinodal (pn, darkest bars), middle (md, intermediate shading) and remote (re, palest bars) adipocytes from the popliteal adipose depots enclosing the stimulated (plain and speckled bars) and unstimulated (striped bars) popliteal lymph nodes, and that of perirenal adipocytes, following three injections per week for 6 weeks of 10 or 20 µg lipopolysaccharide, or saline. (A) Mean ± SE of adipocyte volume (nL). (B) Mean ± SE of the ratios of the volumes of popliteal adipocytes to that of perirenal adipocytes of the same rat. Each group, N = 18 large male rats. Differences between homologous samples from stimulated and unstimulated legs of the same rats significant at: *P < 0.05; **P < 0.01; ***P < 0.001.
All adipocytes were significantly smaller in the rats that had been stimulated with the high dose of LPS than in homologous samples of the saline-injected controls (Fig. 1A), although there were no differences in body mass or body composition (Table 1). Much of the variance in Fig. 1A is probably due to differences in the rats’ total fatness (Table 1), so in Fig. 1B the data from the popliteal depots are normalized to the volumes of the perirenal adipocytes of the same rat. This analysis shows that, following the high-dose regime, all the popliteal adipocytes became smaller relative to those in the (nodeless) perirenal depot. The differences between values for homologous samples from the stimulated and unstimulated legs were small for the perinodal adipocytes (28%), but highly significant for the middle and remote samples. Although the adipocytes in the popliteal adipose tissue enclosing the stimulated node were consistently smaller (Fig. 1A), the depot's total mass was slightly larger (Table 1), so the number of mature adipocytes must have increased.
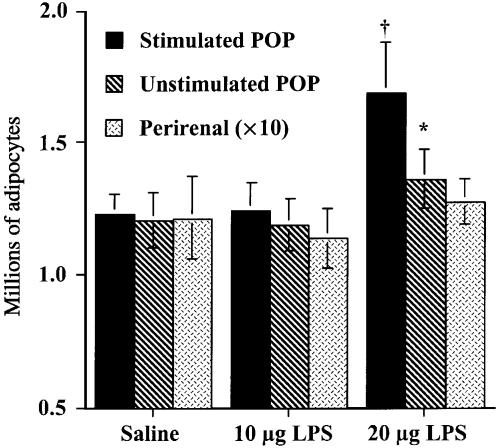
Figure 2 shows the approximate adipocyte complement of the depots studied. As expected from Fig. 1, the popliteal depot surrounding the lymph node stimulated with 20 µg of LPS contained significantly more adipocytes than its homologue in the other leg, and the corresponding depot injected with the lower dose of LPS or with sterile saline (t = 2.2780, d.f. = 34, P < 0.05). This method is not sensitive enough to reveal significant differences in the adipocyte complements of the perirenal or the unstimulated popliteal depot expected from the small changes in their adipocyte volumes reported in Fig. 1.
Fig. 2.
Numbers (means ± SE) of mature adipocytes (millions) in the popliteal adipose depots enclosing the stimulated (plain bars) and unstimulated (striped bars) popliteal lymph nodes, and in the perirenal depot, following three injections per week for 6 weeks of 10 or 20 µg lipopolysaccharide, or saline. Note that the values for the large perirenal depot have been divided by 10 to fit the data to the scale. Each group, N = 18 large male rats. Asterisk shows difference between homologous samples from stimulated and unstimulated legs of the same rats significant at P < 0.05; †, value significantly different at P < 0.05 from homologous sample of saline-injected controls.
Chemical composition of adipose tissue
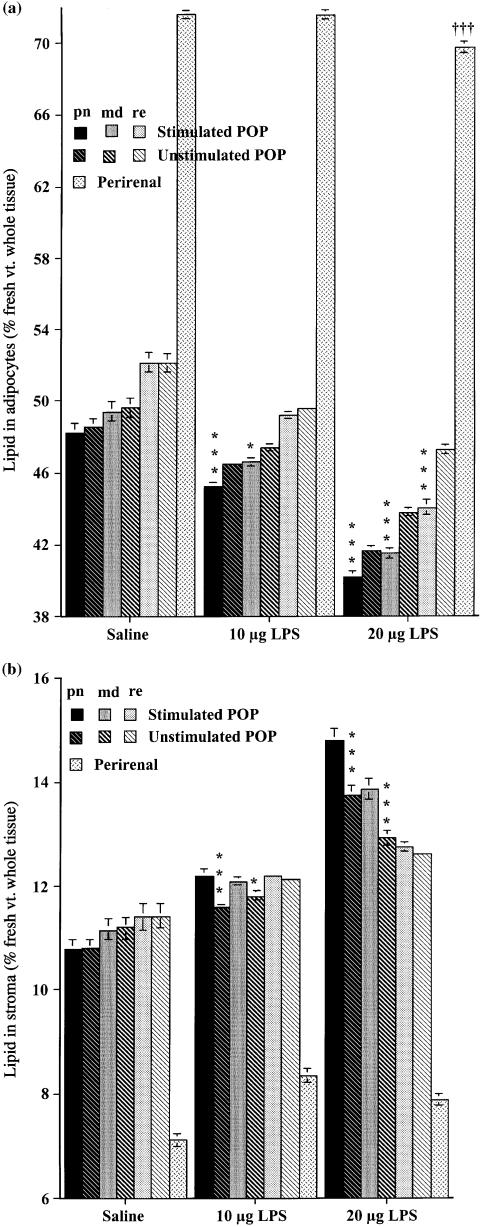
Figure 3 shows the proportions of extractable lipid in the adipocytes (all intact cells whose lipid content was high enough for their density to be less than that of HBSS), and stroma, the remaining fraction consisting of vascular and lymphoid cells, extracellular material and any pre-adipocytes too immature to have acquired enough lipid to float. As expected from their larger size (Fig. 1), perirenal adipocytes contained a much higher proportion of lipid (Fig. 3A), which decreased by about 2.7% following stimulation with the high dose of LPS. This technique (Fig. 3A) demonstrated a change in composition of the adipocytes surrounding the lymph node stimulated with the lower dose of LPS (highly significant for perinodal, significant at P < 0.05 for the middle sample) that was not clear from the measurements of cell volume (Fig. 1). There is a close correlation (r = 0.95) between the volume and lipid content of adipocytes for six popliteal samples of the three experimental groups, but data for the perirenal fall far off the regression line for these values; perirenal adipocytes contain proportionately more lipid than those of the node-containing depots.
Fig. 3.
Means ± SE of lipid content (% fresh weight of adipose tissue) of the adipocytes and stroma from adipose tissue surrounding the stimulated and unstimulated popliteal lymph nodes, and that of perirenal adipocytes, following three injections per week for 6 weeks of 10 or 20 µg lipopolysaccharide, or saline. patterns and shading as in Fig. 1. (A) Isolated adipocytes; (B) stroma of adipose tissue. Each group, N = 18 large male rats. Differences between homologous samples from stimulated and unstimulated legs of the same rats significant at: *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 3B shows similar measurements from the stromal fraction. In the saline-injected controls, all the popliteal stroma contained more lipid than the perirenal, but significantly (t = 5.24, P < 0.001) less was measured in the perinodal than the remote from node samples from the popliteal depots. Following the lower dose of LPS, this pattern persisted in the popliteal adipose stroma around the unstimulated lymph node, but was abolished in the stroma from adipose tissue surrounding the stimulated node, producing significant differences between homologous samples from the two legs. With the higher dose, similar patterns of within-depot differences were measured in both popliteal depots, but the quantities of stromal lipid were significantly higher in the perinodal and middle samples from the stimulated leg, reaching values of more than twice as much as in perirenal samples of the saline-injected controls.
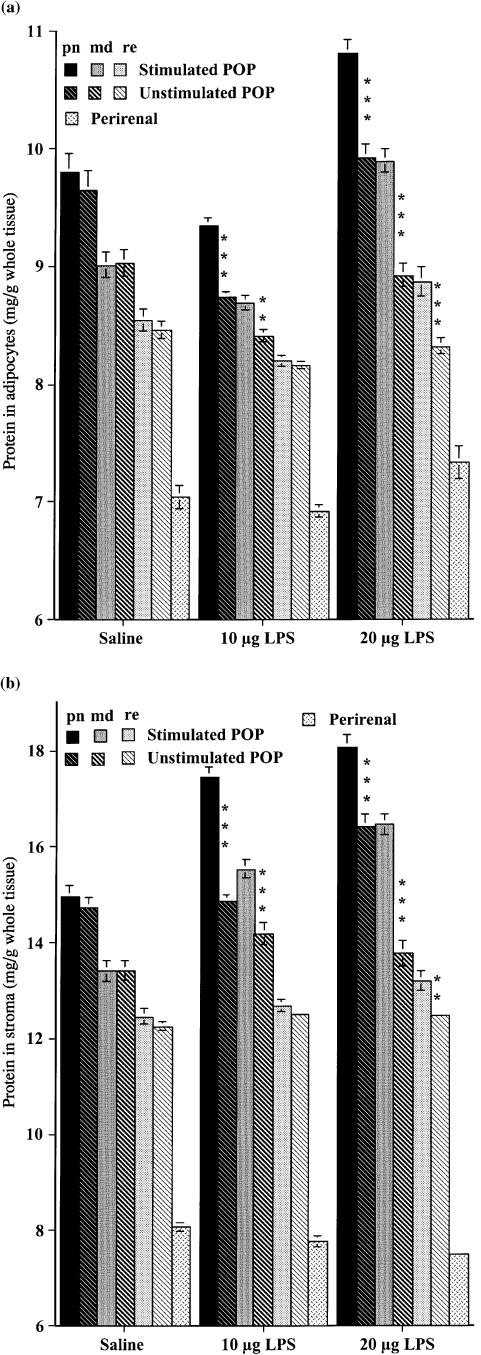
Figure 4 shows that the protein contents of both adipose tissue fractions were higher in the node-containing depot than the nodeless perirenal depot at rest, with the highest values measured from perinodal samples. The protein contents of all adipocytes (Fig. 4A) from the rats on the low-dose LPS were slightly lower than those of the controls, but significantly more protein was found in both adipocytes and stroma (Fig. 4B) of the perinodal and middle samples following this regime. This effect extended throughout the popliteal depot after the high-dose regime, and relative to the saline-injected controls, there was more protein in all popliteal samples studied, except remote from node of the unstimulated leg.
Fig. 4.
Means ± SE of protein content (mg/g fresh weight adipose tissue) of the adipocytes and stroma from the adipose tissue surrounding the stimulated and unstimulated popliteal lymph nodes, and that of perirenal adipocytes, following three injections per week for 6 weeks of 10 or 20 µg lipopolysaccharide, or saline. Bar patterns and shading as in Figs 1 and 3. (A) Isolated adipocytes; (B) stroma of adipose tissue. Each group, N = 18 large male rats. Differences between homologous samples from stimulated and unstimulated legs of the same rats significant at: *P < 0.05; **P < 0.01; ***P < 0.001.
The data from the chemical analyses (Figs 3 and 4) can be used to calculate the water content of the tissues. All the popiteal samples contained significantly (P < 0.001) more water following stimulation of the left leg with the high dose of LPS: the water content of the perinodal increased by 10%, middle by 13% and remote by 20% over those of the saline-injected controls, to values of 41–42% water in the whole of both popliteal depots. The low-dose regime produced statistically insignificant trends towards these values. Such oedema would increase tissue mass and may be the origin of the small (14%) enlargement of the popliteal depot in the unstimulated leg (Table 1). However, the locally-stimulated popliteal depot was 29% heavier than the control, of which at least 15% can be attributed to the adipocytes themselves (Fig. 2).
Discussion
The immune stimulation regimes used for these experiments were sufficient for signals to pass from the locally-stimulated lymph node to the adipose tissue surrounding other nodes, as has previously been found (Mattacks et al. 2002; Pond & Mattacks, 2002), but did not produce a systemic immune response involving fever. Even the higher dose of lipopolysaccharide is less than half that required to generate measurable hyperthermia, and 1–10% of that used to generate fever in adult rats (Rosenthal et al. 1996).
At the time this experiment was terminated, all popliteal adipocytes of the rats on the high-dose regime were smaller than the corresponding samples from the other two groups, with those from the stimulated leg consistently smaller than those from the unstimulated leg (Fig. 1). Factors contributing to lowering the average adipocyte volume could include the maturation (and/or formation) of pre-adipocytes into small mature adipocytes, and the depletion of large adipocytes as their lipid is released by lipolysis and taken up by the newly formed adipocytes and/or by macrophages, dendritic cells and other lymphoid cells. The enlargement of the popliteal depot around the stimulated node (Table 1), and its greater adipocyte complement (Fig. 2), indicate that 6 weeks of chronic inflammation has induced the formation of additional mature adipocytes, as predicted by our hypothesis for the origin of HARS (Pond, 2003a). The only known mechanism for adipocyte hyperplasia (Fig. 2) is proliferation of pre-adipocytes followed by their maturation and enlargement to become recognizable adipocytes (Amri et al. 1994; Schling & Löffler, 2002). Vascularization of perinodal adipose tissue increases slowly during chronic inflammation (MacQueen et al. 1999). In adipose tissue, increased blood perfusion is strongly associated with hyperplasia, though not with adipocyte hypertrophy (Crandall et al. 1997; Schling & Löffler, 2002).
The largest differences between the adipocyte volumes of stimulated and unstimulated popliteal depots were found for the middle and remote samples (Fig. 1). This effect is consistent with the finding (Pond & Mattacks, 2002) that only perinodal samples are activated by inflammation of a remote node, but at least in the popliteal, the entire adipose depot responds to local stimulation of a contiguous lymph node. Pond & Mattacks (2002) detected changes in rates of lipolysis and sensitivity to exogenous cytokines within a few hours of the onset of low-level immune stimulation. The differences in outcome between the low-dose and high-dose regimes indicate that weeks of exposure to relatively strong, but still not acute, immune disturbance is necessary for these metabolic effects to bring about changes in tissue structure. The similar but smaller changes in the cellular structure of the perirenal depot (Fig. 1A) suggest that secretions from chronically stimulated lymph nodes enter the general blood circulation and reach the nodeless depots. The mediators of this effect remain to be explored: possibilities include interleukins, particularly IL-6 (Coppack, 2001; Havlir et al. 2001), and adipocyte macrophage colony-stimulating factor (Levine et al. 1998).
Because the tissue was fractionated, the total lipid and protein contents of the fresh tissue are the sums of corresponding values for the adipocytes (Figs 3A and 4A) and stroma (Figs 3B and 4B). Calculated in this way, our results are similar to previous measurements from the retroperitoneal (perirenal) depot of ad libitum fed fully grown rats (Oliver et al. 2001) and guinea-pigs (Mattacks et al. 1987). The site-specific differences in lipid content of adipocytes in the saline-injected control rats were as expected from Fig. 1 and from previous measurements of the homologous depots of wild mammals and laboratory rodents (Mattacks et al. 1987; Pond et al. 1992, 1995). The differences between experimental groups in chemical composition of adipocytes (Figs 3A and 4A) and stroma (Figs 3B and 4B) are consistent with the conclusions reached from those of adipocyte volume (Fig. 1) and number (Fig. 2). The decrease in lipid content of adipocytes (Fig. 3A) following immune stimulation was expected, since activated lymph nodes stimulate lipolysis in adjacent adipocytes (Pond & Mattacks, 1998, 2002), and the cells have become smaller (Fig. 1), thus decreasing the proportion of triacylglycerols, and increasing that of the protein, in the whole tissue. Stromal lipid probably includes substantial amounts of phospholipids from membranes of lymphoid and vascular cells, and extracellular non-esterified fatty acids, as well as triacylglycerols. An increase in non-esterified fatty acids outside adipocytes would promote the maturation and enlargement of pre-adipocytes (Amri et al. 1994), thus increasing adipocyte complement (Fig. 2). The appearance of more lipid in the perinodal and middle samples of stroma (Fig. 3B) following immune stimulation is also consistent with the finding that lymph node lymphoid cells incorporate fatty acids derived from the surrounding adipocytes and with the hypothesis that the proliferation of inflamed lymphoid cells is provisioned by adjacent adipose tissue (Pond et al. 1995).
The changes in protein content of the adipocytes (Fig. 4A) are consistent with the reduced size of the cells, and with an increase in the abundance of cytokines, receptors and enzymes in mature cells that are interacting with the LPS-stimulated lymphoid cells. The protein content of the stroma also increases (Fig. 4B), suggesting that it acquires more vascular and/or lymphoid cells and/or more pre-adipocytes. The stroma of the nodeless perirenal depot contains less than half as much protein as the maximum measured from perinodal tissues around the experimentally stimulated lymph node, and differences between the three groups of rats were minimal, suggesting that lymphoid tissues form a large part of the protein measured in Fig. 4B.
The popliteal lymph nodes harbour numerous dendritic cells (Hill et al. 1990) that could permeate the adjacent adipose tissue, and popliteal adipocytes interact strongly with lymphoid cells (Pond & Mattacks, 1995). In contrast to the changes in the adipocytes, the increase in stromal protein is almost maximal following the lower dose of LPS. More protein-rich lymphoid cells may permeate the adipose tissue, and/or stem cells may divide to form more pre-adipocytes, before inflammation is severe enough to prompt the maturation of more adipocytes, thus enlarging the depot. These observations suggest that prolonged episodes of even mild inflammation may cumulatively facilitate adipose tissue growth. The calculations showing an increase in water content provide evidence for some local oedema, as suggested by clinical observation on chronically inflamed adipose tissue (Ryan, 1989, 1995) but this effect alone cannot explain the increased mass of adipose tissue surrounding the stimulated lymph node.
Implications for HIV-associated lipodystrophy
These findings are consistent with the perinodal adipose tissue hypothesis for the origin of the hypertrophy of node-containing depots in HARS (Pond, 2003a). The data in Figs 1 and 2 show that 6 weeks of chronic inflammation has induced the formation of more adipocytes, especially in the depot contiguous with the stimulated lymph node, and its consequent enlargement. An increase in extra-adipocyte lipids was observed following less immune stimulation than was necessary to induce the formation of additional adipocytes (Fig. 3B). Treating HIV infection with NRTI antiviral drugs would further increase lipids in the stroma: zidovudine, didanosine, lamivudine and stavudine added at therapeutic concentrations (0.1–1 µg mL−1) to co-incubations of adipose explants and lymphoid cells activated with LPS and concanavalin A stimulate adipocyte lipolysis over 48 h (Mattacks et al. 2003).
Certain polymorphisms of the promoter region of the TNFα gene predispose HIV-positive patients to develop lipodystrophy (Maher et al. 2002). TNFα is one the most potent mediators of lymphoid cell-controlled lipolysis (Mattacks & Pond, 1999), and its secretion is sustained over long periods of chronic inflammation (Rennert et al. 1996), so it may be among the mediators of the experimental effects described here, and HARS. The fact that the principal mechanism of selective hypertrophy of adipose depots containing lymphoid tissue seems to be adipocyte hyperplasia, rather than adipocyte enlargement, is consistent with the clinical observation that HIV-associated adipose redistribution fails to reverse, even if drugs are discontinued and/or the accompanying metabolic abnormalities are corrected (Hatano et al. 2000; Negredo et al. 2002). Mature adipocytes are eliminated extremely slowly (Prins & O’Rahilly, 1997), and those associated with lymphoid tissues respond only weakly to the endocrine conditions of fasting (Mattacks & Pond, 1999), so hyperplasia of perinodal adipocytes is likely to produce permanent expansion of the depot.
Implications for other aspects of the biology of adipose tissue
These findings suggest explanations for the wide variation between conspecific individuals in the relationship between the size and abundance of adipocytes that is found in both humans (Sjöström & Björntorp, 1974) and wild and domesticated animals (Pond & Mattacks, 1985). Exposure to chronic inflammation, especially early in life while the adipose tissue is forming, could stimulate adipocyte proliferation more strongly or for longer than normal. ‘Hyperplastic’ obesity in humans, in which more mature adipocytes appear, has long been recognized as more difficult to cure than ‘hypertrophic’ obesity in which existing cells enlarge (Strain et al. 1984; Schling & Löffler, 2002). Seasonal obesity in wild animals appears to be almost always hypertrophic and, although we find much variation between individuals, there is no consistent relationship between adipose tissue cellularity and age (Pond, 1992; Pond & Mattacks, 1998).
Adipocytes in node-containing depots that are controlled by cytokines do not respond as readily to the endocrine conditions of fasting as those in nodeless depots (Mattacks & Pond, 1999), so would be less easily depleted by slimming regimes. These properties suggest another mechanism for the slow accumulation of large adipose depots incorporating much lymphoid tissue, such as the intra-abdominal mesenteric and omental, that eventually leads to abdominal hypertrophy and high waist/hip ratios in human (Björntorp, 1996). Thick waists are common among people of average body mass who smoke heavily, which continually exposes them to toxins and irritants (Seidell et al. 1991), or who are frequently exposed to a wide variety of parasites and pathogens (Singh, 1993). The local hypertrophy of adipose tissue contiguous with chronically inflamed lymphoid tissue may also account for reports of an association between markers of viral infection and enlargement of certain adipose depots in domesticated birds, captive primates and people (Dhurandhar et al. 2000). Such ‘obesity’ may not involve the usual metabolic correlates of obesity and is in fact the slow selective hypertrophy of adipose depots that enclose lymphoid tissue (Dhurandhar et al. 1997, 2000, 2002).
Acknowledgments
This research was supported by a research grant to C.M.P. from Bristol-Myers Squibb (USA). Drs R. H. Colby and J. D. Priddle contributed helpful comments on the manuscript.
References
- Amri E-Z, Ailhaud G, Grimaldi P-A. Fatty acids as signal transducing molecules: involvement in the differentiation of preadipose to adipose cells. J. Lipid. Res. 1994;35:930–937. [PubMed] [Google Scholar]
- Björntorp P. The regulation of adipose-tissue distribution in humans. Int. J. Obesity. 1996;20:291–302. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Ann. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carr A. HIV protease inhibitor–related lipodystrophy syndrome. Clin. Infect. Dis. 2000;30:S135–S142. doi: 10.1086/313854. [DOI] [PubMed] [Google Scholar]
- Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc. Nutr. Soc. 2001;60:349–356. doi: 10.1079/pns2001110. [DOI] [PubMed] [Google Scholar]
- Crandall DL, Hausman GJ, Kral JG. A review of the microcirculation of adipose tissue: Anatomic, metabolic and angiogenic perspectives. Microcirculation. 1997;4:211–232. doi: 10.3109/10739689709146786. [DOI] [PubMed] [Google Scholar]
- Dhurandhar NV, Kulkarni PR, Ajinkya SM, Sherikar AA, Atkinson RL. Association of adenovirus infection with human obesity. Obes. Res. 1997;5:464–469. doi: 10.1002/j.1550-8528.1997.tb00672.x. [DOI] [PubMed] [Google Scholar]
- Dhurandhar NV, Israel BA, Kolesar JM, Mayhew GF, Cook ME, Atkinson RL. Increased adiposity in animals due to a human virus. Int. J. Obesity. 2000;24:989–996. doi: 10.1038/sj.ijo.0801319. [DOI] [PubMed] [Google Scholar]
- Dhurandhar NV, Whigham LD, Abbott DH, Schultz-Darken NJ, Israel BA, Bradley SM, et al. Human adenovirus Ad-36 promotes weight gain in male rhesus and marmoset monkeys. J. Nutr. 2002;132:3155–3160. doi: 10.1093/jn/131.10.3155. [DOI] [PubMed] [Google Scholar]
- Goldrick RB. Morphological changes in the adipocyte during fat deposition and mobilization. Am. J. Physiol. 1967;212:777–782. doi: 10.1152/ajplegacy.1967.212.4.777. [DOI] [PubMed] [Google Scholar]
- Hatano H, Miller KD, Yoder CP, Yanovski JA, Sebring NG, Jones E, et al. Metabolic and anthropometric consequences of interruption of highly active antiretroviral therapy. AIDS. 2000;14:1935–1942. doi: 10.1097/00002030-200009080-00008. [DOI] [PubMed] [Google Scholar]
- Havlir DV, Torriani FJ, Schrier RD, Huang JY, Lederman MM, Chervenak KA, et al. Serum interleukin-6 (IL-6), IL-10, tumor necrosis factor (TNF) α, soluble type II TNF receptor, and transforming growth factor beta levels in human immunodeficiency virus type 1-infected individuals with Mycobacterium avium complex disease. J. Clin. Microbiol. 2001;39:298–303. doi: 10.1128/JCM.39.1.298-303.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S, Edwards AJ, Kimber I, Knight SC. Systemic migration of dendritic cells during contact sensitization. Immunology. 1990;71:277–281. [PMC free article] [PubMed] [Google Scholar]
- John M, Nolan D, Mallal S. Antiretroviral therapy and the lipodystrophy syndrome. Antivir. Therap. 2001;6:9–20. doi: 10.1177/135965350100600102. [DOI] [PubMed] [Google Scholar]
- Kaur A, Rosenzweig M, Johnson RP. Immunological memory and acquired immunodeficiency syndrome pathogenesis. Phil. Trans. R. Soc. Lond. B. 2000;355:381–390. doi: 10.1098/rstb.2000.0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JA, Jensen MD, Eberhardt NL, O'Brien T. Adipocyte macrophage colony-stimulating factor is a mediator of adipose tissue growth. J. Clin. Invest. 1998;101:1557–1564. doi: 10.1172/JCI2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen HA, Pond CM. Immunofluorescent localisation of tumour necrosis factor-α receptors on the popliteal lymph node and the surrounding adipose tissue following a simulated immune challenge. J. Anat. 1998;192:223–231. doi: 10.1046/j.1469-7580.1998.19220223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen HA, Waights V, Pond CM. Vascularisation in adipose depots surrounding immune-stimulated lymph nodes. J. Anat. 1999;194:33–38. doi: 10.1046/j.1469-7580.1999.19410033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madge S, Kinloch-de-Loes S, Mercey D, Johnson MA, Weller IVD. Lipodystrophy in patients naive to HIV protease inhibitors. AIDS. 1999;13:735–737. doi: 10.1097/00002030-199904160-00020. [DOI] [PubMed] [Google Scholar]
- Maher B, Alfirevic A, Vilar FJ, Wilkins EGL, Park BK, Pirmohamed M. TNF-α promoter region gene polymorphisms in HIV-positive patients with lipodystrophy. AIDS. 2002;16:2013–2018. doi: 10.1097/00002030-200210180-00005. [DOI] [PubMed] [Google Scholar]
- Mattacks CA, Sadler D, Pond CM. The effects of exercise on the activities of hexokinase and phosphofructokinase in superficial, intra-abdominal and intermuscular adipose tissue of guinea-pigs. Comp. Biochem. Physiol. 1987;87B:533–542. doi: 10.1016/0305-0491(87)90049-6. [DOI] [PubMed] [Google Scholar]
- Mattacks CA, Pond CM. Interactions of noradrenalin and tumour necrosis factor-α, interleukin-4 and interleukin-6 in the control of lipolysis from adipocytes around lymph nodes. Cytokine. 1999;11:334–346. doi: 10.1006/cyto.1998.0442. [DOI] [PubMed] [Google Scholar]
- Mattacks CA, Sadler D, Pond CM. The effects of dietary lipids on adrenergically stimulated lipolysis in perinodal adipose tissue following prolonged activation of a single lymph node. Br. J. Nutr. 2002;87:375–382. doi: 10.1079/BJNBJN2002557. [DOI] [PubMed] [Google Scholar]
- Mattacks CA, Sadler D, Pond CM. Site-specific differences in the action of NRTI drugs on adipose tissue incubated in vitro with lymphoid cells, and their interaction with dietary lipids. Comp. Biochem. Physiol. 2003;135C:11–29. doi: 10.1016/s1532-0456(03)00024-3. [DOI] [PubMed] [Google Scholar]
- Negredo E, Ribalta J, Paredes R, Ferre R, Sirera G, Ruiz L, et al. Reversal of atherogenic lipoprotein profile in HIV-1 infected patients with lipodystrophy after replacing protease inhibitors by nevirapine. AIDS. 2002;16:1383–1389. doi: 10.1097/00002030-200207050-00010. [DOI] [PubMed] [Google Scholar]
- Oliver P, Picó C, Palou A. Ontogenesis of leptin expression in different adipose tissue depots in the rat. Pflügers Arch. 2001;442:383–390. doi: 10.1007/s004240100540. [DOI] [PubMed] [Google Scholar]
- Orenstein JM, Feinberg M, Yoder C, Schrager L, Mican JM, Schwartzentruber DJ, et al. Lymph node architecture preceding and following 6 months of potent antiviral therapy: follicular hyperplasia persists in parallel with p24 antigen restoration after involution and CD4 cell depletion in an AIDS patient. AIDS. 1999;13:2219–2229. doi: 10.1097/00002030-199911120-00004. [DOI] [PubMed] [Google Scholar]
- Pond CM, Mattacks CA, Sadler D. The effects of food restriction and exercise on site-specific differences in adipocyte volume and adipose tissue cellularity. I. Superficial and intra-abdominal sites. Br. J. Nutr. 1984;51:415–424. doi: 10.1079/bjn19840047. [DOI] [PubMed] [Google Scholar]
- Pond CM, Mattacks CA. Body mass and natural diet as determinants of the number and volume of adipocytes in eutherian mammals. J. Morph. 1985;185:183–193. doi: 10.1002/jmor.1051850204. [DOI] [PubMed] [Google Scholar]
- Pond CM, Mattacks CA, Thompson MC, Sadler D. The effects of age, dietary restriction, exercise and maternity on the abundance and volume of adipocytes in twelve adipose depots of adult guinea-pigs. Br. J. Nutr. 1986;56:29–48. doi: 10.1079/bjn19860083. [DOI] [PubMed] [Google Scholar]
- Pond CM. An evolutionary and functional view of mammalian adipose tissue. Proc. Nutr. Soc. 1992;51:367–377. doi: 10.1079/pns19920050. [DOI] [PubMed] [Google Scholar]
- Pond CM, Mattacks CA, Colby RH, Ramsay MA. The anatomy, chemical composition and metabolism of adipose tissue in wild polar bears (Ursus maritimus) Can. J. Zool. 1992;70:326–341. [Google Scholar]
- Pond CM, Mattacks CA, Prestrud P. Variability in the distribution and composition of adipose tissue in arctic foxes (Alopex lagopus) on Svalbard. J. Zool., Lond. 1995;236:593–610. [Google Scholar]
- Pond CM, Mattacks CA. Interactions between adipose tissue around lymph nodes and lymphoid cells in vitro. J. Lipid. Res. 1995;36:2219–2231. [PubMed] [Google Scholar]
- Pond CM, Mattacks CA. In vivo evidence for the involvement of the adipose tissue surrounding lymph nodes in immune responses. Immunol. Letts. 1998;63:159–167. doi: 10.1016/s0165-2478(98)00074-1. [DOI] [PubMed] [Google Scholar]
- Pond CM. Physiological specialisation of adipose tissue. Progr. Lipid. Res. 1999;38:225–248. doi: 10.1016/s0163-7827(99)00003-x. [DOI] [PubMed] [Google Scholar]
- Pond CM, Mattacks CA. The activation of adipose tissue associated with lymph nodes during the early stages of an immune response. Cytokine. 2002;17:131–139. doi: 10.1006/cyto.2001.0999. [DOI] [PubMed] [Google Scholar]
- Pond CM. Paracrine relationships between adipose and lymphoid tissues: implications for the mechanism of HIV–associated adipose redistribution syndrome. Trends Immunol. 2003a;24:13–18. doi: 10.1016/s1471-4906(02)00004-2. [DOI] [PubMed] [Google Scholar]
- Pond CM. Paracrine interactions of mammalian adipose tissue. J. Exp. Zool. 2003b;295A:99–110. doi: 10.1002/jez.a.10215. [DOI] [PubMed] [Google Scholar]
- Pond CM, Mattacks CA. The source of fatty acids incorporated into proliferating lymphoid cells in immune-stimulated lymph nodes. Br. J. Nutr. 2003;89:375–382. doi: 10.1079/BJN2002784. [DOI] [PubMed] [Google Scholar]
- Prins JB, O'Rahilly S. Regulation of adipose cell number in man. Clin. Sci. 1997;92:3–11. doi: 10.1042/cs0920003. [DOI] [PubMed] [Google Scholar]
- Rennert PD, Browning JL, Mebius R, Mackay F, Hochman PS. Surface lymphotoxin α/β complex is required for the development of peripheral lymphoid organs. J. Exp. Med. 1996;184:1999–2006. doi: 10.1084/jem.184.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal M, Roth J, Störr B, Zeisberger E. Fever response in lean (Fa/-) and obese (fa/fa) Zucker rats and its lack to repeated injections of LPS. Physiol. Behav. 1996;59:787–793. doi: 10.1016/0031-9384(95)02158-2. [DOI] [PubMed] [Google Scholar]
- Ryan TJ. Panniculitis. Clin. Dermatol. 1989;7:120–148. doi: 10.1016/0738-081x(89)90047-3. [DOI] [PubMed] [Google Scholar]
- Ryan TJ. Lymphatics and adipose tissue. Clin. Dermatol. 1995;13:493–498. doi: 10.1016/0738-081x(95)00092-t. [DOI] [PubMed] [Google Scholar]
- Schling P, Löffler G. Cross talk between adipose tissue cells: Impact on pathophysiology. News Physiol. Sci. 2002;17:99–104. doi: 10.1152/nips.01349.2001. [DOI] [PubMed] [Google Scholar]
- Seidell J, Cigolini M, Deslypere J-P, Charzewska J, Ellsinger B-M, Cruz A. Body-fat distribution in relation to physical activity and smoking habits in 38-year-old European men. The European fat distribution study. Am. J. Epidemiol. 1991;133:257–265. doi: 10.1093/oxfordjournals.aje.a115870. [DOI] [PubMed] [Google Scholar]
- Shevitz A, Wanke CA, Falutz J, Kotler DP. Clinical perspectives on HIV–associated lipodystrophy syndrome: an update. AIDS. 2001;15:1917–1930. doi: 10.1097/00002030-200110190-00003. [DOI] [PubMed] [Google Scholar]
- Singh D. Adaptive significance of female physical attractiveness: the role of waist-to-hip ratio. J. Personal. Soc. Psychol. 1993;654:293–307. doi: 10.1037//0022-3514.65.2.293. [DOI] [PubMed] [Google Scholar]
- Sjöström L, Björntorp P. Body composition and adipose tissue cellularity in human obesity. Acta Med. Scand. 1974;195:201–211. doi: 10.1111/j.0954-6820.1974.tb08123.x. [DOI] [PubMed] [Google Scholar]
- Smith JB, Morris B. The response of the popliteal lymph node of the sheep to swine influenza virus. Aust. J. Exp. Biol. Medical. 1970;48:47–55. doi: 10.1038/icb.1970.5. [DOI] [PubMed] [Google Scholar]
- Strain GW, Strain JJ, Zumolf B, Knittle J. Do fat cell morphometrics predict weight loss maintenance? Int. J. Obesity. 1984;8:53–59. [PubMed] [Google Scholar]